Abstract
Electrospinning forms fibers from either an electrically charged polymer solution or polymer melt. Over the past decades, it has become a simple and versatile method for nanofiber production. Hence, it has been explored in many different applications. Commonly used electrospinning assembles fibers from polymer solutions in various solvents, known as solution electrospinning, while melt and near-field electrospinning techniques enhance the versatility of electrospinning. Adaption of additive manufacturing strategy to electrospinning permits precise fiber deposition and predefining pattern construction. This manuscript critically presents the potential of electrospun nanofibers in healthcare applications. Research community drew impetus from the similarity of electrospun nanofibers to the morphology and mechanical properties of fibrous extracellular matrices (ECM) of natural human tissues. Electrospun nanofibrous scaffolds act as ECM analogs for specific tissue cells, stem cells, and tumor cells to realize tissue regeneration, stem cell differentiation, and in vitro tumor model construction. The large surface-to-volume ratio of electrospun nanofibers offers a considerable number of bioactive agents binding sites, which makes it a promising candidate for a number of biomedical applications. The applications of electrospinning in regenerative medicine, tissue engineering, controlled drug delivery, biosensors, and cancer diagnosis are elaborated. Electrospun nanofiber incorporations in medical device coating, in vitro 3D cancer model, and filtration membrane are also discussed.
INTRODUCTION
As reported by “Research and Markets,” the global market for nanofibers can reach 1 billion U.S. dollars by the end of 2021.1 Electrospinning is a simple and versatile process to fabricate micrometer and nanometer scale thickness fibers that contribute to the emerging nanotechnology field. In simple terms, the electrospinning process relies on an electrohydrodynamic principle that a highly electrified polymer solution or melt is forced to stretch and elongate into fibers [Fig. 1(a)]. Electrospraying involves applying high voltage to liquid jets as well, whereas particles are collected instead of fibers [Fig. 1(b)].
FIG. 1.
Schematic illustrations of (a) electrospinning and (b) electrospraying. The charged jet can be kept in a continuous form to produce fibers in electrospinning, whereas it breaks into droplets to form particles in electrospraying. During electrospinning, the ejected jet initially follows a straight line in the near-field zone and undergoes stretching and thinning upon whipping motions in the far-field zone. (c) Schematic illustration of melt electrospinning. Unlike conventional solution electrospinning, a heating device is attached to maintain a molten jet in melt electrospinning. Normally, the jet travels in a straight line and generates micrometer scale fibers. (d) Schematic illustration of near-field electrospinning. The jet deposited on the collector within the straight segment, which shows higher spatial control of fiber placement but larger fiber diameter. (e) Schematic illustration of direct write electrospinning that integrates AM concept to electrospinning. A translational collector is used for predefined pattern construction.
In solution electrospinning where a polymer solution is elongated and thinned by whipping effect, quick evaporation of solvents results in solidification of the polymer jet and nanofiber deposition, whereas in melt electrospinning [Fig. 1(c)], polymers remain in a molten state that exhibit lower electrical conductivity, higher viscosity, and lower density of surface charge than polymer solutions. The solidification of molten jets relies on the heat transfer between the jet and surrounding medium. Such rapid solidification further suppresses the whipping effect and requires stronger electrostatic repulsion to overcome the viscoelastic force.2 Therefore, the electrostatic force mainly contributes to thinning of the molten jet, which is not as sufficient as solution electrospinning with whipping effect and solvent evaporation. The diameter of melt electrospun fibers is typically on the micrometer scale, while the jet travels in a straight path before deposition and solidification, allowing better fiber placement control. Since a heating device is included in the melt electrospinning setup, thermoset polymers, thermally unstable polymers, and some bioactive molecules cannot be incorporated. Conventional electrospinning is usually conducted in the far-field model where the distance between the spinneret and collector ranges from 5 to 15 cm with a high applied voltage (10–20 kV).2 By reducing that distance to 500 μm–1 cm,2,3 near-field electrospinning can be obtained [Fig. 1(d)]. The electric field will be highly concentrated within such short distance that permits substantially reduced applied voltage to several hundred volts (normally 0.6–3 kV).3 The whipping instability is dampened in near-field electrospinning, and the jet is deposited on the collector within straight segments. Similarly, like melt electrospinning, near-field electrospinning allows precisely spatial control of fiber deposition together with fiber diameters on the micrometer scale. Yet with lower applied voltage, near-field electrospinning is more suitable than melt electrospinning for bioactive molecules accommodation.
Recently, researchers have adopted additive manufacturing (AM) concept to electrospinning for more accurate control of fiber deposition.4–6 AM enables fabrication of 3D constructs with customized geometry and structure, whereas the spatial resolution is quite limited. Direct write electrospinning [Fig. 1(e)] is an integration of electrospinning and AM that combines the nanofibrous characteristics of electrospinning and accurate designing potential of AM. In direct write electrospinning, the jet is focused to travel in a straight line via auxiliary electrodes, melt electrospinning, or near-field electrospinning. Together with predefining translational movement of the collector, 3D constructs with designed patterns and accurately controlled features such as pore size7 can be produced.
Initial applications of electrospinning emerged in air filtration and personal protection purposes. Subsequently, efforts are made for diverse applications in medicine and healthcare, water treatment, damage resistant composites, light weight buildings and construction, mitigation of noise pollution, energy generation and storage, photonics, electronics, and wearables. This paper focuses on medicine and healthcare applications.
Bioengineering and biomedical engineering research community drew impetus from the similarity of electrospun nanofibers to the morphology and mechanical properties of fibrous extracellular matrices, extracellular matrix (ECM) of natural human tissues [Table I (Refs. 2, 8, and 9)]. ECM is a collection of extracellular molecules secreted by cells that provide mechanical support and biochemical cues to surrounding cells and tissue.2 It is a complex and heterogeneous network with tissue-specific characteristics. The highly porous structure of ECM allows nutrients and oxygen diffusion and transport. Adequate pore size is critical to support cell–cell and cell-matrix interactions. Collagen is the most abundant fibrous protein in native ECM, which offers structural support and topographic guidance through specific orientations to surrounding cells and facilitate cell–cell interactions. Collagen exists as nanofibers with a diameter of 50–500 nm and accounts for the nanofibrous structure of ECM with specific fiber alignment. Biomolecules residing in the ECM provide intrinsic biochemical cues for regulating cellular functions as well as cell-matrix interaction. Mechanical properties such as stiffness of the ECM can affect cellular activities including adhesion, migration, proliferation, and differentiation. For example, neural stem cells cultured on a stiffer matrix (1–10 kPa) showed glial differentiation, while exhibited neural differentiation on a softer matrix (100–500 Pa).10 Similarly, electrospun nanofibers can assemble into a nanofibrous network with tailorable porosity and pore size. The diameter of nanofibers can be altered accordingly as well. Through various electrospinning setups such as different collectors design, the fiber orientation can be customized. Electrospinning of various materials blends leads to optimal mechanical properties of electrospun fibers that maintain the integrity and match with native ECM.
TABLE I.
Similarities between electrospun fibers and fibrous ECM.
| Characteristics | Natural ECM | Electrospun fibers |
|---|---|---|
| Diameter of fibrous components | 50–500 nm (collagen fibers)2 | Tens to hundreds of nanometers |
| Porosity and pore size | Highly interconnected pores | Highly porous |
| Tissue-specific | ||
| e.g., Ref. 8 Neovascularization: 5 μm | Interconnected pores | |
| Fibroblast ingrowth: 5–15 μm | Tailorable | |
| Bone regeneration: 200–350 μm | ||
| Skin regeneration: 20–125 μm | ||
| Mechanical properties | Tissue-specific | Tailorable |
| e.g., Ref. 9 Cancellous bone: 0.4 GPa (modulus); 7.4 MPa (tensile strength) | Vary across materials selection, porosity control and fiber orientation | |
| Articular cartilage:10.5 MPa (modulus); 27.5 MPa (tensile strength) | ||
| Skin: 0.1–0.2 MPa (modulus); 7.6 MPa (tensile strength) | ||
| Physical architecture | Tissue-specific | Tailorable |
| E.g. Skin: basketweave-like pattern of collagen fibers | ||
| Tendon: parallelly aligned collagen fibers |
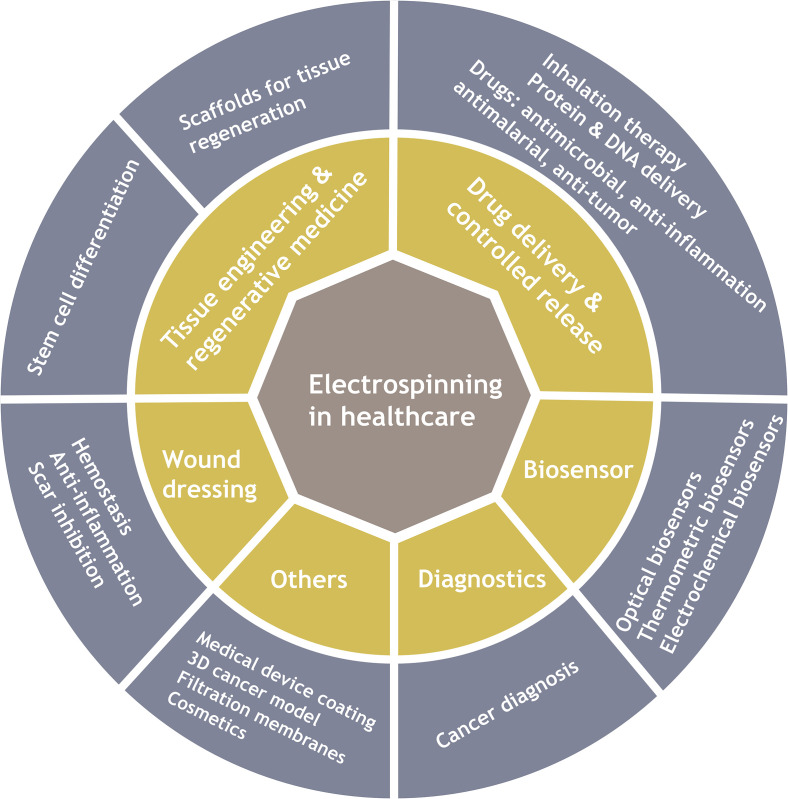
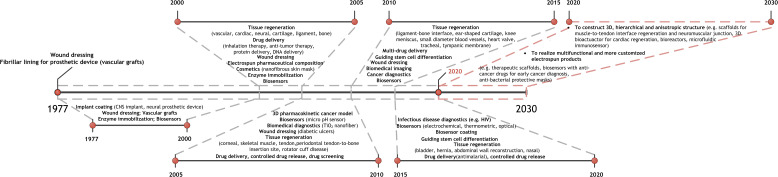
Nanofibers with relatively large surface-to-volume ratio and nanofibrous structure with high porosity make electrospun products potential for tissue engineering and regenerative medicine, drug delivery, biosensors, diagnostics, etc. (Fig. 2). The first patented electrospun product in biomedical applications is a wound dressing mat by Martin et al. in 1977.11 Since then, electrospinning has been widely explored for healthcare applications specifically over the past two decades (Fig. 3). Herein this review, we described recent advances related to biomedical applications of electrospinning through representative examples. We mainly focus on electrospun nanofibers in tissue engineering and regenerative medicine, drug delivery, biosensors, and cancer diagnosis. Other applications including medical device coating, in vitro 3D cancer models, viral and microbial resistant surgical masks, respirators, personal protective equipment, and filtration membranes related to the coronavirus disease 2019 (COVID-19) outbreak are discussed as well. Current challenges in each application are mentioned along with future perspectives.
FIG. 2.
Applications of electrospinning in healthcare.
FIG. 3.
Development of electrospinning in biomedical applications. Electrospun nanofibers have been utilized in biomedical applications mainly as wound dressing and implant coating since 1977. There were broad applications of electrospinning in healthcare in the past two decades. From 2000 to 2020, the key applications of electrospinning in healthcare are summarized and presented at 5-year intervals. From 2020 to 2030, two future trends of applying electrospinning in healthcare are suggested with examples. CNS implant: central nervous system implant.
TISSUE ENGINEERING AND REGENERATIVE MEDICINE
Tissue engineering, as first fully illustrated by Langer and Vacanti,12 is a highly multidisciplinary field for damaged tissue regeneration that accommodates cells into porous scaffolds made of biomaterials and guide their growth to new tissue13,14 (Fig. 4). Electrospinning has been widely explored for nanofibrous scaffolds fabrication and achieve promising replication of native ECM in terms of composition and architecture, whereas the composition and structure of ECM are tissue specific leading to specific requirements in different applications.15 Applications of conventional electrospun scaffolds in tissue engineering are limited because of the mechanical incompliance between scaffolds and native ECM such as in bone and cardiac regeneration, inefficient replication of complex and anisotropic structure such as in tendon and cartilage and tissue-to-tissue interface, and difficulty in providing specific functionality such as electrochemical stimulation to neural and cardiac tissue, anti-thrombogenicity of vascular grafts, and scar inhibition in skin regeneration.
FIG. 4.
Basic concept of tissue engineering approach for tissue regeneration. (a) Tissue engineering is a highly multidisciplinary field that recruits experts from engineering, materials science, life science, and clinical medicine. (b) In tissue engineering, biocompatible scaffolds act as a temporary template for tissue-specific cell growth and proliferation, and are occasionally incorporated with biomolecules for enhanced cell regulation and tissue regeneration. Upon implantation of the engineered tissue, scaffolds will gradually degrade leaving regenerated tissues or organs with restored functionality.
Recent electrospun scaffolds in tissue engineering and regenerative medicine could combine various guidance to address aforementioned problems via a synergistic effect on stem cell differentiation [Table II (Refs. 6 and 16–34)] and tissue regeneration. Basically, topographical and biomechanical cues can be generated via scaffold design and configurations, biological cues can be accomplished by bioactive agents and/or biomolecules incorporation, and electrochemical cues are attained through material selection, fiber chemistry, thickness, and porosity control as needed. Fibrous proteins assemble a 3D network in ECM with specific fiber orientation varying across tissues. Compared to conventional 2D electrospun scaffolds, 3D scaffolds are favored in resembling natural ECM. Electrospinning in specific tissue engineering applications as the potential solution to current limitations is discussed in the section titled Tissue Engineering and Regenerative Medicine.
TABLE II.
Examples of electrospinning for stem cell differentiation. MSCs: mesenchymal stem cells; iPSCs: induced pluripotent stem cells; BMP-2 peptide: bone morphogenic protein-2.
| Electrospun scaffolds features | Stem cell types | Stem cell differentiation |
|---|---|---|
| Topographical and mechanical cues | ||
| Uniaxially aligned nanofibers16,17,27 | Adipose derived MSCs iPSCs derived MSCs | Tenogenic differentiation |
| Human fetal osteoblasts | Osteogenic differentiation | |
| Bone marrow derived MSCs | Neural differentiation | |
| Uniaxially aligned yarns28 | Adipose derived MSCs | Anisotropic soft tissue differentiation |
| Orthogonal layers29 | Bone marrow derived MSCs | Osteoblastic differentiation |
| Coiled nanofibers30 | Bone marrow derived MSCs | Mild myofibroblastic differentiation |
| Honeycomb-compartmented monolayer31 | iPSCs | Cardiac differentiation |
| Netslike nanofibrous mesh32 | Bone mesenchymal stromal cells | Osteogenic differentiation |
| Zonal organized nanofibers6 | Mesenchymal stromal cells | Chondrogenic differentiation |
| Higher degree of roughness | Mesenchymal stromal cells | Osteogenic differentiation |
| Lower degree of roughness33,34 | Chondrogenic differentiation | |
| Lower stiffness18 | Smooth muscle cells | Contractile phenotype |
| Dynamic mechanical stimulation19 | Adipose derived MSCs | Tenogenic differentiation |
| Electrochemical cues | ||
| Electrical pulse application20 | Cardiovascular disease specific iPSCs | Cardiomyocytes |
| Piezoelectric scaffold21 | Bone marrow derived MSCs | Osteogenic differentiation (high voltage) |
| Chondrogenic differentiation (low voltage) | ||
| Biological cues | ||
| Hemin doping22 | iPSCs derived neural stem cells | Neural differentiation |
| Retinoic acid induction23 | Chorion derived MSCs | Neural differentiation |
| Peptide decoration24 | Human PSCs | Osteogenic differentiation |
| BMP-2 peptide25 | Adipose derived MSCs | Osteogenic differentiation |
| Co-culture with chondrocytes26 | Bone marrow derived MSCs | Chondrogenic differentiation |
Electrospinning in bone regeneration
Mechanical properties incompliance between electrospun scaffolds and native bones is a common problem. Polymers are the most commonly electrospun materials, yet exhibit lower modulus than native hard tissue in general.9 The ideal scaffold should offer sufficient mechanical support during bone regeneration and biochemical guidance to induce osteogenesis for healing acceleration. A sponge-like 3D nanofibrous silk fibroin/poly(ε-caprolactone) (PCL) scaffold was mineralized and immobilized with BMP-2 peptide.25 The mineralization improved the compressive modulus of scaffolds significantly (468.5 ± 48.7 kPa vs 109.3 ± 21.8 kPa). However, such modulus is much lower than that of native cancellous bones of 0.4 GPa.9 The authors suggested that those sponge-like scaffolds were potential to calvarial defects. Since the major contents in the bone are type I collagen fibrils and hydroxyapatite nanoparticles, for bone regeneration, biocompatible and biodegradable polymeric scaffolds are always reinforced with inorganic substance such as hydroxyapatite, bioactive glass, and silica. An electrospun scaffold out of silk fibroin/PVA/58S bioglass was developed for bone regeneration.35 The addition of bioactive glass significantly improved the mechanical properties of scaffolds with a raised Young's modulus from 293 ± 64 MPa to 655 ± 151 MPa. Considering relatively high modulus of ceramics and metals, incorporation of such stiffer materials into a polymeric matrix or developing polymer-free ceramic scaffolds for hard tissue regeneration is expected.
Electrospinning in cartilage regeneration
Unlike bone, the major components in cartilage are type II collagen and proteoglycan. There is a zonal organization and distribution of collagen in cartilaginous ECM that the content decreases from the superficial zone to the deep zone. Collagen fibrils are aligned parallel to the articular surface in the superficial zone, while they are oriented perpendicular to the articular surface in the middle and deep zones. For 2D planar mats, the ongoing challenge is to achieve complete cell infiltration through the defects. A 3D nanofibrous scaffold with hierarchical architecture, sufficient compressive strength, and highly interconnected pores is more ideal. Chen et al. combined direct writing with solution electrospinning for hierarchical scaffolds fabrication mimicking the zonal organization of articular cartilage.6 The 3D multiscale fibrous scaffolds promoted chondrogenic differentiation of hMSCs and directed tissue organization in a zone-dependent way.
Electrospinning in tendon and ligament regeneration
Collagen fibers are closely packed in parallel arrays in tendon and ligament. Thus, uniaxially aligned nanofibrous mats are widely applied to tendon and ligament regeneration. Uniaxial aligned nanofibers can be easily collected using a rotating collector or extra parallel electrodes. Adipose derived MSCs and iPSC derived MSCs show tenogenic differentiation on uniaxial nanofibers. However, the bulk of tendon and ligament tissue present highly anisotropic structures. 3D scaffolds with braided, woven, or knitted yarn networks are more favored compared to 2D mats. When including hydrogels into the nanofibrous matrix, it can benefit biomolecule and cell encapsulation. For example, unidirectional PCL nanofibers were once coated with chitosan/hyaluronic acid hydrogel for ligament regeneration.36
Electrospinning in tissue-to-tissue interface regeneration
Currently, regeneration of tissue-to-tissue interface remains challenging. Those interfaces contain soft-to-hard interfaces and soft-to-soft interfaces. In the junctions between a soft matrix and a hard matrix (e.g., tendon/ligament-to-bone, cartilage-to-bone), there are gradual variations in matrix composition, architecture, mineral content, and significant difference in mechanical properties.37 An ideal scaffold should contain a hierarchical structure that allows a transmission in structure and mechanical stress between two mechanically differed tissues. Spatially organized nanofiber structure, material composition with graded distribution, and bioactive agents can attribute to a specialized scaffold for regeneration of such interfaces. A nanofibrous mat with “aligned-to-random” nanofibers was developed to mimic the graded structure in the tendon-to-bone interface.38 The aligned nanofibers represented highly aligned collagen fibers and tendon, while the random portion mimicked the less ordered collagen fibers in bones. A dual-layer organic/inorganic nanofibrous scaffold was fabricated to recapitulate the gradient mineral content within the tendon-to-bone interface.39 The PLLA/nanohydroxyapatite (nHA) nanofibrous mat was synthesized via electrospinning of nHA on the top of the PLLA electrospun mat, which resembled the mineralized and non-mineralized fibrocartilage in the interface, respectively. For cartilage-to-bone interface regeneration, nanofibrous scaffolds are always combined with hydrogels since cartilage is a resilient tissue. Mohan et al. designed a 3D hybrid scaffold that assembled PCL nanofibers with gradients of chondroitin sulfate and bioactive glass into hydrogel.40 The chondroitin sulfate addition promoted glycosaminoglycan-enriched ECM secretion by chondrocytes mimicking the hyaline cartilage, whereas the bioactive glass content enhanced mineralized ECM formation.
Muscle-to-tendon interface and interface at the neuromuscular junction are two typical soft-to-soft tissue interfaces. The myotendinous junction connects dense collagen fibers in tendon to softer muscle fibers. The main difficulty in regeneration of myotendinous junction is a comparable stiffness transition to native junctions. For example, Ladd et al. developed PCL/poly (lactic acid) (PLA) nanofibrous scaffolds which followed the mechanical trend of native myotendinous junctions.41 However, the ratio of tendon to muscle stiffness was 6, which is far from the natural ratio in the range of 179–37 000.42 Studies on neuromuscular junction remodeling are quite limited. The natural neuromuscular junction is essential to support native functionality of motor neurons and skeletal muscles. The scaffolds should recapitulate the innervation in living skeletal muscles and restore the response of muscles to neurotransmitters.42 Culturing stem cells on the scaffolds and guiding them into desired differentiation such as tenogenic, myofibroblastic, and neural differentiation may help with the interface reconstruction.
Electrospinning in cardiac regeneration
It is critical to maintain sufficient mechanical strength in the cardiac tissue to sustain myocardium contraction and relaxation. The perimysial fibers in myocardium exhibit a coiled structure and contribute to the interwoven structure of myocardium. Complete replication of such anisotropic organization and mechanical capability of cardiac tissue remains challenging. Liu et al. developed honeycomb-patterned scaffolds for better mimicking the myocardium architecture.43 The beating rate of cardiomyocytes on patterned scaffolds showed a comparable value to adult or neonatal rats. A study investigated coiled PCL nanofibers together with gold nanoparticles to restore the myocardium functionality.44 The scaffold design is mainly focused on planar scaffolds, which have limited effect on myocardium maturation.45 A 3D scaffold that replicates the architecture, the mechanical properties, and the native function of cardiac tissue is highly desired in the future. Since the heart wall is a multi-layered structure, scaffolds composing of multilayers or a yarn network are suggested for more efficient tissue regeneration.2 Each layer can be individually controlled over the nanofiber orientation and layer pattern. Thus, the interwoven and anisotropic structure of native cardiac tissue can be better emulated. To further restore the functionality of the myocardium, 3D electrospun scaffolds integrated with electrochemical cues are favored. Wang et al. developed a 3D bioactuator in a tubular shape by loading cardiomyocytes on conductive PLA/PANi nanofibers, which combined topographic, biological, and electrochemical cues together.46 Cardiomyocytes on such 3D scaffolds showed higher beating frequency than those cultured on planar scaffolds.
Electrospinning in neural regeneration
Both uniaxially and radially aligned nanofibers have been demonstrated to be effective in neural regeneration. Bone marrow derived MSCs can differentiate into Schwann cells on uniaxially oriented PCL nanofibers.27 Radially aligned nanofibers are attractive for spinal cord injury repair, which recruits cells from the central canal region to lesion site. Li et al. developed radially aligned electrospun collagen/PCL mats with a circular gradient of biomolecules incorporation.47 Such scaffolds directed and promoted neural stem cells migrated from the periphery to center along nanofibers. However, planar scaffolds are mainly used in in vitro neural tissue regeneration studies. A nerve guidance conduit (NGC) with a complex, multitubular structure is widely used in in vivo studies. Currently, including all the topographic and biochemical characteristics of the native nerve tissue in the NGC design remains challenging. The size of intraluminal microchannels in NGC can be controlled via sacrificial templates such as sucrose fibers.48 With a controllable channel, intraluminal fillers can be introduced to obtain additional topographical cues. Poly(lactic-co-glycolic) acid (PLGA) unidirectionally aligned nanofibers with laminin coating have been used as fillers within NGC, which provides topographic and biological guidance to nerve tissue regeneration simultaneously.49 Piezoelectric polymers and conductive polymers can provide electrical stimulation to cells. A conductive polypyrrole (PPy)/silk fibroin NGC was fabricated by 3D printing and electrospinning.50 Unidirectional PPy/silk fibroin fibers were 3D printed and incorporated as intraluminal fillers. A dual-layer of electrospun aligned and random silk fibroin nanofibers assembled the shell of NGC. The topographic and electrical cues of such NGCs allowed proliferation of Schwann cells and in vivo axonal regeneration. Further study can focus on integrating biological, topographic, and electrochemical cues in nanofibers for better neural regeneration due to the synergistic effect of those guidance.
Electrospinning in vascular regeneration
There are commercially available vascular grafts for large-size blood vessels regeneration, yet studies of regenerating blood vessels of small diameter (i.e., <6 mm) are quite limited. Unlike the grafts for large-diameter arteries, the major concern for small-diameter vascular grafts is maintaining the lumen patency.51 Graft occlusion typically occurs in small-size blood vessels due to acute thrombosis and intimal hyperplasia, which significantly impairs vascular grafts function. Construction of a non-thrombogenic scaffold surface and achieving rapid endothelization within the electrospun scaffolds are both critical in avoiding thrombosis. There is limited research investigating the scaffold surface configuration for anti-thrombosis. Current approaches are incorporation of biomolecules into nanofibers such as heparin for anticoagulation and accelerating endothelization within the grafts. Surface modified with biomolecules (e.g., vascular endothelial growth factors) through covalent bonding or the core-sheath structure to promote endothelial cells recruitment52 and local delivery of miRNA to modulate the endothelial cell phenotype have been both investigated.53 Other approaches rely on direct pre-seeding of endothelial cells or endothelial progenitor cells on electrospun grafts.54 Maintaining a contractile phenotype of smooth muscle cells (SMCs) is critical in minimizing intimal hyperplasia. Highly aligned PCL/hyaluronan nanofibers promoted higher contractile gene expression in SMCs.55 It is worth to note that intimal hyperplasia occurs in all vascular grafts with different degrees due to blood flow profiles, which is independent of scaffold materials,51 while compliance mismatch between the scaffolds and native vessels partially results in intimal hyperplasia. Developing a vascular graft to obtain mechanical properties consistent with native vessels is of great importance.
Electrospinning in skin regeneration
In native ECM, the fibrous structure always exhibits a more complex architecture other than simple unidirectional alignment. Collagen fibrils in skin tissue show a mesh-like or a basketweave-like pattern. Therefore, scaffolds with crossed nanofibers showed higher keratinocytes and fibroblasts migration rate, thus better wound healing performance than either random or unidirectionally aligned nanofibers.56 A highly porous cotton-wool-like PCL/chitosan scaffold was developed using emulsion electrospinning and achieved accelerated full-thickness wound healing in 3 weeks in vivo.57 One major concern for wound healing is scar inhibition, which is a long-lasting obstacle in clinical studies. Abnormal fibroblast proliferation and subsequent collagen deposition lead to scar formation. Several cell signaling molecules including basic fibroblast growth factor (bFGF), transforming growth factor-β1 (TGF-β1), and ginsenoside-Rg3 have been studied to address it. Ginsenoside-Rg3 and bFGF can promote the normal function of fibroblast; thus, they have been introduced to PLGA nanofibers by blending electrospinning and surface immobilization,58 whereas TGF-β1 signaling would promote fibroblast abnormal proliferation. TGF-β1 inhibitors were loaded in PCL/gelatin nanofibers via blend electrospinning for hypertrophic scarring inhibition.59 To prevent scar formation, the effect of nanofibers on the cellular signaling mechanisms and biochemical pathways should be elucidated. Whether the nanofiber features such as fiber diameter and fiber alignment would influence the scar inhibition remains to be determined.
DRUG DELIVERY AND CONTROLLED RELEASE
The principle of using polymer nanofibers as drug carriers is that the dissolution rate of drugs increases with the increase in surface area of both drugs and carriers.60 The inherent higher surface-to-volume ratio of electrospun nanofibers affords high drug loading capacity and efficiency. Electrospun nanofibers can achieve controlled drug release with better preserved bioavailability by different drug encapsulation designs. Typical drug loading strategies include post-electrospinning modification, blend electrospinning, coaxial electrospinning, and nanoparticles encapsulation (Fig. 5). Different drug loading strategies lead to different interactions between drugs and nanofibers, which results in different drug releasing kinetics. Initial burst release typically occurs in drug loading avenues. Practically, initial release of specific drugs in clinic can benefit the anti-inflammatory effect in the early stage. It would be more favorable to secure controlled and sustained drug release in complex scenarios. The localized and target-specific delivery of anti-cancer drugs can further eliminate potential damage of chemotherapy to surrounding tissue. Potential strategies to achieve controlled drug release with preserved bioactivity are discussed in the section titled Drug Delivery and Controlled Release.
FIG. 5.
Common approaches of loading drugs into electrospun nanofibers. Post-electrospinning modifications including simple physical adsorption and surface immobilization for more controlled drug release. Blend electrospinning and coaxial electrospinning allow drug encapsulation to as-spun nanofibers. Loading drugs into particles followed by particle incorporation to nanofibers permits a more versatile drug delivery system by tailoring the characteristics of both nanofibers and particles. Characteristics of each approach are presented.
Physical adsorption is the simplest way to introduce drugs to the electrospun matrix, which is prone to an initial burst release and inefficient in sustained drug release.61 Since the post-electrospinning concept can prevent bioactive agents from destabilization and denaturation during the electrospinning process, research on more controlled release with surface localized drugs is highly encouraged. One strategy is surface immobilization of drugs to nanofibers and by controlling the degradation rate of a specific conjugation linker to achieve controlled drugs release. For instance, it is reported that there is an inverse correlation between matrix-metalloproteinase (MMP) levels and diabetic ulcer wound healing rate.62 A complex of linear PEI and DNA was conjugated to a PCL/polyethylene glycol (PEG) scaffold via an MMP cleavable peptide linker for diabetic ulcer healing.63 Compared to direct immobilization of DNA to scaffolds, the modified conjugating system showed an MMP dependent release profile.
Blend electrospinning and emulsion electrospinning of bioactive drugs and polymers are two typical strategies to incorporate drugs in as-spun nanofibers. Electrospinning of drug and polymer blends with a hand-hold device allows for direct deposition of drug loaded fibrous matrix to the wound site. However, one thing worth noted is that the addition of drugs could affect the properties of electrospinning solution such as solution conductivity and viscosity. When loading meloxicam in PVA solutions, the solution viscosity was elevated and, in turn, resulted in thicker fibers as reported.64 Besides, improving the drug-polymer compatibility is considered worth for more controlled drug release. Otherwise, drugs tend to accumulate on the surface of nanofibers leading to a burst release.65 Zeng et al. investigated different release kinetics between doxorubicin base and doxorubicin HCl in electrospun PLLA nanofibers.66 Results revealed that there was a 70% burst release of doxorubicin HCl due to a rapid wash-off from the nanofiber surface where it got accumulated. In contrast, doxorubicin base only showed a 20% burst release since it embedded into the fibers other than residing on the surface.
When processing drugs/polymer blends through electrospinning, preserving the bioactivity of drugs is another challenge. Drugs would be in direct contact with organic solvents, high electric charge, and even mechanical stress during electrospinning. Therefore, the bioactivity of drugs, some labile molecules in particular, will be hindered. To stabilize the drugs, binding them to other molecules may be an option.61 For example, emulsion electrospinning of BMP-2 and PLGA resulted in denaturation of BMP-2.67 When included hydroxyapatite into the formulation, the bioactivity of BMP-2 was retained. Similarly, using bovine serum albumin (BSA) as a stabilizer for electrospinning of NGFs and poly (caprolactone-co-ethylethylene phosphate) led to preserved bioactivity to some extent.68 Blending drugs with polymers (e.g., silk fibroin) that can be electrospun under mild processing conditions can be another approach. Near-field electrospinning exhibits concentrated electric field; thus, it allows for a substantially reduced applied voltage. It can be further investigated for drug delivery since it can minimize the electrically charged effect on bioactivity. Coaxial electrospinning of drugs and polymers provides a more sustained drug release. Normally, drugs are embedded in the core and released through either pores in the sheath or shell polymer degradation. Due to the barrier effect of the sheath structure, an initial burst release can be avoided. The shell polymer solution guarantees the successful fiber formation in coaxial electrospinning, and the electric charge is predominantly found on the sheath.61 Therefore, core-sheath nanofibers are preferred for delivery of labile molecules such as enzymes and growth factors even cells can be included.69
One current research focus is on the construction of a “smart” drug delivery system for controlled release in terms of target-specific and triggerable. Incorporating drug loaded nanoparticles and microspheres into electrospun nanofibers is a common strategy for better control of drug release. The host fiber characteristics as well as the particle properties can be both tailored for specific requirements. The drug delivery system containing drugs encapsulated particles and electrospun nanofibers is highly desired in cancer therapeutics. Localized and controlled chemotherapy can be achieved with higher therapeutic efficiency but lower drugs dosage; hence, there is lower toxicity to the surrounding tissue. A pH-sensitive anti-tumor drug delivery system was constructed by encapsulating CaCO3-capped mesoporous SiO2 nanoparticles in PLLA electrospun fibers.70 Doxorubicin (DOX), an anti-tumor drug, was loaded into nanoparticles. The reaction between CaCO3 and the acidic environment on the tumor site would stimulate the release of DOX, while in the normal tissue with the physiological pH, only a minor release of such anti-tumor drug was detected. That pH-responsive anti-cancer efficacy can last over 40 days. Trametinib loaded hollow copper silicate was introduced to the PCL/poly (D, L-lactic acid) (PDLLA) electrospun matrix for chemo-photothermal therapy of melanoma.71 This anti-tumor drug delivery system was near-infrared irradiation (NIR)-sensitive. Releasing of multiple drugs attributes to a synergistic therapy that exhibits better antitumor efficacy. For example, co-delivery of paclitaxel and RNA interference (RNAi) to brain tumor simultaneously inhibited tumor angiogenesis and suppressed tumor cells proliferation.72 The RNAi and drugs were loaded in PEI nanoparticles and then encapsulated into PLGA electrospun microfibers. Surface immobilized of nanofibers or particles with functional groups allow for higher tumor cells affinity; hence, there is better target efficiency. In one demonstration, folate decorated PCL/PEG micelles were loaded with DOX and processed through coaxial electrospinning with PVA aqueous solution.73 DOX contained micelles resided in the core, while PVA nanofiber composed the outer sheath. Folate can bind to folate receptors that usually overexpressed on a number of solid tumors, which constructs an active-targeting drug delivery system.74
BIOSENSORS AND CANCER DIAGNOSIS
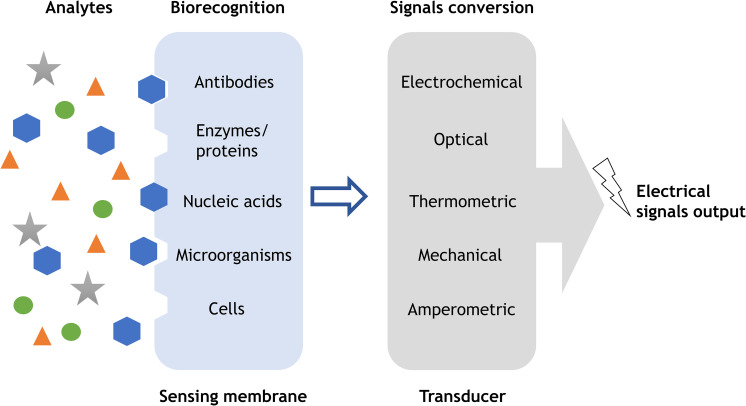
Biosensors are normally composed of biofunctional membranes and transducers for biological substances detection, where the sensing membrane is responsible for substances recognition, and transducer converts it into output signals75 (Fig. 6). The sensing membrane affects the performance of a biosensor including sensitivity, selectivity, reproducibility, and response time. High sensitivity is highly important in successful detection of biological substances at relatively low concentrations, to which electrospun nanofibers could contribute. The large surface-to-volume ratio of nanofibers allows more binding sites for analytes recognition; thus, it ensures an optimal sensitivity of electrospun nanofibers incorporated biosensors. Electrospun nanofibers are typically integrated into biosensors through two avenues.76 Electrospun functional polymers such as PANi can be directly used as the inducing element in biosensors. The other one is using electrospun nanofibers as templates for sensing materials deposition.
FIG. 6.
Schematic illustration of basic concept of a biosensor. Biorecognition elements such as antibodies, enzymes, and nucleic acids are incorporated in the sensing membrane to detect analytes via specific binding mechanisms. The transducer will convert acquired signals such as chemical substances, light, heat, etc., to electrical signals for further processing and analysis.
Incorporation of biomolecules to electrospun nanofibers is common for substance recognition. For example, glucose oxidase is a glucose sensitive enzyme, which has been widely immobilized on electrospun nanofibers for glucose biosensors construction. However, use of enzyme is usually associated with instability and denaturation problems. Therefore, several studies investigated non-enzymatic glucose sensing using carbon, metals, and their oxides as electrodes. In one demonstration, bimetallic nanoparticles CuCo doped electrospun carbon nanofibers were developed for enzyme-free glucose detection.77 Such glucose biosensor showed a high sensitivity, fast response within 2 s, long-term stability, and excellent anti-interference to electroactive molecules.
Electrospun nanofibers incorporating biosensor can be used for early cancer diagnosis. Substances to be detected for early cancer diagnosis include specific biomarkers overexpressed by cancer cells, oxygen level, and circulating tumor cells (CTCs). Specific antibodies, surface functional groups, and oxygen sensitive materials can be incorporated to electrospun nanofibers early diagnosis. For instance, epidermal growth factor receptor 2 (ErbB2)-antibody was conjugated to mesoporous ZnO nanofibers for breast cancer diagnosis.78 Combination between electrospun nanofibers with microfluidic techniques can improve the diagnostics sensitivity and CTC capture efficiency. A microfluidic immunosensor was designed for ErbB2 detection,79 which is a breast cancer biomarker. The immunoelectrode of this biosensor composed of the porous graphene foam modified with electrospun carbon-doped TiO2 nanofibers. This microfluidic biosensor allowed a wide concentration range of target ErbB2 antigen and achieved femtomolar sensitivity. CTCs are cancer cells that shed from the primary tumor and circulated into the bloodstream leading to metastasis.80 A microfluidic chip was fabricated for melanoma CTCs capture via conjugation of anti-146 antibody, a melanoma-specific capture agent, to PLGA nanofibers.81 This biosensor not only exhibited high CTCs capture efficiency, but also enabled specific isolation of single circulating melanoma cells.
OTHER APPLICATIONS
Medical device and implant coating
Electrospun nanofibers can be deposited on medical implants to improve the biocompatibility or acquire additional function. Nanofibrous coating would change the implant surface topography, thus providing topographical cues to surrounding cells and tissue. Incorporating bioactive agents or even cells into nanofibers can achieve multifunctional medical device coating such as drug delivery. Palumbo et al. coated biodegradable magnesium prosthesis with a porous PCL layer through electrospinning.82 Such porous coating could enhance cell adhesion and act as a drug releaser for anti-infection. A commercial coronary stent system, PK Papyrus (Biotronik),83 incorporates polyurethane as a stent coating for higher bending flexibility. A stent for aneurysm treatment was coated with poly (L-lactide-co-ε-caprolactone) (PLCL) nanofibers to achieve anti-coagulation and rapid endothelialization.84 Heparin and vascular endothelial growth factors were encapsulated into the core of PLCL nanofibers. Such core-sheath structure allows a sustained release for 30 days without an initial burst release. The nanofibrous coating can act as an interphase between the implant and host tissue to minimize the stiffness mismatch at the interface, which is quite critical in hard tissue prosthesis failure.60
In vitro 3D cancer models
The in vitro cancer models provide an effective way for drug screening, anti-cancer mechanism, and tumor cell biology. Traditional 2D cancer models employ culturing tumor cell monolayer on 2D flasks, which can reflect cancer cell behavior to a certain degree.85 However, tumor cells behave differently in 2D models and 3D models. 2D cancer models are difficult to mimic cell-cell and cell-matrix interactions, whereas 3D models can offer a comprehensive understanding of the collective behavior between cells and cell-matrix, thus allowing more accurate control of tumor activities.80
Electrospun nanofibers mimic the native structure and composition of ECM for target tissue regeneration or mimic the stem cell niches for stem cell differentiation. Similarly, to construct an in vitro 3D tumor model, electrospun nanofibers play a role in replicating native extracellular environment for the tumor cells. Electrospun nanofibers can realize the biochemical stimuli of native tumor ECM through surface functionalization with biomolecules or drug encapsulation. For instance, perlecan domain IV peptide was conjugated on electrospun PCL/gelatin nanofibers to construct a pharmacokinetic prostate cancer model.86 Tumor ECM yet exhibit a physiological complexity and heterogenous feature.80 Currently, electrospun in vitro 3D tumor models encounter some challenges including failure in mimicking the tumor angiogenesis, heterotypic cell-cell signaling, biomechanical stimuli, and insufficient cell infiltration. Inclusion of bioreactors in model fabrication can provide mechanical stimulus to cancer cells.87 Combining electrospinning with bioprinting technology may help with more sophisticated 3D cancer models development.
Filtration membranes for biomedical applications and wearables
Large surface-to-volume ratio, light weight, high porosity, interconnectivity, and microscale interstitial space make electrospun nanofiber meshes an excellent material for filtration applications in terms of personal protective equipment. Conventional protective clothing is based on full barrier protection that is limited in weight and moisture retention.88 Electrospun nanofibers can contribute to light-weight and breathable fabrics production that permit air and water vapor transport but filter other undesired agents.60 Protective masks are typical used filtration membranes in biomedical applications. Chemical and biological threats such as nerve agents, bacteria, and virus can be blocked and even decomposed through active reagents embedded on the nanofibrous membrane. Electrospun nonwoven PAN nanofibrous mats incorporated with sliver nanoparticles were used for bacterial filtration.89 Such filter possessed 99% bacterial filtration efficiency with promising anti-bacterial activity. There is supply shortage of face masks during the outbreak of COVID-19. Researchers from Korea Advanced Institute of Science and Technology (KAIST) developed washable and reusable nanofiber filtered masks to deal with it.90 The masks contain electrospun orthogonally aligned nanofibers with a diameter of 100–500 nm. Upon 20 repeated bactericidal tests with ethanol, masks maintain more than 94% filtration efficiency and are water resistant without deformation in the membrane structure. Inovenso Ltd. fabricated nanofiber face masks containing non-woven electrospun layers with 99.9% filtration efficiency.91 It is believed that nanofiber masks would be the new generation with their small pore size, large surface area, and light weight.
CONCLUSION
We overviewed the advances of electrospun nanofibers in biomedical applications and highlighted the application in tissue engineering, drug delivery, biosensors, and cancer diagnosis. There are various types of commercial biomedical products based on electrospun nanofibers (Table III). Electrospinning allows the manipulation of material properties down to the nanoscale, which is the most consistent scale to native ECM. Instead of the traditional 2D scaffolds used in those applications, 3D scaffolds are more favored for more efficient resemblance to native ECM. A complete replication of cell-matrix interactions can enhance the regeneration efficiency. Therefore, the influence of nanofibers on cellular signaling mechanisms and biochemical pathways is encouraged to further investigate for a better understanding of cell-nanofiber interactions. Drugs can be encapsulated into nanofibers through post-electrospinning modifications, blend electrospinning as well as coaxial electrospinning, and nanoparticles incorporation. Different drug loading methods result in different drug releasing profiles. Drug releasing from nanofibers following a predictable spatiotemporal profile is more expected in the future, whereas there are a few studies of pharmacokinetics of drug-loaded electrospun nanofibers, which would limit the practical application. Overall, the future opportunities would lie in multifunctional electrospun products development based on combination of various applications. For example, drug releasing nanofibrous scaffolds can simultaneously promote tissue regeneration and achieve therapeutic function or anti-tumor drug embedded biosensors for early cancer diagnosis and chemotherapy. Combining electrospinning with other biofabrication techniques can benefit 3D hierarchical and heterogenous structure construction such as cooperation with microfluidic technique for cancer biosensors and with bioprinting for 3D cancer models.
TABLE III.
Examples of commercial electrospun products for healthcare. β-TCP: β-tricalcium phosphate; SiV: siloxane-containing calcium carbonate.
| Commercial products | Company and country | Stage of products | Electrospun material | Application |
|---|---|---|---|---|
| AVflo™ | Nicast (Israel) | CE certified (2008) | PU | Nanofibrous vascular grafts |
| Bioweb™ | Zeus (USA) | Clinical use | PTFE | Vascular stent coating |
| PK Papyrus | Biotronik (Germany) | FDA approved (Sept. 2018) | PU | PU covered coronary stent system |
| ReBOSSIS | ORTHOREBIRTH (USA) | FDA cleared (2018) | β-TCP/PLLA/SiV | Bone void filler |
| SurgiClot® | St Teresa Medical (USA) | Clinical use | Dextran | Hemostatic wound dressing |
| ReDura™ | MEDPRIN (Germany) | Clinical use | PLLA | Dural substitute patch |
| NeoDura™ | MEDPRIN (Germany) | Clinical use | Synthetic polymers /gelatin | Dural substitute patch |
| Rivelin® patch | Bioinicia (Spain) | Clinical trial (phase 2) | Drug delivery layer: PVP/Eudragit RS100® Hydrophobic backing layer: PCL | Mucoadhesive drug delivery patch |
| HealSmart™ personalized antimicrobial dressings | PolyRemedy® (USA) | Clinical use | Hyaluronic acid | Personalized wound-care system |
| SpinCare™ | Nanomedic (Israel) | CE certified (2017) | … | A portable bedside wound care device |
| Stem cell culture/extract sheet | ORTHOREBIRTH (USA) | … | Bioresorbable polymers | Stem cell culturing for research studies |
| BioPaper™ Technology | Dipole Materials (USA) | … | Various materials (e.g. gelatin, colagen) | Lab tissue culture |
| Cytoweb® sheets | eSpin (USA) | … | PLGA, PLA, PCL, PU | In vitro cell culture |
| NanoAligned™ | Nanofiber solutions (USA) | … | PCL | 3D cell culture |
| Mimetix® | Electrospinning Company (UK) | … | PLLA | Multi-well plates for 3D cell culture |
By optimizing properties, nanofibrous mats in terms of fiber diameter, orientation, morphology, porosity, mechanical properties, and electrospun nanofibrous products can be explored for more specific requirements of different applications. Melt electrospinning and near-field electrospinning can avoid toxic solvents incorporation and high electric field interference, respectively. Inspired by AM, direct writing electrospinning allows development of highly customizable 3D constructs with well-defined features, while those techniques typically generate fibers on micrometer scale, which limits their employment in nanometer scale favored applications. Hence, a complete understanding of electrospinning mechanisms, specifically in the fast acceleration zone that contributes to sufficient thinning of fibers, will benefit the prediction of fiber properties. Simulation and modeling design of such mechanism can help with electrospinning parameters selection prior to the actual experiments. There are concerns associated with the environmental effect of organic solvents during solution electrospinning. How to achieve a “green” process remains to be addressed. Besides, there are difficulties in scale-up production due to the lack of a reliable system for quality control. With a comprehensive understanding of the electrospinning mechanism and better control of nanofiber properties, further developments of electrospinning in healthcare in the following decades can be expected.
NOMENCLATURE
- PCL
poly(ε-caprolactone)
- PLLA
poly(L-lactic acid)
- PDLLA
poly(D, L-lactic acid)
- PLA
poly(lactic acid)
- PLGA
poly(lactic-co-glycolic) acid
- PLCL
poly(L-lactide-co-ε-caprolactone)
- PAN
polyacrylonitrile
- PANi
polyaniline
- PEI
polyethylenimine
- PEG
polyethylene glycolPVApoly(vinyl alcohol)
- PU
polyurethane
- PVP
polyvinylpyrrolidone
- PEO
poly(ethylene oxide)
Contributor Information
Seeram Ramakrishna, Email: .
Xiaoling Liu, Email: .
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Research and Markets, Global Nanofibers Market: Trends Analysis & Forecasts to 2021 ( Infinium Global Research, 2016). [Google Scholar]
- 2. Xue J., Wu T., Dai Y., and Xia Y., “ Electrospinning and electrospun nanofibers: Methods, materials, and applications,” Chem. Rev. 119, 5298–5415 (2019). 10.1021/acs.chemrev.8b00593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang Y. et al. , “ Electrohydrodynamic direct-writing,” Nanoscale 5, 12007 (2013). 10.1039/c3nr04329k [DOI] [PubMed] [Google Scholar]
- 4. Yang G. H., Mun F., and Kim G. H., “ Direct electrospinning writing for producing 3D hybrid constructs consisting of microfibers and macro-struts for tissue engineering,” Chem. Eng. J. 288, 648–658 (2016). 10.1016/j.cej.2015.12.047 [DOI] [Google Scholar]
- 5. Lei T. P., Lu X. Z., and Yang F., “ Fabrication of various micro/nano structures by modified near-field electrospinning,” AIP Adv. 5, 041301 (2015). 10.1063/1.4901879 [DOI] [Google Scholar]
- 6. Chen H. et al. , “ Direct writing electrospinning of scaffolds with multidimensional fiber architecture for hierarchical tissue engineering,” ACS Appl. Mater. Interfaces 9, 38187–38200 (2017). 10.1021/acsami.7b07151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee J., Jang J., Oh H., Jeong Y. H., and Cho D. W., “ Fabrication of a three-dimensional nanofibrous scaffold with lattice pores using direct-write electrospinning,” Mater. Lett. 93, 397–400 (2013). 10.1016/j.matlet.2012.11.124 [DOI] [Google Scholar]
- 8. Yang S., Leong K.-F., Du Z., and Chua C.-K., “ The design of scaffolds for use in tissue engineering. Part I. Traditional factors,” Tissue Eng. 7, 679–689 (2001). 10.1089/107632701753337645 [DOI] [PubMed] [Google Scholar]
- 9. Ramakrishna S., Mayer J., Wintermantel E., and Leong K. W., “ Biomedical applications of polymer-composite materials: A review,” Compos. Sci. Technol. 61, 1189–1224 (2001). 10.1016/S0266-3538(00)00241-4 [DOI] [Google Scholar]
- 10. Saha K. et al. , “ Substrate modulus directs neural stem cell behavior,” Biophys. J. 95, 4426–4438 (2008). 10.1529/biophysj.108.132217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin G. E., Cockshott I. D., and Fildes F. J. T., “ Fibrillar lining for prosthetic device,” U.S. Patent no. US4044404A (1977).
- 12. Langer R. and Vacanti J. P., “ Tissue engineering,” Science 260, 920–926 (1993). 10.1126/science.8493529 [DOI] [PubMed] [Google Scholar]
- 13. Rajesh V. and Katti D. S., “ Nanofibers and their applications in tissue engineering,” Int. J. Nanomedicine 1, 15–30 (2006). 10.2147/nano.2006.1.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhandayuthapani B., Yoshida Y., Maekawa T., and Kumar D. S., “ Polymeric scaffolds in tissue engineering application: A review,” Int. J. Polym. Sci. 2011, 1. 10.1155/2011/290602 [DOI] [Google Scholar]
- 15. Teo W. E., He W., and Ramakrishna S., “ Electrospun scaffold tailored for tissue-specific extracellular matrix,” Biotechnol. J. 1, 918–929 (2006). 10.1002/biot.200600044 [DOI] [PubMed] [Google Scholar]
- 16. Zhang C. et al. , “ Well-aligned chitosan-based ultrafine fibers committed teno-lineage differentiation of human induced pluripotent stem cells for Achilles tendon regeneration,” Biomaterials 53, 716–730 (2015). 10.1016/j.biomaterials.2015.02.051 [DOI] [PubMed] [Google Scholar]
- 17. Wu S. et al. , “ Effect of scaffold morphology and cell co-culture on tenogenic differentiation of HADMSC on centrifugal melt electrospun poly (L-lactic acid) fibrous meshes,” Biofabrication 9, 044106 (2017). 10.1088/1758-5090/aa8fb8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vatankhah E. et al. , “ Phenotypic modulation of smooth muscle cells by chemical and mechanical cues of electrospun tecophilic/gelatin nanofibers,” ACS Appl. Mater. Interfaces 6, 4089–4101 (2014). 10.1021/am405673h [DOI] [PubMed] [Google Scholar]
- 19. Wu S., Wang Y., Streubel P. N., and Duan B., “ Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation,” Acta Biomater. 62, 102–115 (2017). 10.1016/j.actbio.2017.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mohammadi Amirabad L. et al. , “ Enhanced cardiac differentiation of human cardiovascular disease patient-specific induced pluripotent stem cells by applying unidirectional electrical pulses using aligned electroactive nanofibrous scaffolds,” ACS Appl. Mater. Interfaces 9, 6849–6864 (2017). 10.1021/acsami.6b15271 [DOI] [PubMed] [Google Scholar]
- 21. Damaraju S. M. et al. , “ Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation,” Biomaterials 149, 51–62 (2017). 10.1016/j.biomaterials.2017.09.024 [DOI] [PubMed] [Google Scholar]
- 22. Hsu C. C., Serio A., Amdursky N., Besnard C., and Stevens M. M., “ Fabrication of hemin-doped serum albumin-based fibrous scaffolds for neural tissue engineering applications,” ACS Appl. Mater. Interfaces 10, 5305–5317 (2018). 10.1021/acsami.7b18179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Faghihi F. et al. , “ Differentiation potential of human chorion-derived mesenchymal stem cells into motor neuron-like cells in two- and three-dimensional culture systems,” Mol. Neurobiol. 53, 1862–1872 (2016). 10.1007/s12035-015-9129-y [DOI] [PubMed] [Google Scholar]
- 24. Deng Y., Yang Y., and Wei S., “ Peptide-decorated nanofibrous niche augments in vitro directed osteogenic conversion of human pluripotent stem cells,” Biomacromolecules 18, 587–598 (2017). 10.1021/acs.biomac.6b01748 [DOI] [PubMed] [Google Scholar]
- 25. Luo J. et al. , “ 3-D mineralized silk fibroin/polycaprolactone composite scaffold modified with polyglutamate conjugated with BMP-2 peptide for bone tissue engineering,” Colloids Surf., B 163, 369–378 (2018). 10.1016/j.colsurfb.2017.12.043 [DOI] [PubMed] [Google Scholar]
- 26. He X. et al. , “ Electrospun gelatin/polycaprolactone nanofibrous membranes combined with a coculture of bone marrow stromal cells and chondrocytes for cartilage engineering,” Int. J. Nanomedicine 10, 2089–2099 (2015). 10.2147/IJN.S79461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xue J. et al. , “ Differentiation of bone marrow stem cells into Schwann sells for the promotion of neurite outgrowth on electrospun fibers,” ACS Appl. Mater. Interfaces 9, 12299–12310 (2017). 10.1021/acsami.7b00882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu S. et al. , “ Fabrication of aligned nanofiber polymer yarn networks for anisotropic soft tissue scaffolds,” ACS Appl. Mater. Interfaces 8, 16950–16960 (2016). 10.1021/acsami.6b05199 [DOI] [PubMed] [Google Scholar]
- 29. Shao W., He J., Wang Q., Cui S., and Ding B., “ Biomineralized poly(L-lactic-co-glycolic acid)/graphene oxide/Tussah silk fibroin nanofiber scaffolds with multiple orthogonal layers enhance osteoblastic differentiation of mesenchymal stem cells,” ACS Biomater. Sci. Eng. 3, 1370–1380 (2017). 10.1021/acsbiomaterials.6b00533 [DOI] [PubMed] [Google Scholar]
- 30. Berat Taskin M. et al. , “ Three-dimensional polydopamine functionalized coiled microfibrous scaffolds enhance human mesenchymal stem cells colonization and mild myofibroblastic differentiation,” ACS Appl. Mater. Interfaces 8, 15864–15873 (2016). 10.1021/acsami.6b02994 [DOI] [PubMed] [Google Scholar]
- 31. Tang Y. et al. , “ Induction and differentiation of human induced pluripotent stem cells into functional cardiomyocytes on a compartmented monolayer of gelatin nanofibers,” Nanoscale 8, 14530–14540 (2016). 10.1039/C6NR04545F [DOI] [PubMed] [Google Scholar]
- 32. Ren Z. et al. , “ Repairing a bone defect with a three-dimensional cellular construct composed of a multi-layered cell sheet on electrospun mesh,” Biofabrication 9, 025036 (2017). 10.1088/1758-5090/aa747f [DOI] [PubMed] [Google Scholar]
- 33. Chen H. et al. , “ Tailoring surface nanoroughness of electrospun scaffolds for skeletal tissue engineering,” Acta Biomater. 59, 82–93 (2017). 10.1016/j.actbio.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 34. Xu T., Yang H., Yang D., and Yu Z. Z., “ Polylactic acid nanofiber scaffold decorated with chitosan islandlike topography for bone tissue engineering,” ACS Appl. Mater. Interfaces 9, 21094–21104 (2017). 10.1021/acsami.7b01176 [DOI] [PubMed] [Google Scholar]
- 35. Singh B. N. and Pramanik K., “ Development of novel silk fibroin/polyvinyl alcohol/sol-gel bioactive glass composite matrix by modified layer by layer electrospinning method for bone tissue construct generation,” Biofabrication 9, 015028 (2017). 10.1088/1758-5090/aa644f [DOI] [PubMed] [Google Scholar]
- 36. Deepthi S., Jeevitha K., Nivedhitha Sundaram M., Chennazhi K. P., and Jayakumar R., “ Chitosan-hyaluronic acid hydrogel coated poly(caprolactone) multiscale bilayer scaffold for ligament regeneration,” Chem. Eng. J. 260, 478–485 (2015). 10.1016/j.cej.2014.08.106 [DOI] [Google Scholar]
- 37. Armitage O. E. and Oyen M. L., “ Indentation across interfaces between stiff and compliant tissues,” Acta Biomater. 56, 36–43 (2017). 10.1016/j.actbio.2016.12.036 [DOI] [PubMed] [Google Scholar]
- 38. Xie J. et al. , “ Aligned-to-random' nanofiber scaffolds for mimicking the structure of the tendon-to-bone insertion site,” Nanoscale 2, 923–926 (2010). 10.1039/c0nr00192a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li X. et al. , “ Flexible bipolar nanofibrous membranes for improving gradient microstructure in tendon-to-bone healing,” Acta Biomater. 61, 204–216 (2017). 10.1016/j.actbio.2017.07.044 [DOI] [PubMed] [Google Scholar]
- 40. Mohan N., Wilson J., Joseph D., Vaikkath D., and Nair P. D., “ Biomimetic fiber assembled gradient hydrogel to engineer glycosaminoglycan enriched and mineralized cartilage: An in vitro study,” J. Biomed. Mater. Res., Part A 103, 3896–3906 (2015). 10.1002/jbm.a.35506 [DOI] [PubMed] [Google Scholar]
- 41. Ladd M. R., Lee S. J., Stitzel J. D., Atala A., and Yoo J. J., “ Co-electrospun dual scaffolding system with potential for muscle-tendon junction tissue engineering,” Biomaterials 32, 1549–1559 (2011). 10.1016/j.biomaterials.2010.10.038 [DOI] [PubMed] [Google Scholar]
- 42. Banik B. L., Bowers D. T., Fattahi P., and Brown J. L., “ Bio-instructive scaffolds for musculoskeletal interfaces,” Bio-Instructive Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine ( Elsevier Inc, 2017), pp. 203–233. [Google Scholar]
- 43. Liu Y., Xu G., Wei J., Wu Q., and Li X., “ Cardiomyocyte coculture on layered fibrous scaffolds assembled from micropatterned electrospun mats,” Mater. Sci. Eng., C 81, 500–510 (2017). 10.1016/j.msec.2017.08.042 [DOI] [PubMed] [Google Scholar]
- 44. Fleischer S., Shevach M., Feiner R., and Dvir T., “ Coiled fiber scaffolds embedded with gold nanoparticles improve the performance of engineered cardiac tissues,” Nanoscale 6, 9410–9414 (2014). 10.1039/C4NR00300D [DOI] [PubMed] [Google Scholar]
- 45. Han J., Wu Q., Xia Y., Wagner M. B., and Xu C., “ Cell alignment induced by anisotropic electrospun fibrous scaffolds alone has limited effect on cardiomyocyte maturation,” Stem Cell Res. 16, 740–750 (2016). 10.1016/j.scr.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang L., Wu Y., Hu T., Guo B., and Ma P. X., “ Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators,” Acta Biomater. 59, 68–81 (2017). 10.1016/j.actbio.2017.06.036 [DOI] [PubMed] [Google Scholar]
- 47. Li X. et al. , “ Radially aligned electrospun fibers with continuous gradient of SDF1α for the guidance of neural stem cells,” Small 12, 5009–5018 (2016). 10.1002/smll.201601285 [DOI] [PubMed] [Google Scholar]
- 48. Lee D. J., Fontaine A., Meng X., and Park D., “ Biomimetic nerve guidance conduit containing intraluminal microchannels with aligned nanofibers markedly facilitates in nerve regeneration,” ACS Biomater. Sci. Eng. 2, 1403–1410 (2016). 10.1021/acsbiomaterials.6b00344 [DOI] [PubMed] [Google Scholar]
- 49. Wu T. et al. , “ Laminin-coated nerve guidance conduits based on poly(l-lactide-co-glycolide) fibers and yarns for promoting Schwann cells' proliferation and migration,” J. Mater. Chem. B 5, 3186–3194 (2017). 10.1039/C6TB03330J [DOI] [PubMed] [Google Scholar]
- 50. Zhao Y. et al. , “ Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering,” Biomaterials 255, 120164 (2020). 10.1016/j.biomaterials.2020.120164 [DOI] [PubMed] [Google Scholar]
- 51. Shojaee M. and Bashur C. A., “ Compositions including synthetic and natural blends for integration and structural integrity: Engineered for different vascular graft applications,” Adv. Healthcare Mater. 6, 1–14 (2017). 10.1002/adhm.201700001 [DOI] [PubMed] [Google Scholar]
- 52. Seyednejad H. et al. , “ Coaxially electrospun scaffolds based on hydroxyl-functionalized poly(ε-caprolactone) and loaded with VEGF for tissue engineering applications,” Biomacromolecules 13, 3650–3660 (2012). 10.1021/bm301101r [DOI] [PubMed] [Google Scholar]
- 53. Zhou F. et al. , “ Nanofiber-mediated microRNA-126 delivery to vascular endothelial cells for blood vessel regeneration,” Acta Biomater. 43, 303–313 (2016). 10.1016/j.actbio.2016.07.048 [DOI] [PubMed] [Google Scholar]
- 54. Peck M., Gebhart D., Dusserre N., McAllister T. N., and L'Heureux N., “ The evolution of vascular tissue engineering and current state of the art,” Cells Tissues Organs 195, 144–158 (2012). 10.1159/000331406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yuan H. et al. , “ Highly aligned core-shell structured nanofibers for promoting phenotypic expression of vSMCs for vascular regeneration,” Nanoscale 8, 16307–16322 (2016). 10.1039/C6NR05075A [DOI] [PubMed] [Google Scholar]
- 56. Sun L. et al. , “ Enhanced wound healing in diabetic rats by nanofibrous scaffolds mimicking the basketweave pattern of collagen fibrils in native skin,” Biomater. Sci. 6, 340–349 (2018). 10.1039/C7BM00545H [DOI] [PubMed] [Google Scholar]
- 57. Pal P. et al. , “ Nano-/microfibrous cotton-wool-Like 3D scaffold with core-shell architecture by emulsion electrospinning for skin tissue regeneration,” ACS Biomater. Sci. Eng. 3, 3563–3575 (2017). 10.1021/acsbiomaterials.7b00681 [DOI] [PubMed] [Google Scholar]
- 58. Cheng L. et al. , “ Surface biofunctional drug-loaded electrospun fibrous scaffolds for comprehensive repairing hypertrophic scars,” Biomaterials 83, 169–181 (2016). 10.1016/j.biomaterials.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 59. Wang L. et al. , “ Small molecular TGF-β1-inhibitor-loaded electrospun fibrous scaffolds for preventing hypertrophic scars,” ACS Appl. Mater. Interfaces 9, 32545–32553 (2017). 10.1021/acsami.7b09796 [DOI] [PubMed] [Google Scholar]
- 60. Huang Z.-M., Zhang Y.-Z., Kotaki M., and Ramakrishna S., “ A review on polymer nanofibers by electrospinning and their applications in nanocomposites,” Compos. Sci. Technol. 63, 2223–2253 (2003). 10.1016/S0266-3538(03)00178-7 [DOI] [Google Scholar]
- 61. Meinel A. J., Germershaus O., Luhmann T., Merkle H. P., and Meinel L., “ Electrospun matrices for localized drug delivery: Current technologies and selected biomedical applications,” Eur. J. Pharm. Biopharm. 81, 1–13 (2012). 10.1016/j.ejpb.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 62. Liu Y. et al. , “ Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers,” Diabetes Care 32, 117–119 (2009). 10.2337/dc08-0763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim H. S. and Yoo H. S., “ MMPs-responsive release of DNA from electrospun nanofibrous matrix for local gene therapy: In vitro and in vivo evaluation,” J. Controlled Release 145, 264–271 (2010). 10.1016/j.jconrel.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 64. Ngawhirunpat T. et al. , “ Development of meloxicam-loaded electrospun polyvinyl alcohol mats as a transdermal therapeutic agent,” Pharm. Dev. Technol. 14, 73–82 (2009). 10.1080/10837450802409420 [DOI] [PubMed] [Google Scholar]
- 65. Sun Y. et al. , “ Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization,” RSC Adv. 9, 25712–25729 (2019). 10.1039/C9RA05012D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zeng J. et al. , “ Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation,” J. Controlled Release 105, 43–51 (2005). 10.1016/j.jconrel.2005.02.024 [DOI] [PubMed] [Google Scholar]
- 67. Nie H., Beng W. S., Fu Y. C., and Wang C. H., “ Three-dimensional fibrous PLGA/HAp composite scaffold for BMP-2 delivery,” Biotechnol. Bioeng. 99, 223–234 (2008). 10.1002/bit.21517 [DOI] [PubMed] [Google Scholar]
- 68. Chew S. Y., Wen J., Yim E. K. F., and Leong K. W., “ Sustained release of proteins from electrospun biodegradable fibers,” Biomacromolecules 6, 2017–2024 (2005). 10.1021/bm0501149 [DOI] [PubMed] [Google Scholar]
- 69. Huang Z. M. et al. , “ Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning,” J. Biomed. Mater. Res., Part A 77A, 169–179 (2006). 10.1002/jbm.a.30564 [DOI] [PubMed] [Google Scholar]
- 70. Zhao X. et al. , “ Tumor-triggered controlled drug release from electrospun fibers using inorganic caps for inhibiting cancer relapse,” Small 11, 4284–4291 (2015). 10.1002/smll.201500985 [DOI] [PubMed] [Google Scholar]
- 71. Yu Q. et al. , “ Copper silicate hollow microspheres-incorporated scaffolds for chemo-photothermal therapy of melanoma and tissue healing,” ACS Nano 12, 2695–2707 (2018). 10.1021/acsnano.7b08928 [DOI] [PubMed] [Google Scholar]
- 72. Lei C., Cui Y., Zheng L., Kah-Hoe Chow P., and Wang C. H., “ Development of a gene/drug dual delivery system for brain tumor therapy: Potent inhibition via RNA interference and synergistic effects,” Biomaterials 34, 7483–7494 (2013). 10.1016/j.biomaterials.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 73. Yang G. et al. , “ An implantable active-targeting micelle-in-nanofiber device for efficient and safe cancer therapy,” ACS Nano 9, 1161–1174 (2015). 10.1021/nn504573u [DOI] [PubMed] [Google Scholar]
- 74. Norouzi M., Nazari B., and Miller D. W., “ Electrospun-based systems in cancer therapy,” Electrospun Materials for Tissue Engineering and Biomedical Applications: Research, Design and Commercialization ( Elsevier Ltd, 2017), pp. 337–356. [Google Scholar]
- 75. Zhang Y., Chwee T. L., Ramakrishna S., and Huang Z. M., “ Recent development of polymer nanofibers for biomedical and biotechnological applications,” J. Mater. Sci. Mater. Med. 16, 933–946 (2005). 10.1007/s10856-005-4428-x [DOI] [PubMed] [Google Scholar]
- 76. Liu Y. et al. , “ A review on recent advances in application of electrospun nanofiber materials as biosensors,” Curr. Opin. Biomed. Eng. 13, 174–189 (2020). 10.1016/j.cobme.2020.02.001 [DOI] [Google Scholar]
- 77. Li M. et al. , “ Bimetallic MCo (M = Cu, Fe, Ni, and Mn) nanoparticles doped-carbon nanofibers synthetized by electrospinning for nonenzymatic glucose detection,” Sens. Actuators, B 207, 614–622 (2015). 10.1016/j.snb.2014.10.092 [DOI] [Google Scholar]
- 78. Ali M. A., Mondal K., Singh C., Dhar Malhotra B., and Sharma A., “ Anti-epidermal growth factor receptor conjugated mesoporous zinc oxide nanofibers for breast cancer diagnostics,” Nanoscale 7, 7234–7245 (2015). 10.1039/C5NR00194C [DOI] [PubMed] [Google Scholar]
- 79. Ali M. A. et al. , “ Microfluidic immuno-biochip for detection of breast cancer biomarkers using hierarchical composite of porous graphene and titanium dioxide nanofibers,” ACS Appl. Mater. Interfaces 8, 20570–20582 (2016). 10.1021/acsami.6b05648 [DOI] [PubMed] [Google Scholar]
- 80. Chen S., Boda K., Batra S. K., Li X., and Xie J., “ Emerging roles of electrospun nanofibers in cancer research,” Adv. Healthcare Mater. 7, 1701024 (2018). 10.1002/adhm.201701024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hou S. et al. , “ Polymer nanofiber-embedded microchips for detection, isolation, and molecular analysis of single circulating melanoma cells,” Angew. Chem., Int. Ed. 52, 3379–3383 (2013). 10.1002/anie.201208452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Palumbo G. et al. , “ Single point incremental forming and electrospinning to produce biodegradable magnesium (AZ31) biomedical prostheses coated with porous PCL,” in Materials Today: Proceedings ( Elsevier Ltd, 2019), Vol. 7, pp. 394–401. [Google Scholar]
- 83.Biotronik, see https://www.biotronik.com/en-gb/products/coronary/pk-papyrus for Vascular Intervention // Covered Coronary Stent System.
- 84. Wang J. et al. , “ Heparin and vascular endothelial growth factor loaded poly(l-lactide-co-caprolactone) nanofiber covered stent-graft for aneurysm treatment,” J. Biomed. Nanotechnol. 11, 1947–1960 (2015). 10.1166/jbn.2015.2138 [DOI] [PubMed] [Google Scholar]
- 85. Wang C. et al. , “ Three-dimensional in vitro cancer models: A short review,” Biofabrication 6, 022001 (2014). 10.1088/1758-5082/6/2/022001 [DOI] [PubMed] [Google Scholar]
- 86. Hartman O. et al. , “ Biofunctionalization of electrospun PCL-based scaffolds with perlecan domain IV peptide to create a 3-D pharmacokinetic cancer model,” Biomaterials 31, 5700–5718 (2010). 10.1016/j.biomaterials.2010.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Santoro M., Lamhamedi-Cherradi S. E., Menegaz B. A., Ludwig J. A., and Mikos A. G., “ Flow perfusion effects on three-dimensional culture and drug sensitivity of Ewing sarcoma,” Proc. Natl. Acad. Sci. U. S. A. 112, 10304–10309 (2015). 10.1073/pnas.1506684112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ramakrishna S. et al. , “ Electrospun nanofibers: Solving global issues,” Mater. Today 9, 40–50 (2006). 10.1016/S1369-7021(06)71389-X [DOI] [Google Scholar]
- 89. Karthick Selvam A. and Nallathambi G., “ Polyacrylonitrile/silver nanoparticle electrospun nanocomposite matrix for bacterial filtration,” Fibers Polym 16, 1327–1335 (2015). 10.1007/s12221-015-1327-8 [DOI] [Google Scholar]
- 90.KAIST News Center, see http://news.kaist.ac.kr/newsen/html/news/?mode=V&mng_no=6530 for “ Recyclable Nano-Fiber Filtered Face Masks: A Boon for Supply Fiasco ” (2020).
- 91.Inovenso Ltd., see https://www.inovenso.com/nanofiber-facemask/nanofiber-facemask/ for “N95/ N99 Nanofiber Face Mask” (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.