Abstract
Objective:
To provide evidence available in the literature on the role of granulocyte colony stimulating factor (G-CSF) in women submitted to in vitro fertilization, with repeated implantation failure associated with thin endometrium.
Methods:
Systematic review of the use of G-CSF, as part of assisted reproduction techniques in women with repeated embryo implantation failures associated with thin endometrium. The study was carried out in the PubMed, BIREME and Elsevier databases from 2008 to 2018, in English, Spanish and Portuguese.
Results:
We included all the studies, which used intrauterine G-CSF. We found an increase in endometrial thickness in eight of the 10 studies included. Of these, the implantation rate improved significantly in two studies, but the gestation rate increased in only one. We found the highest rates of implantation (32%) and pregnancy (48%) in a non-randomized clinical trial. On the other hand, two other studies did not demonstrate an increase in endometrial thickness and in pregnancy rates in patients with thin endometrium submitted to the assisted reproduction in frozen embryo transfer cycles.
Conclusion:
Studies published so far point to a positive influence on the use of G-CSF in relation to the improvement in endometrial receptivity and pregnancy rates. Therefore, there is a need for further studies to determine whether to use it, as well as the period, route of administration, dosage and duration of treatment.
Keywords: granulocyte colony-stimulating factor, in vitro fertilization, endometrium
INTRODUCTION
Despite advances in assisted reproduction, the rates of a well-succeeded embryo implantation are still low. Embryo quality and endometrial receptivity, apart from a suitable embryo transfer technique, may influence the success of such implantation (Zenclussen & Hämmerling, 2015; Kushnir et al., 2017). However, the morphological quality of an embryo is not a guarantee of a well-succeeded implantation. Therefore, the exclusion of an embryo with a better chance of implantation may occur, just because it was not considered the one with the best morphological aspect at the moment of evaluation (Donadio et al., 2012).
Conversely, it is believed that the adequate endometrial thickness could make the endometrial cells change into decidual easier, as well as the invasion of blastocysts and a timely placenta growth (Zenclussen & Hämmerling, 2015). However, there is no agreement in the literature regarding endometrial thickness to characterize a receptive endometrium. A thin endometrium is seen more often in women aged over 40, probably due to vascularity decrease. A 2.4% to 5% prevalence of thin endometrium has been reported in women under 40 years of age, and 25% in women over 40 in natural cycles (Sher et al., 1991; Kasius et al., 2014).
There are studies which indicate a thickness threshold below 7mm (Mahajan & Sharma, 2016; Cavalcante et al., 2015), yet others report 6mm (Shapiro et al., 1993; Kunicki et al., 2014) or 8mm (Gingold et al., 2015). On the other hand, a study reports clinical pregnancy with 4mm of endometrial thickness (Check et al., 2016), which brings about the possibility that the endometrial receptivity may not necessarily be related to the endometrial thickness. In spite of not having a consensus, endometrial thickness has been used to predict the likelihood of pregnancy in assisted reproduction cycles.
Today, there are pieces of evidence that the embryonic implantation process turned easier by immune cells, growth factors, cytosines, and hormonal changes (Kunicki et al., 2014; Davari-Tanha et al., 2016; Eftekhar et al., 2016a). G-CSF is a hemanopoietic cytosine produced in the reproductive system, at the maternofetal interface, during embryo implanting, which stimulates granulocyte proliferation and differentiation. It has been suggested that this cytosine could, therefore, play a role both on the decidua and the placental function (Salmassi et al., 2004; Li et al., 2014; Cavalcante et al., 2015). G-CSF for clinical use is mainly indicated to reduce neutropenia duration and fevered neutropenia incidence in patients with non-myelogenic neoplasia, undergoing cytotoxic chemotherapy. Besides this, it is also indicated to reduce neutropenia duration and after-effects in patients submitted to myeloablative therapy followed by bone marrow transplant (Wurfel, 2015). Synthetic G-CSF differs from its natural counterpart for presenting an additional N-methionine terminal residue and for the lack of O-Glycogenesis. In Brazil this drug is traded under the name Filgastrim (Granulokine; Roche), presented in pre-bottled syringes holding 0.5mL injectable solution, containing 300µg, which comprises 30 million units.
The first evidence of improvement on in vitro fertilization embryo implanting rates and higher G-CSF concentration in follicular liquid was reported in 2005 (Salmassi et al., 2005). Since that time, some studies have evaluated G-CSF usage in a systemic form via subcutaneous injection or directly in the endometrium via intrauterine injection (Kunick et al., 2014, Li et al., 2014), in women with recurrent spontaneous abortion and repeated implantation failures. Others show pregnancy improved results (Santjohanser et al., 2013; Eftekhar et al., 2016b), even in those with thin endometrium (Gleicher et al., 2013; Lucena & Moreno-Ortiz, 2013). Thus, the objective of this study was to systematize the literature evidence on the use of G-CSF and pregnancy rates in women submitted to assisted reproduction techniques (ART) with repeated failures associated with thin endometrium.
MATERIALS AND METHODS
This systematic review study included papers published in English, Spanish, and Portuguese, which investigated the use of intrauterine or subcutaneous G-CSF in cases of implantation failure, associated with thin endometrium in the context of human assisted reproduction. We searched in PubMed, Bireme and Elsevier databases, using the following keywords: "granulocyte colony-stimulating factor” [MeSH Terms] AND "endometrium"[MeSH Terms] AND ("humans"[MeSH Terms] AND (English [lang] OR Portuguese[lang] OR Spanish[lang])) in a-10 year period (from January 2008 to March 2018).
Two independent authors read the titles and abstracts in order to check for duplicates and to meet the pre-established inclusion criteria. Afterwards, they read the potentially eligible papers entirely. Those papers, which despite reporting the use of G-CSF in human reproduction did not evaluate its impact on endometrial thickness and on pregnancy rates, we discarded. The data was extracted from the text, tables, and graphs in the studies included. We collected information such as study type, place and year of publication, number and age of participants, the timeframe and G-CSF administration via, endometrial thickness before and after G-CSF, and pregnancy rates.
RESULTS
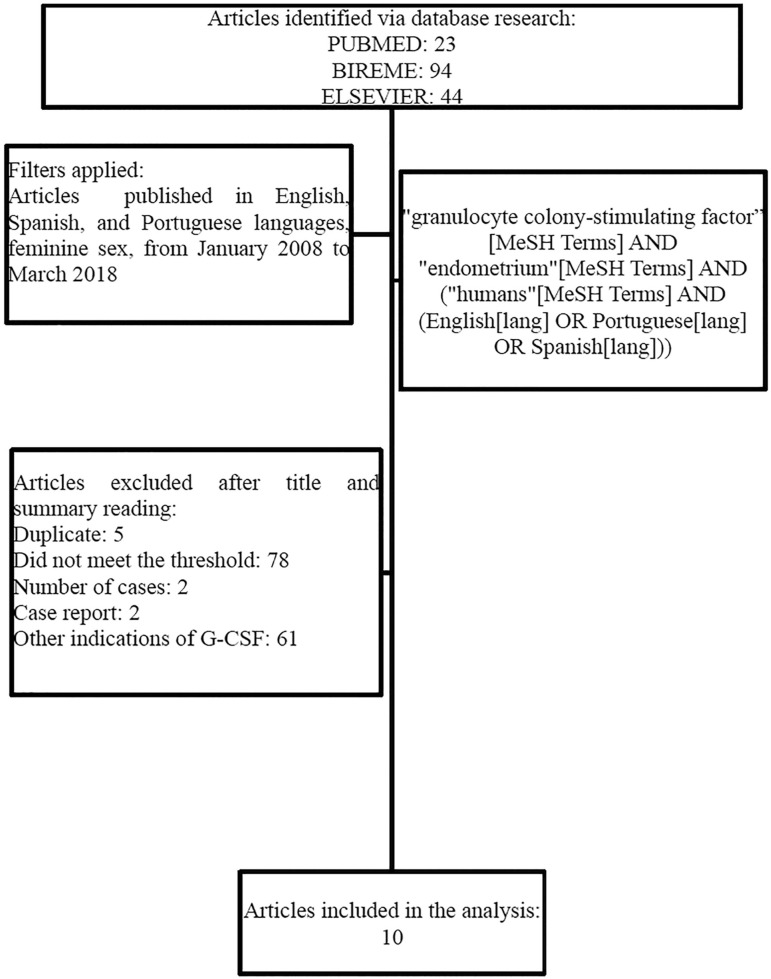
We selected 161 papers: 23 from PubMed, 94 from Bireme, and 44 from Elsevier (Figure 1). Five papers were taken off for being duplicated, 78 for not addressing the G-CSF regarding repeated failures associated with thin endometrium, and 68 for addressing other indications regarding the use of G-CSF. In our study, 10 papers were included, namely, two randomized clinical trials (Eftekhar et al., 2016b; Sarvi et al., 2017), three not-randomized clinical trials (Xu et al., 2015; Tehraninejad et al., 2015; Eftekhar et al., 2014), three prospective cohort studies (Shah et al., 2014; Gleicher et al., 2011; 2013) and two cross-sectional studies (Mishra et al., 2016; Kunicki et al., 2014).
Figure 1.
Flowchart of the papers included in the systematic review study
Out of these 10 studies, seven were published in the Asian continent, one in the European, and two in the American continent (Chart 1). These papers were published from 2011 to 2017 and included 475 participants with thin endometrium and repeated failures in the assisted reproduction techniques to whom, G-CSF was employed. The average age range of the participants included in the published studies was from 30.5 to 40.5. All of the 10 studies included utilized G-CSF via uterine at a 300mcg dosage. The G-CSF application day concerning the menstrual cycle period varied among the studies (Chart 1).
Chart 1.
List of the studies that evaluated the use of G-CSF in women submitted to assisted fertilization, which held thin endometrium and repeated failures
| First author/Year Geographic Region | Study Type | Participants number | Average age | G-CSFmethod of use Date | Endometrium thikness after G-CSF (average) | Pregnancy rates (%) |
|---|---|---|---|---|---|---|
| Sarvi et al., 2017 Iran | RCT | TG: 13 CG: 15 | TG:31.2 CG: 31.6 | TG: 300 mcg IU GC: Saline | TG: 5±1.4 mm | CG: 20 TG: 15.3 ** |
| Eftekhar et al., 2016b Iran | RCT | TG: 44 CG: 45 | TG: 32.5 CG: 31.7 | 300 mcg IU | CG: 8.8 TG: 9.1 ** | TG: 28.8 CG: 1.3 * |
| Xu B et al., 2015 China | Non-randomized CT | TG: 41 CG: 65 | TG: 31.4 CG: 32.0 | 300 mcg IU | 5.7mm antes 8.4 mm após* | TG: 48 CG: 25* |
| Tehraninejad et al., 2015 Iran | Non-randomizedCT | 15 | 35.13 | 300 mcg IU eggs collecting day | 3.6mm antes 7.1mm após* | 20 |
| Eftekhar et al., 2014 Iran | Non-randomized CT | TG: 34 CG: 34 | TG: 30.8 CG: 28.6 | 300 mcg IU 12th to 13th day | 5.6 CG 5.8: TG ** | CG:28.6 TG:30.8 ** |
| Shah et al., 2014 India | Prospective cohort study | EG: 231 NEG: 117 | 33.5 | 300 mcg IU 10 days from estrogen onset | < 8mm before 10.9mm after * | 37 |
| Gleicher et al., 2013 USA | Prospective cohort study | 21 | 40.5 | 300 mcg IU on hCG day | 5.7mm before 9.3mm after* | 19.1 |
| Gleicher et al., 2011 USA | Prospective cohort study | 4 | 38.3 | 300mcg IU 48 h beforeET | 4.9mm before 8.7mm after* | 100 (1 ectopic) |
| Mishra et al., 2016 India | Cross-sectional study | 35 | 30.5 | 300 mcg IU on 14th day of cycle | 5.9mm before 6.6mm after * | Zero |
| Kunicki et al., 2014 Poland | Cross-sectional study | 37 | 34.7 | 300 mcg IU on hCG day | 6.7 before 8.4 after * | 19.1 |
RCT: Randomized clinical trial. IU: intrauterine; ET: Embryo Transfer; CT: Clinical Trial;
SD: Standard Deviation; IR: implantation Rate;
Significant;
not significant.
TG: Treated Group.
CG: Control Group, EG: Exposed Group; NEG: Non-Exposed Group.
Three out of the five clinical trials evidenced significant increases on the endometrial thickness with the use of G-CSF (Sarvi et al., 2017; Xu et al., 2015; Tehraninejad et al., 2015); three prospective cohorts (Shah et al., 2014; Gleicher et al., 2011; 2013) and two out of the cross-sectional studies (Mishra et al., 2016; Kunicki et al., 2014) included in the present review. The pregnancy rates in the studies, which showed endometrial thickness significant increase, ranged from 19.1% to 37.0% (Chart 1).
Three non-randomized clinical trials (Eftekhar et al., 2014; Tehraninejad et al., 2015; Xu et al., 2015) and two randomized clinical studies (Eftekhar et al., 2016b; Sarvi et al., 2017) showed implantation rate increase. Despite the small number of participants involved in the included randomized trials, the implantation rate ranged from 10 to 17% and the pregnancy was 29% after G-CSF treatment. Three out of the ten included studies did not evidence improvements in pregnancy rates (Eftekhar et al., 2014; Mishra et al., 2016; Sarvi et al., 2017). As for the other seven studies, the rates ranged from 19 to 37% (Gleicher et al., 2011; 2013; Kunicki et al., 2014; Shah et al., 2014; Tehraninejad et al., 2015; Xu et al., 2015; Eftekhar et al., 2016b). The highest clinical pregnancy rate (28.8%) was found in the randomized clinical trial (Eftekhar et al., 2016b) and, the rate in the implantation group, treated with G-CSF, was 17%; whereas in the control group, it was 5% (p<0.05).
DISCUSSION
Intrauterine G-CSF infusion in assisted reproduction cycles aims at increasing the endometrial receptivity and, thus, reshape and increase endometrium thickness. Therefore, it would help the embryo transfer and clinical pregnancy rates (Eftekhar et al., 2014; Kunicki et al., 2014; Eftekhar et al., 2016a; b). Nonetheless, literature data is not conclusive regarding what it considers as thin or unresponsive endometrium. Some studies state that pregnancy takes place when the endometrium reaches over 7mm (Gleicher et al., 2013; Gingold et al., 2015), and others say that more than 9mm is required (Kasius et al., 2014; Mahajan & Sharma, 2016). Besides this, there is strong evidence that the thin endometrium is not necessarily a factor, which hinders, a well-succeeded embryo implanting. There is evidence that the thin endometrium is not necessarily a factor preventing successful embryo implantation, although it may negatively affect pregnancy after embryo transfers (Gingold et al., 2015; Mahajan & Sharma, 2016).
The studies included in this review, demonstrated an effect considered moderate, on the unresponsive endometrium treatment, with low implantation and pregnancy rates. G-CSF use as an additive in assisted reproduction treatment, aiming at enhancing endometrial receptivity, is new. It is expected, though, that G-CSF will be an outstanding agent in assisted reproduction (Eftekhar et al., 2016a; Sarvi et al., 2017).
Randomized clinical trials are taken as standard of excellence to evaluate the efficacy of an intervention. So, the results presented by Eftekhar et al. (2016b) and Sarvi et al. (2017), concerning thin endometria and low implantation and pregnancy rates, provide resources to carry out trials with higher number of participants aiming at evaluating the G-CSF efficacy. Another important factor to be noticed is that there was no homogeneity on the treatment utilized in the evaluated trials, neither with regards to intrauterine infusion day nor number and dose applied. Therefore, there is a need for better evidence about number and dose, and on the cycle phase they should be administered. In addition to this, the timeframe between intrauterine infusion and endometrium evaluation is not well-grounded in literature. Gleicher et al. (2011), did the re-evaluation at 48h, time much shorter than the one reported by Kunicki et al. (2014).
One of the earlier publications regarding its use dates back to 2011 (Gleicher et al., 2011). However, over these seven years, few clinical essays were performed with few participants, which constraints the conclusion with regards to the benefit or not of its use, in assisted reproduction. Thus, despite improvements in endometrial thickness associated with increases in pregnancy rates, confirmed in eight studies in the present review (Gleicher et al., 2011; 2013; Shah et al., 2014; Kunicki et al., 2014; Tehraninejad et al., 2015; Xu et al., 2015; Mishra et al., 2016; Sarvi et al., 2017), the results are not sufficient yet to provide a robust evidence for the use of G-CSF in patients with thin endometria, as well as repeated failures both at implantation and pregnancy rates. In a cohort study, all of the treated patients improved their endometrial thickness at an average of 3.54 mm upon treatment and, at the moment of the publishing they had an ongoing pregnancy (Gleicher et al., 2011).
Thus, the best moment for this evaluation still remains as a question to be answered. Likewise, there is a need for long-term studies to evaluate the impact of the treatment on the future health of the babies born. Amongst the results limitation, we point out the small number of studies, all of them with small sampling, and the variability of evaluated criteria, as well as low levels of evidence, based on the study design of the included papers. Thus, in order to indicate the clinical use of the G-CSF in patients with implantation failure due to thin endometrium, there is a need for further studies.
CONCLUSION
The pieces of evidence in the literature suggest a positive influence of G-CSF on improving endometrial receptivity and pregnancy rates. Notwithstanding, the literature evidence is conflicting and of hard comparison because of the small number of studies addressing the theme, as well as for the different types of studies. There is a must for more controlled randomized studies involving a larger number of participants to make it possible to establish the correct prescription, as well as the suitable dose and the treatment timeframe.
ACKNOWLEDGEMENTS
In this study, I counted on the support and collaboration of very special people, who became even more important in my professional carrier. For them, I express my recognition; above all, for the incentive and the support to overcome great limits and difficulties, at this time of my living.
Footnotes
CONFLICT OF INTERESTS
There is not any conflict of interests on the proposed study we carried out here.
REFERENCES
- Cavalcante MB, Costa Fda S, Barini R, Araujo E., Júnior Granulocyte colony-stimulating factor and reproductive medicine: A review. Iran J Reprod Med. 2015;13:195–202. [PMC free article] [PubMed] [Google Scholar]
- Check JH, Choe JK, Summers-Chase D. Failure to increase the thickness of thin endometria with intrauterine infusion of granulocyte colony stimulating factor (G-CSF) Clin Exp Obstet Gynecol. 2016;43:332–333. [PubMed] [Google Scholar]
- Davari-Tanha F, Shahrokh Tehraninejad E, Ghazi M, Shahraki Z. The role of G-CSF in recurrent implantation failure: A randomized double blind placebo control trial. Int J Reprod Biomed (Yazd) 2016;14:737–742. doi: 10.29252/ijrm.14.12.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadio NF, Dzik A, Cavagna M, Donadio N. Fertilização. In: Dzik A, Donadio NF, Esteves SC, Nagy ZP, editors. Atlas de Reprodução umana. São Paulo: Segmento Farma; 2012. pp. 123–129. Vitro. [Google Scholar]
- Eftekhar M, Sayadi M, Arabjahvani F. Transvaginal perfusion of G-CSF for infertile women with thin endometrium in frozen ET program: A non-randomized clinical trial. Iran J Reprod Med. 2014;12:661–666. doi: 10.29252/ijrm.14.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhar M, Hosseinisadat R, Baradaran R, Naghshineh E. Effect of granulocyte colony stimulating factor (G-CSF) on IVF outcomes in infertile women: An RCT. Int J Reprod Biomed (Yazd) 2016a;14:341–346. doi: 10.29252/ijrm.14.11.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhar M, Miraj S, Farid Mojtahedi M, Neghab N. Efficacy of Intrauterine infusion of granulocyte colony stimulating factor on patients with history of implantation failure: A randomized control trial. Int J Reprod Biomed (Yazd) 2016b;14:687–690. [PMC free article] [PubMed] [Google Scholar]
- Gingold JA, Lee JA, Rodriguez-Purata J, Whitehouse MC, Sandler B, Grunfeld L, Mukherjee T, Copperman AB. Endometrial pattern, but not endometrial thickness, affects implantation rates in euploid embryo transfers. Fertil Steril. 2015;104:620–628. doi: 10.1016/j.fertnstert.2015.05.036. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Vidali A, Barad DH. Successful treatment of unresponsive thin endometrium. Fertil Steril. 2011;95:e13–e17. doi: 10.1093/humrep/des370. 2123. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Kim A, Michaeli T, Lee HJ, Shohat-Tal A, Lazzaroni E, Barad DH. A pilot cohort study of granulocyte colony-stimulating factor in the treatment of unresponsive thin endometrium resistant to standard therapie. Hum Reprod. 2013;28:172–177. doi: 10.1093/humrep/des370. [DOI] [PubMed] [Google Scholar]
- Kasius A, Smit JG, Torrance HL, Eijkemans MJ, Mol BW, Opmeer BC, Broekmans FJ. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:530–541. doi: 10.1093/humupd/dmu011. [DOI] [PubMed] [Google Scholar]
- Kunicki M, Łukaszuk K, Woclawek-Potocka I, Liss J, Kulwikowska P, Szczyptańska J. Evaluation of granulocyte colony-stimulating factor effects on treatment-resistant thin endometrium in women undergoing in vitro fertilization. Biomed Res Int. 2014;2014:913235–913235. doi: 10.1155/2014/913235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod Biol Endocrinol. 2017;15:6–6. doi: 10.1186/s12958-016-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Pan P, Chen X, Li L, Li Y, Yang D. Granulocyte colony-stimulating factor administration for infertile women with thin endometrium in frozen embryo transfer program. Reprod Sci. 2014;21:381–385. doi: 10.1177/1933719113497286. [DOI] [PubMed] [Google Scholar]
- Lucena E, Moreno-Ortiz H. Granulocyte colony-stimulating factor (G-CSF): a mediator in endometrial receptivity for a patient with polycystic ovary (PCO) undergoing in vitro maturation (IVM) BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2012-008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan N, Sharma S. The endometrium in assisted reproductive technology: How thin is thin? J Hum Reprod Sci. 2016;9:3–8. doi: 10.4103/0974-1208.178632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra VV, Choudhary S, Sharma U, Aggarwal R, Agarwal R, Gandhi K, Goraniya N. Effects of Granulocyte Colony-Stimulating Factor (GCSF) on Persistent Thin Endometrium in Frozen Embryo Transfer (FET) Cycles. J Obstet Gynaecol India. 2016;66:407–411. doi: 10.1007/s13224-015-0775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmassi A, Schmutzler AG, Huang L, Hedderich J, Jonat W, Mettler L. Detection of granulocyte colony-stimulating factor and its receptor in human follicular luteinized granulosa cells. Fertil Steril. 2004;81:786–791. doi: 10.1016/j.fertnstert.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Salmassi A, Schmutzler AG, Schaefer S, Koch K, Hedderich J, Jonat W, Mettler L. Is granulocyte colony-stimulating factor level predictive for human IVF outcome? Hum Reprod. 2005;20:2434–2440. doi: 10.1093/humrep/dei071. [DOI] [PubMed] [Google Scholar]
- Santjohanser C, Knieper C, Franz C, Hirv K, Meri O, Schleyer M, Würfel W, Toth B. Granulocyte-colony stimulating factor as treatment option in patients with recurrent miscarriage. Arch Immunol Ther Exp (Warsz) 2013;61:159–164. doi: 10.1007/s00005-012-0212-z. [DOI] [PubMed] [Google Scholar]
- Sarvi F, Arabahmadi M, Alleyassin A, Aghahosseini M, Ghasemi M. Effect of Increased Endometrial Thickness and Implantation Rate by Granulocyte Colony-Stimulating Factor on Unresponsive Thin Endometrium in Fresh In Vitro Fertilization Cycles: A Randomized Clinical Trial. Obstet Gynecol Int. 2017;2017:3596079–3596079. doi: 10.1155/2017/3596079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J, Gangadharan A, Shah V. Effect of intrauterine instillation of granulocyte colony-stimulating factor on endometrial thickness and clinical pregnancy rate in women undergoing in vitro fertilization cycles: an observational cohort study. Int J Infertil Fetal Med. 2014;5:100–106. doi: 10.5005/jp-journals-10016-1090. [DOI] [Google Scholar]
- Shapiro H, Cowell C, Casper RF. The use of vaginal ultrasound for monitoring endometrial preparation in a donor oocyte program. Fertil Steril. 1993;59:1055–1058. doi: 10.1016/S0015-0282(16)55927-5. [DOI] [PubMed] [Google Scholar]
- Sher G, Herbert C, Maassarani G, Jacobs MH. Assessment of the late proliferative phase endometrium by ultrasonography in patients undergoing in-vitro fertilization and embryo transfer (IVF/ET) Hum Reprod. 1991;6:232–237. doi: 10.1093/oxfordjournals.humrep.a137312. [DOI] [PubMed] [Google Scholar]
- Tehraninejad E, Davari Tanha F, Asadi E, Kamali K, Aziminikoo E, Rezayof E. G-CSF Intrauterine for Thin Endometrium, and Pregnancy Outcome. J Family Reprod Health. 2015;9:107–112. [PMC free article] [PubMed] [Google Scholar]
- Wurfel W. Treatment with granulocyte colony-stimulating factor in patients with repetitive implantation failures and/or recurrent spontaneous abortions. J Reprod Immunol. 2015;108:123–135. doi: 10.1016/j.jri.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Xu B, Zhang Q, Hao J, Xu D, Li Y. Two protocols to treat thin endometrium with granulocyte colony-stimulating factor during frozen embryo transfer cycles. Reprod Biomed Online. 2015;30:349–358. doi: 10.1016/j.rbmo.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Hämmerling GJ. Cellular Regulation of the Uterine Microenvironment That Enables Embryo Implantation. Front Immunol. 2015;6:321–321. doi: 10.3389/fimmu.2015.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]