Abstract
The pedunculopontine nucleus (PPN) is located in the mesopontine tegmentum and is best delimited by a group of large cholinergic neurons adjacent to the decussation of the superior cerebellar peduncle. This part of the brain, populated by many other neuronal groups, is a crossroads for many important functions. Good evidence relates the PPN to control of reflex reactions, sleep-wake cycles, posture and gait. However, the precise role of the PPN in all these functions has been controversial and there still are uncertainties in the functional anatomy and physiology of the nucleus. It is difficult to grasp the extent of the influence of the PPN, not only because of its varied functions and projections, but also because of the controversies arising from them. One controversy is its relationship to the mesencephalic locomotor region (MLR). In this regard, the PPN has become a new target for deep brain stimulation (DBS) for the treatment of parkinsonian gait disorders, including freezing of gait. This review is intended to indicate what is currently known, shed some light on the controversies that have arisen, and to provide a framework for future research.
Keywords: Arousal, basal ganglia, blink reflex, brainstem, calcium channels, CaMKII, cAMP, gait, gamma activity, N-type, P/Q-type, posture, prepulse inhibition, sleep, startle reflex
Introduction
The reticular formation of the brainstem is composed of a large number of mixed neuron groups with profuse connections and likely profound effects. One such group is the pedunculopontine nucleus (PPN) which has recently received renewed attention because it is now a target for deep brain stimulation (DBS) for the treatment of parkinsonian gait disorders. The attention has revealed a whole series of controversies. Exactly where is the PPN and how should it be defined? What are its connections? What are the other neuron groups in the same region? What are the functions of the different nuclei, and, specifically, can one of them be recognized as the mesencephalic locomotor region (MLR)? Does the PPN play more of a role in sleep control and arousal than locomotion? Does DBS of the PPN region actually help parklinsonian gait disorders, and, if it does, can knowledge of the anatomy and physiology help improve the therapy? This review attempts to deal with these questions.
1. Anatomy of the reticular formation and the PPN (Saper, Rye)
The pedunculopontine tegmental nucleus (PPN) was originally defined by Jacobsohn in human brains in 1906 as a cluster of large, darkly staining neurons adjacent to the decussation of the superior cerebellar peduncle, which had been previously described, but left unnamed, by Kölliker (Jacobsohn, 1909). Over the years, this same population of neurons was pointed out in other species, but little was known concerning its chemical composition, connections, or functions. In 1966, as the first paper in the first issue of Brain Research, Nauta and Mehler described the projections of the globus pallidus in rhesus monkey brains with Nauta’s silver degeneration method (Nauta et al., 1966). They found terminations in a region that included the middle portion of the superior cerebellar peduncle just caudal to its decussation. This was confirmed by Malcolm Carpenter and his colleagues 10 years later using anterograde transport of tritiated amino acids injected into the internal segment of the globus pallidus (Kim et al., 1976). Both papers equated this terminal field with the PPN, although neither showed Nissl photomicrographs that would have confirmed the presence of the characteristic large, darkly staining PPN neurons in this region.
The beginnings of chemical neuroanatomy took root around the same time, with the publication of papers showing the distribution of monoaminergic neurons using histofluorescence (Dahlstrom et al., 1964), and of the distribution of neurons staining with acetycholinesterase (AChE) histochemistry (Schute et al., 1967). The latter identified neurons along the lateral margin of the superior cerebellar peduncle in rats that were AChE positive, and which were called the “cuneiform nucleus.” Shortly afterward, however, the finding that many AChE-expressing neurons (such as the substantia nigra pars compacta) were not cholinergic, reduced enthusiasm for that approach.
It was not until the early 1980’s that antibodies against choline acetyltransferase (ChAT) were developed that could reliably define cholinergic neurons (Eckenstein et al., 1983; Levey et al., 1983). These stained the same cell group that Schute and Lewis had called the “cuneiform nucleus,” and for the first time recognized that these were, in fact, the same neurons that had been described as the PPN by Jacobsohn (Armstrong et al., 1983). Subsequent studies confirmed this localization in both monkeys (Mesulam et al., 1984), and humans (Mesulam et al., 1989; Manaye et al., 1999), and since that time, the cholinergic population in the PPN (although not the only cell type) has been used to define the borders of this nucleus.
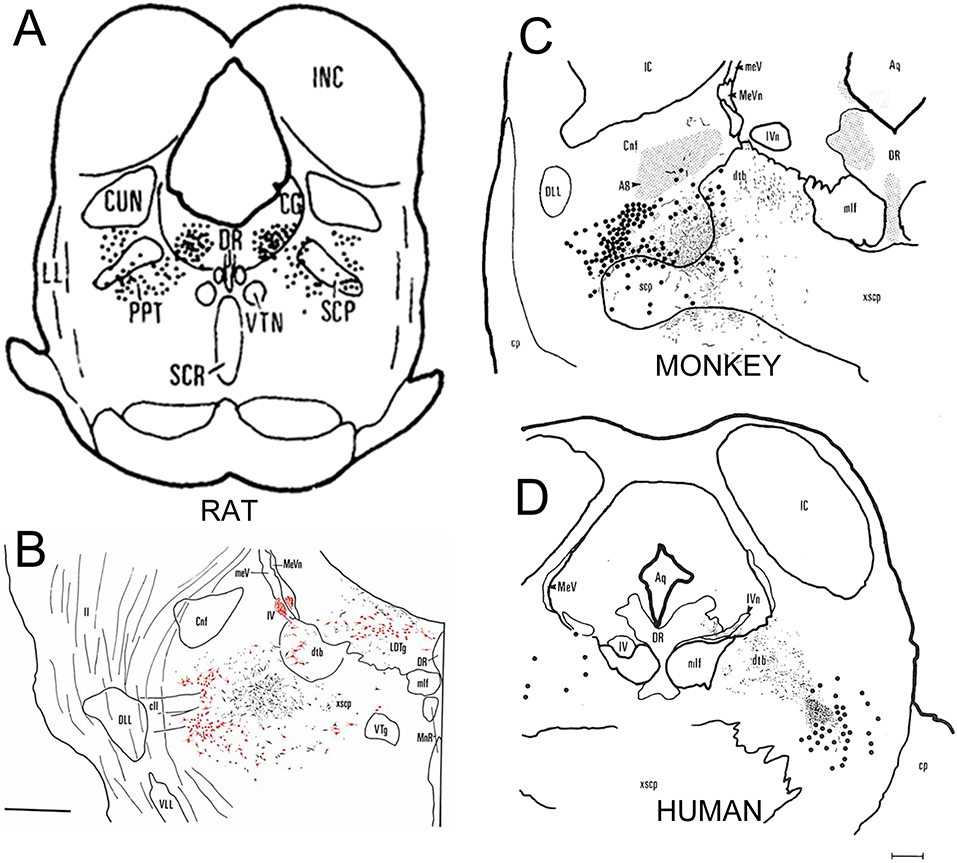
After the borders of the PPN were defined by chemical neuroanatomy, it became clear that the actual terminal field of the descending projection from the globus pallidus is medial to the borders of the PPN, in a region along the middle third of the superior cerebellar peduncle just before the decussation, exactly as Nauta and Mehler and Kim and Carpenter had described (Nauta et al., 1966, Kim et al., 1976). However, it did not correspond to the PPN. This region, just medial to the PPN was identified by Rye and colleagues as the “midbrain extrapyramidal area” or MEA, to distinguish it from the cholinergic neurons concentrated just lateral and caudal to it (Rye et al., 1987). This finding has been repeatedly confirmed both in rodents (e.g., Sherman et al., 2016), and in non-human primates (Rye et al., 1996, 1997). Rye and colleagues even demonstrated this projection in a human brain from a man who died five months after a pallidotomy for Parkinson’s disease, using silver degeneration (Rye et al., 1996). These authors confirmed that the pallidal terminal field in humans is located just medial to the PPN, in the middle portion of the superior cerebellar peduncle, while the PPN cholinergic neurons lie along the lateral margin of the peduncle (Figure 1).
Figure 1. A montage showing the relationship of the pedunculopontine tegmenal nucleus (PPN), as identified by cholinergic neurons, and the terminal zone for the descending pallidal projection.
In panels A,C, and D the cholinergic neurons, stained immunohistochemically for choline acetyltransferase (ChAT), are shown as large black dots; in B the ChAT-immunostaining is red. In B-D the descending pallidal terminals are shown as black stippling. Panel A, from (Armstrong et al., 1983) is the earliest drawing that identified the ChAT-immunoreactive neurons as the PPN (marked as PPT in panel A). A higher magnification drawing of the same area in B, shows that the ChAT-immunoreactive neurons are just lateral to descending terminals from the substantia nigra reticulate (equivalent to the internal segment of the globus pallidus in rodents (Albin et al., 1989)) in the rat (Rye et al., 1987). In panel C, the descending pallidal projection is shown to occupy an almost identical position, medial to but not overlapping the PPN, in a rhesus macaque monkey (Rye, 1997). Panel D shows a section from the brain of a man who died five months after receiving a pallidotomy for Parkinson’s disease (Rye et al., 1996). The pallidal terminals are labeled with the de Olmos silver degeneration method, and again are seen to be medial to the ChAT-immunoreactive PPN neurons. All images reproduced with permission.
PPN neurons have also been thought to mark the site of the midbrain locomotor region (MLR). The MLR was originally identified by Shik and Orlovsky as a region in which electrical stimulation in decorticate cats would elicit locomotion (Shik et al.,, 1976). Careful studies by Garcia-Rill found a region in the vicinity of the PPN that could produce locomotion with low threshold for electrical stimulation (Garcia-Rill et al., 1987). While these studies suggested that the MLR might be associated with the PPN, there remained several problems. First, when the projections of the cholinergic neurons in the PPN were assessed immunohistochemically, the bulk of the projection was upstream, to the thalamus, hypothalamus and basal forebrain, not descending. In addition, although some PPN projections did descend into the medulla, no cholinergic projections from the nucleus have been confirmed in the spinal cord (Rye et al., 1987, 1998; Yasui et al., 1990).
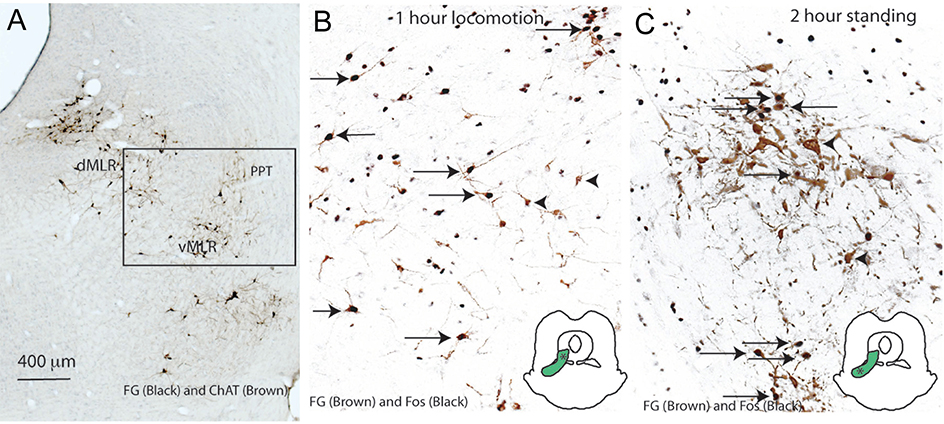
To help resolve these issues, Sherman and colleagues re-examined neurons in the region of the PPN that project directly to the spinal cord, and which might be involved in initiating locomotion (Sherman et al., 2016). They found a population of glutamatergic neurons (as demonstrated by expression of the vesicular glutamate 2 transporter, Vglut2), which project to the ventral horn of the spinal cord. These neurons are adjacent to, and project dendrites into, the MEA, and many of them express receptors either for the D2 dopamine receptor or for the peptide orexin (which is involved in both maintaining upright posture and in exploring the environment). They then examined cFos expression in neurons of that region in animals that were subjected either to forced locomotion or forced standing. They demonstrated that spinally-projecting neurons in the dorsal portion of this region differentially express cFos during forced locomotion, while neurons in the ventral part of the region show cFos expression during forced standing on their hindlegs. Chemical activation of these neurons by micro injection of a glutamate analog caused awake rats to ambulate like automatons, until they ran into a wall, at which point they would try to scale the wall, in an upright posture (Figure 2).
Figure 2. Just medial to the PPN is a population of glutamatergic neurons that project to the spinal cord and are activated during locomotion and standing.
In panel A, the PPN and LDT neurons are stained brown for ChAT, and spinally projecting neurons are immunostained black. In panels B and C, the spinally projecting neurons are labeled brown, and show cFos immunoreactivity (black) in their nucleus if they are activated during forced locomotion (panel B) or forces standing (panel C). Insets show that the locomotion-related neurons are more dorsal than those related to maintaining upright posture. From (Sherman et al., 2015), with permission.
If the MLR neurons are actually glutamatergic bulbospinal neurons medial to the PPN, which receive the bulk of the descending output of the internal pallidal segment, what do the PPN neurons do? An important clue may come from the dense innervation of the thalamus, including the relay nuclei and the reticular nucleus, as well as the intralaminar nuclei, by the PPN and the adjacent laterodorsal tegmental nucleus (which also contains similar cholinergic neurons) (Rye et al., 1987). In addition, early recordings by Sakai and their colleagues in cats had shown neurons in this region that fired specifically during wake or during rapid eye movement (REM) sleep (Sakai et al., 1991). Boucetta and colleagues recently reported juxtacellular recordings from neurons in the laterodorsal tegmentum (PPN-LDT region) in rats in which they had been able to characterize the physiology in awake (although head constrained) animals, across the wake-sleep cycle, and then to chemically characterize many of them. They found that cholinergic (vesicular acetylcholine transporter immunoreactive) neurons in the PPN-LDT were maximally active during both waking and REM sleep (periods of EEG desynchronization). Putative GABAergic neurons (glutamic acid decarboxylase immunoreactive) in the PPN-LDT reached maximal activity during REM sleep (although some were also active during waking). Some of the presumably glutamatergic neurons, containing neither of the other markers, and some containing Vglut2, fired maximally in waking, or in REM, or in both. Thus, the PPN neurons of all three known chemical classes appear to be either waking- or REM sleep-promoting. Follow-up studies in mice combining juxta-cellular recording with optogenetic stimulation of PPN cholinergic neurons showed that photoactivation of the latter shifted the EEG toward the theta range (characteristic of REM sleep) but did not cause changes in nuchal muscle tone (Cisse et al., 2018).
Recent chemogenetic experiments, done in freely behaving mice, have been able to stimulate selectively each of these cell types (Kroeger et al., 2017). These used adeno-associated viral vectors encoding the hM3Dq excitatory receptor in a Cre-dependent cassette, in Vglut2-IRES-Cre, Vgat-IRES-Cre, and Chat-IRES-Cre mice. When the glutamatergic neurons in the PPN were activated by the ligand, clozapine-N-oxide (CNO), animals showed about four hours of excessive wakefulness, and suppression of both NREM and REM sleep. Chemogenetic inhibition of the PPN glutamatergic neurons using the hM4Di vector caused a small decrease in waking. Stimulation of the cholinergic PPN neurons decreased the quantity of slow waves in the sleep EEG, without changing total waking, NREM or REM sleep time. Activation of the GABAergic neurons in the PPN caused a small decrease in REM sleep.
The early reports suggesting that the MLR was associated with the PPN have resulted in the PPN being used as a target for deep brain stimulation (DBS). The data reviewed here, however, suggests that the likely site for the MLR in a human brain is probably about 1–2 mm medial to the location of the PPN cholinergic neurons (still the defining signature of the nucleus), and possible 1–2 mm deeper. On the other hand, as will be discussed below, the state of the art in DBS stimulation electrodes is that they are typically about 1 mm wide, and stimulate an area about 1 mm on either side of the contact. In addition, there are usually three contacts, covering a total of 5 mm of depth, and the current and site of the contact is adjusted to produce the optimal clinical response. Thus, it is likely that a large region is stimulated including the more medial neurons. Even so, reports of motor responses, particularly gait improvement, using the current coordinates that target the PPN, are neither consistent nor encouraging (Thevathasan et al., 2018).
There have been many reports of the effects of PPN DBS on sleep. In some subjects, turning the stimulator on causes sleepiness or even rapid transition into sleep (Arnulf et al., 2010). In others, it increases the amount of REM sleep (Lim et al 2009). In fact, it has been suggested that the motor improvements in patients with PPN DBS may be secondary to improved sleep (Alessandro et al. 2010). Given that the stimulators are optimized for motor response, and that the goal of the stimulation is improved motor ability, not increased sleep, it seems likely that these sleep effects are due to the stimulation (not placebo). It might also be that using target coordinates for the PPN cholinergic neurons may bring the DBS electrode near the MLR, but not into it.
In our view, adjusting the target coordinates medially, ventrally, and slightly rostrally, may result in more robust, consistent, and favorable motor responses, without the unwanted sleep complications.
2. Arousal and motor functions of the PPN (Garcia-Rill)
In this section, we will address its role in sleep-wake control and its connections to posture and locomotion, especially their physiological activity.
2.1. Sleep-wake control
The PPN is made up of three of each kind. As noted in Section 1, it has three types of cells, cholinergic, glutamatergic, and GABAergic (Wang and Morales, 2009). It also has three in vitro electrophysiology types of cells, type I have T-type currents and the cells are non-cholinergic, type II cells have a-type currents and two thirds are cholinergic, and type III cells have both T- and a-type currents and one third are cholinergic (reviewed in Garcia-Rill et al., 2008). The region of the PPN, best visualized in sagittal sections, has three types of in vivo firing patterns in relation to sleep and waking, manifesting, (1) “REM on” cells that are active during REM sleep, (2) “Wake-REM on” cells that are active during both waking and REM sleep, and (3) “Wake on” cells that are active during waking (Sakai et al., 1990; Steriade, 1999; Kayama et al., 1992; Datta and Siwek, 2002; Datta et al., 2009; Boucetta et al., 2014). We will see below how there are also three cell types in terms of expression of N- and/or P/Q-type calcium channels. In keeping with the manifestation of threes in the PPN, we will address three of the most important discoveries in the field of sleep and waking control in the past ten years, the presence in the PPN of, (1) electrical coupling, (2) intrinsic gamma band activity, and (3) gene transcription in relation to waking.
2.1.1. Electrical coupling
Gap junctions are blocked by fast-acting anesthetics such as halothane and propofol (Evans and Boitano, 2001; He and Burt, 2000), suggesting that such anesthesia may rapidly disrupt the coherence in activity that electrical coupling provides. In addition, agents that block gap junctions have soporific effects, such as the antiulcer agent carbenoxolone and the antimalarial agent mefloquine (Rozental et al., 2001). Approximately 7–10% of PPN neurons are electrically coupled through gap junctions (Garcia-Rill et al., 2007, 2008). Modafinil is an atypical stimulant used for the treatment of narcolepsy and excessive daytime sleepiness (Ballon and Feifel, 2006). Modafinil was found to increase electrical coupling between GABAergic cortical interneurons, thalamic reticular neurons, and inferior olive neurons (Urbano et al., 2007). The theory for its mechanism of action is that by increasing electrical coupling, input resistance is decreased in GABAergic cells, thereby decreasing GABA release and thus disinhibiting the entire circuit. Modafinil decreased the resistance of GABAergic PPN cells, suggesting a mechanism for increasing, through disinhibition, firing rates, thus promoting arousal (Garcia-Rill et al., 2007, 2008). In summary, agents that block gap junctions down regulate coherence essential for waking while those that increase gap junctions may up regulate waking. The discovery of this novel mechanism for sleep-wake control provides a host of new therapeutic targets that could modulate gap junction function.
2.1.2. Intrinsic gamma band activity
The PPN is active during waking and REM sleep, the two states of high frequency (beta/gamma) EEG activity. Gamma band activity is present in the cat when the animal is active in both the cortical EEG and simultaneously in PPN neurons (Steriade et al., 1990). Gamma activity is also evident in the region of the PPN in humans during stepping, but not when they are at rest (Fraix et al., 2013). In the primate, PPN neurons fired at low frequencies ~10 Hz at rest, but increased firing to gamma frequencies upon waking or when the monkey walked on a treadmill (Goetz et al., 2016). Thus, the same cells were involved in both arousal and motor control in the PPN in vitro, in vivo, and across species, including man. But, what generates all of that gamma frequency activity?
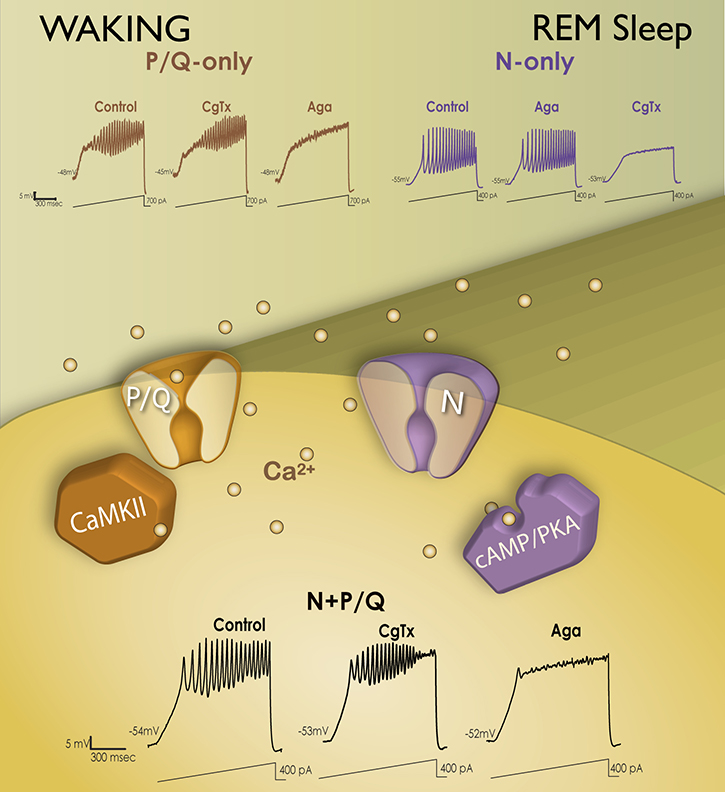
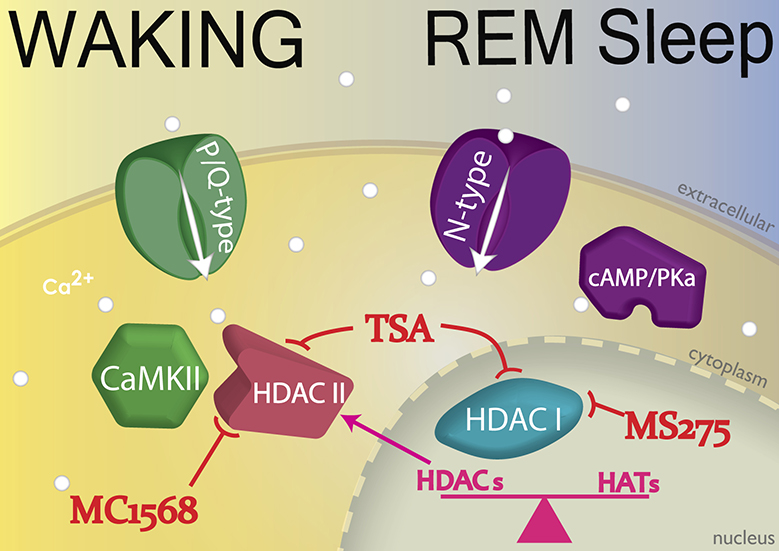
Garcia-Rill and colleagues discovered that, (1) every PPN neuron, regardless of transmitter type, fired maximally at beta/gamma frequencies (Simon et al., 2010), (2) all PPN neurons manifested gamma frequency intrinsic membrane oscillations (Urbano et al., 2012), making this the natural frequency for activating the PPN, accounting for the optimal frequencies of stimulation for inducing locomotion on a treadmill in animals (see below), and for PPN DBS in humans (Garcia-Rill et al., 2014a, 2018), and (3) these intrinsic oscillations were mediated by high threshold, voltage-dependent N- and/or P/Q-type calcium channels (Kezunovic et al., 2011). Calcium imaging revealed that, (4) these channels were distributed along the dendrites of PPN neurons (Hyde et al., 2013), and (5) some cells exhibited both N- and P/Q-type calcium channels, some had only N-type channels, and some had only P/Q-type channels (Luster et al., 2015, 2016). Thus these three populations expressing different calcium channel types actually represented neurons previously named “Wake-REM on” (active in relation to waking and REM sleep and thus expressed both N- and P/Q-type channels), “REM on” cells (active only during REM sleep and expressed only N-type channels), and “Wake-on” cells (active only during waking and expressed only P/Q-type channels) (Garcia-Rill et al., 2014b; Luster et al., 2015, 2016). Figure 3 shows how these PPN neurons were identified. If ramp-induced oscillations were not affected by the N-type channel blocker conotoxin but were blocked by agatoxin, then the cell was labeled a P/Q-only neuron (~25%). On the other hand, if ramp-induced oscillations were not affected by agatoxin but were completely blocked by conotoxin, the cell was labeled a N-only neuron (~25%). Finally, if conotoxin only partially reduced oscillations and agatoxin blocked the remainder of the oscillations, then the cell was labeled a N+P/Q neuron (~50%).
Figure 3. Differential modulation of waking vs REM sleep by two different calcium channel types.
Intracellularly, P/Q-type calcium (Ca2+) channels (brown channel) are regulated by CaMKII (brown sextagon), while N-type channels (purple channel) are regulated by cAMP/PKA (purple structure). If ramp-induced oscillations were not affected by the N-type channel blocker ω-Conotoxin-GVIA (CgTx), but were entirely blocked by ω-Agatoxin-IVA (Aga), a specific P/Q-type channel blocker (brown records top left), the cell was labeled as a P/Q-only neuron (~25%). If ramp-induced oscillations were not affected by Aga but were completely blocked by CgTx (purple records top right), the cell was labeled as a N-only neuron (~25%). If CgTx only partially reduced oscillations and Aga blocked the remainder of the oscillations (black records bottom), the cell was labeled as a N+P/Q neuron (~50%).
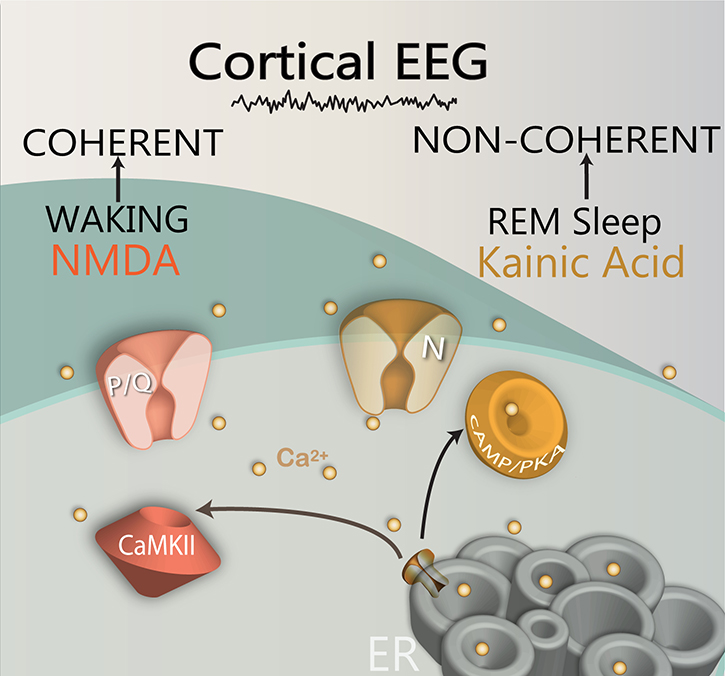
Injections of glutamate into the PPN of freely moving animals were reported to increase both waking and REM sleep (Datta et al., 2001). However, injections of NMDA increased only waking (Datta et al., 2002), and injections of kainic acid (KA) increased only REM sleep (Datta et al. 2001, 2002). Protein kinase C (PKC) modulates KA receptors and was found to enhance N-type channel activity, but to have no effect on P/Q-type channels (Stea et al., 1995). On the other hand, CaMKII modulates NMDA receptors as well as P/Q-type channel function (Jiang et al., 2008). Taken together, these results suggest that the two calcium channel types are modulated by different pathways, N-type channels by the cAMP/PK pathway, and P/Q-type channels through the CaMKII pathway. Therefore, it appears that there is a “Waking” pathway mediated by CaMKII and P/Q-type channels and a “REM sleep” pathway mediated by cAMP/PK and N-type channels (Garcia-Rill, 2015, 2017; Garcia-Rill et al., 2014a,b; Urbano et al., 2012). Figure 4 shows the identified intracellular pathways modulating the two types of high threshold, voltage-dependent calcium channels, the P/Q-type channels by CaMKII, and the N-type channels by cAMP/PKA. These results provide potential treatment options for the differential control of waking vs REM sleep, namely the ability to modulate the CaMKII vs the cAMP/PKA pathways independently.
Figure 4. Differential regulation of two calcium channels by different intracellular pathways.
The CaMKII (brown structure) intracellular pathway regulates P/Q-type calcium channels, while the cAMP/PKA (orange structure) pathway regulates N-type calcium channels. The CaMKII/P/Q-type channel pathway modulates beta/gamma band activity during waking, while the cAMP/PKA/N-type channel pathway modulates beta/gamma band activity during REM sleep. In addition, NMDA receptor activation modulates P/Q-type channel activity, while kainic acid receptor activation modulates N-type channel activity. The gamma band oscillations generated by the PPN during waking ultimately lead to gamma activity in the cortical EEG with coherence across distant sites, while gamma activity during REM sleep leads to a similar fast cortical EEG but without coherence across distant sites. (Ca2+, light brown dots)
2.1.3. Gene transcription
Histone post-translational modification and DNA methylation, basic epigenetic mechanisms, are involved in the regulation of gene expression in response to environmental stimuli. Evaluating whether inhibition of histone deacetylation affected PPN intrinsic gamma oscillations involved in arousal, it could be demonstrated that, (1) in vitro exposure to the histone deacetylation inhibitor trichostatin A (TSA) eliminated the manifestation of PPN intrinsic membrane oscillations in the gamma band range, but not lower frequency oscillations, (2) long-term exposure to TSA also decreased PPN gamma band oscillations, and (3) significantly reduced calcium currents (Urbano et al., 2018). These results pointed to a specific effect on gamma oscillations when histone deacetylation was blocked, suggesting that, without such blockade, arousal-related gamma oscillations modulate gene transcription. A preliminary analysis of the proteins modulated by TSA showed effects on neuronal calcium sensor protein-1 (NCS-1), calcineurin, and other intracellular calcium concentration control proteins. These findings provide multiple and significant implications for new research directions. The potential role of gene transcription resulting from the manifestation of gamma oscillations can now be discussed in terms of daily remodeling of PPN neurons and updating of sensory experience (Urbano et al., 2018).
Figure 5 summarizes the effects following exposure to deacetylation inhibitors. P/Q-type channels are normally controlled by CaMKII to generate gamma band oscillations. TSA blocked PPN oscillations and reduced ICa by inhibiting HDAC I and II. KN-93 decreased the effects of TSA, implying that HDAC II may act on CaMKII to induce transcription following P/Q-type channel-mediated gamma oscillations. The HDAC I inhibitor MS275 or the HDAC IIb inhibitor Tubastatin A had no effect on PPN oscillations or ICa. On the other hand, the HDAC IIa inhibitor MC1568 had the same effect as TSA in reducing PPN oscillations and ICa. These agents acted on cells with P/Q-type channels (N+P/Q and P/Q-only cells) but had little effect on N-only cells. These findings point to many potentially fruitful areas of research on gene transcription that need to be pursued.
Figure 5. Epigenetic mechanisms of gamma oscillations.
CaMKII modulates P/Q-type channels to produce gamma oscillations (green structures). These oscillations were blocked and ICa reduced in PPN cells after inhibiting HDAC I and II (brown and blue structures) using TSA. The CaMKII blocker KN-93 was found to partially blunt the effects of TSA, suggesting that HDAC II effects on CaMKII and its modulation of P/Q-type channels are required for gene transcription. There were no effects on oscillations or ICa following inhibition of HDAC I using MS275, or of HDAC IIb using tubastatin A. On the other hand, HDAC IIa inhibition using MC1568 had the same effect as TSA in reducing oscillations and ICa. These effects were evident in cells with P/Q-type channels (N+P/Q and P/Q-only cells) but not on N-only cells that are modulated by cAMP/PKA (purple structures).
2.2. Ascending modulation of arousal
Ascending PPN output induced by auditory sensory input is manifested in the P50 potential that in human is recorded at the vertex (Garcia-Rill et al., 2001). Using magnetoencephalography, the magnetic equivalent of the P50 potential is the M50 response which is manifested in the region of the vertex (Garcia-Rill et al., 2008). The P50 potential is a midlatency auditory evoked response following a click stimulus occurring at 50–70 msec latency. The P50 potential is, (1) sleep state-dependent, that is, it is present during waking and REM sleep, e.g. is manifested during arousal states when PPN is active (and is not present during slow wave sleep), (2) blocked by low concentrations of the muscarinic cholinergic antagonist scopolamine and by inactivation of the PPN in animals, that is, the P50 potential is generated by cholinergic projections of the PPN, and (3) rapidly habituating, that is, it is reticular in origin with low synaptic security (reviewed in Garcia-Rill et al., 2001). Lesions of the PPN or injections of inhibitory agents into the PPN in animals were found to block the equivalent vertex-recorded potential (P13 in the rodent, “wave a” in the feline), demonstrating its origin as the PPN (reviewed in Garcia-Rill et al., 2001). Briefly, the P50 potential is an arousal-related waveform in the human generated at least in part by the PPN. Parkinson’s disease (PD) patients showed decreased habituation of the P50 midlatency auditory evoked potential using a paired stimulus paradigm (Teo et al., 1997). In addition, in PD patients with a bilateral pallidotomy that alleviated their motor symptoms, habituation of the P50 potential returned to normal levels (Teo et al., 1998). Similar studies showed that decreased habituation indicative of a sensory gating deficit was present in posttraumatic stress disorder, depression, and Huntington’s disease (Skinner et al., 1999; Uc et al., 2003). The P50 potential is therefore a valuable noninvasive measure of ascending sensory activation of the cortex by the PPN in neurological disorders. In the next section, we will address another measure of sensory gating, prepulse inhibition.
2.3. Descending modulation of posture and locomotion
Much of the misunderstanding of the role of the PPN in locomotion stems from early rigid requirements for inducing “controlled locomotion” (Shik and Orlovsky, 1966; Armstrong, 1986; Garcia-Rill, 1986). These characteristics arose from the need to identify locomotion-specific brain regions demanding the ability to, (1) induce locomotion at very low current amplitudes, (2) induce a transition from a walk to a trot to a gallop with increasing current levels, i.e. controlling stepping, (3) elicit stepping only when stimulation was applied (eliminating arousal, appetitive, escape, and other factors), and (4) identify the optimal frequency (40–60 Hz) and duration of pulses for inducing locomotion on a treadmill in the decerebrate animal (reviewed in Garcia-Rill, 1986, 1991). Of necessity, the preparations used were typically decerebrate so that stepping could be selectively assessed in the absence of descending modulation from the cortex, basal ganglia, and thalamus. Identification of “controlled locomotion” also required alternation between antagonists in the same limb, alternation between agonists in opposite limbs, and a proximo-distal delay in contraction of muscles in the hip, knee, and ankle joints during walking, demanding electromyographic (EMG) recordings. Locomotion “purists” did not recognize stepping activated in intact animals because of potential confounding factors, e.g. pain, escape responses, etc., and variable processes related to behavioral acts, e.g. rearing, turning, short episodes of walking, etc. that could originate in higher brain regions. Even using strict criteria in decerebrate animals, Garcia-Rill and colleagues found more than one stimulation site in the mesopontine region that could elicit “controlled locomotion”, including the PPN. These sites were located dorsal to the PPN, in the region of the ventral inferior colliculus at the edge of the cuneiform nucleus, but we could never induce controlled locomotion on a treadmill could not be produced following stimulation of more medial sites such as the laterodorsal tegmental nucleus, or more ventral sites in the area of the midbrain extrapyramidal region (MEA) or substantia nigra. Because they could identify the PPN region stimulated using labeling (Garcia-Rill et al., 1987), they could record PPN neurons in relation to locomotion (Garcia-Rill et al., 1983), and because they could chemically activate the same site to induce locomotion thus eliminating effects of fibers of passage (Garcia-Rill et al., 1985), they concentrated on the PPN, leaving sites in the cuneiform and edge of the inferior colliculus to others. These findings led to the implementation years later of PPN DBS for the treatment of gait and postural disorders in PD. Of particular concern were regions immediately posterior to PPN close to pain and micturition pathways that could affect responses to stimulation (Garcia-Rill et al., 1991; Reese et al., 1995). Given our discovery that every PPN cell manifests intrinsic gamma oscillations (see above), it became clear why the optimal frequencies for inducing locomotion on a treadmill were 40–60 Hz, and why this is also the optimal frequency for some of the beneficial effects of PPN DBS (Garcia-Rill et al., 2014a, 2018). It needs to be emphasized that the enduring beneficial effects of PPN DBS in decreasing falls, improving stepping, and sleep and cognitive effects are seen best when using beta/gamma (20–60 Hz) frequency of stimulation. However, the resting frequency of PPN neurons is ~10 Hz, that is, in the alpha range (Simon, et al., 2010; Kezunovic, et al., 2011). The latest MRI-based study of the optimal area for PPN DBS has shown it to be centered on the PPN (Goetz et al., 2018). These studies also showed that, as far as freezing of gait was concerned, stimulation frequencies in the 10–30 Hz. Previous studies had shown that these lower frequencies are best for maintaining posture and reducing freezing of gait (Fraix et al., 2008). Recent results showed that 30 Hz stimulation of the PPN improved the clinical balance impairment in PD (Perera et al., 2018). These results taken together may suggest that postural maintenance may require lower frequencies (10–30 Hz) than those inducing stepping (20–60 Hz). Interestingly, in two patients stimulated at low frequencies, stimulation of the region induced sleep (Arnulf et al., 2010). This effect needs to be replicated but may mean that stimulation at low frequencies in the delta range may facilitate sleep rather that arousal and movement.
Over the years, however, the term “locomotion” has come to be applied much more loosely to describe almost any type of progression and mostly in intact animals, so that it has been very difficult to identify the specific role of the PPN, or of any other region for that matter, in such events. Since the PPN is part of the reticular activating system (RAS) and participates in arousal and gait and posture control, as long as the region of the PPN is activated in intact animals to induce progression, doubts will always arise that the effects are not specific to locomotor control but rather arousal and its attendant processes such as escape. Moreover, many workers make conclusions about sites related to locomotion without ever physiologically identifying those sites, basing results purely on anatomy or without ever recording an alternating EMG pattern.
Because of results showing more than one potential locomotion-inducing site and the incongruity of a locomotion-specific site being located in the brainstem, the use of such terminology as “mesencephalic locomotor region” (MLR) to Garcia-Rill and colleagues became inaccurate and unsupported by the results (Garcia-Rill and Skinner, 1988; Skinner and Garcia-Rill, 1990). One alternative approach was to adopt a more general stance, suggesting that, rather than being a “locomotion-specific” region, the PPN was more of a rhythmogenic region, stimulation of which at its natural frequency would lead to locomotion. The original requirements of using 40–60 Hz stimulation can now be better understood given the more recent discovery that all PPN neurons manifest intrinsic gamma band oscillations as discussed above, supporting the notion that it is stimulation at its natural gamma frequency that would induce stepping as well as arousal. Early studies proposed that locomotion was not so much induced as “recruited” (Garcia-Rill and Skinner, 1988; Skinner and Garcia-Rill, 1990), and studies described above on how intrinsic gamma oscillations are recruited by ramps help explain many of these original and peculiar stimulation parameters for activating the so-called “MLR”. That PPN stimulation could lead to locomotion is further supported by the properties of its descending projections.
The PPN sends descending projections to the pons and medulla, but only minor efferents to the spinal cord (reviewed in Garcia-Rill, 1991; Reese et al., 1995). The PPN sends projections to a REM sleep-inducing region, the SubCoeruleus dorsalis (SubCD) nucleus, which is activated by cholinergic agonists to elicit paradoxical sleep with atonia (Baghdoyan et al., 1984). Lesions of the SubCD are known to produce REM sleep without atonia (Sanford et al., 1994). Therefore, during REM sleep, the PPN and SubCD may activate reticulospinal systems that hyperpolarize motoneurons, thus inducing the atonia of REM sleep (Chase and Morales, 1994). Descending projections from the PPN to the medioventral medulla instead produce increases in stepping. Medioventral medulla reticulospinal outputs in turn activate spinal pattern generators to induce locomotion (Garcia-Rill and Skinner, 1991, 2004; Reese et al., 1995). Thus, the pontomedullary region is heterogeneous, such that electrical or chemical (with cholinergic agonists) stimulation of the pontomedullary reticular formation can induce decreased muscle tone at some sites and stepping movements at other sites (Garcia-Rill et al., 2001; Reese et al., 1995). Descending projections from the PPN were found to induce long lasting hyperpolarization in large reticulospinal neurons presumably involved in the atonia of REM sleep, and to depolarize medium size interneurons that then activate spinal pattern generators via reticulospinal pathways, thus having a push-pull effect in driving stepping (Garcia-Rill et al., 2001; Mamiya et al., 2005). Therefore, given the extensive evidence, the PPN will modulate both posture and locomotion, in parallel with arousal, in keeping with its role as part of the RAS (Garcia-Rill et al., 2014a, 2018). Moreover, the inhibition of PPN outputs to large reticulospinal neurons (Mamiya et al., 2005) may be involved in modulating the auditory startle reflex, as discussed in the following section. Because of the presence of spinal central pattern generators (CPGs), direct projections to the spinal cord are not needed to elicit stepping, the only requirement being to trigger CPGs via reticulospinal projections.
In the interest of clarity, the Garcia-Rill et al. decerebrate locomotion studies had to comply with the very rigid standards for eliciting “controlled” locomotion (walk-trot-gallop with increasing current amplitude) so that the fact that such stimulation did not induce stepping when applied to such regions as the MEA does not mean that the MEA does not modulate locomotion, it just suggests that stimulation such as that described above was not optimal for activating whatever role the MEA may exert on stepping. Locomotion and posture are modulated by multiple reticulospinal pathways, so that it is not unreasonable to assume some redundancy in triggering stepping and stability. By the same token, PPN DBS in humans is quite different than studies on decerebrate animals, so that the possible beneficial effects of PPN DBS in alleviating falls, improving gait, and even improving sleep and cognition (Garcia-Rill et al., 2014a), may arise from activation of different regions to which the PPN projects, and perhaps at different frequencies. It may be most critical that, it is stimulation at the natural frequency of the PPN, of activating all of its cells which manifest intrinsic gamma band oscillations regardless of transmitter type, that induces its beneficial consequences by propagating gamma band activity throughout the brain. More will be said about DBS below, but it appears that stimulation in the range of the natural frequencies of oscillations that produces the best clinical outcomes.
3. Prepulse Inhibition (Kofler)
3.1. Definitions
Prepulse Inhibition (PPI) is a neurophysiological phenomenon, involving the PPN, in which a weak sensory stimulus causes a decrease in the magnitude of a subsequent test response (Graham, 1975; Koch et al., 1993; Swerdlow et al., 1993b; Inglis et al., 1995). The amount of inhibition is a function of both intensity and timing of the prepulse (Blumenthal, 2015). Inhibition is mostly seen with interstimulus intervals of 30 to 500 ms, while at shorter and longer interstimulus intervals, respectively, prepulse facilitation may be observed (Valls-Solé et al., 1999; Putnam et al., 1999; Swerdlow et al., 1999). PPI appears to reflect the activation of hard-wired neural circuitry that mediates behavioral gating. According to Braff et al. (Braff et al., 1990), the degree to which a startle reflex is inhibited by a prepulse is a measure of the amount of sensorimotor gating.
Two prototypical types of sensorimotor gating may be differentiated, (1) prepulse modulation, which comprises facilitatory and inhibitory effects upon reflex responses, in which the prepulse stimulus is typically insufficient for generation of an overt reflex response by itself, and (2) gating, by which inter-sensory interference of two suprathreshold stimuli (“sensory collision”) typically exerts an effect on non-reflex responses; the sum of the individual responses is larger than the response to both stimuli together.
3.2. History
From an historical perspective, PPI was first described by two independent groups in the context of auditory startle reactions (Hoffman et al., 1965; Buckland et al., 1969; Ison et al., 1971). When reviewing the available literature, the latter authors came across a Russian monograph describing findings related to prepulse inhibition already some 100 years earlier (Sechenov, 1863). Later, PPI was also studied by different groups using blink reflexes to supraorbital nerve stimulation (Rossi et al., 1992; Valls-Solé et al., 1994; Gomez-Wong et al., 1998). Meanwhile, PPI has been well established as an operational measure of sensorimotor gating from rodents to primates, used as an endophenotype for genomic studies, and as a biomarker for brain circuitry, which may even predict sensitivity to psychotherapeutics (Swerdlow et al., 2016).
3.3. Functional anatomy
The functional anatomy of PPI is not completely known, although there is evidence that the PPN may be involved (Swerdlow et al., 1993b; Koch et al., 1993; Inglis et al., 1995; Reese et al., 1995; Blumenthal, 2015). The PPN is the main structure within the first level of higher order hierarchical circuits governing the primary auditory startle circuit (Kofler et al., 2006). In rats, PPN lesions increase baseline startle reflex amplitude and abolish PPI without affecting long-term habituation (Swerdlow et al., 1993b; Kodsi et al., 1997). PPI is likely mediated by a cholinergic pathway, since direct injections of the cholinergic muscarinic antagonist scopolamine into the nucleus reticularis pontis caudalis in rats increased the basal startle reflex amplitude and reduced PPI (Fendt et al., 1999). The PPN was found to hyperpolarize large reticulospinal neurons throught to be involved in the startle reflex (Mamiya et al., 2005), and receives basal ganglia inputs mainly from the substantia nigra pars reticulata and the globus pallidus (Parent et al., 1995), providing the basis for a circuit linking basal ganglia function with the mechanisms that control the startle reaction. This startle modulation circuit is further regulated by higher brain circuitry via the pontine tegmentum which involves limbic cortical inputs to the ventral striatum, striatal connections with the pallidum, and pallidal inputs to the pontine tegmentum (Swerdlow et al., 2000).
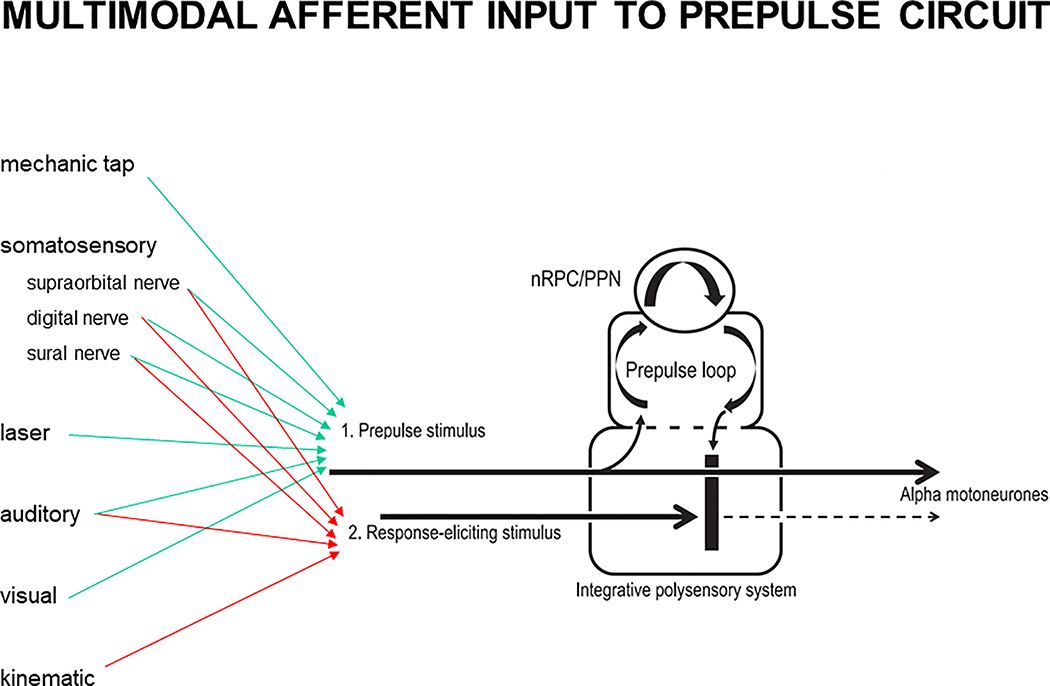
PPI is effective also on the blink reflex elicited by electrical stimulation of the supraorbital nerve (Rossi et al., 1992; Valls-Solé et al., 1994). Low intensity stimuli delivered through deep brain stimulation electrodes inserted in the subthalamic nucleus of PD patients were capable of inducing PPI at very short intervals in the order of 5 ms, suggesting that the structures mediating this effect were very close, most likely the connections between the internal pallidum and the PPN, adjacent to the subthalamic nucleus (Costa et al., 2006). Valls-Solé et al. (Valls-Solé et al., 2008) proposed a prepulse circuit located within the brainstem, which mediates PPI, based on research on both blink reflex and startle reaction. Similarities of auditory and somatosensory prepulse effects at interstimulus intervals exceeding 100 ms on auditory and somatosensory blink reflexes (Valls-Solé et al., 1999) suggest that the neuronal circuitry which mediates PPI is at least in part independent of stimulus modality. A schematic illustration of the PPI circuit and its potential cross-modal input channels is depicted in Figure 6.
Figure 6.
Multimodal afferent input to proposed prepulse circuit (modified from Valls-Solé et al., 2008; and Frauscher et al., 2012). Prepulse inhibition is conveyed by the first modality pathway to the brainstem, where it enters two different circuits: one circuit leading to activation of facial and/or limb motoneurones (straight line), and another “prepulse loop” circuit, which involves the pedunculopontine nucleus (PPN) and, in case of a startle reaction, also the nucleus reticularis pontis caudalis (nRPC). This prepulse loop induces inhibition (vertical thick black line) that will prevent impulses in the second modality pathway from entering the integrative polysensory system for further sensorimotor integration processes.
PPI has been shown to be independent of the StartReact effect (Valls-Sole et al., 2005). When a startling auditory stimulus is delivered during preparation for a ballistic movement in a simple reaction time task experiment, two things happen: (1) a startle response is elicited, and (2) the reaction time is shortened, leading to accelerated release of the preprogrammed ballistic movement (i.e., the StartReact effect). A prepulse reduces the magnitude of the startle response, but does not influence concomitant movement acceleration.
A very recent study has applied sophisticated laboratory techniques in zebrafish, including large scale calcium imaging, optogenetics, and laser ablation, and reported the first cellular resolution circuit for prepulse inhibition in a vertebrate, revealing a central role for presynaptic gating of sensory information to a brainstem motor circuit, while sparing signaling to other brain regions (Tabor et al., 2018).
3.4. Neuropharmacology of PPI
Numerous experimental and human studies have investigated the role of various neurotransmitters and of pharmacological substances on PPI (Swerdlow et al., 1999; Braff et al., 2001). The most important ones seem to be acetylcholine, acting via nicotinic receptors, thereby enhancing PPI (Kumari et al., 1997), and via muscarinic receptors, thereby reducing PPI (Fendt et al., 1999), and dopamine, which suppresses PPI (Mansbach et al., 1988). Recently, Kumru et al. (Kumru et al., 2010, 2011, 2012) demonstrated GABAergic influence on PPI in patients with spinal cord injury who were treated with intrathecal baclofen, a powerful GABAB agonist. The role of inhibitory cholinergic projections from the PPN to the startle reflex circuit in mediating PPI has recently been challenged: in an experimental rat model, transient optogenetic activation of cholinergic PPN neurons did not inhibit startle responses, but rather enhanced startles, concurring with their role in arousal (Azzopardi et al., 2018). These findings led the authors to conclude that non-cholinergic PPN neurons mediate PPI. However, it is not known if the optogenetic method alters the proper workings of the calcium channels in PPN neurons responsible for high frequency activity.
3.5. Physiological purpose of PPI
PPI is considered an operational measure of sensorimotor gating that has been well validated in animal and human studies (Geyer et al., 2001). Sensorimotor gating refers to the process by which a neural system screens or ‘gates’ external (sensory) and internal (cognitive, motor) information from higher order processing and subsequent responses, presumably to enable uninterrupted processing of the most salient aspects of the external and internal environment. In other words, by means of PPI, the PPN serves as one of the “secretaries” of the brain (the “antechamber” to the thalamus), governing the amount of afferent information flow to the brain. This influence on information flow is sensibly linked to the control of posture and of sleep-wakefulness, (1) human beings usually sleep in the supine position as opposed to being upright or even during walking, (2) they are most awake and alert when standing upright, therefore, verticalization is one of the key aspects in neurorehabilitation, and (3) even when sleeping human beings want to become aware of salient stimuli, e.g., when the baby cries, or when the house is burning. Therefore, the PPN is an ideal brainstem center combining these central aspects in human life, suppressing less critical and distracting information, but allowing for critical information, while at the same time controlling posture and the wakefulness state.
3.6. Physiological modulation of PPI
Gender is an important modulator of PPI of the acoustic startle reaction, as differences between men and women became apparent early on (Swerdlow et al., 1993a). Later observations revealed specific changes in PPI in women across the menstrual cycle with significantly less suppression in the luteal phase, while in the follicular phase PPI did not differ significantly from PPI in men (Swerdlow et al., 1997; Jovanovic et al., 2004). Gender also influences PPI of the blink reflex in a similar manner (Kofler et al., 2013). Thus, information on hormonal state as well as the use of oral contraceptives is important and underscores the importance of sex matching comparison groups in studies of sensorimotor gating. Another factor to observe when testing PPI relates to smoking habits, as nicotine has an impact on PPI via nicotinic receptors (Kumari et al., 1996; Della Casa et al., 1998). Recently, a significant influence of peripersonal versus extrapersonal space on PPI of the blink reflex has been reported, concurring with top-down corticobulbar modulation of the neural circuitry underlying PPI (Kiziltan et al., 2018b).
3.7. Pathophysiological considerations related to PPI
Schizophrenia was the first disease in which deficient PPI was suggested to play a pathophysiological key role (Braff et al., 1978). Schizophrenic patients have extensive deficits in both intramodal and cross-modal sensorimotor gating (Braff et al., 1992). Reduced PPI may lead to “stimulus overload”, increased distractibility, and cognitive fragmentation. In fact, schizophrenic patients report oversensitivity to sensory stimulation that theoretically correlates to stimulus overload (Braff et al., 1992). When taking into account sex differences and hormonal effects, PPI may be considered a valid candidate for an endophenotypic marker in genetic studies of schizophrenia (Kumari, 2011). The role of nicotinic receptors in PPI is supported by the self-medication hypothesis, based on a two- to four-fold rate of smoking in patients with schizophrenia compared to the general population, with a tendency to favor stronger cigarettes and to extract more nicotine from their cigarettes than other smokers (Kumari et al., 2005). Early in the era of PPI investigations, patients comorbid for tics and attention-deficit hyperactivity disorder were shown to have significantly reduced PPI compared to patients with attention-deficit hyperactivity disorder alone and to healthy controls. These findings were interpreted as being due to deficient pallidal inhibition in Tourette syndrome (Castellanos et al., 1996).
With respect to other movement disorders, Gomez-Wong et al. reported deficient PPI of the blink reflex in patients with dystonic blepharospasm, in particular those without a sensory trick or ‘geste antagonistique’ (Gomez-Wong et al., 1998). Later, PPI was applied to distinguish EMG activity related to the auditory startle reaction from abnormally prolonged EMG activity following the startle reflex in patients with primary cervical dystonia: the former was suppressed by some 30%, while the latter remained unaffected by a somatosensory prepulse (Muller et al., 2003). Reduced PPI of the blink reflex in the same range of 30% in patients versus blink reflex R2 suppression by 70% in healthy subjects has recently been confirmed in idiopathic cervical dystonia (Ozturk et al., 2016). PPI of the blink reflex following an auditory or somatosensory prepulse was reduced in the majority of patients with idiopathic PD and, similarly, in those with Huntington’s disease, in spite of the two disorders showing opposite blink reflex excitability recovery abnormalities to paired pulse supraorbital nerve stimulation. This indicates that the two tests assess different brainstem circuitry (Valls-Solé et al., 2004).
Conversely, Leon-Sarmiento et al. (Leon-Sarmiento et al., 2015) described normal PPI of the blink reflex following either auditory or tactile stimulation applied to the lower limbs in patients with restless legs syndrome, concurring with common pathways for both intramodal and cross-modal prepulse effects, as previously suggested (Valls-Sole et al., 1999; Valls-Sole et al.. 2008). Because forebrain interneurons are presumably involved in mediating prepulse effects, they seem to be unaffected and not involved in the disordered sensorimotor interaction associated with restless legs syndrome (Leon-Sarmiento et al., 2015).
A significant reduction in blink reflex PPI has recently been reported in hemifacial spasm and postparalytic facial syndrome (Kiziltan et al., 2018a). Interestingly, PPI was similar on symptomatic and asymptomatic sides in both patient groups. However, PPI was significantly more reduced in hemifacial spasm, and less so in postparalytic facial syndrome, compared to healthy controls. In both disorders, sensory input on both sides of the face is obviously less filtered at the brainstem level, presumably in order to better monitor altered facial movements bilaterally following unilateral facial nerve lesion (Kiziltan et al., 2018a).
Based on reduced PPI, insufficient filtering of afferent information flow to the brain at the brainstem level with subsequent “stimulus overload”, possibly causing oversensitivity to sensory stimulation, has been suggested to contribute to altered sensory perception and allodynia in patients with fibromyalgia (Kofler et al., 2014). Recently, pain has also been addressed in patients with migraine, who presented with significantly less PPI than healthy controls (Uygunoglu et al., 2017). Furthermore, PPI was significantly less common in migraineurs with allodynia than in those without. It seems that tonic brainstem activity related to filtering capacity is reduced during migraine headache, and that allodynia is related to impaired sensory modulation of the brainstem, similar to what was earlier proposed in fibromyalgia (Kofler et al., 2014).
A dissociation of PPI from blink reflex excitability recovery – similar to the findings in Huntington’s disease (Valls-Solé et al., 2004) – was also observed in patients with narcolepsy. Frauscher et al. (Frauscher et al., 2012) postulated a dysfunction of hypothalamic control over the PPN by demonstrating deficient PPI of the blink reflex despite normal unconditioned blink reflexes and normal blink reflex excitability recovery in patients with narcolepsy compared to healthy controls. PPI alterations, however, did not correlate with the Epworth Sleepiness Scale or Ullanlinna Narcolepsy Scale scores (Frauscher et al., 2012). Alterations in PPI of the blink reflex have been utilized to provide evidence of reorganization at the brainstem level, and modulation thereof by the GABAB agonist baclofen, following spinal cord injury (Kumru et al., 2010, 2011, 2012). Related to spasticity and its assessment, neurophysiological experiments in healthy subjects documented the existence of startle responses to passive lower limb movements induced with an exoskeleton, which is used for locomotion training in neurorehabilitation, and for measurements of muscle stiffness as a surrogate marker for spasticity. These kinematic startle reflexes, which may “contaminate” measurements of muscle stiffness, were amenable to suppression by means of PPI (Castellote et al., 2017). Work on PPI also suggested the existence of a somatosensory startle response superimposed on the post-inhibitory EMG rebound following nociceptive stimulation of a peripheral cutaneous nerve, known as the cutaneous silent period. In addition, a prepulse served to reduce the level of discomfort associated with noxious electrical nerve stimulation applied in cutaneous silent period testing (Kumru et al., 2009).
In conclusion, reduced PPI has been demonstrated in various clinical conditions, while to date abnormally enhanced PPI has not yet been reported. It remains to be elucidated, however, whether the observed findings are rather cause or consequence of a specific disease. While it is well accepted and seems reasonable that deficient PPI is one pathophysiological mechanism in schizophrenia, the situation is certainly less clear in fibromyalgia and other chronic pain syndromes. In movement disorders, reduced PPI could as well be an epiphenomenon, because complex alterations of sensorimotor loops are often at play in these diseases. At any rate, the PPN is accepted to be the main structure mediating PPI, among other basic functions, in mice and men.
4. The PPN and freezing of gait (Nonnekes)
As outlined in the above section, the mesencephalic locomotor region (MLR), including the region of the PPN, is thought to be crucial in normal locomotion. It is therefore not surprising that its possible role in freezing of gait (FOG) – one of the most mysterious gait disturbances – has received considerable attention in recent years.
4.1. Freezing of gait: a mysterious symptom
FOG is an episodic gait impairment in patients with PD and atypical parkinsonism (Nutt et al., 2011). During an episode of FOG, patients are unable to move forward, and have the feeling that their feet are suddenly glued to the floor. Turning and gait initiation are the strongest provoking circumstances, but FOG is also frequently triggered when performing a concomitant task while walking, such as when passing through doorways, and when under the pressure of time (Nonnekes et al., 2015c). Its relationship with dopaminergic medication is complex; FOG is typically more common OFF-dopaminergic medication compared to ON-dopaminergic medication (Schaafsma et al., 2003), but long-term levodopa treatment has been postulated to increase the occurrence of FOG (Nonnekes et al., 2018). To make it even more complex, three phenotypes of FOG have been described: freezing with total akinesia, freezing with alternating leg trembling, and freezing with small shuffling steps (Schaafsma et al., 2003). It is unknown whether these phenotypes share the same pathophysiological substrate.
The exact underlying mechanism of FOG needs to be unraveled and, in fact, there are likely to be multiple mechanisms. At least in some cases, a possible role for the PPN has been hypothesized. Start-React effects - the acceleration of reaction times by a startling acoustic stimulus - have been reported to be absent or reduced in freezers, whereas Start-React effects are intact in non-freezers (Thevathasan et al., 2011, Nonnekes et al., 2014, Nonnekes et al., 2015b). PPN stimulation restored the Start-React effects in freezers (Thevathasan et al., 2011), which suggests that PPN dysfunction might be present in these patients (Nonnekes et al., 2015a). Moreover, as both turning and gait initiation (the strongest provoking factors for FOG) require precise coordination of stepping movements and postural control, and the PPN may be involved in these processes, several lines of research in animals and humans have investigated whether dysfunction of PPN contributes to the occurrence of FOG.
4.2. PPN and freezing of gait: evidence from animal work
Until the 1980s, the lack of an appropriate animal model hampered the investigation of the mechanisms underlying FOG (and PD in general). In 1982, several young adults developed severe and irreversible parkinsonism, shortly after they had injected themselves with a ‘new synthetic drug’ (Ballard et al., 1985), which appeared to consist of almost pure 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). The MPTP discovery enabled the development of a non-human primate model of PD (Burns et al., 1984, Langston et al., 1984, Bloem et al., 1990), that was unavailable at the time.
Despite extensive use of the MPTP-animal model, the number of studies that evaluated FOG in non-human primates is sparse. In a study by Revuelta and colleagues, 29 macaque monkeys with MPTP-induced parkinsonism were evaluated for the presence of FOG (Revuelta et al., 2012). Approximately half of these monkeys (48%) appeared to have alterations in gait affecting mainly their hind limbs, resembling episodes of FOG in humans: they were episodic in nature, lasted several seconds to a minute, were accompanied by alternating limb movements with a frequency of 4–10 Hz, and were most common OFF-dopaminergic medication. However, preliminary data on those primates with FOG showed no significant neuronal loss in the PPN (Snijders et al., 2016). Hence, this model shows that there is no direct (or primary) role of the PPN in the occurrence of FOG.
4.3. PPN and freezing of gait: evidence from imaging studies in humans
We will now focus on evidence derived from fMRI studies in humans. As locomotion is not possible within a MRI scanner, paradigms have been developed to study the mechanisms involved in gait, and FOG in particular. The first paradigm involves motor imagery of gait. In one study investigating motor imagery of gait, Snijders et al. (2011) found that freezers (n = 12) had higher activity dorsally within the MLR (including the PPN), compared to non-freezers (n = 12). However, this could not be replicated by Peterson et al. (2014), who, after doing the same study in 9 patients in each group, found a trend towards the opposite, i.e., hypoactivity in the MLR of freezers compared to non-freezers. A disadvantage of these motor imagery paradigms is that they study gait planning and not gait execution, and focus on gait rather than on FOG (Snijders et al. 2016). A study by Shine and colleagues tried to overcome these limitations, by studying alternating leg movements in a VR-environment (Shine et al., 2013). In their study, hypoactivity dorsally within the MLR was observed during motor blocks.
Although these imaging data are partly conflicting, they might suggest that the PPN region fulfills a compensatory role during locomotion in freezers, and that FOG emerges when this compensation fails.(Snijders et al., 2016) Hence, in line with the evidence from primates with FOG, this implies that the PPN region has not a primary role in FOG, but rather a secondary or compensatory contribution. Future work is needed to study this hypothesis further. These future studies should ideally disentangle the subtypes of FOG, as their underlying mechanisms (and the role of the PPN) might differ. Moreover, prospective studies are needed, as the possible (compensatory) role of the PPN in FOG might hypothetically change over time.
5. Deep Brain Stimulation of the Pedunculopontine Nucleus Area (Lozano)
5.1. Introduction
As disease progresses, gait disturbances become more prominent in patients with PD, being an increasingly important source of disability and decline in quality of life (Schrag et al., 2000; Hely et al., 2008). For many advanced PD patients, medications and surgical treatments to alleviate gait disturbances provide insufficient benefit even if other motor deficits are ameliorated. For this reason, there is an important need to develop novel strategies to treat this problem. The interest in surgical interventions in brainstem locomotor areas gained attention in the early 2000’s with a reappraisal of the functional anatomy and physiology of the region (Pahapill and Lozano, 2000), and early non-human primate deep brain stimulation experiments (Nandi et al., 2012). These were rapidly followed by first-in-man clinical trials of deep brain stimulation for gait disturbances headed by groups in Rome (Mazzone et al., 2005) and Bristol (Plaha and Gill, 2005) With advances in imaging and electrophysiology, it has been possible to identify the neuronal populations within the PPN area and to target them. As noted earlier, the region has multiple nuclei and exactly which population of neurons is most relevant cannot be determined with human studies. Such work in patients with PD has shown that neurons in the PPN region respond to both active and fictive movement (Weinberger et al., 2008; Tattersall et al., 2014). In addition, functional imaging studies have found activation of the PPN area with fictive locomotion in humans (Karachi et al., 2010). These observations have driven a number of clinical trials of DBS in the PPN area.
The Movement Disorder Society has recently convened an expert review of the role of the PPN area in locomotion in the context of PD and has compiled the clinical data and reviewed the results of studies of PPN DBS (Hamani et al., 2016a, Hamani et al., 2016b, Thevathasan et al., 2018a). While the results across studies have shown some variability, the overall consensus is that there is reduced freezing of gait and reduced falls in PD patients who undergo PPN DBS surgery. This finding has been consistent across several studies, each albeit with a relatively low number of patients (reviewed in Thevathasan et al., 2018a, Thevathasan et al., 2018b). The clinical benefits also appear to extend to other aspects of PD, including some aspects of balance (Perera et al., 2018) and posture, including Pisa syndrome (Shih et al., 2013). There have several attempts of PPN DBS surgery in patients with other parkinsonian syndromes including progressive supranuclear palsy (PSP), with limited success. A recent autopsy report in PSP patients has confirmed the position of the electrodes in the PPN area (Scelzo et al., 2017). The clinical benefits also appear to extend to other aspects of PD, including Pisa syndrome (Shih et al., 2013).
Among the most controversial aspects of PPN DBS are what region(s) and corresponding neural elements, both cell bodies and axons, in the PPN vicinity should be targeted and how to validate the target intraoperatively. Indeed, it makes good sense to designate the surgical target as the “PPN area” until better identification of the precise clinically beneficial target(s) is defined. Additional challenges relate to the selection of optimal stimulating parameters and the possible variable acute and delayed latencies between the application of stimulation and the appearance of clinical effects. These features and the paradoxical and often unpredictable occurrence of freezing phenomena in PD patients (see Section 4 above) makes the selection and timing of appropriate clinical instruments to evaluate the impact of DBS an issue that has to be carefully considered.
The PPN area is complex and has multiple pathways running though it (see Section 1 above). Consequently, a number of stimulation-related adverse effects have been reported including oscillopsia, improved attention and cognition, increased REM, inducing sleep, paresthesias, pain and jaw clenching, and plus-minus syndrome (Soh et al., 2018). There are several possible mechanisms of action of how stimulation in the PPN area could improve gait (Table 1). Among the possibilities are local activation of brainstem locomotor areas (MLR), descending activation of spinal central pattern generators, and effects through the connectivity of PPN area structures to the subthalamic nucleus (STN), substantia nigra pars compacta or cerebellum. There could be an ascending effect through the reticular activating system to midline thalamic nuclei with increased arousal and vigilance (Ballanger et al., 2009; see Section 2 above). There could be also an ascending effect with stimulation of the medial lemniscus and disruption of pathological oscillations or phase amplitude coupling in the cortex and subcortical structures. Additional clinical findings have included improvements in REM sleep (Lim et al., 2009) with decreased latency and increases in the amount of REM which correlated, and also the ability to induce sleep behaviours with stimulation in the PPN region (Arnulf et al., 2010).
Table 1.
Some possible mechanisms of action of deep brain stimulation in the pedunculopontine area.
| Local activation of brainstem locomotor areas |
| Effects on subthalamic nucleus (STN), substantia nigra pars compacta (SNpc) or cerebellum |
| Descending activation of spinal central pattern generators |
| Ascending effects of arousal and vigilance |
| Ascending stimulation of the medial lemniscus and disruption of pathological oscillations in the cortex and subcortical structures |
5.2. Conclusions
There is limited but increasing experience with DBS in the PPN area. The neural elements that are being stimulated and mediating the effects have not been entirely isolated and identified. The overall results suggest that PPN DBS may improve postural instability and gait. This means that the possible clinical indications for PPN DBS could be PD patients with gait disturbances that are refractory to medication or refractory to conventional STN or globus pallidus interna (GPi) DBS. There is increasing evidence that there maybe also a potential role for PPN DBS in other parkinsonian disorders, including PSP.
One of the main challenges is the variable effects of PPN DBS, and this may be related to the heterogeneity in the pathophysiology of gait disturbances with respect to cognitive akinesias, rigidity, postural instability, falls, gait ignition, gait maintenance, postural reflexes and sensory feedback disturbances all contributing. These difficulties combined with a heterogeneity in targeting and the complex anatomy and functional consequences of stimulation in these areas increase the complexity of this treatment. In the future, improved targeting, improved imaging and the availability of directional leads are potential advances. While stimulation of the PPN area is complex, DBS may represent a promising target in patients with treatment-resistant Parkinson’s gait disorders but much work remains to be done.
6. Final remarks (Hallett and Valls-Sole)
Anatomically, the PPN can be defined and located by a group of large cholinergic neurons in the mesopontine tegmentum, lying close to the superior cerebellar peduncle, even though there are other neurons intermixed, especially glutamatergic and GABAergic cells. There are other important nuclei in its neighborhood, and there is still work needed on all the connections and physiological functions of all these nuclei. and for many purposes, particularly DBS, it is likely best at this juncture to refer to the PPN region which would encompass several nuclei. The PPN has an astounding contribution to many functions. These include apparently very disparate processes such as reflex reactions, sleep-waking, posture, and gait. There are several neurophysiological techniques potentially useful for the study of PPN functions, such as Prepulse inhibition (PPI) that may reveal its role in modulation over the startle reaction that may be adequate to characterize descending projections to locomotor centers, or the P50 potential that may be used to assess ascending projections modulating arousal. Many of these tests may be abnormal in patients with basal ganglia disorders, as would be expected because of the innervation that the PPN receives from the internal pallidum and substantia nigra reticulata. Descending PPN projections do influence pontomedullary reticulospinal projections.
The MLR is likely located in the same territory. It is unlikely that the PPN is the same as the MLR, but the PPN may still have some influence on gait. The evidence for influence on sleep-wake cycles and REM sleep in particular seems the strongest for a functional role for the PPN. The PPN may also modulate reflexes via its influence on reticulospinal systems, identifiable with PPI studies, and perhaps this also relates to its ascending modulation of arousal and attention, the latter playing an important role in gait. Recently, the PPN has been used for deep brain stimulation treatment of gait dysfunction in parkinsonism, a problem affecting the quality of life of many PD patients due to its resistance to “standard” treatment. In the scarce evidence gathered so far, PPN DBS has consistently reduced falls and freezing of gait in PD patients. At present, though, whether modulation of the PPN region or other approaches will be the best remains to be determined. In the meantime, we are learning much more about this interesting nucleus, the reticular formation, and the critical functions of the brainstem.
Highlights.
In May 2018, the Brainstem Society celebrated its 7th and last meeting. It was held as a satellite meeting just before the 31st International Congress of Clinical Neurophysiology and it was, as the previous 6 meetings of the Society, a very fruitful event. The pedunculopontine nucleus (PPN) was the focus of a vibrant session, where keynote researchers in the topic presented nicely documented state of the art data on the many aspects in which the PPN is an important hub. This review arises out of that session.
Acknowledgements
This work was supported by NIH grants HL095491 and NS085477 (Saper, Rye). Supported by NIH awards R01 NS20246 and P30 GM110702 from the IDeA program at NIGMS (Garcia-Rill). This work was supported by a Tier 1 Canada Research Chair in Neuroscience and the RR Tasker Chair in Functional Neurosurgery at University Health Network (Lozano). Dr. Hallett is supported by the NINDS Intramural Program.
Conflict of Interest
The authors certify that he has no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. Other full disclosures: Dr. Lozano is a consultant for Medtronic, Boston Scientific, and Abbott Laboratories. Dr. Hallett may accrue revenue on US Patent #6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent #7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil); in relation to the latter, he has received license fee payments from the NIH (from Brainsway) for licensing of this patent. He is on the Medical Advisory Boards of CALA Health and Brainsway. He is on the Editorial Board of approximately 15 journals and receives royalties and/or honoraria from publishing from Cambridge University Press, Oxford University Press, Springer, and Elsevier. Dr. Hallett’s research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds have been granted by Merz for treatment studies of focal hand dystonia, Allergan for studies of methods to inject botulinum toxins, Medtronic, Inc. for a study of DBS for dystonia, and CALA Health for studies of a device to suppress tremor.
Contributor Information
E. Garcia-Rill, Center for Translational Neuroscience, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
C.B. Saper, Department of Neurology, Division of Sleep Medicine and Program in Neuroscience, Harvard Medical School, Boston, MA, USA.
David B. Rye, Department of Neurology, Division of Sleep Medicine and Program in Neuroscience, Harvard Medical School, Boston, MA, USA.
M. Kofler, Department of Neurology, Hochzirl Hospital, Zirl, Austria.
J. Nonnekes, Radboud University Medical Centre; Donders Institute for Brain, Cognition and Behaviour; Department of Rehabilitation; Nijmegen, The Netherlands.
A. Lozano, Division of Neurosurgery, University of Toronto and Krembil Neuroscience Centre, University Health Network, Toronto, Canada.
J. Valls-Solé, Neurology Department, Hospital Clínic, University of Barcelona, IDIBAPS (Institut d’Investigació Biomèdica August Pi i Sunyer), Barcelona, Spain
M. Hallett, Human Motor Control Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–75. [DOI] [PubMed] [Google Scholar]
- Alessandro S, Ceravolo R, Brusa L, Pierantozzi M, Costa A, Galati S, et al. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive domains. J Neurol Sci. 2010;289:44–8. [DOI] [PubMed] [Google Scholar]
- Armstrong DM. Supraspinal contributions to the initiation and control of locomotion. Prog Neurobiol 1986;26:273–361. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983;216:53–68. [DOI] [PubMed] [Google Scholar]
- Arnulf I, Ferraye M, Fraix V, Benabid AL, Chabardes S, Goetz L, et al. Sleep induced by stimulation in the human pedunculopontine nucleus area. Ann Neurol. 2010;67:546–9. [DOI] [PubMed] [Google Scholar]
- Azzopardi E, Louttit AG, DeOliveira C, Laviolette SR, Schmid S. The role of cholinergic midbrain neurons in startle and prepulse inhibition. J Neurosci 2018;38:8798–8808.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res 1984;306:39–52. [DOI] [PubMed] [Google Scholar]
- Ballanger B, Lozano AM, Moro E, van Eimeren T, Hamani C, Chen R, et al. Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson’s disease: a [(15)O] H2O PET study. Hum Brain Mapp. 2009;30:3901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard PA, Tetrud JW, Langston JW. Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): seven cases. Neurology. 1985;35:949–56. [DOI] [PubMed] [Google Scholar]
- Ballon JS, Feifel D. A systematic review of Modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiat 2006;67:554–566. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Irwin I, Buruma OJ, Haan J, Roos RA, Tetrud JW, et al. The MPTP model: versatile contributions to the treatment of idiopathic Parkinson’s disease. J Neurol Sci. 1990;97:273–93. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD. Presidential Address 2014: The more-or-less interrupting effects of the startle response. Psychophysiology 2015;52:1417–31. [DOI] [PubMed] [Google Scholar]
- Boucetta S, Cisse Y, Mainville L, Morales M, Jones BE. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci. 2014;34:4708–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology 1978;15:339–43. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry 1990;47:181–8. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–58. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry 1992;49:206–15. [DOI] [PubMed] [Google Scholar]
- Buckland G, Buckland J, Jamieson C, Ison JR. Inhibition of startle response to acoustic stimulation produced by visual prestimulation. J Comp Physiol Psychol 1969;67:493–6. [DOI] [PubMed] [Google Scholar]
- Burns RS, Markey SP, Phillips JM, Chiueh CC. The neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the monkey and man. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 1984;11:166–8. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biol Psychiatry 1996;39:33–41. [DOI] [PubMed] [Google Scholar]
- Castellote JM, Kofler M, Mayr A, Saltuari L. Evidence for startle effects due to externally induced lower limb movements: implications in neurorehabilitation. BioMed Research International 2017;2017:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase MH, Morales FR. The control of motoneurons during sleep In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. WB Saunders, London, 1984. P. 163–176. [Google Scholar]
- Cisse Y, Toossi H, Ishibashi M, Mainville L, Leonard CS, Adamantidis A, et al. Discharge and Role of Acetylcholine Pontomesencephalic Neurons in Cortical Activity and Sleep-Wake States Examined by Optogenetics and Juxtacellular Recording in Mice. eNeuro. 2018;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J, Valls-Solé J, Valldeoriola F, Pech C, Rumia J. Single subthalamic nucleus deep brain stimuli inhibit the blink reflex in Parkinson’s disease patients. Brain 2006;129:1758–67. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand. 1964;62, suppl 232:1–55. [PubMed] [Google Scholar]
- Datta S, Patterson EH, Spoley EE. Excitation of the pedunculopontine tegmental nmda receptors induces wakefulness and cortical activation in the rat. J Neurosci Res 2002;66:109–116. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res 2002;70:79–82. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Stack EC. Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic adenosine monophosphate response element-binding protein as a function of rapid eye movement sleep. Neurosci 2009;163:397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Amer J Physiol Reg Integ Comp Physiol 2001;280:R752–R759. [DOI] [PubMed] [Google Scholar]
- Della Casa V, Hofer I, Weiner I, Feldon J. The effects of smoking on acoustic prepulse inhibition in healthy men and women. Psychopharmacology (Berl) 1998;137:362–8. [DOI] [PubMed] [Google Scholar]
- Eckenstein F, Sofroniew MV. Identification of central cholinergic neurons containing both choline acetyltransferase and acetylcholinesterase and of central neurons containing only acetylcholinesterase. J Neurosci. 1983;3:2286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WH, Boitano S. Connexin mimetic peptides: specific inhibitors of gap junctional intercellular communication. Bioch Soc Trans 2001;29:606–612. [DOI] [PubMed] [Google Scholar]
- Fendt M, Koch M. Cholinergic modulation of the acoustic startle response in the caudal pontine reticular nucleus of the rat. Eur J Pharmacol 1999;370:101–7. [DOI] [PubMed] [Google Scholar]
- Fraix V, Bastin F, David O, Goetz L, Ferraye M, Benabid AL, et al. Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PLoS ONE 2008,8:e83919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauscher B, Loscher WN, Ehrmann L, Gschliesser V, Brandauer E, Hogl B, et al. Narcolepsy-cataplexy: deficient prepulse inhibition of blink reflex suggests pedunculopontine involvement. J Sleep Res 2012;21:495–501. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E The basal ganglia and the locomotor regions. Brain Res Rev 1986;11:47–63. [PubMed] [Google Scholar]
- Garcia-Rill E The pedunculopontine nucleus. Prog Neurobiol 1991;36:363–389. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E Waking and the Reticular Activating System. Academic Press, New York, 2015, pp. 330. [Google Scholar]
- Garcia-Rill E Bottom-up gamma and stages of waking. Med Hypoth 2017;104:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Charlesworth A, Heister D, Ye M, Hayar A. The developmental decrease in REM sleep: The role of transmitters and electrical coupling. Sleep 2008;31:673–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep 2007;30:1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Homma Y, Skinner RD 2004 Arousal mechanisms related to posture and movement. I. Descending modulation. In: Mori S, Stuart DG, Wiesendanger M, editors, Brain Mechanisms for the Integration of Posture and Movement, Prog Brain Res 2004;143:283–290. [PubMed] [Google Scholar]
- Garcia-Rill E, Houser CR, Skinner RD, Smith W, Woodward DJ. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res Bull 1987;18:731–738. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Hyde J, Kezunovic N, Urbano FJ, Petersen E. The physiology of the pedunculopontine nucleus- implications for deep brain stimulation. J Neural Transm 2014a;122:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Kezunovic N, D’Onofrio S, Luster B, Hyde J, Bisagno V, et al. Gamma band activity in the RAS- intracellular mechanisms. Exptl Brain Res 2014b;232:1509–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Mahaffey S, Hyde JR, Urbano FJ. Bottom-up gamma in various disorders. In: Neurobiology of Disease: Pedunculopontine Nucleus Deep Brain Stimulation. Neurobiol Dis 2018;S0969–9961(18)30016–0. [Google Scholar]
- Garcia-Rill E, Moran K, Garcia J, Findley WM, Walton K, Strotman B, et al. Magnetic sources of the M50 response are localized to frontal cortex. Clin Neurophysiol 2008;119:388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. Modulation of rhythmic function in the posterior midbrain. Neurosci 1988;17:639–654. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. Modulation of rhythmic functions by the brainstem In: Shimamura M, Grillner S, Edgerton VR, editors. Neurobiology of Human Locomotion, Japan Sci Soc Press, Tokyo, 1991, p. 137–158. [Google Scholar]
- Garcia-Rill E, Skinner RD. The sleep state-dependent P50 midlatency auditory evoked potential In: Sleep Medicine, Lee-Chiong TL, Carskadon MA, Sateia MJ, editors. Hanley & Belfus, Philadelphia, 2001, p. 697–704. [Google Scholar]
- Garcia-Rill E, Skinner RD, Fitzgerald JA. Activity in the mesencephalic locomotor region during locomotion. Exptl Neurol 1983;82:606–622. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, Fitzgerald JA. Chemical activation of the mesencephalic locomotor region. Brain Res 1985;330:43–54. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, Clothier J, Dornhoffer J, Uc E, Fann A, et al. The sleep state-dependent midlatency auditory evoked P50 potential in various disorders. Thal & Related Syst 2002;2:9–19. [Google Scholar]
- Garcia-Rill E, Skinner RD, Miyazato H, Homma Y. Pedunculopontine stimulation induces prolonged activation of pontine reticular neurons. Neurosci 2001;104:455–465. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR . Measurement of startle response, prepulse inhibition, and habituation. Curr Protoc Neurosci 2001;Chapter 8:Unit 8 7. [DOI] [PubMed] [Google Scholar]
- Goetz L, Piallat B, Bhattacharjee M, Mathieu H, David O, Chabardes S. The primate pedunculopontine nucleus region: towards a dual role in locomotion and waking state. J Neural Transm 2016;123:667–678. [DOI] [PubMed] [Google Scholar]
- Goetz L, Bhattacharjee M, Ferraye MU, Fraiz V, Maineri C, Nosko D, et al. Deep brain stimulation of the pedunculopontine nucleus area in Parkinson’s disease: MRI-based anatomical correlations and optimal target. Neurosurg. 2018;84:506–518. [DOI] [PubMed] [Google Scholar]
- Gomez-Wong E, Marti MJ, Tolosa E, Valls-Solé J. Sensory modulation of the blink reflex in patients with blepharospasm. Arch Neurol 1998;55:1233–7. [DOI] [PubMed] [Google Scholar]
- Graham FK. Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology 1975;12:238–48. [DOI] [PubMed] [Google Scholar]
- Hamani C, Aziz T, Bloem BR, Brown P, Chabardes S, Coyne T, et al. Pedunculopontine Nucleus Region Deep Brain Stimulation in Parkinson Disease: Surgical Anatomy and Terminology. Stereotact Funct Neurosurg. 2016a;94:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Lozano AM, Mazzone PA, Moro E, Hutchison W, Silburn PA, et al. Pedunculopontine Nucleus Region Deep Brain Stimulation in Parkinson Disease: Surgical Techniques, Side Effects, and Postoperative Imaging. Stereotact Funct Neurosurg. 2016b;94:307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DS, Burt JM. Mechanism and selectivity of the effects of halothane on gap junction channel function. Circ Res 2000;86:1–10. [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–44. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic Variables in the Modification of Startle Reaction in the Rat. J Comp Physiol Psychol 1965;60:53–8. [DOI] [PubMed] [Google Scholar]
- Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E. Spatiotemporal properties of high speed calcium oscillations in the pedunculopontine nucleus. J Appl Physiol 2013;115:1402–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis WL, Winn P. The pedunculo-pontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol 1995;47:1–29. [DOI] [PubMed] [Google Scholar]
- Ison JR, Hammond GR. Modification of the startle reflex in the rat by changes in the auditory and visual environments. J Comp Physiol Psychol 1971;75:435–52. [DOI] [PubMed] [Google Scholar]
- Jacobsohn L Uber die kernes des menshlichen hirnstamms. Berlin: Verlag der Konigl Akademie der Wisenschaftern; 1909. [Google Scholar]
- Jiang X, Lautermilch NJ, Watari H, Westenbroek RE, Scheuer T, Caterall WA. Modulation of Cav2.1 channels by Ca+/calmodulin-dependent kinase II bound to the C-terminal domain. Proc Natl Acad Sci USA 2008;105:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Szilagyi S, Chakravorty S, Fiallos AM, Lewison BJ, Parwani A, et al. Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology 2004;41:401–6. [DOI] [PubMed] [Google Scholar]
- Karachi C, Grabli D, Bernard FA, Tande D, Wattiez N, Belaid H, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest. 2010;120:2745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayama Y, Ohta M, Jodo E. Firing of ‘possibly’ cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res 1992;569:210–220. [DOI] [PubMed] [Google Scholar]
- Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, Garcia-Rill E. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN). Eur J Neurosci 2011;34:404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Nakano K, Jayaraman A, Carpenter MB. Projections of the globus pallidus and adjacent structures: an autoradiographic study in the monkey. J Comp Neurol. 1976;169:263–90. [DOI] [PubMed] [Google Scholar]
- Kiziltan ME, Gunduz A. Reorganization of sensory input at brainstem in hemifacial spasm and postparalytic facial syndrome. Neurol Sci 2018a;39:313–9. [DOI] [PubMed] [Google Scholar]
- Kiziltan ME, Ozturk O, Gunduz A. Differential modulation of prepulse inhibition of the blink reflex in peripersonal versus extrapersonal space. Neurophysiol Clin 2018b;48:181–5. [DOI] [PubMed] [Google Scholar]
- Koch M, Kungel M, Herbert H. Cholinergic neurons in the pedunculopontine tegmental nucleus are involved in the mediation of prepulse inhibition of the acoustic startle response in the rat. Exp Brain Res 1993;97:71–82. [DOI] [PubMed] [Google Scholar]
- Kodsi MH, Swerdlow NR. Regulation of prepulse inhibition by ventral pallidal projections. Brain Res Bull 1997;43:219–28. [DOI] [PubMed] [Google Scholar]
- Kofler M, Halder W. Alterations in excitatory and inhibitory brainstem interneuronal circuits in fibromyalgia: evidence of brainstem dysfunction. Clin Neurophysiol 2014;125:593–601. [DOI] [PubMed] [Google Scholar]
- Kofler M, Kumru H, Schaller J, Saltuari L. Blink reflex prepulse inhibition and excitability recovery: influence of age and sex. Clin Neurophysiol 2013;124:126–35. [DOI] [PubMed] [Google Scholar]
- Kofler M, Müller J, Valls-Solé J. Auditory startle responses as a probe of brainstem function in healthy subjects and patients with movement disorders In: Cruccu G, Hallett M, editors. Suppl Clin Neurophysiol. Brainstem Function and Dysfunction: Elsevier Science B.V.; 2006. p. 232–48. [DOI] [PubMed] [Google Scholar]
- Kroeger D, Ferrari LL, Petit G, Mahoney CE, Fuller PM, Arrigoni E, et al. Cholinergic, Glutamatergic, and GABAergic Neurons of the Pedunculopontine Tegmental Nucleus Have Distinct Effects on Sleep/Wake Behavior in Mice. J Neurosci. 2017;37:1352–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V Sex differences and hormonal influences in human sensorimotor gating: implications for schizophrenia. Curr Top Behav Neurosci 2011;8:141–54. [DOI] [PubMed] [Google Scholar]
- Kumari V, Checkley SA, Gray JA. Effect of cigarette smoking on prepulse inhibition of the acoustic startle reflex in healthy male smokers. Psychopharmacology (Berl) 1996;128:54–60. [DOI] [PubMed] [Google Scholar]
- Kumari V, Cotter PA, Checkley SA, Gray JA. Effect of acute subcutaneous nicotine on prepulse inhibition of the acoustic startle reflex in healthy male non-smokers. Psychopharmacology (Berl) 1997;132:389–95. [DOI] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: The self medication hypotheses. Neurosci Biobehavioral Rev 2005;29:1021–1034. [DOI] [PubMed] [Google Scholar]
- Kumru H, Kofler M. Effect of spinal cord injury and of intrathecal baclofen on brainstem reflexes. Clin Neurophysiol 2012;123:45–53. [DOI] [PubMed] [Google Scholar]
- Kumru H, Opisso E, Valls-Solé J, Kofler M. The effect of a prepulse stimulus on the EMG rebound following the cutaneous silent period. J Physiol 2009;587:587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumru H, Stetkarova I, Schindler C, Vidal J, Kofler M. Neurophysiological evidence for muscle tone reduction by intrathecal baclofen at the brainstem level. Clin Neurophysiol 2011;122:1229–37. [DOI] [PubMed] [Google Scholar]
- Kumru H, Vidal J, Kofler M, Portell E, Valls-Solé J. Alterations in excitatory and inhibitory brainstem interneuronal circuits following severe spinal cord injury. J Neurotr 2010;27:721–8. [DOI] [PubMed] [Google Scholar]
- Langston JW, Langston EB, Irwin I. MPTP-induced parkinsonism in human and non-human primates--clinical and experimental aspects. Acta neurol Scand Suppl 1984;100:49–54. [PubMed] [Google Scholar]
- Leon-Sarmiento FE, Peckham E, Leon-Ariza DS, Bara-Jimenez W, Hallett M. Auditory and lower limb tactile prepulse inhibition in primary restless legs syndrome: clues to its pathophysiology. J Clin Neurophysiol 2015;32:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Armstrong DM, Atweh SF, Wainer BH. Monoclonal antibodies to choline acetyltranferase; production, specificity, and immunohistochemistry. J Neurosci. 1983;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Moro E, Lozano AM, Hamani C, Dostrovsky JO, Hutchison WD, et al. Selective enhancement of rapid eye movement sleep by deep brain stimulation of the human pons. Ann Neurol. 2009;66:110–4. [DOI] [PubMed] [Google Scholar]
- Luster B, D’Onofrio S, Urbano FJ, Garcia-Rill E. High-Threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN). Physiol Rep 2015;3:e12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster B, Urbano FJ, Garcia-Rill E. Intracellular mechanisms modulating gamma band activity in the pedunculopontine nucleus (PPN). Physiol Rep 2016;4:e12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya N, Bay K, Skinner RD, Garcia-Rill E. Induction of long-lasting depolarization in medioventral medulla (MED) neurons by cholinergic input from the pedunculopontine nucleus (PPN). J App Physiol 2005;99:1127–1137. [DOI] [PubMed] [Google Scholar]
- Manaye KF, Zweig RM, Wu D, Hersh LB, De Lacalle S, Saper CB, et al. Quantification of cholinergic and select non-cholinergic neuronal populations in the human brain. Neurosci. 1999;89:759–70. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology (Berl) 1988;94:507–14. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinsion’s disease. Neuroreport 2005;16:1877–1881. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Geula C, Bothwell MA, Hersh LB. Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J Comp Neurol 1989;283:611–33. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Mufson EJ, Levey AI, Wainer BH. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinersterase histochemistry. Neurosci 1984;12:669–86. [DOI] [PubMed] [Google Scholar]
- Muller J, Kofler M, Wenning GK, Seppi K, Valls-Solé J, Poewe W. Auditory startle response in cervical dystonia. Mov Disord 2003;18:1522–6. [DOI] [PubMed] [Google Scholar]
- Nandi D, Liu X, Winter JL, Aziz TZ, Stein JF. Deep brain stimulation of the pedunculopontine region in the normal non-human primate. J Clin Neurosci 2002;9:170–174. [DOI] [PubMed] [Google Scholar]
- Nauta WJ, Mehler WR. Projections of the lentiform nucleus in the monkey. Brain Res. 1966;1:3–42. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, Carpenter MG, Inglis JT, Duysens J, Weerdesteyn V. What startles tell us about control of posture and gait. Neuroscience and biobehavioral reviews. 2015a;53:131–8. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, de Kam D, Oude Nijhuis LB, van Geel K, Bloem BR, Geurts A, et al. StartReact effects support different pathophysiological mechanisms underlying freezing of gait and postural instability in Parkinson’s disease. PloS one. 2015b;10:e0122064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnekes J, Geurts AC, Nijhuis LB, van Geel K, Snijders AH, Bloem BR, et al. Reduced StartReact effect and freezing of gait in Parkinson’s disease: two of a kind? J Neurol. 2014;261:943–50. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, Post B, Tetrud JW, Langston JW, Bloem BR. MPTP-induced parkinsonism: an historical case series. Lancet Neurology. 2018;17:300–1. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, Snijders AH, Nutt JG, Deuschl G, Giladi N, Bloem BR. Freezing of gait: a practical approach to management. Lancet Neurol. 2015c;14:768–78. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk O, Gunduz A, Kiziltan ME. Deficient median nerve prepulse inhibition of the blink reflex in cervical dystonia. Clin Neurophysiol 2016;127:3524–8. [DOI] [PubMed] [Google Scholar]
- Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain. 2000;123 ( Pt 9):1767–83. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev 1995;20:91–127. [DOI] [PubMed] [Google Scholar]
- Perera T, Tan JL, Cole MH, Yohanandan SAC, Silberstein P, Cook R, et al. Balance control systems in Parkinson’s disease and the impact of pedunculopontine area stimulation. Brain 2018;141:3009–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DS, Pickett KA, Duncan R, Perlmutter J, Earhart GM. Gait-Related Brain Activity in People with Parkinson Disease with Freezing of Gait. PloS one. 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. Neuroreport 2005;16:1883–1887. [DOI] [PubMed] [Google Scholar]
- Putnam LE, Vanman EJ. Long lead interval startle modification In: Dawson ME, Schell AM, Böhmelt AH, editors. Startle modification. Cambridge: Cambridge University Press; 1999. p. 72–92. [Google Scholar]
- Reese NB, Garcia-Rill E, Skinner RD. The pedunculopontine nucleus-auditory input, arousal and pathophysiology. Prog Neurobiol 1995;47:105–133. [DOI] [PubMed] [Google Scholar]
- Revuelta GJ, Uthayathas S, Wahlquist AE, Factor SA, Papa SM. Non-human primate FOG develops with advanced parkinsonism induced by MPTP Treatment. Experimental neurology. 2012;237:464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Scarpini C. Gating of trigemino-facial reflex from low-threshold trigeminal and extratrigeminal cutaneous fibres in humans. J Neurol Neurosurg Psychiatry 1992;55:774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozental R, Srinivas M, Spray DC. How to close a gap junction channel. Methods Mol Biol 2001;154:447–477. [DOI] [PubMed] [Google Scholar]
- Rye DB. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20:757–88. [DOI] [PubMed] [Google Scholar]
- Rye DB, Lee HJ, Saper CB, Wainer BH. Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol 1988;269:315–341. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: Cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol. 1987;259:483–528. [DOI] [PubMed] [Google Scholar]
- Rye DB, Turner RS, Vitek JL, Bakay RAE, Crutcher MD, DeLong MR. Anatomical investigations of the pallidotegmental pathway in monkey and man In: Ohye CK M; McKenzie JS, editor. The Basal Ganglia V. New York: Plenum; 1996. p. 59. [Google Scholar]
- Sakai K Some anatomical and physiological properties of ponto-mesencephalic tegmental neurons with special reference to the PGO waves and postural atonia during paradoxical sleep in the cat In: Hobson JA, Brazier MAB, editors. The Reticular Formation Revisited: Raven, New York; 1980. p. 427–47. [Google Scholar]
- Sakai K, El Mansari M, Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res 1990;527:213–223. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Morrison AR, Mann GL, Harris JS, Yoo L, Ross RJ. Sleep patterning and behaviour in cats with pontine lesions creating REM without atonia. J Sleep Res 1994;3:233–240. [DOI] [PubMed] [Google Scholar]
- Scelzo E, Lozano AM, Hamani C, Poon YY, Aldakheel A, Zadikoff C, et al. Peduncolopontine nucleus stimulation in progressive supranuclear palsy: a randomised trial. J Neurol Neurosurg Psychiatry. 2017;88:613–6. [DOI] [PubMed] [Google Scholar]
- Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur J Neurol. 2003;10:391–8. [DOI] [PubMed] [Google Scholar]
- Schute CCD, Lewis PR. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967;90:497–520. [DOI] [PubMed] [Google Scholar]
- Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;69:308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechenov I Refleksy golovnogo mozga [“Reflexes of the brain“]. Meditsinsky vestnik 1863:47–8. [Google Scholar]
- Sherman D, Fuller PM, Marcus J, Yu J, Zhang P, Chamberlin NL, et al. Anatomical Location of the Mesencephalic Locomotor Region and Its Possible Role in Locomotion, Posture, Cataplexy, and Parkinsonism. Front Neurol. 2015;6:140. doi: 10.3389/fneur.2015.00140. eCollection;%2015.:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih LC, Vanderhorst VG, Lozano AM, Hamani C, Moro E. Improvement of pisa syndrome with contralateral pedunculopontine stimulation. Mov Disord. 2013;28:555–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovskii GN. Control of walking and running by means of electric stimulation of the midbrain. Biofizika 1966;11:659–666. [PubMed] [Google Scholar]
- Shik ML, Orlovsky GN. Neurophysiology of locomotor automatism. Physiol Rev. 1976;56:465–501. [DOI] [PubMed] [Google Scholar]
- Shine JM, Matar E, Ward PB, Bolitho SJ, Pearson M, Naismith SL, et al. Differential neural activation patterns in patients with Parkinson’s disease and freezing of gait in response to concurrent cognitive and motor load. PloS one. 2013;8:e52602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Kezunovic N, Ye M, Hyde J, Hayar A, Williams DK, et al. Gamma band unit activity and population responses in the pedunculopontine nucleus. J Neurophysiol 2010;104:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner RD, Garcia-Rill E. Brainstem modulation of rhythmic functions and behaviors, In: Klemm WR, Vertes RP, editors. Brainstem Mechanisms of Behavior, John Wiley & Sons, New York, 1990, pp. 419–445. [Google Scholar]
- Skinner RD, Rasco LM, Fitzgerald J, Karson CN, Matthew M, Williams DK, et al. 1999 Reduced sensory gating of the P1 potential in rape victims and combat veterans with post traumatic stress disorder. Dep Anx 1999;9:122–130. [DOI] [PubMed] [Google Scholar]
- Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, et al. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain. 2011;134:59–72. [DOI] [PubMed] [Google Scholar]
- Snijders AH, Takakusaki K, Debu B, Lozano AM, Krishna V, Fasano A, et al. Physiology of freezing of gait. Annals of Neurology. 2016;80:644–59. [DOI] [PubMed] [Google Scholar]
- Soh D, Algarni M, Wong A, Lozano AM, Fasano A. Stimulation-induced reversed plus-minus syndrome: Insights into eyelid physiology. Brain Stimul. 2018;11:951–2. [DOI] [PubMed] [Google Scholar]
- Stea A, Soomg TW & Snutch TP Determinants of PKC-dependent modulation of a family of neuronal Ca2+ channels. Neuron 15 (1995) 929–940. [DOI] [PubMed] [Google Scholar]
- Steriade M Cellular substrates of oscillations in corticothalamic systems during states of vigilance In: Lydic R, Baghdoyan HA, editors. Handbook of Behavioral State Control. Cellular and molecular mechanisms, CRC Press, New York, 1999, pp. 327–347. [Google Scholar]
- Steriade M, Paré D, Datta S, Oakson D, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci 1990;10:2560–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol Psychiatry 1993a;33:298–301. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol 2000;11:185–204. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Sensorimotor gating of the startle reflex: what we said 25 years ago, what has happened since then, and what comes next. J Psychopharmacol (Oxford, England) 2016;30:1072–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Prepulse inhibition of acoustic startle in rats after lesions of the pedunculopontine tegmental nucleus. Behav Neurosci 1993b;107:104–17. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Neurophysiology and neuropharmacology of short lead interval startle modification In: Dawson ME, Schell AM, Böhmelt AH, editors. Startle Modification Implications for Neuroscience, Cognitive Science, and Clinical Science. Cambridge: Cambridge University Press; 1999. p. 114–33. [Google Scholar]
- Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: implications for neuropsychiatric disorders. Biol Psychiat 1997;41:452–60. [DOI] [PubMed] [Google Scholar]
- Tabor KM, Smith TS, Brown M, Bergeron SA, Briggman KL, Burgess HA. Presynaptic inhibition selectively gates auditory transmission to the brainstem startle circuit. Curr Biol 2018;28:2527–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall TL, Stratton PG, Coyne TJ, Cook R, Silberstein P, Silburn PA, et al. Imagined gait modulates neuronal network dynamics in the human pedunculopontine nucleus. Nat Neurosci. 2014;17:449–54. [DOI] [PubMed] [Google Scholar]
- Teo C, Rasco Al, Al-Mefty K, Skinner RD, Garcia-Rill E Decreased habituation of midlatency auditory evoked responses in Parkinson’s disease, Movement Disord 1997;12:655–664. [DOI] [PubMed] [Google Scholar]
- Teo C, Rasco Al, Skinner RD, Garcia-Rill E Disinhibition of the sleep state-dependent P1 potential in Parkinson’s disease- improvement after pallidotomy, Sleep Res Online 1998;1:62–70. [PubMed] [Google Scholar]
- Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Bogdanovic M, Coyne TJ, et al. A block to pre-prepared movement in gait freezing, relieved by pedunculopontine nucleus stimulation. Brain. 2011;134:2085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevathasan W, Debu B, Aziz T, Bloem BR, Blahak C, Butson C, et al. Pedunculopontine nucleus deep brain stimulation in Parkinson’s disease: A clinical review. Mov Disord. 2018;33:10–20. [DOI] [PubMed] [Google Scholar]
- Thevathasan W, Debu B, Aziz T, Bloem BR, Blahak C, Butson C, et al. Pedunculopontine nucleus deep brain stimulation in Parkinson’s disease: A clinical review. Mov Disord. 2018a;33:10–20. [DOI] [PubMed] [Google Scholar]
- Uc E, Skinner RD, Rodnitzky L, Garcia-Rill E. The midlatency auditory evoked potential P50 is abnormal in Huntington’s Disease. J Neurol Sci 2003;212:1–5. [DOI] [PubMed] [Google Scholar]
- Urbano FJ, Kezunovic N, Hyde J, Simon C, Beck P, Garcia-Rill E. Gamma band activity in the reticular activating system (RAS). Frontiers in Neurology; Sleep and Chronobiology 2012;3:6,1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygunoglu U, Gunduz A, Ertem HD, Uluduz D, Saip S, Goksan B, et al. Deficient prepulse inhibition of blink reflex in migraine and its relation to allodynia. Clin Neurophysiol 2017;47:63–8. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Cammarota A, Alvarez R, Hallett M. Orbicularis oculi responses to stimulation of nerve afferents from upper and lower limbs in normal humans. Brain Res 1994;650:313–316. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Kofler M, Kumru H, Castellote JM, Sanegre M. Startle-induced reaction time shortening is not modified by prepulse inhibition. Exp Brain Res 2005;165:541–548. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Kumru H, Kofler M. Interaction between startle and voluntary reactions in humans. Experimental Brain Research 2008;187:497–507. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Muñoz JE, Valldeoriola F. Abnormalities of prepulse inhibition do not depend on blink reflex excitability: a study in Parkinson’s disease and Huntington’s disease. Clin Neurophysiol 2004;115:1527–36. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Valldeoriola F, Molinuevo JL, Cossu G, Nobbe F. Prepulse modulation of the startle reaction and the blink reflex in normal human subjects. Exp Brain Res 1999;129:49–56. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contan distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci 2009;29:340–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Hamani C, Hutchison WD, Moro E, Lozano AM, Dostrovsky JO. Pedunculopontine nucleus microelectrode recordings in movement disorder patients. Exp Brain Res. 2008;188:165–74. [DOI] [PubMed] [Google Scholar]