Abstract
Objectives:
To assess the effects of recombinant human soluble thrombomodulin treatment on 28-day all-cause mortality in subgroups categorized by baseline coagulation biomarker levels (prothrombin fragment 1.2, thrombin-antithrombin complex, d-dimer) in patients with sepsis-associated coagulopathy in the Sepsis Coagulopathy Asahi Recombinant LE Thrombomodulin trial (SCARLET) (NCT01598831).
Design:
Post hoc, subgroup analysis of a randomized, double-blind, placebo-controlled, multinational, multicenter phase 3 study.
Setting:
ICUs at 159 sites in 26 countries.
Patients:
Eight-hundred adults with sepsis-associated coagulopathy defined as international normalized ratio greater than 1.40 and platelet count between 30 × 109/L and 150 × 109/L or greater than 30% decrease within 24 hours with concomitant cardiovascular and/or respiratory failure.
Interventions:
Patients randomized and treated with recombinant human soluble thrombomodulin (0.06 mg/kg/d; n = 395) or equivalent placebo (n = 405) for 6 days.
Measurements and Main Results:
Recombinant human soluble thrombomodulin did not significantly reduce 28-day all-cause mortality in the Sepsis Coagulopathy Asahi Recombinant LE Thrombomodulin trial: absolute risk reduction was 2.55% (p = 0.32) in patients with sepsis-associated coagulopathy. In this post hoc analysis, mortality steadily increased with increasing baseline prothrombin fragment 1.2 and thrombin-antithrombin complex levels in the placebo group; for those values exceeding the upper limit of normal, the mortality increases in the recombinant human soluble thrombomodulin group were lower or negligible with increasing baseline prothrombin fragment 1.2 and thrombin-antithrombin complex. Consequently, absolute risk reductions were greater in subgroups with higher baseline prothrombin fragment 1.2 or thrombin-antithrombin complex. Absolute risk reductions were also greater in subgroups with baseline coagulation biomarker levels at or above median of the entire study population, ranging from 4.2% (95% CI, –5.0% to 13.4%) to 5.5% (95% CI, –4.0% to 14.9%).
Conclusions:
Compared with patients receiving placebo, patients treated with recombinant human soluble thrombomodulin having higher baseline thrombin generation biomarker levels had lower mortality. Further research regarding the predictive role of coagulation biomarkers for recombinant human soluble thrombomodulin treatment response in sepsis-associated coagulopathy is warranted to evaluate clinical relevance.
Keywords: international normalized ratio, platelet count, prothrombin fragment 1.2, recombinant human soluble thrombomodulin, sepsis, thrombin-antithrombin complex
Sepsis can lead to life-threatening multiple organ dysfunction that is estimated to contribute to approximately 5 million deaths globally per year (1, 2). It is caused by a dysregulated response of the body to infection and is often complicated by coagulopathy and endothelial dysfunction (1, 3, 4). Disseminated intravascular coagulation (DIC) is a key predictor of clinical outcomes in patients with sepsis and is associated with aberrantly enhanced thrombin generation (5, 6). Generated thrombin is primarily neutralized by antithrombin, thereby forming thrombin-antithrombin complexes (TAT). Prothrombin fragment 1.2 (F1.2) is an activation peptide generated when prothrombin is converted to thrombin (7). New criteria for DIC from the Japanese Society on Thrombosis and Hemostasis (JSTH) include these biomarkers of thrombin generation, TAT, and F1.2 to improve the sensitivity and specificity of the DIC criteria. If any of these values are greater than or equal to two-fold of the respective upper limit of normal, then a point is added to the JSTH diagnostic score (8).
There is an incremental increased risk of mortality with an increase in the severity of sepsis-associated coagulopathy (SAC), as defined by prolonged prothrombin time-international normalized ratio (PT-INR) and reduced platelet count (9). In SAC, a systemic inflammatory response to the infection is triggered, which leads to coagulation activation (10). Activating the coagulation cascade also leads to excess formation of fibrin, the primary component of a thrombus. A key enzymatic degradation product of this process is d-dimer (11). Endothelial expression of the pivotal receptor of the crucial regulatory protein C pathway, thrombomodulin, is severely reduced (10, 12), leading to reduced activated protein C (APC), which functions to proteolytically degrade coagulation factors Va and VIIIa, exerting an antithrombogenic effect (13). In addition, the APC pathway has anti-inflammatory properties (10, 12, 14). An initial phase 3 study of recombinant human APC (rhAPC; Xigris, Eli Lilly, Indianapolis, IN) showed that rhAPC decreased mortality in patients with severe sepsis (15). However, subsequent studies showed less-favorable results and rhAPC has been withdrawn from the market (16).
Recombinant human soluble thrombomodulin (ART-123) exerts anticoagulant and anti-inflammatory effects (17). Unlike exogenously administered rhAPC, ART-123 binds circulating thrombin molecules to serve as an activation complex to convert endogenous protein C to endogenous APC, which is the underlying mechanism for the anticoagulant effect of ART-123 (17). Thus, the complex formed by the binding of ART-123 with thrombin is the initial step in exerting the anticoagulant and anti-inflammatory effects of ART-123. However, plasma APC activity is not increased in patients with sepsis-associated DIC who are treated with ART-123. It is possible that APC acts locally and does not systemically circulate (18). Thus, endogenously generated APC concentrations are very low and nearly undetectable. In addition, ART-123 inhibits inflammation and organ injury caused by damage-associated molecular patterns, such as high mobility group box protein 1 and histones (19–21).
ART-123 was evaluated in a global phase 2b clinical study in patients with sepsis and DIC (NCT00487656) (22). The 28-day mortality rates were 17.8% in the ART-123 treatment group and 21.6% in the placebo group (p = 0.27) (22). Biomarker analysis in this study showed that ART-123–treated patients exhibited a significant decrease in the levels of coagulation biomarkers F1.2, TAT, and d-dimer compared with patients who received placebo at day 7 (p < 0.05) (23).
Sepsis Coagulopathy Asahi Recombinant LE Thrombomod ulin (SCARLET) (ClinicalTrials.gov identifier: NCT01598831) was a randomized, double-blind, placebo-controlled, multinational, multicenter, parallel-group phase 3 trial of patients with organ dysfunction and coagulopathy due to severe sepsis to assess the safety and efficacy of ART-123 treatment. ART-123 did not significantly reduce 28-day all-cause mortality (primary efficacy endpoint) versus placebo—the absolute risk reduction (ARR) of 28-day all-cause mortality in ART-123–treated patients was 2.55% (p = 0.32). However, in post hoc analyses, 28-day all-cause mortality rates in the subgroup of patients who maintained the protocol-specified coagulopathy criteria at baseline (after randomization and before the first dose of study drug; n = 634; PT-INR > 1.40 and platelet count > 30 × 109/L) were 26.7% (82/307) for ART-123 and 32.1% (105/327) for placebo—a difference of 5.40% (95% CI, –1.7% to 12.5%). In the post hoc analysis, a significant change from baseline was observed in each biomarker during the 6-day treatment period among those receiving ART-123 versus those assigned to placebo (p < 0.05), despite the reduction in 28-day all-cause mortality not being significant in the ART-123 treatment group. Thrombin generation markers F1.2 and TAT appeared to undergo significantly more rapid reduction in the ART-123 group as compared with the placebo group. Similarly, plasma levels of d-dimer were lowered after treatment with ART-123 compared with placebo (24).
The objective of this post hoc analysis is to present the 28-day all-cause mortality data for patients treated with ART-123 categorized by baseline coagulation biomarker levels (F1.2, TAT, and d-dimer) in patients with SAC in the SCARLET trial.
MATERIALS AND METHODS
Study Design
A total of 816 patients who were critically ill with SAC were randomized in a 1:1 ratio to allocate to ART-123 (n = 402) or placebo (n = 414). Among these patients, 800 individuals in the full analysis set (FAS) were randomized and treated with either IV ART-123 (n = 395) or equivalent placebo (n = 405). ART-123 was dosed at 0.06 mg/kg/d up to a maximum dose of 6.0 mg/d for 6 days, as described previously (24). For each of the participating institutions, the institutional review board or local ethics committee reviewed and approved the study. Informed consent was obtained from all participants prior to performing any study-specific procedures to conform to all applicable local, regulatory, and ethical requirements. This study was conducted in compliance with the International Conference on Harmonisation Good Clinical Practice guideline (25).
Patient Population
Patients greater than or equal to 18 years old who were critically ill with SAC from a bacterial source and receiving treatment with IV antibiotics, with WBC counts greater than 12,000/mm3 or less than 4,000/mm3 or bandemia greater than 10%, temperature of less than 36°C or fever greater than 38°C, with a concurrent diagnosis of cardiovascular dysfunction (requiring both adequate fluid resuscitation and vasopressors) and/or respiratory dysfunction (requiring mechanical ventilation for hypoxemia), and coagulopathy were included in the study. Coagulopathy was characterized by 1) PT-INR greater than 1.40 without other known etiology (e.g., anticoagulant therapy, chronic liver disease) and 2) platelet count between 30 × 109/L and 150 × 109/L or greater than 30% decrease in platelet count within 24 hours. Subcutaneous heparin for thromboprophylaxis was permitted at doses per the manufacturer recommendations in the package inserts approved for each country. Wide variability was observed across countries in the heparin dose administered and percentage of enrolled patients receiving prophylactic heparin at baseline.
Approximately 80% of patients (634/800) maintained protocol-specified eligibility for coagulopathy of PT-INR greater than 1.40 and platelet count greater than 30 × 109/L before initiating the first dose of study drug (baseline). In the remaining patients, PT-INR was less than or equal to 1.40 and/or platelet count less than 30 × 109/L at baseline after initial confirmation of eligibility. The subset of patients with PT-INR greater than 1.40 and platelet count greater than 30 × 109/L at baseline will be referred to as having “confirmed coagulopathy” in this article.
Measurement and Analysis of Coagulation Biomarkers
Citrate- or ethylenediaminetetraacetic acid-anticoagulated whole blood was drawn from patients. Plasma was prepared from the blood and frozen at –70°C or –20°C and shipped to the central laboratory (PPD, Wilmington, NC) Plasma samples for F1.2 and TAT measurements were analyzed on a monthly basis using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Siemens, Berlin, Germany) at Loyola University Chicago, Hemostasis and Thrombosis Research Laboratories. The coefficient of variation (CV) between assays for F1.2 was 4.8% and the CV for TAT was 4.1%. The plasma samples for d-dimer measurements were analyzed on a weekly basis using commercially available ELISA kits (Instrumentation Laboratory, Bedford, MA) at PPD. The CV for d-dimer intra-assay variation was 7.0%.
Mortality Data for Baseline Coagulation Biomarker Subgroups
In this post hoc analysis, the placebo and ART-123 groups were categorized by baseline values of F1.2, TAT, and d-dimer (≤ upper limit of normal range, > upper limit, ≥ 1.5-fold upper limit, ≥ two-fold upper limit, and ≥ 2.5-fold upper limit; categories were based on normal range values from the package inserts of the assays for each of the three coagulation biomarkers), consistent with the new JSTH DIC criteria described previously (7). Mortality rates in the FAS population and the confirmed coagulopathy subgroup were evaluated according to baseline coagulation biomarker levels. According to the assay kit package inserts, the normal range for F1.2 was 69 to 229 pmol/L, and TAT was 1.0 to 4.1 μg/L; for d-dimer, the upper limit was 243 ng/mL.
Statistical Analysis
CIs were calculated using traditional 95% two-sided intervals and all calculations were performed using SAS Version 9.3 or 9.4 (SAS Institute, Cary, NC).
RESULTS
Patients
Baseline demographics and characteristics for all study patients were described previously (24). In summary, baseline characteristics were well balanced between the ART-123 and placebo groups in the FAS population. The mean age of the patients was 61.0 years in the ART-123 group and 60.5 years in the placebo group and approximately 55% of patients in each group were male. The demographics and baseline characteristics for patients with F1.2 and TAT biomarker levels below the median value and patients with biomarker levels at or above the median value were well balanced in the ART-123 and placebo groups (Tables 1 and 2). In patients with baseline thrombin generation markers at or above the median value, mean INR was slightly higher, platelet counts were slightly lower, and Acute Physiology and Chronic Health Evaluation II scores were slightly higher. In addition, standard-of-care prophylactic heparin use was lower at baseline in these patients.
TABLE 1.
Patient Characteristics and Baseline Coagulation Biomarker Levels by Subgroups Based on Prothrombin Fragment 1.2 Levels in the Full Analysis Set of the Sepsis Coagulopathy Asahi Recombinant LE Thrombomodulin Trial
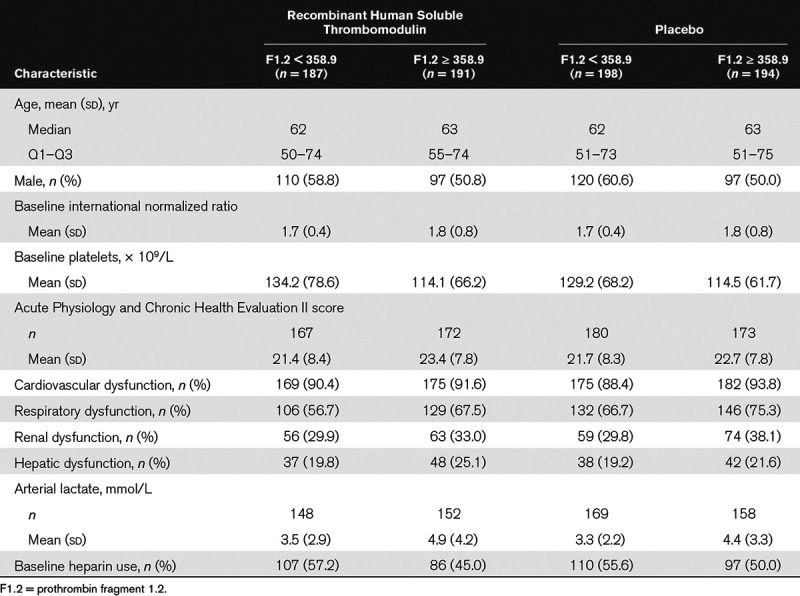
TABLE 2.
Patient Characteristics and Baseline Coagulation Biomarker Levels by Subgroups Based on Thrombin-Antithrombin Complex Levels in the Full Analysis Set of the Sepsis Coagulopathy Asahi Recombinant LE Thrombomodulin Trial
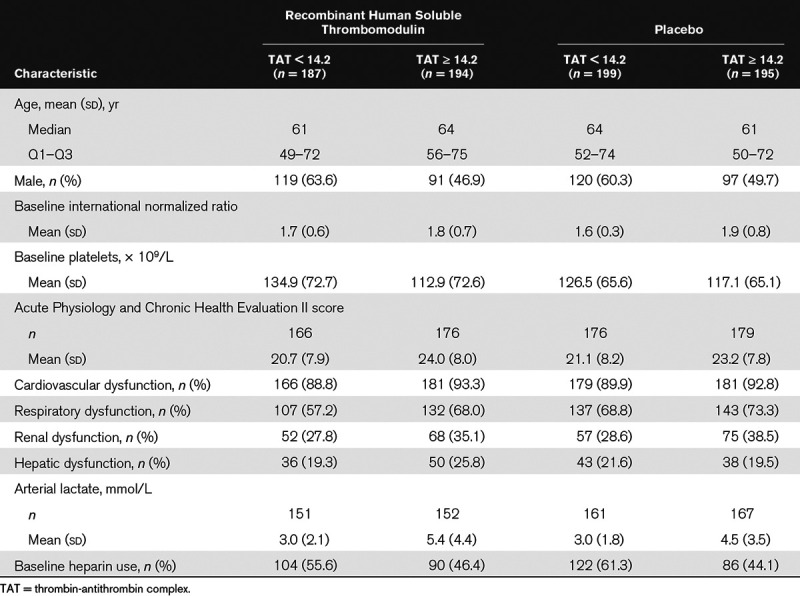
Mortality Data for Baseline Coagulation Biomarker Subgroups
As previously reported, the change from baseline of each coagulation biomarker (F1.2, TAT, d-dimer) during the treatment period (6 d) was statistically significantly greater in the ART-123 group than the placebo group (24). The baseline values of these coagulation biomarkers and 28-day all-cause mortality rates were also investigated in patients who were randomized and treated with ART-123 (n = 395) or placebo (n = 405; Fig. 1). These results were not statistically significant in this post hoc analysis. As the baseline plasma levels of F1.2 and TAT increased in the placebo group, the 28-day all-cause mortality rates increased from 20.0% and 19.2% in the group with biomarker levels less than or equal to the upper limit to 38.9% and 36.3% in the group with greater than or equal to the 2.5-fold upper limit, respectively. However, except for the group with baseline biomarker levels less than or equal to the upper limit, the 28-day all-cause mortality rates ranging from 28.8% to 31.8% and 27.0% to 29.4% were consistently lower or not apparent in patients treated with ART-123 versus those assigned to placebo, whose mortality rates ranged from 33.1% to 38.9% and 29.6% to 36.3%, across the range of increasing baseline F1.2 and TAT levels, respectively. Consequently, ARRs were greater in the subgroups with higher baseline F1.2 or TAT levels. Analysis of d-dimer levels did not show a change in the 28-day all-cause mortality rates for either group with increasing baseline levels. ARRs were also greater in the subgroups with baseline coagulation biomarker levels at or above median values of the entire study population: 4.7% (95% CI, –4.7% to 14.1%), 5.5% (95% CI, –4.0% to 14.9%), and 4.2% (95% CI, –5.0% to 13.4%) for F1.2, TAT, and d-dimer, respectively (Table 3). However, none of the ARRs indicate statistical significance.
Figure 1.
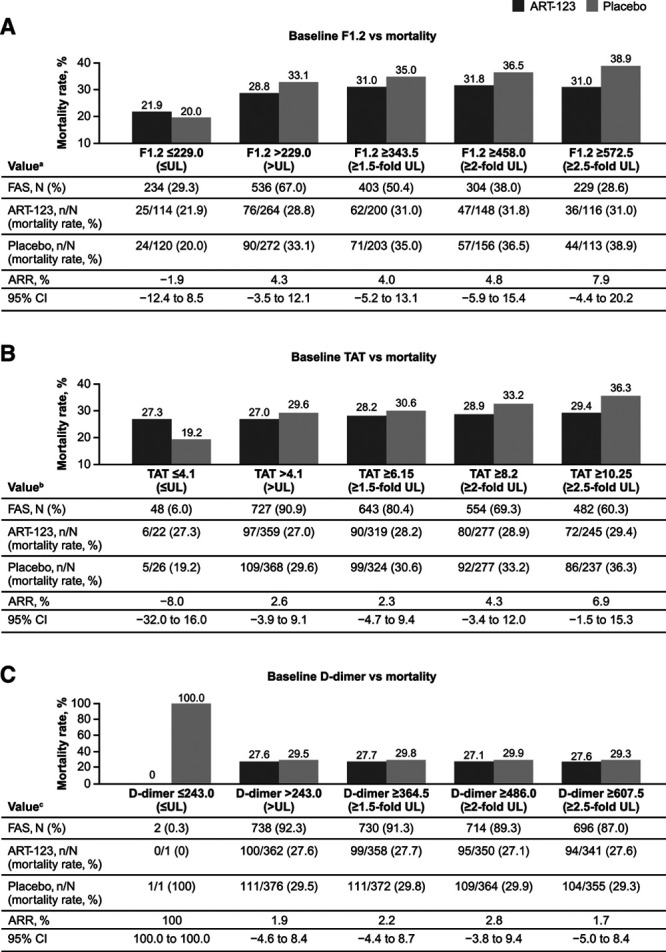
Mortality rates in coagulation biomarker subgroups. A, Prothrombin fragment 1.2 (F1.2), B, Thrombin-antithrombin complex (TAT), C, d-dimer, and 28-d all-cause mortality in patients treated with recombinant human soluble thrombomodulin (ART-123) or placebo. aData were unavailable for 30 patients. bData were unavailable for 25 patients. cData were unavailable for 60 patients. ARR = absolute risk reduction, FAS = full analysis set, UL = upper limit.
TABLE 3.
Association of Baseline Coagulation Biomarkers With 28-Day All-Cause Mortality in the Post Hoc Analysis in the Full Analysis Set Population of the Sepsis Coagulopathy Asahi Recombinant LE Thrombomodulin Trial
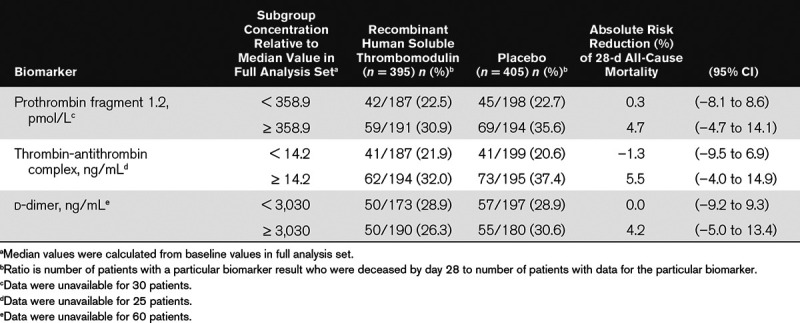
A separate post hoc analyses was also performed in patients with confirmed coagulopathy (PT-INR > 1.40 and platelet count > 30 × 109/L) at baseline who were treated with ART-123 (n = 308) and placebo (n = 326). In this subset of patients with confirmed coagulopathy and baseline coagulation biomarker levels at or above median values of the FAS population, ARRs were 10.3% (95% CI, –0.2% to 20.8%), 8.1% (95% CI, –2.3% to 18.6%), and 7.5% (95% CI, –2.8% to 17.8%) for F1.2, TAT, and d-dimer, respectively, versus 2.55% for the FAS population and 5.40% for the patients with confirmed coagulopathy.
DISCUSSION
Coagulopathy is prevalent in patients with sepsis and likely plays a key role in multiple organ dysfunction (26, 27). SAC is correlated with an increased risk of mortality and is defined by a prolonged PT-INR and reduced platelet count. In this post hoc analysis, patients in the SCARLET trial were sorted by the baseline values of coagulation biomarkers (≤ upper limit, > upper limit, ≥ 1.5-fold upper limit, ≥ two-fold upper limit, and ≥ 2.5-fold upper limit), referring to the new JSTH DIC criteria, which include values of F1.2 or TAT (8). The upper limits of normal range were based on values from the package inserts for each of the three coagulation biomarkers. Findings from this post hoc analysis show that mortality rates steadily increased, with increasing levels of baseline thrombin generation markers, F1.2 and TAT, in the placebo group (Fig. 1). However, except for the group with F1.2 and TAT levels at or below the upper limit of normal, the increases in the 28-day all-cause mortality rates in the ART-123 group were lower or negligible with increasing baseline F1.2 and TAT levels. Consequently, ARRs were greater in the subgroups with higher baseline F1.2 or TAT levels. These results are consistent with the known anticoagulant effects of ART-123 exerted by binding with thrombin and modifying thrombin activity (17). The discrepancies between the observations with F1.2 or TAT levels and d-dimer levels are possibly due to F1.2 and TAT being pure markers of thrombin generation, whereas d-dimer is a marker of fibrin degradation (fibrinolysis). These d-dimer data may be a reflection of the impaired fibrinolysis known to occur in sepsis due to elevated levels of plasminogen activator inhibitor-1 (PAI-1), which inhibits plasmin formation and subsequently the degradation of fibrin. High levels of PAI-1 are correlated with the severity of sepsis and are associated with increased mortality (28). The higher levels of PAI-1 in patients with severe sepsis could have affected the relationship between mortality and d-dimer levels in this analysis. In the previous post hoc analyses, 28-day all-cause mortality rates in the subgroup of patients who maintained the protocol-specified coagulopathy criteria at baseline (after randomization and before the first dose of study drug; n = 634, PT-INR > 1.40 and platelet count > 30 × 109/L) were 26.7% (82/307) with ART-123 and 32.1% (105/327) with placebo—a difference of 5.40% (95% CI, –1.7% to 12.5%), exceeding the 2.55% ARR of the FAS (p = 0.32) (24). This post hoc analysis shows the mortality data for ART-123–treated patients with SAC and higher coagulation markers (F1.2, TAT, and d-dimer). The ARRs of these subgroups increased with higher baseline levels of thrombin generation biomarkers.
Results from a phase 1 study showed that the inhibition of thrombin generation was directly correlated with the concentration of ART-123 (29), and the pharmacokinetic profile of ART-123 showed that dosing at 0.06 mg/kg/d for 6 consecutive days caused a gradual increase in the plasma concentration of ART-123 up to 1,600 ng/mL at day 6, after which the levels slowly decreased (30). Similar outcomes were noted in the analysis of coagulation biomarker levels in patients in the SCARLET trial in which consistent reductions from baseline were observed in the plasma levels of each of the coagulation biomarkers F1.2, TAT, and d-dimer during the treatment period (6 d) and the reductions were greater with ART-123 than with placebo (24).
SAC is a heterogeneous condition, and there are limitations for applying the findings of this clinical study across all patients with this condition. Variations in standard of care for the treatment of SAC may exist across sites because characteristics of ICUs vary among countries and regions (31). Such potential differences include use of prophylactic heparin. Data from this post hoc subgroup analysis should be reviewed with consideration that the sample size analyzed here limits the ability to draw robust conclusions. The findings from the post hoc analysis provide a solid rationale for pursuing additional studies with larger sample sizes to confirm these findings.
CONCLUSIONS
This post hoc analysis shows mortality data for patients with SAC according to baseline coagulation marker levels. Compared with patients treated with placebo, the subgroup of ART-123–treated patients with SAC who had higher baseline levels of thrombin generation biomarkers had lower mortality. Further research regarding the predictive role of coagulation biomarkers for ART-123 treatment response in patients with SAC is warranted to evaluate clinical relevance.
ACKNOWLEDGMENTS
Medical writing and medical editing were provided under the direction of the authors by Rajni Parthasarathy, PhD, and Sherri Damlo, ELS, of MedThink SciCom, Cary, NC, funded by Asahi Kasei Pharma America Corporation.
Footnotes
All authors have materially participated in the conception, design, execution, and writing of the article.
This study was funded by Asahi Kasei Pharma America Corporation.
Significance of present work: Sepsis is a host response to infection leading to activation of inflammation and coagulation and is associated with high mortality rates. Recombinant human soluble thrombomodulin (ART-123) has distinct anticoagulant and anti-inflammatory effects. Primary results from the randomized, double-blind, placebo-controlled, multinational phase 3 Sepsis Coagulopathy Asahi Recombinant LE Thrombomodulin trial (SCARLET) (NCT01598831) showed that ART-123 did not significantly reduce 28-day all-cause mortality (absolute risk reduction [ARR], 2.55% [p = 0.32]), in patients with sepsis-associated coagulopathy. Herein we report that the ARR values with ART-123 were higher in patients with higher baseline thrombin generation biomarker levels in the SCARLET trial.
Dr. Levi is a member of the program advisory board of Asahi Kasei Pharma America Corporation. Dr. Vincent reported receiving grants from Asahi Kasei Pharma America Corporation during the conduct of the study. s. Tanaka, Ms. Radford, Kayanoki, and Dr. Fineberg are employees of Asahi Kasei Pharma America Corporation. Dr. Hoppensteadt is principal coordinator of the biomarker-profiling studies at Loyola University related to the Sepsis Coagulopathy Asahi Recombinant LE Thrombomodulin trial and has received consulting fees from Asahi Kasei Pharma America Corporation for the development of recombinant thrombomodulin. Dr. Fareed is a member of an advisory board for and has received consulting fees from Asahi Kasei Pharma America Corporation for the development of recombinant thrombomodulin.
The work for the Sepsis Coagulopathy Asahi Recombinant LE Thrombomodulin trial, a multinational, multicenter study, was performed in 27 countries (320 sites), of which 26 countries enrolled patients (159 enrolling sites) spanning across Europe (including Israel), North and South Americas, Russia, and the Asia-Pacific region.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NK, et al. ; International Forum of Acute Care Trialists: Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193:259–272 [DOI] [PubMed] [Google Scholar]
- 3.Levi M, Keller TT, van Gorp E, et al. Infection and inflammation and the coagulation system. Cardiovasc Res 2003; 60:26–39 [DOI] [PubMed] [Google Scholar]
- 4.Vincent JL. Microvascular endothelial dysfunction: A renewed appreciation of sepsis pathophysiology. Crit Care 2001; 5:S1–S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi M. Current understanding of disseminated intravascular coagulation. Br J Haematol 2004; 124:567–576 [DOI] [PubMed] [Google Scholar]
- 6.Fourrier F, Chopin C, Goudemand J, et al. Septic shock, multiple organ failure, and disseminated intravascular coagulation. Compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest 1992; 101:816–823 [DOI] [PubMed] [Google Scholar]
- 7.Gibson NS, Sohne M, Gerdes VE, et al. Clinical usefulness of prothrombin fragment 1 + 2 in patients with suspected pulmonary embolism. Thromb Res 2010; 125:97–99 [DOI] [PubMed] [Google Scholar]
- 8.Asakura H, Takahashi H, Uchiyama T, et al. ; DIC subcommittee of the Japanese Society on Thrombosis and Hemostasis: Proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J 2016; 14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyons PG, Micek ST, Hampton N, et al. Sepsis-associated coagulopathy severity predicts hospital mortality. Crit Care Med 2018; 46:736–742 [DOI] [PubMed] [Google Scholar]
- 10.Scarlatescu E, Tomescu D, Arama SS. Sepsis-associated coagulopathy. J Crit Care Med (Targu Mures) 2016; 2:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sathe PM, Patwa UD. D Dimer in acute care. Int J Crit Illn Inj Sci 2014; 4:229–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levi M. The coagulant response in sepsis. Clin Chest Med 2008; 29:627–642, viii [DOI] [PubMed] [Google Scholar]
- 13.de Pont AC, Bakhtiari K, Hutten BA, et al. Recombinant human activated protein C resets thrombin generation in patients with severe sepsis - a case control study. Crit Care 2005; 9:R490–R497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iba T, Thachil J. Clinical significance of measuring plasminogen activator inhibitor-1 in sepsis. J Intensive Care 2017; 5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levi M. Activated protein C in sepsis: A critical review. Curr Opin Hematol 2008; 15:481–486 [DOI] [PubMed] [Google Scholar]
- 16.Ranieri VM, Thompson BT, Barie PS, et al. ; PROWESS-SHOCK Study Group: Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 2012; 366:2055–2064 [DOI] [PubMed] [Google Scholar]
- 17.Saito H, Maruyama I, Shimazaki S, et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: Results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost 2007; 5:31–41 [DOI] [PubMed] [Google Scholar]
- 18.Arishima T, Ito T, Yasuda T, et al. Circulating activated protein C levels are not increased in septic patients treated with recombinant human soluble thrombomodulin. Thromb J 2018; 16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito T, Kawahara K, Okamoto K, et al. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol 2008; 28:1825–1830 [DOI] [PubMed] [Google Scholar]
- 20.Morser J. Thrombomodulin links coagulation to inflammation and immunity. Curr Drug Targets 2012; 13:421–431 [DOI] [PubMed] [Google Scholar]
- 21.Nakahara M, Ito T, Kawahara K, et al. Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS One 2013; 8:e75961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent JL, Ramesh MK, Ernest D, et al. A randomized, double-blind, placebo-controlled, phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med 2013; 41:2069–2079 [DOI] [PubMed] [Google Scholar]
- 23.Hoppensteadt D, Tsuruta K, Cunanan J, et al. Thrombin generation mediators and markers in sepsis-associated coagulopathy and their modulation by recombinant thrombomodulin. Clin Appl Thromb Hemost 2014; 20:129–135 [DOI] [PubMed] [Google Scholar]
- 24.Vincent JL, Francois B, Zabolotskikh I, et al. ; SCARLET Trial Group: Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: The SCARLET randomized clinical trial. JAMA 2019; 321:1993–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon JR., Jr The International Conference on Harmonization good clinical practice guideline. Qual Assur 1998; 6:65–74 [DOI] [PubMed] [Google Scholar]
- 26.Anas AA, Wiersinga WJ, de Vos AF, et al. Recent insights into the pathogenesis of bacterial sepsis. Neth J Med 2010; 68:147–152 [PubMed] [Google Scholar]
- 27.Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med 1999; 340:207–214 [DOI] [PubMed] [Google Scholar]
- 28.Tipoe TL, Wu WKK, Chung L, et al. Plasminogen activator inhibitor 1 for predicting sepsis severity and mortality outcomes: A systematic review and meta-analysis. Front Immunol 2018; 9:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moll S, Lindley C, Pescatore S, et al. Phase I study of a novel recombinant human soluble thrombomodulin, ART-123. J Thromb Haemost 2004; 2:1745–1751 [DOI] [PubMed] [Google Scholar]
- 30.Tsuruta K, Yamada Y, Serada M, et al. Model-based analysis of covariate effects on population pharmacokinetics of thrombomodulin alfa in patients with disseminated intravascular coagulation and normal subjects. J Clin Pharmacol 2011; 51:1276–1285 [DOI] [PubMed] [Google Scholar]
- 31.Sakr Y, Moreira CL, Rhodes A, et al. ; Extended Prevalence of Infection in Intensive Care Study Investigators: The impact of hospital and ICU organizational factors on outcome in critically ill patients: Results from the Extended Prevalence of Infection in Intensive Care study. Crit Care Med 2015; 43:519–526 [DOI] [PubMed] [Google Scholar]


