Abbreviations
- AGPG
actin gamma 1 pseudogene
- APC/C
anaphase‐promoting complex
- ESCC
esophageal squamous cell carcinoma
- lncRNA
long non‐coding RNA
- PFKFB3
6‐Phosphofructo‐2‐Kinase/Fructose‐2,6‐Biphosphatase 3
- siRNA
small interfering RNA
Over the years, thousands of long non‐coding RNAs (lncRNAs) have been identified to be exclusively expressed in specific cancer types and for their unique functions in tumorigenesis. This has led to an increasing interest in elucidating the vital roles [1] and underlying mechanism [2, 3] of such non‐coding genome in driving cancerous phenotypes. A number of studies have pinpointed the key functions of lncRNAs in diverse biological events including chromatin interactions, transcriptional regulation, RNA processing, mRNA stabilization, signal transduction, and metabolic regulation; highlighting their essential roles in both physiology and diseases such as cancer [4].
A classic hallmark of cancer is the reprogramming of glucose metabolism that occurs to redirect glycolytic intermediates toward the biosynthetic production of macromolecules needed for cancer progression [5, 6, 7]. The metabolic role of lncRNAs has been also discovered [8, 9], while the mechanistic details on how lncRNAs regulate metabolic processes remain to be investigated. Notably, a recent study conducted by Liu and colleagues [10] revealed a novel lncRNA, named as actin gamma 1 pseudogene (AGPG), as a key regulator of 6‐Phosphofructo‐2‐Kinase/Fructose‐2,6‐Biphosphatase 3 (PFKFB3), in driving glycolysis and cell cycle progression, and a biomarker in esophageal squamous cell carcinoma (ESCC; Figure 1).
FIGURE 1.
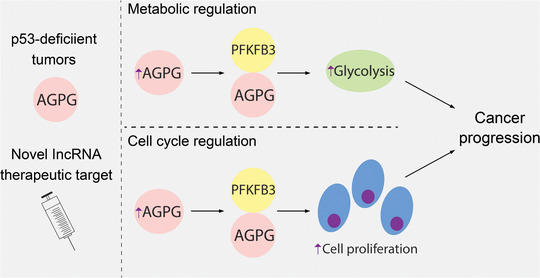
Illustration of the oncogenic role of lncRNA AGPG in driving p53‐deficient ESCC development. Loss of p53 promotes AGPG expression, which in turn forms a complex with PFKFB3 to induce glycolysis and cell cycle progression. This AGPG‐dependent oncogenic alteration contributes to the ESCC development and could be used as a therapeutic target for ESCC treatment
1. AGPG IS A NOVEL STIMULATOR OF GLYCOLYSIS AND CANCER DEVELOPMENT
In that study [10], the authors aimed to identify oncogenic lncRNAs in ESCC with a focus on their involvement in glucose metabolism. To achieve this, they conducted a small interfering RNA (siRNA) screening by taking cell viability and lactate production as readouts, where AGPG stood out as one of the top candidates. The authors further validated their findings by measuring the glycolytic flux and found that glycolysis was significantly diminished when AGPG was depleted. Additionally, the downregulation of AGPG inhibited cancer cell proliferation and cell cycle progression. Discovery of such a novel lncRNA adds to the developing body of literature, showing the importance of lncRNAs in metabolic regulation. However, a lack of understanding in the field is how specific can lncRNAs mechanistically modulate cancer metabolism. To address this, the authors further performed mass spectrometry analysis and successfully discovered PFKFB3 as a putative binding partner for AGPG.
2. AGPG ACTS THROUGH PFKFB3 INTERACTION
Through a variety of in vitro and in vivo experiments, the authors successfully elucidated the functional significance of AGPG and PFKFB3 binding in reprogramming glucose metabolism. So far, this is the first study to report the lncRNA binding partner of PFKFB3, making it possible to study PFKFB3 from a new perspective. Mechanistically, AGPG sterically blocks the association between anaphase‐promoting complex (APC/C) and PFKFB3, ultimately halting the ubiquitination and degradation of PFKFB3. As previously reported, PFKFB3 is an important target for cancer therapeutics because of its role in driving glycolysis and cell proliferation in cancer cells [11]. Therefore, such AGPG‐mediated PFKFB3 stabilization nicely explained how AGPG is involved in these two oncogenic processes. Notably, a previous study reported a lncRNA‐PFKFB2 complex in promoting metastasis through the alteration of glycolysis [12]. Together with current findings for the AGPG‐PFKFB3 complex, it would be interesting to see if a general lncRNA‐based regulation of the PFKFB family of enzymes could exist. Taken together, these data provide the first step for understanding the intricate mechanism of the fundamental concepts missing regarding the stabilization of PFKFB3 and its novel regulator, AGPG, in metabolic remodeling.
3. AGPG SERVES AS A NON‐CODING LINKER BETWEEN P53 AND GLUCOSE METABOLISM
To explore the upstream regulation of AGPG, the authors further discovered p53 as a putative transcription factor that negatively regulates AGPG expression. This intriguing finding not only connected AGPG with various cellular stresses signaling via p53, but also explained the oncogenic upregulation of AGPG in ESCC with p53 deficiency. Given the critical role of p53 in controlling glucose metabolism [13], the discovery of AGPG as a non‐coding transcript of p53 provides a novel insight into this process.
4. AGPG IS A BIOMARKER AND A THERAPEUTIC TARGET FOR ESCC
While metabolic reprogramming in cancer is vital for cancer initiation and progression, efficacious therapeutics are limited. Despite the numerous available therapies, ESCC is currently the foremost cause of cancer associated deaths, thus calling for a need to identify novel biomarkers [14]. Pathologically, the authors showed that the overexpression of AGPG was correlated with poor survival rates in ESCC patients. Notably, upon depletion of AGPG using an optimized lncRNA inhibitor, a dramatic decrease in ESCC patient‐derived xenograft tumor growth was observed. Therefore, identification of novel cancer‐associated lncRNAs, such as, AGPG, makes it an attractive biomarker and a therapeutic target in ESCC. With this important information, it would be also interesting to see AGPG exploited pharmaceutically into inhibitors for personalized cancer therapy.
DECLARATIONS
COMPETING INTERESTS
The authors declare that they have no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This work was supported in part by an NIH grant (GM126048) and a Research Scholar Grant (RSG‐18‐009‐01‐CCG) from the American Cancer Society to WW, and an NIH‐IMSD training grant (GM055246) to REV.
AUTHORS' CONTRIBUTIONS
REV and WW wrote the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
Not applicable.
Vargas RE, Wang W. Significance of long non‐coding RNA AGPG for the metabolism of esophageal cancer. Cancer Communications. 2020;40:313–315. 10.1002/cac2.12035
REFERENCES
- 1. Agostini M, Ganini C, Candi E, Melino G. The role of noncoding RNAs in epithelial cancer. Cell death discovery. 2020;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schmitt AM, Chang HY. Long Noncoding RNAs in Cancer Pathways. Cancer Cell. 2016;29(4):452‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lian Y, Yang J, Lian Y, Xiao C, Hu X, Xu H. DUXAP8, a pseudogene derived lncRNA, promotes growth of pancreatic carcinoma cells by epigenetically silencing CDKN1A and KLF2. Cancer Commun (Lond). 2018;38(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell metabolism. 2016;23(1):27‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Xia Y, Lu Z. Metabolic features of cancer cells. Cancer Commun (Lond). 2018;38(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang YP, Lei QY. Metabolic recoding of epigenetics in cancer. Cancer Commun (Lond). 2018;38(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shankaraiah RC, Veronese A, Sabbioni S, Negrini M. Non‐coding RNAs in the reprogramming of glucose metabolism in cancer. Cancer Letters. 2018;419:167‐74. [DOI] [PubMed] [Google Scholar]
- 9. Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu J, Liu ZX, Wu QN, Lu YX, Wong CW, Miao L, et al. Long noncoding RNA AGPG regulates PFKFB3‐mediated tumor glycolytic reprogramming. Nature communications. 2020;11(1):1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu L, Chen Y, Zhu Y. The molecular basis of targeting PFKFB3 as a therapeutic strategy against cancer. Oncotarget. 2017;8(37):62793‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao L, Ji G, Le X, Wang C, Xu L, Feng M, et al. Long Noncoding RNA LINC00092 Acts in Cancer‐Associated Fibroblasts to Drive Glycolysis and Progression of Ovarian Cancer. Cancer Research. 2017;77(6):1369‐82. [DOI] [PubMed] [Google Scholar]
- 13. Cheung EC, Vousden KH. The role of p53 in glucose metabolism. Current Opinion in Cell Biology. 2010;22(2):186‐91. [DOI] [PubMed] [Google Scholar]
- 14. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. New England Journal of Medicine. 2012;366(22):2074‐84. [DOI] [PubMed] [Google Scholar]


