Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of an ongoing pandemic, with increasing deaths worldwide. To date, documentation of the histopathological features in fatal cases of the disease caused by SARS-CoV-2 (COVID-19) has been scarce due to sparse autopsy performance and incomplete organ sampling. We aimed to provide a clinicopathological report of severe COVID-19 cases by documenting histopathological changes and evidence of SARS-CoV-2 tissue tropism.
Methods
In this case series, patients with a positive antemortem or post-mortem SARS-CoV-2 result were considered eligible for enrolment. Post-mortem examinations were done on 14 people who died with COVID-19 at the King County Medical Examiner's Office (Seattle, WA, USA) and Snohomish County Medical Examiner's Office (Everett, WA, USA) in negative-pressure isolation suites during February and March, 2020. Clinical and laboratory data were reviewed. Tissue examination was done by light microscopy, immunohistochemistry, electron microscopy, and quantitative RT-PCR.
Findings
The median age of our cohort was 73·5 years (range 42–84; IQR 67·5–77·25). All patients had clinically significant comorbidities, the most common being hypertension, chronic kidney disease, obstructive sleep apnoea, and metabolic disease including diabetes and obesity. The major pulmonary finding was diffuse alveolar damage in the acute or organising phases, with five patients showing focal pulmonary microthrombi. Coronavirus-like particles were detected in the respiratory system, kidney, and gastrointestinal tract. Lymphocytic myocarditis was observed in one patient with viral RNA detected in the tissue.
Interpretation
The primary pathology observed in our cohort was diffuse alveolar damage, with virus located in the pneumocytes and tracheal epithelium. Microthrombi, where observed, were scarce and endotheliitis was not identified. Although other non-pulmonary organs showed susceptibility to infection, their contribution to the pathogenesis of SARS-CoV-2 infection requires further examination.
Funding
None.
Introduction
In December, 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in Wuhan, China from a cluster of severe pneumonia cases.1 The virus and the disease it causes (COVID-19) have now spread globally and are responsible for an ongoing pandemic that has claimed hundreds of thousands of lives. In the months after its emergence, the community of health-care workers and researchers acted quickly to sequence the virus, establish transmission chains, elucidate the receptor, and test therapeutics.2, 3
These efforts have revealed similarities and differences between SARS-CoV-2 and the related virus, severe acute respiratory syndrome coronavirus (SARS-CoV). Both viruses have similar clinical presentations, with the highest viral load identified in lower respiratory samples.4, 5 Viral RNA has also been detected in blood, stool, and urine samples, suggesting the potential for extrapulmonary spread and multiorgan involvement.6, 7 The viruses also share a common cellular entry receptor, angiotensin converting enzyme 2 (ACE2).3 Despite their similarities, SARS-CoV was responsible for a restricted disease outbreak with high mortality, whereas SARS-CoV-2 has caused a greater number of infections with relatively lower mortality.8
Post-mortem studies have shown pulmonary, renal, and small vessel injury, with particles resembling virus observed in the kidney by electron microscopy.9, 10, 11, 12 One study described two complete autopsies without tissue-based methods for detection of SARS-CoV-2.9 Our study expands the literature by documenting a series of 14 fatal COVID-19 cases that occurred in Washington State during February and March, 2020. Systematic evaluation of all major organs was done by a combination of light microscopy, immunohistochemistry, electron microscopy, and quantitative RT-PCR. Our findings serve as a basis for generating further discussion related to the tropism, mechanisms of dissemination, and pathophysiology of severe SARS-CoV-2 infection.
Research in context.
Evidence before this study
We searched PubMed and MEDLINE for peer-reviewed articles published between database inception and May 1, 2020, that described the histopathological features of severe COVID-19 infections, with the search terms “SARS-CoV-2”, “COVID-19”, “autopsy”, “postmortem”, and “histology”. Our search was restricted to studies published in English. Of the eight studies identified by our search, one documented complete histopathological findings from all major organs in two autopsies. Other series showed evidence of diffuse alveolar damage, endothelial injury, and viral particles within renal cells. Tissue quantitative RT-PCR (RT-qPCR) was used as an ancillary technique to identify virus in one study.
Added value of this study
This study provides crucial information related to the natural history of fatal COVID-19 from early in the US outbreak. Our analysis used multiple methods, including clinical chart review, histopathological evaluation, electron microscopy, immunohistochemistry, and quantitative RT-qPCR to examine all major organ systems. To our knowledge, no previous studies have used all these techniques simultaneously. Our results support previous studies, which suggested that diffuse alveolar damage is the major source of pulmonary injury in COVID-19; however, we found no evidence of widespread microvascular injury. Additional investigations raised the possibility of extrapulmonary involvement in renal, intestinal, cardiac, and lymphoid tissues.
Implications of all the available evidence
In addition to the results of previous studies, our findings provide a histological explanation for physiological derangements observed by clinicians in patients who died with COVID-19. Further investigation is required to characterise the extent of extrapulmonary injury caused by severe acute respiratory syndrome coronavirus 2 infection.
Methods
Patient selection and autopsy procedures
In this case series, patients with a positive antemortem or post-mortem SARS-CoV-2 result were considered eligible for enrolment. Preliminary testing for SARS-CoV-2 was done at the Washington State Department of Health Public Health laboratory (Shoreline, WA, USA). Confirmatory testing was done by the US Centers for Disease Control and Prevention (CDC) in Atlanta (GA, USA). Both locations used the CDC-designed 2019 nCoV real-time RT-PCR assay for virus detection. Autopsies were done at the King County Medical Examiner's Office (Seattle, WA, USA) and Snohomish County Medical Examiner's Office (Everett, WA, USA) in negative-pressure isolation suites during February and March, 2020. Given safety concerns, in-situ dissection was done for patients 2, 3, 4, 5, 6, 7, and 9. Patients 1, 8, 10, 11, 12, 13, and 14 were examined by standard autopsy procedure. Three to 23 blocks were submitted per autopsy case. Limited autopsies submitted two blocks containing sections from the left and right lung lobes, whereas complete autopsies submitted at least four blocks of lung-containing sections from central and peripheral locations of both lungs. Resource limitations led to fresh tissue collection for patients 8, 13, and 14 only.
Institutional Review Board approval was requested and waived for this study (STUDY00009856).
Histological examination
Autopsy material was fixed in 10% neutral buffered formalin and submitted for standard processing with haematoxylin and eosin staining. Select kidney sections were stained with periodic acid Schiff and Jones methenamine silver. Evaluation of haematoxylin and eosin sections was done by consensus agreement by four board-certified forensic pathologists (NY, TW, JML, and DAM) with expert guidance provided in cardiothoracic pathology (HX and GD) and renal pathology (BN).
Immunohistochemistry
Immunohistochemical staining was done on formalin-fixed, paraffin-embedded 5-μm sections following citrate pH 6·0 antigen retrieval, endogenous biotin, and peroxidase block. Immunohistochemistry for SARS-CoV-2 was done using a monoclonal antibody to the spike protein (1:250; GeneTex; Irvine, CA, USA) on the Ventana BenchMark ULTRA IHC (Roche Diagnostics; Basel, Switzerland). Images were visualised and captured with a digital camera mounted on a Nikon Eclipse 80i microscope using NIS-Elements Advanced Research Software version 4.13 (Nikon Instruments; Tokyo, Japan).
Ultrastructural examination
Samples from patients 8 and 13 were placed in half-strength Karnovsky fixative. Tissue was then post-fixed in 1% osmium tetroxide, processed according to standard transmission electron microscopy procedures, and embedded in PolyBed 812 (Polyscience; Warrington PA, USA). Suitable sections were identified by toluidine blue staining. Thin sections were examined using a Tecnai G2 Spirit Bio-Twin transmission electron microscope (FEI; Hillsboro, OR, USA) and digital images and measurements were acquired using AMT image capture software (version 602.446).
Molecular detection of viral RNA in tissue
RNA was extracted from 0·5 μg of tissue using the Direct-zol RNA Miniprep Plus kit (Zymo Research; Irvine, CA, USA). Complementary DNA was synthesised using the iScript cDNA kit (BioRad; Hercules, CA, USA). Samples were tested in triplicate using iTAq Universal Probes Supermix (BioRad) with the CDC 2019 nCoV N1 and N2 primer/probe set and Sarbeco E gene primer/probe set (IDT; Coralville, IA, USA). Cycle threshold values less than 40 were considered positive. The limit of detection was estimated to be 700 copies per reaction based on serial dilutions of a positive control plasmid (COV019; Exact Diagnostics; Fort Worth, TX, USA). Negative control reactions included on each plate reproducibly showed no amplification. PCR was done on patients 8, 13, and 14. Tissues examined included lung, trachea, subcarinal lymph node, heart, spleen, liver, large intestine, kidney, and whole blood.
Role of the funding source
There was no funding source for this study.
Results
The median age of our cohort was 73·5 years (range 42 to 84; IQR 67·5–77·25). Seven members of the cohort were part of a single cluster from a long-term care facility.13 All patients had clinically significant comorbidities, the most common being hypertension, chronic kidney disease, obstructive sleep apnoea, and metabolic disease, including diabetes and obesity (table 1 ).
Table 1.
Patient characteristics, comorbidities, symptoms, radiographic findings, select initial laboratory findings, and cause of death
| Age, years | Sex | Comorbidities | Initial symptoms | Time from symptom onset to admission, days | Time from symptom onset until intubation, days | Time from symptom onset to death, days | Radiographic findings | Elevated creatinine | Elevated troponin | Additional respiratory pathogens | Lymphopenia | Cause of death (ICD-10 code); other significant causes | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | Male | End-stage renal disease, type 2 diabetes, hypertension, obstructive sleep apnoea, obesity | Cough, fever, chills, loose stool, fatigue, respiratory distress | 4 | 4 | 10 | Bilateral multifocal patchy airspace opacities | Yes | No | No | Yes | Cause A: COVID-19 pneumonia (U07.1); OSC: diabetes, end-stage renal disease on dialysis, hypertension |
| 2 | 74 | Female | Type 2 diabetes, obstructive sleep apnoea, atrial fibrillation, pulmonary hypertension, chronic kidney disease, obesity | Acute renal failure, altered mental status, cough, acute cardiomyopathy, acute respiratory distress | 2 | 2 | 2 | Increased pulmonary artery and interstitial markings | Yes | No | No | NA | Cause A: Cardiomyopathy (I25.5), cause B: COVID-19 (U07.1); OSC: diabetes, pulmonary hypertension, immunosuppression |
| 3 | 54 | Male | Traumatic brain injury with secondary neurological dysfunction and dysphagia | 40°C fever, respiratory distress, tachycardia | 1 | NA* | 2 | Bilateral patchy opacities | No | No | No | NA | Cause A: aspiration pneumonia and sepsis (J15, A41), cause B: COVID-19 infection (U07.1); OSC: dysphagia due to blunt force head injury |
| 4 | 74 | Male | Heart failure with preserved ejection fraction, frontotemporal dementia, hypertension, obstructive sleep apnoea | Cough, tactile fever, body aches, respiratory distress | 1 | 1 | 1 | Bilateral diffuse scattered opacities | No | No | No | NA | Cause A: adult respiratory distress syndrome (J80), cause B: viral pneumonia (J12.8), cause C: COVID-19 (U07.1); OSC: chronic renal disease |
| 5 | 73 | Female | Type 2 diabetes, hypertension, congestive heart failure, hypothyroidism, obesity, schizoaffective disorder, bipolar disorder | Cough, respiratory distress, fever | 5 | 5 | 13 | Widespread bilateral opacities | No | No | No | NA | Cause A: adult respiratory distress syndrome (J80), cause B: viral pneumonia (J12.8), cause C: COVID-19 (U07.1); OSC: morbid obesity, hypertension, diabetes |
| 6 | 84 | Female | Chronic obstructive pulmonary disease, congestive heart failure, atrial fibrillation, aortic stenosis, hypertension, chronic kidney disease, osteoporosis | Respiratory distress, altered mental status | 1 | NA | 2 | Bibasilar atelectasis or consolidations with small pleural effusions | No | No | No | Yes | Cause A: adult respiratory distress syndrome (J80), cause B: viral pneumonia (J12.8), cause C: COVID-19 (U07.1); OSC: chronic obstructive pulmonary disease, atrial fibrillation, aortic stenosis, mitral stenosis |
| 7 | 71 | Male | Hypertension, hyperlipidaemia, coronary artery disease, atrial fibrillation, end-stage renal disease, obstructive sleep apnoea | Acute respiratory distress, cough | 7 | NA | 13 | Multifocal bilateral opacities | Yes | No | Pseudomonas aeruginosa | Yes | Cause A: viral pneumonia (J12.8), cause B: COVID-19 (U07.1), cause C: immunosuppression (Z92.25), cause D: renal transplant (T86.1); OSC: end-stage renal disease, coronary artery disease, cerebral vascular accident |
| 8 | 76 | Female | Hyperlipidaemia, osteoporosis | Respiratory distress, hypotension, tachycardia, fever | 3 | 3 | 7 | Multifocal bilateral opacities | Yes | Yes | Meticillin-sensitive Staphylococcus aureus and influenza A | Yes | Cause A: acute respiratory distress syndrome (J80), cause B: viral pneumonia (J12.8), cause C: COVID-19 (U07.1); OSC: influenza A, staphylococcal pneumonia, myocarditis, cardiomyopathy, septic shock |
| 9 | 75 | Female | Hyperlipidaemia, type 2 diabetes, coronary artery disease, congestive heart failure, chronic kidney failure, chronic obstructive pulmonary disease, previous deep vein thrombosis | Altered mental status, respiratory distress, fever | 3 | NA | 12 | Bilateral interstitial opacities and asymmetric oedema on the right | No | No | No | Yes | Cause A: Adult respiratory distress syndrome (J80), cause B: pneumonia (J12.8), cause C: COVID-19 (U07.1); OSC: chronic kidney disease, diabetes, venous thromboembolism |
| 10 | 84 | Male | Chronic kidney disease, chronic obstructive pulmonary disease, hyperlipidaemia, obstructive sleep apnoea, mitral regurgitation, complete heart block, chronic pain, arthritis, obesity, hypertension | Respiratory distress, altered mental status, hypotension | 1 | NA | 1 | Complete opacification of the left hemithorax with additional right lower lobe and middle lobe opacities | Yes | No | No | Yes | Cause A: acute or chronic hypoxic and hypercarbic respiratory failure (J96.2), cause B: pulmonary emphysema (J43); OSC: COVID-19, hypertensive cardiovascular disease, mitral valve regurgitation, and stage 3 chronic kidney disease |
| 11 | 81 | Female | Hypertension, hyperlipidaemia, breast cancer, chronic kidney disease, demyelinating neuropathy, lacunar infarcts, recent pneumonia in January, 2020, Alzheimer's disease | Fever, cough, nausea and vomiting | 1 | 5 | 7 | Bilateral multifocal opacities | No | Yes | No | Yes | Cause A: acute hypoxic respiratory failure (J96.01), cause B: adult respiratory distress syndrome (J80), cause C: co-incident viral and bacterial pneumonia (J12, J15), cause D: COVID-19 (U07.1); OSC: hypertensive cardiovascular disease |
| 12 | 42 | Female | Lobular breast cancer status post-bilateral mastectomy and neoadjuvant chemotherapy and anaemia | Fever, headache, leukopenia | 5 | 12 | 14 | Bilateral multifocal opacities | No | No | No | Yes | Cause A: adult respiratory distress syndrome (J80), cause B: COVID-19 (U07.1); OSC: history of lobular carcinoma of the breast status—post-bilateral mastectomies and adjuvant radiation and anti-oestrogen therapy |
| 13 | 71 | Male | Coronary artery disease ischaemic cardiomyopathy, hypertension, aortic stenosis, end-stage renal disease on dialysis, pulmonary fibrosis, previous cerebellar cardiovascular accident | Shortness of breath, bradycardia, heart block, delirium | 1 | NA | 4 | Low lung volumes, diffuse lung disease, probably reflecting underlying fibrosis | Yes | Yes | No | Yes | Cause A: ventricular fibrillation (I49.01), cause B: acute respiratory distress syndrome (J80), cause C: COVID-19 respiratory infection (U07.1); OSC: cardiac conduction system disease, cardiac amyloidosis, ischaemic heart disease, hypertension, aortic stenosis status—post transcatheter aortic valve replacement pulmonary fibrosis, end-stage renal disease, cervical spinal stenosis with recent treated osteomyelitis |
| 14 | 73 | Female | Hypertension, asthma, diabetes, hyperlipidaemia, obesity | Shortness of breath, respiratory distress | 2 | 2 | 23 | Low lung volumes, diffuse bilateral lung disease | No | No | No | No | Cause A: acute respiratory distress syndrome (J80), cause B: COVID-19 pneumonia (U07.1); OSC: hypertension, asthma, type 2 diabetes, paroxysmal atrial fibrillation, and WHO class 3 obesity |
Elevated troponin defined by >0·4 ng/mL, elevated creatinine defined by 1·5 mg/dL, fever defined by >37·5°C, and lymphopenia defined as lymphocyte count <1·5 × 109 per L. ICD-10=International Classification of Diseases version 10. NA=not applicable. OSC=other significant conditions.
Case 3 had a tracheostomy.
The most frequent presenting symptoms were respiratory distress, fever, and cough. Less commonly encountered initial symptoms included altered mental status and gastrointestinal symptoms (nausea, vomiting, or diarrhoea). During hospital admission, six patients had acute kidney injury shown by elevated creatinine and three patients had elevated troponins. Additional respiratory pathogens were isolated from two patients—patient 8 had influenza A and meticillin-sensitive Staphylococcus aureus and patient 7 had sputum cultures positive for Pseudomonas aeruginosa. Excluding those who declined resuscitative measures (six patients), all patients were intubated. Patient 12 was electively extubated less than a day before death. The median time from symptom onset to intubation was 3·5 days (IQR 2–5; eight patients), with most intubations occurring at the time of admission. Time to death after symptom onset varied from 1 day to 23 days, with a median time of 7 days (IQR 2–13).
For patients 8, 13, and 14, fresh tissue was collected at the time of autopsy for molecular and ultrastructural examination. Patient 8 was a 76-year-old woman who presented with cough and malaise for 3 days after returning from international travel. On admission, the patient was hypoxic with elevated troponins and leukocytosis. Her x-ray showed extensive bilateral opacities and testing was positive for SARS-CoV-2, influenza A, and meticillin-sensitive S aureus. The patient had increasing oxygen demands and died 7 days after admission. Patient 13 was a 71-year-old man with a complex cardiovascular history, end-stage renal disease, and chronic pulmonary fibrosis who presented with shortness of breath, delirium, and new onset atrioventricular node block. Given his respiratory symptoms and lymphopenia, SARS-CoV-2 testing was done and returned a positive result. 4 days after admission, the patient developed worsening hypoxia followed by sudden cardiac arrest and died. Patient 14 was a 73-year-old woman who presented after 2 days of progressive shortness of breath while travelling abroad. On admission, she had clinically significant respiratory distress with hypoxia and signs of shock with elevated lactate, leukocytosis, and initial normal troponin. Her chest x-ray showed diffuse lung disease and SARS-CoV-2 testing was positive. The patient's clinical course was complicated by multifactorial encephalopathy, new atrial fibrillation, and presumed ventilator-associated pneumonia and she died 21 days after admission.
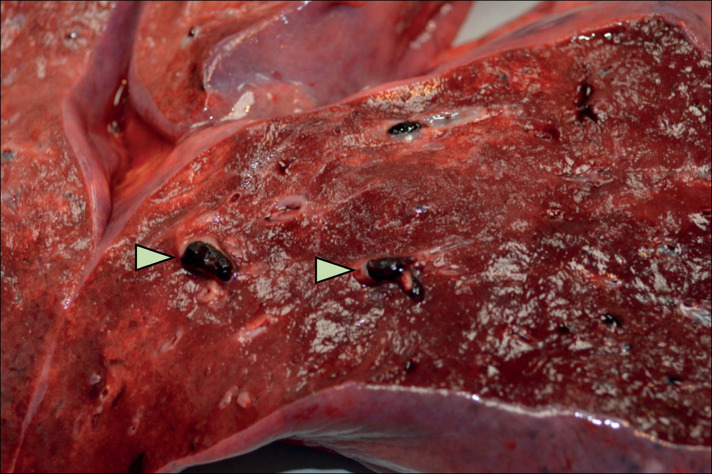
All seven decedents examined by gross standard autopsy had heavy, oedematous lungs, with an average weight of 975 g for the right lung and 700 g for the left lung. Intraparenchymal haemorrhage was observed in patient 6 and pulmonary consolidation was present in patient 14. The volume of pleural fluid was highly variable and ranged from 0 mL to 450 mL per pleural space. Patients 10 and 12 had evidence of central pulmonary emboli (figure 1 ). Splenomegaly was observed in patients 8 and 13 (350 g and 505 g, respectively), whereas splenic atrophy was observed in patient 13 (63 g). Scattered punctate subarachnoid haemorrhages were observed in the brain of patient 8. Additional gross findings showed changes including varying degrees of cardiac and atherosclerotic disease, hypertensive renal surface changes, and hepatic congestion in most patients. Organ weights and gross observations are available in the appendix (p 1).
Figure 1.
Lung with subsegmental pulmonary embolism (indicated by arrowheads) in patient 12
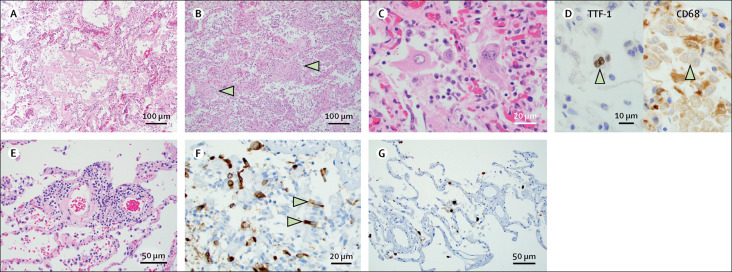
Histopathological examination of the pulmonary system showed a spectrum of diffuse alveolar damage in 12 (86%) of 14 patients, which was evidenced by the presence of intra-alveolar fibrin, hyaline membranes, or loosely organising connective tissue in the alveolar septal walls. 11 (92%) of 12 patients with diffuse alveolar damage showed acute or acute and organising diffuse alveolar damage (figure 2A, B ). For patient 2, only organising diffuse alveolar damage was observed. Airways and alveolar spaces contained large, reactive multinucleated cells that stained positive for the epithelial marker TTF-1 and negative for the macrophage marker CD68 (figure 2C, D). Microscopic haemorrhage was identified with diffuse alveolar damage in patients 7, 11, and 13. Most patients showed variable degrees of chronic interstitial inflammation, with some having more prominent perivascular lymphocytic inflammation (figure 2E). Areas of neutrophilic inflammation were common, with nine patients showing at least focal bronchial or bronchiolar inflammation, including three patients with acute bronchopneumonia. No definite evidence of vasculitis or endotheliitis was identified. Background chronic pulmonary disease in the form of emphysematous change was observed in patients 3, 6, and 10, and patient 13 had extensive chronic interstitial fibrosis. Immunohistochemical staining for SARS-CoV-2 spike protein was done on lung sections from patients 8, 10, 11, and 12. Positive cells were present to a varying degree and observed in all patients tested. SARS-CoV-2 immunohistochemical stain highlighted alveolar pneumocytes along with sloughed ciliated respiratory epithelium in the bronchioles (figure 2F, G).
Figure 2.
Lung pathology of fatal COVID-19 infections
(A) Hyaline membranes in patient 4. Haematoxylin and eosin; magnification ×100. (B) Diffuse alveolar damage, organising phase, in patient 2. Arrowheads indicate fibroblast proliferations. Haematoxylin and eosin; magnification ×100. (C) Multinucleated giant cells and pleomorphic, reactive pneumocytes in patient 5. Haematoxylin and eosin; magnification ×400. (D) Pleomorphic multinucleated giant cells stained positive for pneumocyte marker TTF-1 and negative for macrophage marker CD68 by immunohistochemistry in patient 5. Magnification ×600. (E) Perivascular lymphocytic inflammation in patient 10. Haematoxylin and eosin; magnification ×200. (F) Reactive airway cells and bronchial epithelium (indicated by arrowheads) positive for SARS-CoV-2 spike protein in patient 10. Haematoxylin and eosin; magnification ×400. (G) Pneumocytes and alveolar macrophages positive for SARS-CoV-2 spike protein by immunohistochemistry in patient 10. Magnification ×200. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
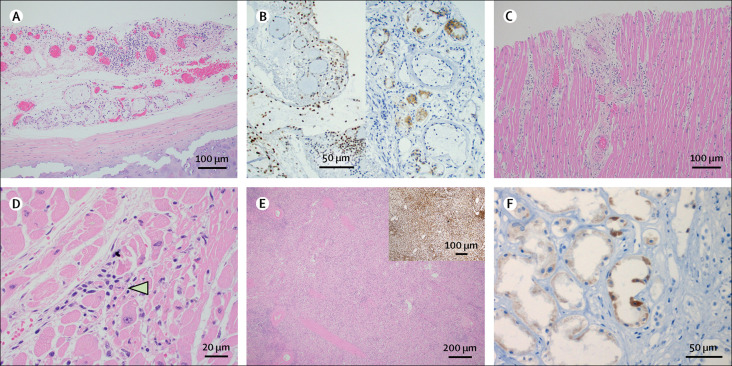
Evaluation of the tracheal mucosa was restricted by post-mortem sloughing of epithelial cells in most patients. However, mild inflammatory changes of the submucosa were observed and typified by oedema with small lymphocytic aggregates (figure 3A ). In patient 8, SARS-CoV-2 immunohistochemical staining showed a positive signal in the submucosal glands and lymphocytes (figure 3B). Four additional patients had focal acute tracheitis.
Figure 3.
Organ findings in fatal COVID-19 infections
(A) Trachea with submucosal lymphocytic inflammation in patient 8. Haematoxylin and eosin; magnification ×100. (B) Lymphocytes (left panel) and submucosal glands (right panel) in the trachea stained positive for SARS-CoV-2 spike protein by immunohistochemistry in patient 8. Magnification ×200. (C) Heart with lymphocytic myocarditis and associated myocyte damage in patient 8. Haematoxylin and eosin; magnification ×40. (D) Heart with lymphocytic myocarditis and necrotic myocyte (indicated by arrowhead) in patient 8. Haematoxylin and eosin; magnification ×400. (E) Spleen with decreased white pulp in patient 9. Haematoxylin and eosin; magnification ×40. Inset image shows CD45 immunohistochemistry. (F) Renal tubular epithelium positive for SARS-CoV-2 spike protein by immunohistochemistry in patient 8. Magnification ×400. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Cardiac findings were mostly non-specific and associated with pre-existing comorbidities. The most common changes observed were fibrosis in 14 (100%) patients and myocyte hypertrophy in 13 (93%) patients. In patient 8, myocarditis was present with aggregates of lymphocytes surrounding necrotic myocytes (figure 3C, D). SARS-CoV-2 S protein immunohistochemistry was negative in patient 8. Amyloid was identified in small vessels in patient 7 and within the myocardium in patient 13.
Patients 5, 9, and 12 had splenic white pulp depletion, which was confirmed by CD45 staining in patient 9 (figure 3E). Patient 8 had a small sub-centimetre splenic infarction. Subcarinal lymph nodes showed normal follicular architecture with haemophagocytosis observed in patients 8, 11, and 14. SARS-CoV-2 immunohistochemical staining was negative in the subcarinal lymph node from patient 12 and spleen from patient 10. Lymphoid tissue from additional patients was not available for testing.
The kidneys showed mild to severe arterionephrosclerosis and diabetic nephropathy. Although restricted by autolysis, features suggestive of acute tubular injury, including extensive tubular epithelial vacuolisation, were identified in 11 patients. In patient 14, chronic inflammation of the renal parenchyma and focal segmental glomerulosclerosis were present. SARS-CoV-2 immunohistochemical testing in patients 8 and 10 showed patchy, granular cytoplasmic staining of the renal tubular epithelial cells (figure 3F). Virus was not visualised in the endothelial cells or glomeruli by immunohistochemistry.
Pathological findings in the liver showed predominantly chronic changes associated with pre-existing comorbidities, with variable degrees of acute congestion. Centrilobular necrosis consistent with hypoperfusion injury was identified in four patients (patients 3, 8, 12, and 14). Liver inflammation was not prominent, although some patients had mild periportal lymphocytic inflammation. Weak SARS-CoV-2 immunohistochemical staining in the liver was indistinguishable from background. Samples of the thyroid gland, pituitary gland, adrenal glands, and pancreas were largely unremarkable. Post-mortem autolysis prevented thorough investigation of the gastrointestinal system and assessment by SARS-CoV-2 immunohistochemical staining.
Brain examination was done in five patients (patients 1, 8, 10, 11, and 12). Patient 8 had acute pathology with scattered punctate subarachnoid haemorrhages and rare microhaemorrhage in the brainstem. Neuropathological examinations were otherwise unremarkable.
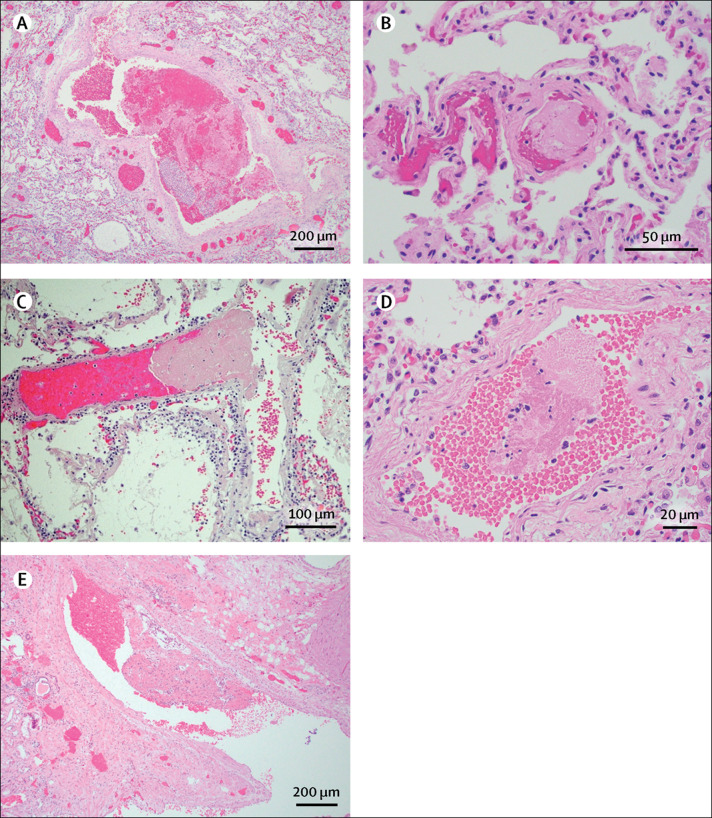
Focal pulmonary microthrombi were identified in five patients (patients 3, 8, 9, 13, 14; figure 4 ). Patient 3 and patient 9 also had microthrombi in the trachea, although in patient 3 this was qualified by chronic tracheostomy. An organising thrombus was identified within a small renal vein in patient 13. Microscopic involvement by grossly observed pulmonary emboli was seen in patient 10 and patient 12. Pathological findings for individual patients are provided in table 2 .
Figure 4.
Coagulopathy of fatal COVID-19 infections
(A) Small vessel thrombus in patient 12. Haematoxylin and eosin; magnification ×40. (B) Pulmonary microthrombus in patient 3. Haematoxylin and eosin; magnification ×200. (C) Pulmonary microthrombus in patient 8. Haematoxylin and eosin; magnification ×200. (D) Pulmonary microthrombus in patient 14. Haematoxylin and eosin; magnification ×400. (E) Renal vein organising thrombus in patient 13. Haematoxylin and eosin; magnification ×40.
Table 2.
Postmortem findings by organ system by patient
| Lungs | Heart | Liver | Kidney | Spleen | Trachea | Subcarinal lymph node | Gastrointestinal | Brain | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pulmonary oedema, acute phase diffuse alveolar damage, multinucleated giant cells, reactive pneumocytes | Interstitial fibrosis, myocyte hypertrophy | Steatosis, periportal lymphocytic inflammation | Moderate to severe arterionephrosclerosis, diabetic changes, scattered tubular casts | No diagnostic alterations | Oedema, chronic (lymphocytic) tracheitis | Not sampled | Multifocal gastric haemorrhages | No diagnostic alterations |
| 2 | Organising phase diffuse alveolar damage, reactive pneumocytes, acute bronchiolitis, alveolar septal thickening | Interstitial fibrosis, myocyte hypertrophy, replacement fibrosis | Steatosis, congestion | Moderate to severe arterionephrosclerosis, diabetic changes | No diagnostic alterations | Oedema, chronic (lymphocytic) tracheitis | Not sampled | No diagnostic alterations | Not sampled |
| 3 | Pulmonary oedema, reactive pneumocytes, acute bronchiolitis, background emphysematous change, microthrombi | Interstitial fibrosis, myocyte hypertrophy | Periportal lymphocytic inflammation, centrilobular necrosis | Mild arterionephrosclerosis, scattered tubular casts | Evaluation limited by autolysis | Acute (neutrophilic) tracheitis, fibrosis and ossification, microthrombi | Not sampled | No diagnostic alterations | Not sampled |
| 4 | Pulmonary oedema, acute phase diffuse alveolar damage, multinucleated giant cells, reactive pneumocytes, alveolar septal thickening, patchy perivascular lymphocytic inflammation | Interstitial fibrosis, myocyte hypertrophy, replacement fibrosis | Steatosis, congestion, features of toxic or metabolic disease | Mild to moderate arteriolosclerosis, scattered tubular casts | Evaluation limited by autolysis | Acute (neutrophilic) tracheitis | Not sampled | No diagnostic alterations | Not sampled |
| 5 | Pulmonary oedema, acute phase diffuse alveolar damage, multinucleated giant cells, alveolar septal thickening, perivascular and interstitial lymphocytic inflammation | Interstitial fibrosis, myocyte hypertrophy | Steatosis, congestion, lobar neutrophilic inflammation | Mild arterionephrosclerosis, scattered tubular casts, diabetic changes | White pulp depletion | Oedema, chronic (lymphocytic) tracheitis | Not sampled | No diagnostic alterations | Not sampled |
| 6 | Acute phase diffuse alveolar damage, reactive pneumocytes, pulmonary haemorrhage, acute bronchopneumonia, background emphysematous changes | Interstitial fibrosis, myocyte hypertrophy | Congestion, portal lymphocytic inflammation | Mild to moderate arterionephrosclerosis, scattered tubular casts | No diagnostic alterations | Oedema, chronic (lymphocytic) tracheitis | Not sampled | No diagnostic alterations | Not sampled |
| 7 | Pulmonary oedema, acute and organising diffuse alveolar damage, reactive pneumocytes, alveolar septal thickening, pulmonary haemorrhage | Interstitial fibrosis, myocyte hypertrophy, vascular predominant amyloid | Congestion | Severe arterionephrosclerosis, vascular predominant amyloid | No diagnostic alterations | Oedema, acute (neutrophilic) tracheitis | Not sampled | No diagnostic alteration | Not sampled |
| 8 | Pulmonary oedema, acute and organising phase diffuse alveolar damage, reactive pneumocytes, multinucleated cells, alveolar septal thickening, acute bronchiolitis, perivascular and interstitial lymphocytic inflammation, microthrombi | Interstitial fibrosis, myocyte hypertrophy, replacement fibrosis, myocarditis | Steatosis, centrilobular necrosis | Mild arterionephrosclerosis, scattered tubular casts, reactive tubular epithelium, chronic (lymphocytic) interstitial inflammation | Splenic infarction | Oedema, chronic (lymphocytic) tracheitis | Rare haemophagocytosis | No diagnostic alterations | Punctate subarachnoid haemorrhages, punctate microhaemorrhages in brainstem |
| 9 | Oedema, acute phase diffuse alveolar damage, reactive pneumocytes, acute bronchiolitis, microthrombi | Interstitial fibrosis, myocyte hypertrophy | Steatosis, congestion | Moderate to severe arterionephrosclerosis | White pulp depletion | Oedema, chronic (lymphocytic) tracheitis, microthrombi | Not sampled | No diagnostic alterations | Not sampled |
| 10 | Pulmonary oedema, focal acute phase diffuse alveolar damage, reactive pneumocytes, acute and chronic bronchitis, perivascular and interstitial lymphocytic inflammation, background emphysematous changes, subsegmental pulmonary embolus | Interstitial fibrosis, myocyte hypertrophy, replacement fibrosis | Congestion | Mild to moderate arterionephrosclerosis, reactive tubular epithelium | No diagnostic alterations | Oedema, chronic (lymphocytic) tracheitis | No diagnostic alterations | No diagnostic alterations | No diagnostic alterations |
| 11 | Acute and organising diffuse alveolar damage, reactive pneumocytes, multinucleated giant cells, acute bronchopneumonia, pulmonary haemorrhage | Interstitial fibrosis | Steatosis, congestion | Mild to moderate, arterionephrosclerosis, scattered tubular casts | No diagnostic alterations | Oedema, acute (neutrophilic) tracheitis | Haemophagocytosis | No diagnostic alterations | No diagnostic alterations |
| 12 | Pulmonary oedema, acute and organising phase diffuse alveolar damage, reactive pneumocytes, multinucleated giant cells, acute bronchiolitis, subsegmental pulmonary emboli | Interstitial fibrosis, myocyte hypertrophy, replacement fibrosis | Steatosis, congestion, centrilobular necrosis | Mild to moderate arteriolosclerosis, scattered granular casts | White pulp depletion | Oedema | Not sampled | No diagnostic alterations | No diagnostic alterations |
| 13 | Pulmonary oedema, acute phase diffuse alveolar damage, pulmonary haemorrhage, chronic fibrosis, microthrombi | Interstitial fibrosis, myocyte hypertrophy, replacement fibrosis, myocardial amyloid | Congestion | Severe arterionephrosclerosis, scattered tubular casts, reactive tubular epithelium, renal vein organising thrombus | No diagnostic alterations | Sloughed epithelium | No diagnostic alterations | No diagnostic alterations | Pending |
| 14 | Pulmonary oedema, acute bronchopneumonia, perivascular and interstitial lymphocytic infiltrate, microthrombus, reparative fibrosis and neovascularisation, vascular disease | Interstitial fibrosis, myocyte hypertrophy, replacement fibrosis | Steatosis, congestion, centrilobular necrosis, portal lymphocytic inflammation | Mild to moderate arterionephrosclerosis, reactive tubular epithelium, tubular casts, chronic inflammation, focal segmental glomerulosclerosis | No diagnostic alterations | Oedema, chronic (lymphocytic) tracheitis, haemorrhage, ulceration, epithelial sloughing | Haemophagocytosis | No diagnostic alterations | Not sampled |
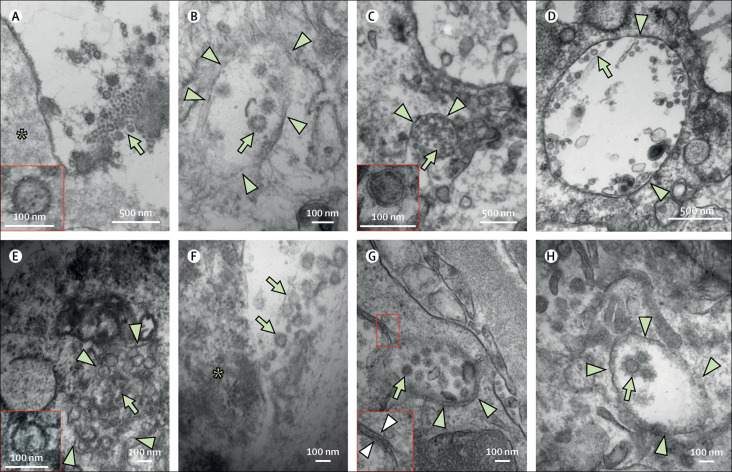
By electron microscopy, aggregates of uniform, round enveloped particles ranging in size from around 70 nm to 100 nm with peripheral spike-like projections consistent with the morphology described for SARS-CoV-2 were observed in the lung, trachea, kidney, and large intestine of patient 8 and patient 13.14, 15 When confined to vesicles or in the extracellular space, structures with these characteristics were designated coronavirus-like particles. For simplicity, we refer to these coronavirus-like particles as viral particles. Viral particles were present both inside tracheal epithelial cells and the extracellular space adjacent to the cell membrane or mixed with the luminal mucus (figure 5A, B ). In the lung, extensive sloughing of pneumocytes into alveolar spaces was observed. Some of these pneumocytes contained abundant autophagosomes, and occasionally viral particles were observed within the vesicles. Viral particles were seen both in type 2 and type 1 pneumocytes (figure 5C, D). Aggregates of viral particles were found in enterocytes; however, the membranes of the surrounding vesicles were often disrupted and the tissue affected by post-mortem artefact (figure 5E, F). In the kidney, viral particles were observed in tubular epithelial cells, and more rarely in endothelial cells (figure 5G, H). Some viral particles were associated with double membranes, and resembled double membrane vesicles (figure 5G).16 Rare ill-defined round particles were also observed in podocytes. Definitive viral particles were not observed in the other examined organs, including the heart, spleen, and liver.
Figure 5.
Ultrastructural features in fatal COVID-19 infections
Ultrastructural finding of viral particles in tracheal epithelial cells (A and B) in patient 13, lung pneumocytes (C and D) in patient 13, enterocytes (E and F) in patient 13, and kidney endothelial cells (G) in patient 8 and proximal tubular epithelial cells (H) in patient 13. Viral particles (indicated by green arrows) were observed either outside cells (A and F) in close proximity to the cell membrane or inside the cells (B, C, D, E, G, and H) in aggregates confined within vesicles (indicated by green arrowheads). Some of the particles were associated with double membranes (indicated by white arrowheads) resembling double membrane vesicles. Asterisks in (A) and (F) mark the cells adjacent to the viral particles in the extracellular space.
SARS-CoV-2 RNA was detected in the lung, trachea, subcarinal lymph node, kidney, large intestine, and spleen of all three tested patients (patients 8, 13, and 14). Viral RNA was also detected in the liver, heart, and blood for patient 8 and patient 13. In all three patients, the lungs and trachea had the lowest cycle threshold values.
Discussion
The spectrum of pathological findings in people who die from COVID-19 is only beginning to emerge.9, 10, 11, 12 We present a case series of autopsy findings in 14 patients who died after SARS-CoV-2 infection. Our results show the central role of lung damage and provide evidence that suggests extrapulmonary involvement of SARS-CoV-2 during severe infection.
The major histopathological observation in our series of patients who died with COVID-19 was diffuse alveolar damage-type lung injury in the acute or organising phases (12 [86%] of 14 patients). Lung tissue from most decedents showed pulmonary oedema, prominent reactive type 2 pneumocytes, intra-alveolar fibrin, and hyaline membranes. These findings are similar to those described during the 2002–03 SARS-CoV outbreak and more recent COVID-19 studies.9, 11, 17, 18, 19 In contrast to SARS-CoV, in which organising diffuse alveolar damage was predominantly observed in those with longer hospitalisations (>10 to 14 days), we found evidence of organising diffuse alveolar damage in a patient with COVID-19 who died 2 days after symptom onset (patient 2) and observed acute and organising diffuse alveolar damage in patients 8 and 11, who died within a week of symptom onset.20 Compared with the study by Franks and colleagues,20 our patient cohort also had a shorter interval from symptom onset to death (median 7 days vs 12·3 days). We hypothesise that there was a subclinical period during which lung injury was occurring in COVID-19 patients with organising diffuse alveolar disease who died in the week after symptom onset. The hypothesis that lung injury might occur in COVID-19 patients before symptom onset is supported by evidence of abnormal pulmonary CT findings in asymptomatic patients.21 These findings could have implications for screening patients at the time of admission to identify and aggressively manage those with pre-existing diffuse alveolar disease-type injury. Additionally, we noted mostly focal SARS-CoV-2 immunohistochemical staining in the lungs of tested patients. In areas with less severe diffuse alveolar damage the virus was more readily visualised in pneumocytes. The absence of strong diffuse viral staining might indicate that most cytotoxic damage caused by SARS-CoV-2 occurs early on in infection, with diffuse alveolar damage seen later as part of an exuberant host response.
Whether COVID-19 patients are at increased risk for endothelial injury causing pulmonary microthrombi has become central to the discussion of patient management.22 This theory is based on the observed mismatch between lung compliance and oxygen saturation in ventilated patients.23 These results have led to the proposal that COVID-19 acute respiratory distress syndrome (ARDS) might represent a novel type of lung injury. An early pathology report documenting three patients with COVID-19 found active endotheliitis and endothelial cells containing coronavirus-like particles, supporting the claims of microvascular damage during infection.15 However, the observation of virally infected endothelial cells has been called into question.24 Although our findings document coronavirus-like particles in the endothelial cells of the kidney, we did not observe endothelial cell infection in other organs surveyed by electron microscopy or immunohistochemistry. Additionally, no histological evidence of endotheliitis was observed in our cohort. Given the scarcity of these findings, we propose that the lung injury observed in our cohort presented the typical ARDS lung phenotype and not a novel type of injury.
Infection of endothelial cells by SARS-CoV-2 is hypothesised to cause dysregulation of the clotting system, which particularly affects small vessels and leads to pulmonary microthrombi and altered ventilatory patterns in intubated patients.22, 23 Around a third of our cohort (five patients) had infrequent microthrombi. As no formal grading scale for the severity of microthrombi in tissue exists, we would classify the presence of microthrombi in our cohort as less than one per low power (10×) field and within the scope of what is seen in other causes of diffuse alveolar damage. Most microthrombi were seen when a full autopsy was done, and we suspect rare microthrombi might also be present in our cohort of limited autopsies. However, this factor is unlikely to have changed our overall findings or final cause of death. Macroscopic pulmonary arterial thrombi were identified in two patients (patient 10 and patient 12). Patient 10 showed evidence of organisation with adherence to the pulmonary artery wall, indicating an event that probably predated COVID-19 infection. An organising thrombus was also found in a small renal vein on microscopic evaluation of patient 13 and was considered probably unrelated to COVID infection.
Cardiac injury in COVID-19 is common, although our results do not provide direct evidence of myocardial injury by SARS-CoV-2. In a study that documented the clinical course of patients in the intensive care unit in Kirkland, WA,25 33% of patients had cardiomyopathy of unclear cause. Previous post-mortem examinations have detected viral RNA in cardiac tissue from a single patient, although histopathological evidence of myocarditis was not present.10 In our cohort, patients 8, 11, and 13 had elevated troponin; however, only patient 8 had histologically apparent lymphocytic myocarditis. Myocardial tissue from this patient was positive for viral RNA by PCR, but immunohistochemistry and electron microscopy results were negative. As the patient was also viraemic, the low RNA level detected in the cardiac tissue might represent contamination by circulating virus rather than direct infection. Patient 8 also tested positive for influenza A, a known cause of viral myocarditis, before death.26 Contamination by viral RNA could also explain the results in the liver and spleen, where no definitive virus was identified by electron microscopy or immunohistochemistry.
Tubular epithelial cells, endothelial cells, and podocytes express ACE2, making kidneys a candidate target for SARS-CoV-2 infection.27 Direct infection of kidney cells by the virus has been proposed as a mechanism for acute kidney injury observed during SARS-CoV-2 infection.28 Two studies reported SARS-CoV-2 in tubular epithelial cells and podocytes.14, 15 These studies depended on ultrastructural findings, which carry a risk of confusion with cellular structures if used without immunolabelling. Therefore, we labelled the observed structures as coronavirus-like particles, although positive PCR (three of three patients) and immunohistochemistry (two of four patients) data support our assertion that infection of renal cells occurs by COVID-19.
Multiple orthogonal approaches, including PCR, immunohistochemistry, and electron microscopy were used to help identify tissues that harboured viral particles. The pattern of virus distribution seen in our cohort raises questions about the mechanism of viral dissemination during severe infections, specifically whether SARS-CoV-2 can be transported by lymphocytes. Current data support this hypothesis, with viral RNA detected in blood samples and in vitro data showing pseudotyped SARS-CoV-2 capable of infecting lymphocytes.6, 29 In our study, two of three whole blood samples and three of three lymph nodes tested positive by PCR, in addition to SARS-CoV-2 immunohistochemistry highlighting lymphocytes in the tracheal submucosa of patient 8. If SARS-CoV-2 is capable of productive infection in lymphocytes, this could provide a mechanistic explanation for the poor survival and cytokine derangements of severe COVID-19 cases with lymphopenia.30
The extent to which our findings of extrapulmonary involvement of SARS-CoV-2 are generalisable to non-severe COVID-19 infections is uncertain. As SARS-CoV-2 has been detected in urine and stool of non-severe COVID-19 cases, there is evidence suggesting productive infection of non-pulmonary sites.7, 31 These questions raise concerns for susceptible groups, including those with chronic renal injury or inflammatory bowel disease. Whether these patients are at increased risk for more serious complications during SARS-CoV-2 infection requires close monitoring. Another patient group that requires close monitoring are those undergoing organ transplantation. Although extrapulmonary infection might be less common in mild or subclinical disease, it is unclear whether active extrapulmonary infection can exist in a patient without concurrent respiratory infection.
Our study has several limitations. Electron microscopy was limited to patients 8 and 13, PCR to patients 8, 13, and 14, and immunohistochemical testing to patients 8, 10, 11, and 12. Future studies will reveal whether the relatively consistent findings in this small number of patients are borne out in larger samples. As safety limitations in place during February and early March, 2020, precluded complete autopsies of seven patients, our ability to detect rare findings was restricted for part of the cohort. In some situations, there was an extended post-mortem interval before examination. As post-mortem autolysis reduces the sensitivity of electron microscopy and PCR, patient samples collected several days after death might generate false negative results. However, we could detect viral particles and viral RNA from specimens up to 140 h post mortem (patient 13). Extended post-mortem intervals should not be an absolute criterion for exclusion in future studies.
In conclusion, our findings show the central role of diffuse alveolar damage-type lung injury in patients with severe COVID-19. Microthrombotic disease and endothelial injury were not as pronounced as reported in previous studies. We found broad tropism for SARS-CoV-2 with coronavirus-like particles identified in the pulmonary system, kidneys, and gastrointestinal tract. Our results also raise the question as to whether SARS-CoV-2 can cause direct myocardial injury and whether direct infection of lymphocytes promotes viral dissemination and immune dysregulation. These findings provide histological context for clinical observations and help characterise the pathophysiology of SARS-CoV-2, hopefully leading to novel treatment strategies.
This online publication has been corrected. The corrected version first appeared at thelancet.com on July 30, 2020
Acknowledgments
Acknowledgments
We thank the technical staff of King County Medical Examiner's Office (Seattle, WA, USA) and Snohomish Medical Examiner's Office (Everett, WA, USA) for their assistance with autopsy procedures. We thank Jennifer Swicord, Gianni Niolu, and the Electron Microscopy Laboratory at the University of Washington (Seattle, WA, USA) for preparation of electron microscopy specimens.
Contributors
BTB conceived and designed the study. HM, RJ, and IC contributed to clinical data collection. HM, BN, and BTB contributed to figure design. NY, TW, JML, DAM, RJ, IC, and BTB did the post-mortem examinations. NY, TW, JML, DAM, RJ, IC, HM, HX, BN, GD, and BTB contributed to histopathological evaluation of tissue. GD contributed to immunohistochemical studies. BN and BTB contributed to electron microscopy images. BTB and SLF contributed to molecular testing. BTB and HM wrote the manuscript. All authors contributed to data analysis, data interpretation, and editing the manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Zhu N, Zhang D, Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Yu F, Yan L, Wang N. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa345. https://doi.org.10.1093/cid/ciaa345 published online March 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Xu Y, Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling Y, Xu S-B, Lin Y-X. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee N, Hui D, Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 9.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian S, Xiong Y, Liu H. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Z, Shi L, Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magro C, Mulvey JJ, Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMichael TM, Currie DW, Clark S. Epidemiology of COVID-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su H, Yang M, Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varga Z, Flammer AJ, Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi Y, Bowman JW, Jung JU. Autophagy during viral infection—a double-edged sword. Nat Rev Microbiol. 2018;16:341–354. doi: 10.1038/s41579-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao S-Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu J, Gong E, Zhang B. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholls JM, Poon LLM, Lee KC. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franks TJ, Chong PY, Chui P. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol. 2003;34:743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi H, Han X, Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323:2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 23.Gattinoni L, Chiumello D, Caironi P. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldsmith CS, Miller SE, Martines RB, Bullock HA, Zaki FR. Electron microscopy of SARS-CoV-2: a challenging task. Lancet. 2020;395:e99. doi: 10.1016/S0140-6736(20)31188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arentz M, Yim E, Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowles NE, Ni J, Kearney DL. Detection of viruses in myocardial tissues by polymerase chain reaction. Evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- 27.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batlle D, Soler MJ, Sparks MA. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31:1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Xu W, Hu G. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0424-9. published online April 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Li S, Liu J. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing Y-H, Ni W, Wu Q. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 2020;53:473–480. doi: 10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.