Abstract
We conducted a seroprevalence study of a large ongoing outbreak of human adenovirus type 55 (HAdV-55) among the military in South Korea. Serum samples were collected between 2018 and 2019 from military-exposed (military group) and non-exposed (non-military group) populations. The plaque reduction neutralization test (PRNT) was used to assess neutralization activity against HAdV-55. A total of 100 sera was collected from the non-military group, of which 18.8% showed HAdV-55 neutralizing antibody activity. Ninety-six sera were tested from the military group, which had significantly higher prevalence of neutralizing antibodies (56.0%, P <0.001). A significantly higher proportion of the military group had PRNT titers ≥1:1,000 than the non-military group (85.7% vs. 50.0%, P = 0.004). Among the military group, 48.9% of active-duty soldiers had PRNT titers ≥1:5,000, while none of the discharged civilians did (P = 0.007). In conclusion, Koreans were exposed to HAdV-55 in their communities, but the exposure risk was higher among people in military service.
Introduction
Human adenovirus type 55 (HAdV-55) is an emerging recombinant HAdV strain of types 11 and 14, causing a potentially fatal acute respiratory illness (ARI) in adult populations [1, 2]. Since the first Chinese outbreak of HAdV-55 in 2006 [3], sporadic severe cases and multiple outbreaks in civilian and military communities have been reported in China [1–5]. A Chinese seroprevalence study evaluating healthy blood donors in Guangzhou in 2015 reported seropositive rates for HAdV-55 at 22.4% with gradual increases associated with age, suggesting that HAdV-55 became a strain circulating among the Chinese community [6].
In South Korea, HAdV-55 has been detected among severe pneumonia patients in the military since 2012 [7–9]. In the winter of 2014, HAdV-55 caused a large ongoing outbreak within the military and is currently considered a major pathogen of severe pneumonia among military personnel [10, 11]. However, a molecular typing study of respiratory HAdV in the Korean civilian community has not been conducted since 2010 [12], and the current epidemiologic status of HAdV-55 among Koreans who have been discharged from military service is unknown. In May 2017, the first HAdV-55 outbreak among the Korean civilian community was reported from a high school, emphasizing the need for an epidemiologic investigation of HAdV-55 in Korea [13]. Thus, we investigated seroprevalence and distribution of neutralizing antibodies (nAb) against HAdV-55 and HAdV-4 among healthy civilian volunteers and military personnel in various settings.
Methods
Study population and serum samples
To evaluate HAdV-55 seroprevalence in Korean society, we collected serum samples from four distinct populations: 1) healthy civilian volunteers, including male citizens discharged from obligatory military service; 2) new military recruits collected during the entrance medical examination; 3) active-duty soldiers admitted to a military hospital for ARI caused by respiratory viruses other than HAdV; and 4) active-duty soldiers after one year of service, collected during routine medical examinations.
In South Korea, male citizens are conscripted in their early 20s and serve for approximately two years. Because HAdV-55 was first detected in 2012 and then another military outbreak, which remains ongoing, followed two years later [8–10], individuals who entered military service after 2012 are more likely to have nAb against HAdV-55. To evaluate seroprevalence according to military exposure status, we categorized populations into those who had been exposed to the military environment since 2012 (military group) and those who had not (non-military group). Grouping details are presented in Table 1. This study was approved the Institutional Review Boards of the Armed Forces Medical Command (AFMC-18004-IRB-18-005) and Yonsei University (1041849-201808-BM-084-02). Written informed consent was obtained from each volunteer and patient.
Table 1. Study population characteristics according to military status.
| Category | Study population, number | Serum collection date | Age, median (IQR) | Notes |
|---|---|---|---|---|
| Non-military group | New recruits, before initiation of military training, n = 24 | July 2018 | 20 (20–22) | Serum samples were collected on the 2nd day of military training. |
| Healthy male civilian volunteers, without military service experience after 2012, n = 13 | March-May 2019 | 22 (21–25) | Includes male citizens who were not yet conscripted, were waived for service, and who completed service before 2012. | |
| Healthy female civilian volunteers, n = 59 | March-May 2019 | 21 (20–23) | Female citizens are not mandatorily conscripted for military service, and none of volunteers had served in the military as occupational soldiers. | |
| Military group | Soldiers admitted to a military hospital for ARI, excluding HAdV infections, n = 31 | March 2018-January 2019 | 21 (20–21) | All patients were tested with a multiplex respiratory virus panel and those infected with HAdV were excluded. |
| Soldiers after a year of service, n = 50 | May 2019 | 21 (21–22) | Serum samples were collected during routine medical examinations. | |
| Healthy male civilian volunteers, with military service experience after 2012, n = 19 | March-May 2019 | 24 (23–25) | All completed their service after 2012. |
Abbreviations: IQR, interquartile range.
Laboratory procedures
The plaque reduction neutralization test (PRNT) was used to assess neutralization activity against HAdV-55 in the collected sera. All samples were aliquoted and frozen at –80°C after collection. Before the neutralizing assays, the serum samples were heat inactivated at 56°C for 30 min, and then stored at 4°C. Neutralization activity against HAdV-4, a community-circulating HAdV strain, was also evaluated as a control [12, 14]. We used a rabbit immunization model to establish PRNTs against HAdV-55 and HAdV-4 and confirmed the no cross-reactivity between HAdV-4 and HAdV-55 sera. HAdV-55 (NCCP43158) and HAdV-4 (NCCP43156) were distributed by the National Culture Collection for Pathogens in the Korean Centers for Disease Control and Prevention. A549 human lung carcinoma cells (ATCC, Manassas, VA, USA) were maintained at 37°C under 5% CO2 in Eagle's minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin. A549 cells were plated at a density of 7x105 cells per well in 6-well plates. HAdVs (100 plaque-forming units (PFU) per well) were incubated for 1 h at 37°C either alone or together with serial dilutions of human sera or rabbit antisera. The positive control were used 2-fold dilution of the rabbit sera beginning with a 1:20 dilution (1:80; 1:160; 1:320; 1:640 and 1:1280), the human sera were used 5 or 10-fold dilution beginning with a 1:100 dilution (1:100; 1:500; 1:1000; 1:5000 and 1:10000). Subsequently, each sample was added to the 6 well plates and incubated at 37°C for 5 hr. The supernatant was then aspirated and overlaid with 3 mL per well of MEM with 2% heat-inactivated fetal bovine serum containing 0.8% SeaPlaque agarose (Takara). Following a 5 day incubation at 37°C, virus plaques were counted following crystal violet staining. nAb titers were determined by calculating the reciprocal of the serum dilution that inhibited 50% of the HAdV plaque compared with the negative control. To assess nAb presence, all collected sera were tested from a 1:20 dilution with a log2 dilution series, and those with a titer ≥1:80 were considered positive. Sera positive for HAdV-55 were titrated with 1:100, 1:500, 1:1000, 1:5000, and 1:10000 dilutions to assess the degree of neutralization activity.
Statistical analyses
To compare prevalences among the different groups, either the chi-square or Fisher’s exact test was used. All P-values were two-tailed, and P<0.05 was considered statistically significant. IBM SPSS Statistics version 20.0 (IBM, Armonk, NY, USA) was used for all statistical analyses.
Results
Seroprevalence of HAdV-55 and HAdV-4, according to military status
A total of 196 sera was collected, and 74 (37.8%) were positive for HAdV-55 and 116 (59.2%) were positive for HAdV-4 (Table 2). Eighteen sera from the non-military group (18.8%) were positive for HAdV-55, and the positivity rate was significantly higher in the military group (56.0%, P <0.001). However, HAdV-4 seropositivity rates were not different between the non-military and military groups (63.5% and 55.5%, P = 0.247) (Fig 1). Similarly, HAdV-55 seropositivity rates were not different between the HAdV-4 positive and negative sera (65.3% and 62.0%, P = 0.296) (S1 Fig). The HAdV seropositivity rates among the sub-populations of both groups were not statistically different.
Table 2. Seroprevalence of neutralization antibodies against HAdV-55 and HAdV-4 in Korean society according to recent military exposure.
| Non-military group | Military group | ||||
|---|---|---|---|---|---|
| Population | Positive/tested sera (%) | Population | Positive/tested sera (%) | ||
| HAdV-55 | HAdV-4 | HAdV-55 | HAdV-4 | ||
| New recruits, before training | 5/24 (20.8) | 14/24, (58.3) | ARI Soldiers, HAdV infection excluded | 15/31 (48.4) | 19/31 (61.3) |
| Male civilians, without recent military exposure | 2/13 (15.4) | 7/13 (53.8) | Soldiers, routine medical examination | 32/50 (64.0) | 25/50 (50.0) |
| Female civilians | 11/59 (18.6) | 40/59 (67.8) | Male civilians, military service after 2012 | 9/19 (47.4) | 11/19 (57.9) |
| Total | 18/96 (18.8) | 61/96 (63.5) | Total | 56/100 (56.0) | 55/100 (55.0) |
Fig 1. Seroprevalence of neutralization antibodies against HAdV-55 and HAdV-4 in Korean society according to recent military exposure.
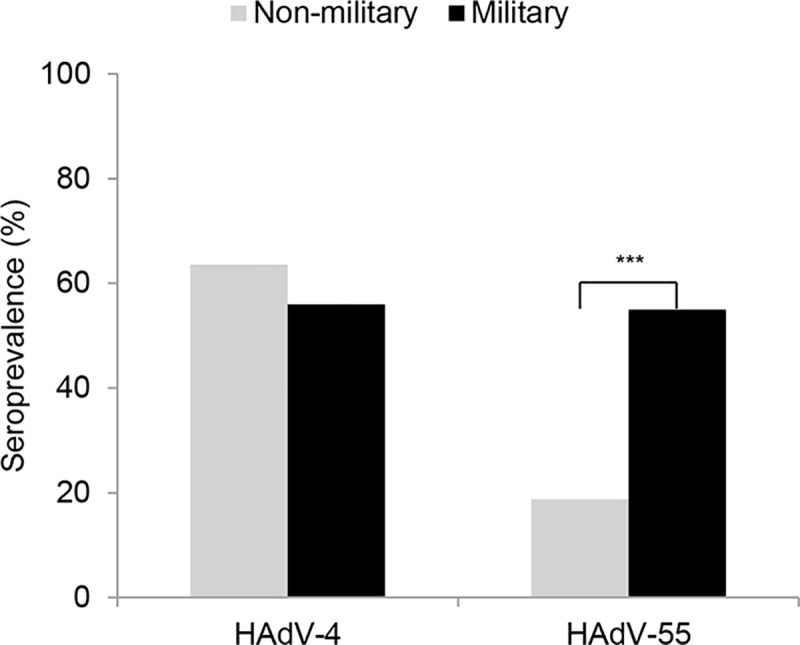
The data were analyzed with chi-square test. ***P<0.001.
PRNT titers against HAdV-55 among seropositive people
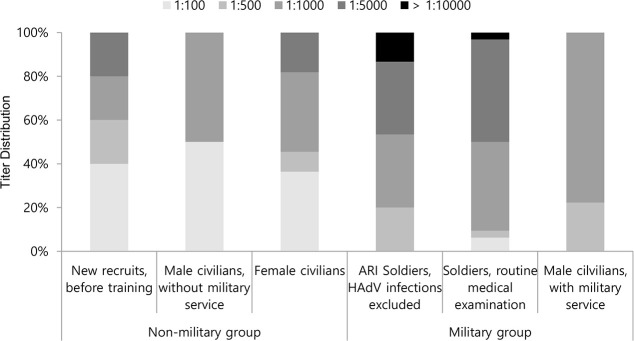
PRNT titration from 1:100 to 1:10,000 dilutions were conducted on the 74 HAdV-55-positive sera (Fig 2). Nine samples (12.2%) showed PRNT titers of 1:100, 8 (10.8%) showed titers of 1:500, 31 (41.9%) showed titers of 1:1000, 23 (31.1%) showed titers of 1:5000, and 3 (4.1%) showed titers ≥1:10000. The military group had a significantly higher proportion of PRNT titers ≥1:1000 (48 sera, 85.7%) compared with the non-military group (9 sera, 50.0%; P = 0.004). The distribution of PRNT titers in each sub-population is shown in Fig 3. Among the military group, 23 sera from active-duty soldiers (48.9%) showed PRNT titers ≥1:5000, while none of the discharged civilian sera had similar titers (P = 0.007). The distribution of PRNT titers was not different between the non-military subgroups. HAdV-55 titer distribution were not significantly different between the HAdV-4 positive and negative sera (S1 Fig).
Fig 2. Distribution of neutralizing antibody titer against HAdV-55 according to military status.
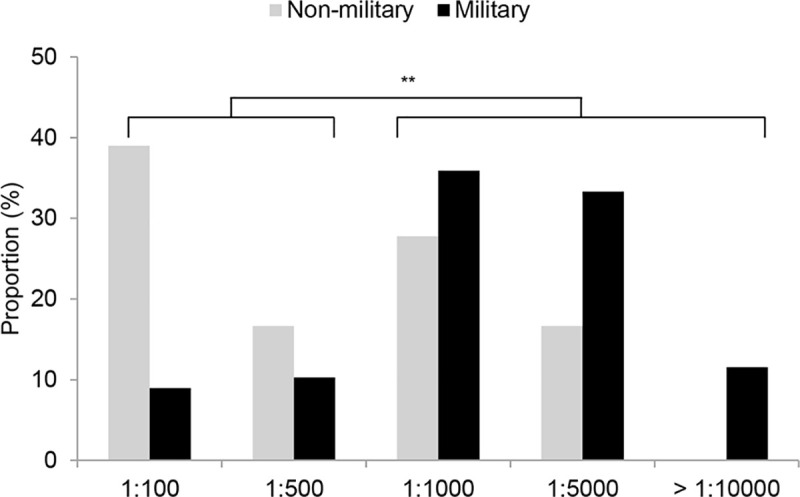
The data were analyzed with chi-square test. **P<0.01.
Fig 3. Titer distribution of neutralizing antibody against HAdV-55 according to population groups.
Discussion
Currently, the only epidemiologic investigation of HAdV-55 in a civilian community has been conducted in China, where several fatal HAdV-55 pneumonia cases have occurred among civilians [2, 6]. Several HAdV-55-associated ARIs have been reported in other countries, including France, Israel, and Singapore [15–17]. In South Korea, a military outbreak of HAdV-55 occurred in 2014 and remains ongoing [10, 11]. Because soldiers reside closely in barracks and contact with people outside the platoon is limited, the HAdV-55 outbreak was distinctly observed within the military population and it had a distinct epidemiologic pattern from that in the civilian community [11]. However, as approximately 300,000 Korean male citizens either enter the military or return to civilian society every year, transmission of HAdV-55 between the Korean military and civilian society is a great concern.
Seroprevalence of anti-HAdV-55 antibodies among the non-military exposed population was 18.8%. Although the kinetics of anti-HAdV-55 nAb have not been evaluated, the presence of PRNT titers ranging from 1:100 to 1:5,000 in this young population with a median age of 21 years suggests recent and remote exposure during adolescence. Molecular typing of respiratory HAdVs in Korea has not been reported since Lee et al., which reported 0.1% HAdV-55 prevalence among HAdV-positive throat swab samples collected in 2010 [12]. Our seroprevalence data imply that HAdV-55 spread widely into the Korean community after 2010. This is the first evidence that HAdV-55 became a community-circulating respiratory HAdV strain imported from China, and our results also suggest that a seroprevalence study or molecular typing of respiratory HAdVs is needed in neighboring countries and at-risk communities, including US military troops living in South Korea.
HAdV-55 seroprevalence was significantly higher in the military group compared with the non-military group, while HAdV-4 seroprevalence was not different between the two groups. More serum samples from the military groups had PRNT titers ≥1:1000 compared with the non-military group and more samples of active-duty soldiers had titers ≥1:5000 compared with discharged civilians. This finding is consistent with a previous HAdV-55 outbreak report and suggests that a proportion of the soldiers who participated in this study experienced a HAdV-55 infection during their military service. High PRNT titers ≥1:1000 in healthy blood donors were also observed in the Chinese seroprevalence study [6], where the researchers also found that strong nAb responses were more frequently observed against HAdV-55 than HAdV-14 and about half of HAdV-55-positive sera had titers ≥1:1000. By titrating sera up to a 1:10000 dilution, our study additionally showed that patients with recent HAdV-55 infections yielded nAb titers over 1:5000, which suggests that high titration is required for an antibody kinetics study of HAdV-55-infected patients.
In this study, most sera were collected from young people in their early 20s and we did not evaluate seroprevalence according to a range of age groups. However, if we did, we could have more accurately compared the serologic status of the non-military population with the military population, among whom a large HAdV-55 outbreak persists. Although approximately 100 samples were collected from the military and non-military groups each, the sample sizes for each sub-population were limited. However, by collecting serum samples from various populations, including both male and female civilians, and newly recruited, active-duty, and discharged soldiers, we were able to compare the effect of military exposure in greater detail.
In conclusion, seroprevalence of the non-military exposed population was 18.8%, suggesting that HAdV-55 has become a circulating respiratory HAdV strain among the Korean civilian community. Seroprevalence in the military population was also significantly higher than in the non-exposed sub-populations, with notably high titers (>1:5,000). Further epidemiologic studies across different age groups and neighboring communities are needed.
Supporting information
The data were analyzed with chi-square test.
(TIF)
Acknowledgments
We deeply appreciate the civilian and military volunteers who readily donated their blood samples for this research.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by a grant (18172MFDS255) from Ministry of Food and Drug Safety in 2018.
References
- 1.Walsh MP, Seto J, Jones MS, Chodosh J, Xu W, Seto D. Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J Clin Microbiol. 2010;48(3):991–993. 10.1128/JCM.01694-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun B, He H, Wang Z, Qu J, Li X, Ban C, et al. Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: a prospective observational study. Crit Care. 2014;18(4):456 10.1186/s13054-014-0456-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Z, Zhang Y, Xu S, Yu P, Tian X, Wang L, et al. Outbreak of acute respiratory disease in China caused by B2 species of adenovirus type 11. J Clin Microbiol. 2009;47(3):697–703. 10.1128/JCM.01769-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao B, Huang GH, Pu ZH, Qu JX, Yu XM, Zhu Z, et al. Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia. Chest. 2014;145(1):79–86. 10.1378/chest.13-1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu QB, Tong YG, Wo Y, Wang HY, Liu EM, Gray GC, et al. Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in China, 2009–2012. Influenza Other Respir Viruses. 2014;8(3):302–308. 10.1111/irv.12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng X, Rong X, Feng Y, Sun X, Li L, Wang Q, et al. Seroprevalence of neutralizing antibodies against adenovirus type 14 and 55 in healthy adults in Southern China. Emerg Microbes Infect. 2017;6(6):e43 10.1038/emi.2017.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heo JY, Lee JE, Kim HK, Choe KW. Acute lower respiratory tract infections in soldiers, South Korea, April 2011-March 2012. Emerg Infect Dis. 2014;20(5):875–877. 10.3201/eid2005.131692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heo JY, Noh JY, Jeong HW, Choe KW, Song JY, Kim WJ et al. Molecular epidemiology of human adenovirus-associated febrile respiratory illness in soldiers, South Korea. Emerg Infect Dis. 2018;24(7):1221–1227. 10.3201/eid2407.171222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SJ, Kim K, Park SB, Hong DJ, Jhun BW. Outcomes of early administration of cidofovir in non-immunocompromised patients with severe adenovirus pneumonia. PLoS One. 2015;10(4):e0122642 10.1371/journal.pone.0122642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo H, Gu SH, Jung J, Song DH, Yoon C, Hong DJ et al. Febrile respiratory illness associated with human adenovirus type 55 in South Korea military, 2014–2016. Emerg Infect Dis. 2017;23(6):1016–20. 10.3201/eid2306.161848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko JH, Woo HT, Oh HS, Moon SM, Choi JY, Lim JU et al. Ongoing outbreak of human adenovirus-associated acute respiratory illness in the Republic of Korea military, 2013–2018. Korean J Intern Med. 2019092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee WJ, Jung HD, Cheong HM, Kim K. Molecular epidemiology of a post-influenza pandemic outbreak of acute respiratory infections in Korea caused by human adenovirus type 3. J Med Virol. 2015;87(1):10–17. 10.1002/jmv.23984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song J, Lee H, Cho E. Epidemiological investigation of the outbreak of acute respiratory infection caused by adenovirus type B55 in a physical education school in 2017. Infect Chemother. 2019;51(2):119–129. 10.3947/ic.2019.51.2.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye X, Xiao L, Zheng X, Wang J, Shu T, Feng Y, et al. Seroprevalence of neutralizing antibodies to human adenovirus type 4 and 7 in healthy populations from Southern China. Front Microbiol.2018;9:3040 10.3389/fmicb.2018.03040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafolie J, Mirand A, Salmona M, Lautrette A, Archimbaud C, Brebion A, et al. Severe pneumonia associated with adenovirus type 55 infection, France, 2014. Emerg Infect Dis. 2016;22(11):2012–2014. 10.3201/eid2211.160728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salama M, Amitai Z, Amir N, Gottesman-Yekutieli T, Sherbany H, Drori Y, et al. Outbreak of adenovirus type 55 infection in Israel. J Clin Virol. 2016;78:31–35. 10.1016/j.jcv.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 17.Kajon AE, Dickson LM, Metzgar D, Houng HS, Lee V, Tan BH. Outbreak of febrile respiratory illness associated with adenovirus 11a infection in a Singapore military training cAMP. J Clin Microbiol. 2010;48(4):1438–1441. 10.1128/JCM.01928-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The data were analyzed with chi-square test.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.