Introduction
Cases of meningoencephalitis have increased in the zoonotic hyperendemia of sporotrichosis in the state of Rio de Janeiro, Brazil [1–2]. The gold standard for the diagnosis of sporotrichosis is the isolation of Sporothrix spp. from clinical specimens [3], not always possible from the cerebrospinal fluid (CSF) of these patients, since fungus recovery in this specimen is difficult in most cases [4]. This limitation led us to pursue a new approach on central nervous system (CNS) sporotrichosis diagnosis based on existing molecular methodologies for the detection of Sporothrix spp. in skin samples [5–9]. Kano and colleagues (2003) designed species-specific primers for polymerase chain reaction (PCR) based on Sporothrix schenckii sensu lato chitin synthase 1 (CHS1) gene sequence and applied it in skin biopsy paraffin block [5]. Hu and colleagues (2003) used a nested PCR in human clinical samples and samples from infected mice, with the 18S rRNA gene sequence as target [6]. The assay was successfully used to detect S. schenckii DNA from strains from different areas of the world [7]. However, Mendoza and colleagues [8] compared the previously described nested PCR with conventional diagnostic methods, and the molecular methodology presented lower efficacy. Liu and colleagues [9], using the primer pair S2-R2 targeting the CHS1 gene in the PCR of biopsy tissue, verified positive results in 25 out of 30 cases (83.3%). The nested PCR targeting the partial sequence of the 18S rRNA gene was the best choice in terms of sensitivity due to a low fungal burden in CSF [6–7].
Since the beginning of the hyperendemic sporotrichosis in 1998, patients with disseminated sporotrichosis followed up at the Instituto Nacional de Infectologia Evandro Chagas (INI), Fundação Oswaldo Cruz (Fiocruz), undergo a protocol with lumbar puncture because of the possible neurotropism of S. brasiliensis, the main involved species in this region. Thereby, our main purpose was to apply the nested PCR assay proposed by Hu and colleagues [6], slightly modified, for the diagnosis of CNS sporotrichosis.
Methods
Study site and samples
INI-Fiocruz is a national reference center for infectious diseases, located in Rio de Janeiro, Brazil. Samples of CSF from 5 patients with advanced AIDS and sporotrichosis, collected during a routine clinical investigation, were used in the analyses.
Ethical aspects
All patients were included in a cohort of a study approved by the institutional Research Ethics Committee of the INI-Fiocruz, Brazil, approval number 3.095.183, and the data were analyzed anonymously.
DNA extraction from clinical samples
Two hundred microliters of the CSF were used for DNA extraction using the QIAamp DNA mini kit (QIAGEN, Hilden, Germany), following all the manufacturer’s instructions. NanoDrop (Thermo Fisher Scientific, Waltham, Massachusetts, United States of America) was used to analyze the DNA concentration.
DNA extraction control in reaction
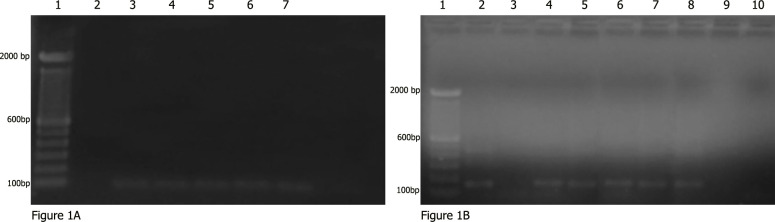
To validate the quality control for DNA extraction, the human β-globin gene was amplified in a separate PCR using the primers β-glob F (5’-GCAAGAAAGTGCTCGGTGC-3’) and β-glob R (5’-CACTCAGTGTGGCAAAGGTG-3’), according to a previous protocol (Fig 1A) [10]. S. brasiliensis DNA extracted from CBS120339 (former IPEC16490) strain was used in every batch of PCR as a positive control (Fig 1B). To avoid contamination, all steps of the preparation of PCR mixes were carried out in a laminar flow hood with aseptic techniques. In order to ensure no cross-reaction with fungi frequently found in cases involving CNS, Cryptococcus neoformans DNA was included as well (Fig 1B).
Fig 1.
PCR amplification products in agarose gel electrophoresis (A) Detection of human β-globin DNA in all 5 patients (3 to 7) by the control PCR. (1) Molecular marker DNA ladder, 100 bp (Invitrogen), (2) negative control (Blank), and (3–7) DNA of the patient’s CSF. (B) Detection of Sporothrix by nested PCR obtained for the DNA of the patient’s CSF. (1) Molecular marker DNA ladder, 100 bp (Invitrogen), (2) positive control (DNA of Sporothrix spp.), (3) negative control (Blank), (4–8) DNA of the patients CSF, (9) negative control (DNA Cryptococcus neoformans), and (10) negative control (Blank). CSF, cerebrospinal fluid.
Nested PCR
This reaction was performed according to the method described previously, to target the 18S rRNA gene [6], with slight modifications. Briefly, the reaction mixture of the first-round PCR consisted of 5 μl of DNA extract in a total volume of 50 μl, with final concentrations of 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 1.5 mM MgCl2, 10μM concentrations of primers SS1 (5´-CTCGTTCGGCACCTTACACG-3´) and SS2 (5´-CGCTGCCAAAGCAACGCGGG-3´), 1.5 U of Taq polymerase (Invitrogen, USA), and a 200 μM concentration of each deoxynucleotide triphosphate (Invitrogen, USA). The reaction mixture of the nested PCR was identical, except that 3 μl of the first reaction product and the inner primer pair SS3 (5´-ACTCACCAGGTCCAGACACGATG-3´) and SS4 (5´-CGCGGGCTATTTAGCAGGTTAAG-3´) were used. Briefly, the PCR reaction was 95°C for 5 min and 40 cycles of 1 min at 95°C, 1 min at 68°C, and 1 min at 72°C, followed by 10 min at 72°C. PCR products were loaded onto agarose 2% w/v gels for electrophoresis and the gels stained with 0.5 mg per l of ethidium bromide. The first round amplified a 305 bp fragment and the second one, a 152bp fragment. The digital images were captured, and each experiment was repeated at least 3 times to ensure reproducibility.
Results
Four patients were men, and one was woman, with ages from 25 to 44 years. All but one had neurological symptoms. All had CSF inflammatory parameters compatible with chronic meningoencephalitis, with no mass lesions. The cluster of differentiation 4 (CD4)+ T-cell count ranged from 11 mm3 to 302 mm3. Lumbar punctures were performed for all patients, with negative cultures in 4 cases for bacteria and fungi, and positive culture for Sporothrix spp. in only one case. Applying the mentioned nested PCR technique with the mentioned adjusts, we were able to detect the 152bp fragment from CSF of all the 5 patients tested, suggesting the presence of Sporothrix sensu lato, as previously described [6]. Negative control showed no amplification as well as positive controls for C. neoformans. In just one case, there was the isolation of Sporothrix sensu lato. No other agents were detected from the routine microbiological and immunological investigation.
Discussion
CNS sporotrichosis is a challenge and is associated with a worsening of prognosis due to the difficult CSF sterilization [2]. Thus, it is pivotal a faster and more effective method for recognizing the fungus dissemination than mycological culture.
We are presenting an efficient approach for direct detection of Sporothrix DNA in specimens from sporotrichosis patients (Table 1). The paucity of pathogens, due to a low fungal burden in cases of CNS sporotrichosis, probably contributes to negative culture [4]. The nested PCR assay employed in our study provides a highly specific method to detect the Sporothrix sensu lato in CSF. It is important to highlight that the nested amplification of the 18S rRNA gene fragment can detect all Sporothrix species of the Sporothrix complex. Thus, our clinical sample had the presence of a Sporothrix sensu lato. The definition of the species depends on the development of new molecular strategies, which may be the aim for further studies.
Table 1. Advantages and disadvantages.
| Advantages | Disadvantages |
|---|---|
| Fast diagnosis of sporotrichosis cases affecting CSF | Need laboratory structure for development of molecular technique |
| Early treatment start | Expensive DNA extraction kit |
| Avoid inappropriate treatment | Does not allow characterization at species level of the pathogen |
| High sensitivity and specificity to detect CSF sporotrichosis cases | Requires technical accuracy to perform the reaction |
CSF, cerebrospinal fluid
This approach for a known technique is innovative and has the benefit to improve diagnosis and early treatment in patients with meningoencephalitis due to Sporothrix sensu lato.
Funding Statement
RMZ-O was supported in part by Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (http://www.cnpq.br/) (304976/2013-0) and by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro - FAPERJ (E-26/103.157/2011). MMEO was supported in part by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro - FAPERJ (http://www.faperj.br/) - Grants: INST E-26/010.001784/2016; JCNE E-26/203.301/2017. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Freitas DF, do Valle AC, da Silva MBT, Campos DP, Lyra MR, de Souza RV, et al. Sporotrichosis: An Emerging Neglected Opportunistic Infection in HIV-Infected Patients in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2014; 8(8):3110 10.1371/journal.pntd.0003110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freitas DF, Lima MA, Almeida-Paes R, Lamas CC, do Valle AC, Oliveira MM, et al. Sporotrichosis in the central nervous system caused by Sporothrix brasiliensis. Clin Infect Dis. 2015; 61(4):663–664. 10.1093/cid/civ361 [DOI] [PubMed] [Google Scholar]
- 3.Zancopé-Oliveira RM, Almeida-Paes R, Oliveira MME, Freitas DFS, Gutierrez-Galhardo MC. New diagnostic applications in sporotrichosis In: Khopkar U, editor. Skin biopsy-perspectives. Rijeka: InTech Europe; 2011. p. 53–72. [Google Scholar]
- 4.Galhardo MC, Silva MT, Lima MA, Nunes EP, Schettini LE, de Freitas RF, et al. Sporothrix schenckii meningitis in AIDS during immune reconstitution syndrome. J Neurol Neurosurg Psychiatry. 2010; 81(6):696–699. 10.1136/jnnp.2009.173187 [DOI] [PubMed] [Google Scholar]
- 5.Kano R, Matsuoka A, Kashima M, Nakamura Y, Watanabe S, Mizoguchi M, et al. Detection of Sporothrix schenckii chitin synthase 1 (CHS1) gene in biopsy specimens from human patients with sporotrichosis. J Dermatol Sci. 2003; 33:73–74. 10.1016/s0923-1811(03)00153-1 [DOI] [PubMed] [Google Scholar]
- 6.Hu S, Chung WH, Hung SI, Ho HC, Wang ZW, Chen CH, et al. Detection of Sporothrix schenckii in clinical samples by a nested PCR assay. J Clin Microbiol. 2003; 41:1414–1418. 10.1128/jcm.41.4.1414-1418.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu TH, Lin JP, Gao XH, Wei H, Liao W, Chen HD. Identification of Sporothrix schenckii of various mtDNA types by nested PCR assay. Med Mycol. 2010; 48:161–165. 10.3109/13693780903117481 [DOI] [PubMed] [Google Scholar]
- 8.Mendoza M, Brito A, Schaper DA, Spooner VA, Alvarado P, Castro A, et al. Evaluación de la técnica PCR anidada para el diagnóstico de la esporotricosis experimental. Rev Iberoam Micol. 2012; 29:120–125. 10.1016/j.riam.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Zhang Z, Hou B, Wang D, Sun T, Li F, et al. Rapid identification of Sporothrix schenckii in biopsy tissue by PCR. J Eur Acad Dermatol Venereol. 2013; 27(12):1491–1497. 10.1111/jdv.12030 [DOI] [PubMed] [Google Scholar]
- 10.Eschevarria-Lima J, Rumjanek VM, Kyler-Cezar F, Harab RC, Leite ACCB, et al. HTLV-I alters the multidrug resistance associated protein 1 (ABCC1/MRP1) expression and activity in human T cells. J Neuroim. 2007; 185:175–181 [DOI] [PubMed] [Google Scholar]