Abstract
The putative active metabolite of aeruginascin, a naturally occurring tryptamine of “magic mushrooms,” has been synthesized and structurally characterized. Competitive radioligand binding assays demonstrate that it has a high affinity at human serotonin receptors 5-HT1A, 5-HT2A, and 5-HT2B, though it does not bind at the 5-HT3 receptor, where activity was previously predicted.
Introduction
More than 200 species of fungi, collectively known as “magic mushrooms,” including those from the genus Psilocybe, are known to contain psychoactive tryptamine compounds.1 Components of “magic mushrooms” (i.e., psilocybin/psilocin) have incredible potential for treating intractable mental and physical conditions.2 These drugs show promise in the treatment of disorders, including addiction,3,4 anxiety,5 depression,6 and post-traumatic stress disorder.7 Of note, psilocybin was granted the “breakthrough therapy” designation by the US Food and Drug Administration (FDA).8 This FDA designation has cleared the way for clinical trials of psilocybin to treat major depressive disorders and treatment-resistant depression.
One of the biggest concerns in using these compounds as pharmaceuticals for humans is the potential for a “bad trip” or dysphoric experience.9 The extracts of “magic mushrooms” demonstrate the same clinical effects as pure psilocybin at dosages that are an order of magnitude smaller,10 suggesting important activity by other psychoactive molecules or the presence of an entourage effect.11 To have a better understanding of how “magic mushroom” extracts function, it is important to understand the properties of the minor active components of “magic mushrooms,” alone and in combination with psilocybin.
New psychoactive tryptamines have been identified in “magic mushrooms” as recently as 2017.12 Until this year, there was no general synthetic method for producing useful amounts of the minor psychoactive tryptamines.13 One of these minor components is aeruginascin,14 the N-trimethyl analogue of psilocybin. Documented accounts of human exposure to Inocybe aeruginascens, a species of mushroom containing aeruginascin, describe hallucinations that exhibited only euphoric experiences.15 These limited reports about aeruginascin are interesting, given that dysphoria or a “bad trip” is a significant concern associated with consuming psilocybin or mushrooms containing it. Despite these observations, the pharmacological activity of aeruginascin has remained unexplored.14
The active metabolite of psilocybin is its hydrolysis product psilocin; it is likely that aeruginascin undergoes similar hydrolysis to generate its active metabolite, 4-hydroxy-N,N,N-trimethyltryptamine (4-HO-TMT).13,14 This compound is closely related to bufotenidine, the N-trimethyl analogue of serotonin found naturally in toad venom. Bufotenidine is a selective 5-HT3 agonist,16 with strong binding to that receptor (Ki = 17 nM).17 The leading hypothesis for aeruginascin has been that it is similarly active at 5-HT3 and inactive at 5-HT2 receptors. To assess this leading hypothesis on aeruginascin, we set out to synthesize its putative active metabolite and examine its binding affinity at human serotonin receptors.
Results and Discussion
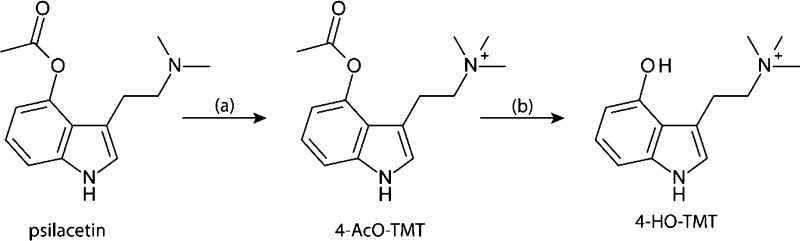
In the case of psilocybin, its phosphate group is hydrolyzed during metabolism to generate psilocin in the body, which functions as the active psychedelic (Figure 1).18 A well-known functional analogue of psilocybin is psilacetin, or 4-acetoxy-N,N-dimethyltryptamine (4-AcO-DMT), the 4-acetoxy derivative of psilocybin, which is similarly hydrolyzed to generate psilocin.19 To synthesize the active metabolite of aeruginascin, psilacetin (4-AcO-DMT) fumarate was used as the starting material and methylated in the presence of excess iodomethane. The resulting compound, 4-acetoxy-N,N,N-trimethyltryptammonium (4-AcO-TMT) iodide, was generated in a good yield (53%). In an analogy to psilacetin, 4-AcO-TMT would be expected to serve as a convenient source of 4-HO-TMT, which is consistent with our experimental observations. In aqueous acetic acid, the 4-AcO-TMT iodide is hydrolyzed to generate 4-HO-TMT iodide (Scheme 1). The material was purified by recrystallization from a methanolic solution (60% yield).
Figure 1.
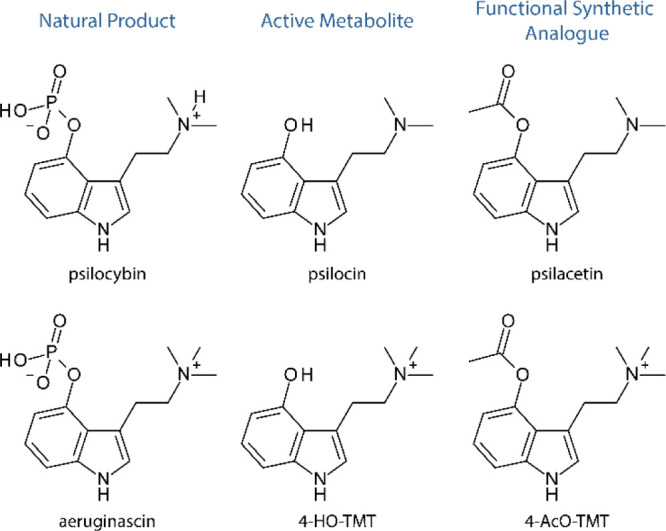
Analogy of psilocybin and aeruginascin derivatives.
Scheme 1. Synthesis of the Active Metabolite of Aeruginascin Where (a) MeI/MeOH and (b) AcOH/H2O.
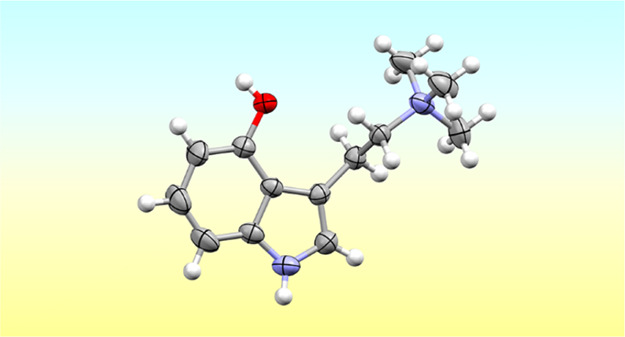
The compounds were both recrystallized from water to obtain them in a single-crystalline form. The molecular structures for both compounds are shown in Figure 2. These are the first two quaternary tryptammonium salts ever characterized by single-crystal diffraction. The presence of such structural data is helpful for modeling studies to probe their activity at receptors. NMR data and elemental analyses further demonstrate the high purity of these compounds as synthesized.
Figure 2.
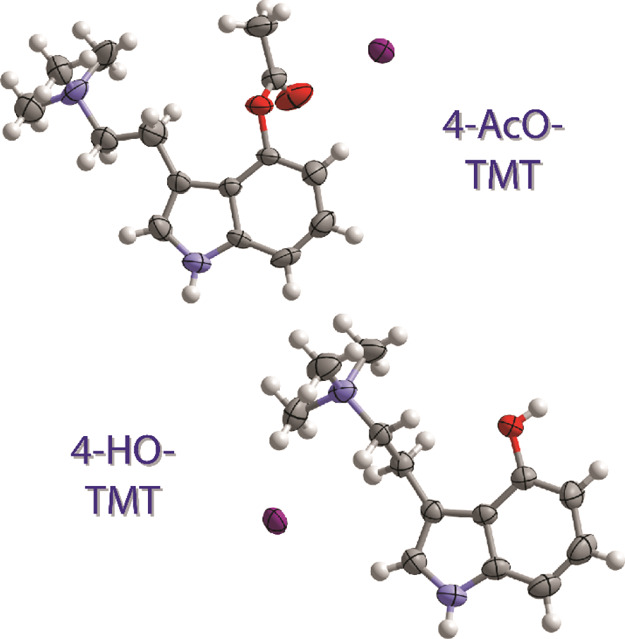
Crystal structures of the iodide salts of 4-AcO-TMT (top) and 4-HO-TMT (bottom) with thermal ellipsoids shown at the 50% probability level.
The two compounds were screened for binding at the orthosteric sites of human serotonin receptors 5-HT1A, 5-HT2A, 5-HT2B, and 5-HT3.20 Competitive radioligand binding assays were used to assess the affinity of the compounds for the receptors. Binding is reported as the Ki for the inhibition of binding well-characterized orthosteric ligands (Table 1 and Supporting Information). The aeruginascin active metabolite, 4-HO-TMT, shows binding at 5-HT1A, 5-HT2A, and 5-HT2B receptors. Counter to the prevailing theory that aeruginascin should function as a powerful 5-HT3 agonist, there is no binding (Ki > 10,000 nM) observed at this receptor. The aeruginascin functional analogue, 4-AcO-TMT, shows no binding affinity at any of the receptors. For comparison, psilocybin, the prodrug of psilocin, shows no activity at 5-HT1A, 5-HT2A, or 5-HT3 but does show itself to bind strongly at 5-HT2B. Psilocin, its active metabolite, shows activity at 5-HT1A and 5-HT2A that has a greater, though comparable, binding affinity to 4-HO-TMT. It is two orders of magnitude more potent than 4-HO-TMT at the 5-HT2B receptor, and in fact, psilocybin is more active at this receptor as well.
Table 1. Inhibition Constants (Ki) in nM Units.
Despite its close structural relationship to bufotenidine, 4-HO-TMT does not exhibit binding at the serotonin 5-HT3 receptor. The results of receptor screening show that this metabolite has unexpected binding affinity at the serotonin 5-HT2A receptor which is associated with psychotropic activity. The quaternary ammonium functionality makes it less likely that this charged species will cross the blood–brain barrier. However, quaternary ammonium salts have been known to cross the blood–brain barrier through transporters; therefore, psychotropic activity remains a possibility.21 It has been speculated that an inability to cross the blood–brain barrier might lead to the different observed effects from this compound.14,22 Also of note is that it shows significantly less binding than psilocin at the serotonin 5-HT2B receptor, where activation is tied to valvular heart disease.23
Conclusions
In summary, the putative active metabolite of one of the naturally occurring tryptamines found in at least one species of “magic mushrooms” (aeruginascin) has been synthesized and characterized for the first time. Its binding affinity at serotonergic receptors has been assayed, demonstrating that it is not active at the 5-HT3 receptor, as previously predicted, but shows strong binding at the 5-HT2 receptors which was unexpected. In the last year, over 100 U.S. cities launched initiatives to decriminalize “magic mushrooms” despite having limited scientific information about many of the tryptamines contained in the fungi. The study of this and other natural products in “magic mushrooms” will be important to understand their effects and to avoid dangerous peripheral consequences.
Acknowledgments
Ki determinations for psilocybin and psilocin were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program [Contract #HHSN-271-2018-00023-C (NIMH PDSP)]. The NIMH PDSP is Directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda MD, USA. The crystal data were collected on a diffractometer funded by a National Science Foundation instrumentation grant (CHE-1429086). Funding for this research was provided by CaaMTech, Inc., Issaquah, WA, USA.
Glossary
Abbreviations
- FDA
United States Food and Drug Administration
- 5-HT
5-hydroxytryptamine receptor
- DMT
N,N-dimethyltryptamine
- 4-AcO-DMT
4-acetoxy-N,N-dimethyltryptamine
- 4-AcO-TMT
4-acetoxy-N,N,N-trimethyltryptammonium
- 4-HO-TMT
4-hydroxy-N,N,N-trimethyltryptammonium
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02208.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
CaaMTech, Inc. National Science Foundation (CHE-1429086).
The authors declare the following competing financial interest(s): Andrew R. Chadeayne and Brian G. Reid report ownership interests in CaaMTech, Inc., which owns U.S. and worldwide patent applications, covering new tryptamine compounds, compositions, formulations, novel crystalline forms, and methods of making and using the same.
Supplementary Material
References
- Stamets P.Psilocybin Mushrooms of the World: An Identification Guide; Ten Speed Press: Berkeley, CA, 1996. [Google Scholar]
- Matsushima Y.; Eguchi F.; Kikukawa T.; Matsuda T. Historical overview of psychoactive mushrooms. Inflammation Regener. 2009, 29, 47–56. 10.2492/inflammregen.29.47. [DOI] [Google Scholar]
- Bogenschutz M. P.; Forcehimes A. A.; Pommy J. A.; Wilcox C. E.; Barbosa P.; Strassman R. J. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J. Psychopharmacol. 2015, 29, 289–299. 10.1177/0269881114565144. [DOI] [PubMed] [Google Scholar]
- Johnson M. W.; Garcia-Romeu A.; Cosimano M. P.; Griffiths R. R. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J. Psychopharmacol. 2014, 28, 983–992. 10.1177/0269881114548296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob C. S.; Danforth A. L.; Chopra G. S.; Hagerty M.; McKay C. R.; Halberstadt A. L.; Greer G. R. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch. Gen. Psychiatry 2011, 68, 71–78. 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- dos Santos R. G.; Osório F. L.; Crippa J. A. S.; Riba J.; Zuardi A. W.; Hallak J. E. C. Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years. Ther. Adv. Psychopharmacol. 2016, 6, 193–213. 10.1177/2045125316638008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer M. C.; Grob C. S.; Brewerton T. D. Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA. Lancet Psychiat. 2016, 3, 481–488. 10.1016/s2215-0366(15)00576-3. [DOI] [PubMed] [Google Scholar]
- Feltman R.The FDA is fast-tracking a second psilocybin drug to treat depression; Popular Science, 2019. https://popsci.com/story/health/psilocybin-magic-mushroom-fda-breakthrough-depression/ (accessed March 2020).
- Horgan J.Psychedelic therapy and bad trips; Scientific American, 2016. https://blogs.scientificamerican.com/cross-check/psychedelic-therapy-and-bad-trips/ (accessed March 2020).
- Zhuk O.; Jasicka-Misiak I.; Poliwoda A.; Kazakova A.; Godovan V.; Halama M.; Wieczorek P. Research on acute toxicity and the behavioral effects of methanolic extract from psilocybin mushrooms and psilocin in mice. Toxins 2015, 7, 1018–1029. 10.3390/toxins7041018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E. B. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz C.; Wick J.; Hoffmeister D. Identification of ω-N-Methyl-4-hydroxytryptamine (Norpsilocin) as a Psilocybe Natural Product. J. Nat. Prod. 2017, 80, 2835–2838. 10.1021/acs.jnatprod.7b00407. [DOI] [PubMed] [Google Scholar]
- Sherwood A. M.; Halberstadt A. L.; Klein A. K.; McCorvy J. D.; Kaylo K. W.; Kargbo R. B.; Meisenheimer P. Synthesis and biological evaluation of tryptamines found in hallucinogenic mushrooms: norbaeocystin, baeocystin, norpsilocin, and aeruginascin. J. Nat. Prod. 2020, 83, 461–467. 10.1021/acs.jnatprod.9b01061. [DOI] [PubMed] [Google Scholar]
- Jensen N.; Gartz J.; Laatsch H. Aeruginascin, a Trimethylammonium Analogue of Psilocybin from the Hallucinogenic MushroomInocybe aeruginascens. Planta Med. 2006, 72, 665–666. 10.1055/s-2006-931576. [DOI] [PubMed] [Google Scholar]
- Gartz J. Analysis of aeruginascin in fruit bodies of the mushroom Inocybe aeruginascens. Int. J. Crude Drug Res. 1989, 27, 141–144. 10.3109/13880208909053954. [DOI] [Google Scholar]
- Glennon R. A.Strategies for the development of selective serotonergic agents. In The Serotonin Receptors; Roth B. L., Ed.; Humana Press: Totowa, NJ, 2006. [Google Scholar]
- Glennon R. A.; Peroutka S. J.; Dukat M.. Binding characteristics of a quaternary amine analog of serotonin: 5-HTQ. In Serotonin: Molecular Biology, Receptors and Functional Effects; Fozard J. R., Saxena P. R., Eds.; Birkhauser Verlag: Basel, Switzerland, 1991. [Google Scholar]
- Dinis-Oliveira R. J. Metabolism of psilocybin and psilocin: clinical and forensic toxicological relevance. Drug Metab. Rev. 2017, 49, 84–91. 10.1080/03602532.2016.1278228. [DOI] [PubMed] [Google Scholar]
- Nichols D. E.; Frescas S. Improvements to the synthesis of psilocybin and a facile method for preparing the O-acetyl prodrug of psilocin. Synthesis 1999, 935–938. 10.1055/s-1999-3490. [DOI] [Google Scholar]
- Roth B. L.; Lopez E.; Patel S.; Kroeze W. K. The multiplicity of serotonin receptors: uselessly diverse molecules or an embarrassment of riches?. Neuroscientist 2000, 6, 252–262. 10.1177/107385840000600408. [DOI] [Google Scholar]
- Zacny J. P.; Wroblewski K.; Coalson D. W. Methylnaltrexone: its pharmacological effects alone and effects on morphine in healthy volunteers. Psychopharmacology 2015, 232, 63–73. 10.1007/s00213-014-3637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne N.; Thomsen P.; Mølgaard Knudsen N.; Rubaszka P.; Kristensen M.; Borodina I. Metabolic engineering of Saccharomyces cerevisiae for the de novo production of psilocybin and related tryptamine derivatives. Metab. Eng. 2020, 60, 25–36. 10.1016/j.ymben.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman R. B.; Baumann M. H.; Savage J. E.; Rauser L.; McBride A.; Hufeisen S. J.; Roth B. L. Evidence for Possible Involvement of 5-HT 2B Receptors in the Cardiac Valvulopathy Associated With Fenfluramine and Other Serotonergic Medications. Circulation 2000, 102, 2836–2841. 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.