Abstract
Background
In patients with adrenal incidentalomas (AIs), there is uncertainty on how to rule out hypercortisolism. The occurrence of postsurgical (unilateral adrenalectomy) hypocortisolism (PSH) has been proposed as a proof of the presence of presurgical hypercortisolism in AI patients. The aim of this study was to define the thresholds of cortisol level after the 1 mg overnight dexamethasone suppression test (F-1mgDST), urinary free cortisol (UFC), midnight serum cortisol (MSC), and adrenocorticotropin (ACTH) to predict the absence of PSH in AI patients undergoing surgery.
Methods
In 60 patients who underwent AI excision, cortisol secretion was assessed by a low-dose corticotropin stimulation test or insulin tolerance test when needed. We searched for the lowest presurgical value of F-1mgDST, UFC, and MSC and the highest value for ACTH in AI patients with PSH as indexes of normal cortisol secretion.
Results
The lowest values of F-1mgDST, UFC, and MSC and the highest value for ACTH in PSH patients were 1.2 µg/dL (33 nmol/L), 10.4 µg/24 hours (29 nmol/24 hours), 1.2 µg/dL (33 nmol/L), and 26.9 pg/mL (6 pmol/L), respectively, but only F-1mgDST <1.2 µg/dL (33 nmol/L) was able to predict the absence of PSH. Among AI patients with F-1mgDST <1.2 µg/dL (33 nmol/L) no subjects had diabetes mellitus and/or metabolic syndrome, and these subjects tended to have a better metabolic profile than those with F-1mgDST ≥1.2 µg/dL (33 nmol/L)
Conclusion
In AI patients a F-1mgDST <1.2 µg/dL (33 nmol/L) rules out PSH and could be used to exclude hypercortisolism in AI patients.
Keywords: cortisol, 1 mg overnight dexamethasone suppression test, adrenal incidentalomas, hypocortisolism
In recent years several studies have shown that in patients with incidentally discovered adrenal masses (AIs) but without the classical signs and symptoms of cortisol excess, the presence of a less severe hypercortisolism (previously called autonomous cortisol excess, possible autonomous cortisol excess, or subclinical hypercortisolism) was associated with increased mortality, mainly due to an elevated risk of cardiovascular events [1-5]. In fact, these findings were somewhat expected, since in the last decades many studies have shown an increased frequency of cardiovascular risk factors [5] and their reduction after adrenalectomy [6] in AI patients with less severe hypercortisolism.
Nevertheless, several works have reported an increased risk of cardiovascular disease [7-11] or diabetes mellitus [12] even in patients with nonfunctioning AI (NFAI). Recently, mortality associated with the presence of NFAI has been found to be similar to that in AI patients with less severe hypercortisolism [13], and a meta-analysis has shown that adrenalectomy could reduce the risk of hypertension and diabetes mellitus even in patients with NFAI [6].
The unexpected finding of increased cardiovascular risk even in NFAI patients was suggested to be due to an exaggerated cortisol response to stress [11], increased secretion of steroid precursors [14-16], cyclic cortisol secretion [17], and to variations in individual cortisol sensitivity [18]. Alternatively, it has been proposed that these tumors have subtle cortisol (hyper)secretion [19, 20], which cannot be disclosed by the currently used parameters of hypothalamic–pituitary–adrenal (HPA) axis activity. If this were the case, the current cut-offs used for the definition of hypercortisolism in AI patients may be inadequate. This idea is in keeping with the fact that cortisol after a dexamethasone suppression test with a cut-off set at 1.4 to 1.5 µg/dL (39–41 nmol/L), rather than at 1.8 µg/dL (50 nmol/L, as currently used), had the highest accuracy in predicting cardiovascular risk [11, 21] or the incidence of diabetes mellitus [12] in AI patients.
By definition, AI patients are asymptomatic for the classic signs and symptoms of hypercortisolism. However, the occurrence of postsurgical hypocortisolism (PSH) has been proposed as an indirect proof of the presence of autonomous cortisol secretion by these tumors [5, 22, 23].
Therefore, the aim of the present study was to define the absence of hypercortisolism in AI by assessing the cut-offs of the 1 mg overnight dexamethasone suppression test (F-1mgDST) and other parameters of HPA axis activity able to predict the absence of PSH in AI patients undergoing surgery.
1. Patients and Methods
A. Patients
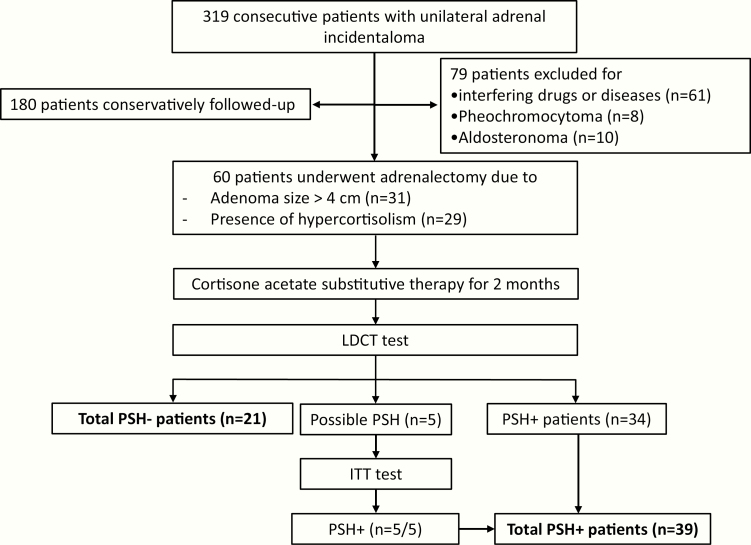
The current study is based on the population of a previous study aimed to evaluate whether, in AI patients undergoing unilateral adrenalectomy, PSH could be predicted by the parameters of HPA axis function [22]. In that study, performed from December 2003 to December 2008, 319 consecutive AI patients with unilateral AI and without signs and/or symptoms specific to cortisol excess (moon facies, striae rubrae, skin atrophy, buffalo hump) were enrolled. In all patients, a unilateral adrenal mass was incidentally found by noninvasive imaging methods of the abdomen performed for other causes. No subject had evidence of metastatic diseases. At computed tomography all adrenal masses were consistent with the diagnosis of adrenocortical adenomas (ie, homogeneous, hypodense, <10 Hounsfield units, and with well-shaped features). We excluded 61 patients taking drugs or affected with diseases influencing cortisol metabolism and/or secretion (ie, rheumatologic or hematologic diseases, depression, alcoholism, eating disorders, bowel diseases, thyrotoxicosis, and chronic renal and/or hepatic disease) and 18 patients with pheochromocytoma (n = 8) or aldosterone-secreting adenoma (n = 10). Among the remaining 240 AI patients, 60 underwent surgical removal of the adrenal mass due to the presence of hypercortisolism and/or on the basis of the size of the lesion and were included in the study. Figure 1 summarizes the protocol used.
Figure 1.
Flow chart reporting the inclusion criteria, reasons for adrenalectomy, assessments, and protocol. A total of 319 consecutive patients with unilateral incidentally found adrenal adenoma (adrenal incidentaloma) and without signs and/or symptoms specific of cortisol excess (moon facies, striae rubrae, skin atrophy, buffalo hump) have been enrolled. Interfering diseases: rheumatologic or haematological diseases, depression, alcoholism, eating disorders, bowel diseases, thyrotoxicosis and chronic renal and/or hepatic disease. Presence of hypercortisolism [ie presence of ≥3 criteria out the following: (1) Urinary free cortisol levels >60 mg/24h (165 nmol/24h, the 97th percentile of a reference population), (2) cortisol after 1 mg-overnight dexamethasone suppression test >3.0 mg/dL (83 nmol/L), (3) adrenocorticotroph hormone levels <10 pg/mL (2.2 pmol/L), (4) midnight serum cortisol levels >5.4 mg/dL (149 nmol/L)]. Possible PSH [ie baseline cortisol >5 mg/dL (138 nmol/L) and LDCT stimulated cortisol levels between 16 mg/dL (441 nmol/L) and 22 mg/dL (600 nmol/L)]. Abbreviations: ITT, insulin tolerance test; LDCT, low dose corticotropin stimulation test; PSH, post-surgical hypocortisolism (PSH–: PSH absence, PSH+: PSH presence).
In all patients, at baseline, the following parameters were measured at least once: basal morning (08.00 hours) adrenocorticotropin (ACTH), urinary free cortisol (UFC), midnight serum cortisol (MSC), and F-1mgDST levels. For each subject, the mean values of these parameters were reported if more than 1 determination was available during the presurgical follow-up, which lasted from 15 days to 24 months.
In 31 patients, surgery was considered the best therapeutic option on the basis of increasing dimensions (>1 cm increase during 12 months of follow-up) or size >4 cm at diagnosis. None of these patients had clearly fluctuating cortisol secretion. In 29 patients, even though completely asymptomatic for hypercortisolism, the surgical operation was decided on the basis of biochemical data strongly suggesting the presence of cortisol excess, such as the presence of ≥3 criteria out the following: (1) UFC levels >60 µg/24 hours (165 nmol/24 hours, the 97th percentile of a reference population for UFC), (2) F-1mgDST >3.0 µg/dL (83 nmol/L), (3) ACTH levels <10 pg/mL (2.2 pmol/L), (4) MSC >5.4 µg/dL (149 nmol/L), as suggested by other authors [24].
Laparoscopic or laparotomic adrenalectomy was decided by the endocrine surgeon, depending on the size of the adrenal adenoma and the patients’ clinical characteristics. In all cases, histology was consistent with adrenocortical adenoma. No patient had perioperative and postoperative complications.
Since adrenal failure occurs more frequently during stressful events, and following the recommendations of the Italian Association of Clinical Endocrinologists [24], we administered in all patients precautionary steroid therapy during surgery with hydrocortisone (100 mg intravenously on the basis of the anesthesiologic protocol used in our hospital at the time of the study) and immediately after operation with cortisone acetate orally (at weight-related dosing ranging between 25 and 37.5 mg/day in 3 subdivided doses during the day corresponding to 20-30 mg/day hydrocortisone), in order to avoid the possible consequences of undiagnosed hypoadrenalism [24]. The starting cortisone acetate dose was weight adjusted (corresponding to ∼0.3 mg/kg per day hydrocortisone). The commonly used cortisone acetate dose was 25 mg/day (corresponding to hydrocortisone 20 mg/day, 51 patients), while higher doses of 31.3 and 37.5 mg/day (corresponding to 25 and 30 mg/day hydrocortisone) were used in 7 and 2 patients, respectively.
In all patients, after about 60 days, cortisol secretion was assessed by a low-dose corticotropin stimulation test (LDCT), after 24-hour steroid therapy withdrawal [25]. The timing of the assessment of HPA axis function had been decided to avoid the persistence of a postoperative stress condition, as in this setting LDCT has not been validated [26, 27]. At the time of the study we used the cut-offs of 16 µg/dL (440 nmol/L) and 22 µg/dL (600 nmol/L) for diagnosing PSH and for ruling out PSH, respectively, on the basis of data showing that LDCT-stimulated cortisol values <16 µg/dL (440 nmol/L) best predicted adrenal insufficiency, whereas values >22 µg/dL (600 nmol/L) best predicted normal adrenal function with good accuracy (area under the curve 0.94, 95% confidence interval 0.90-0.94) [26]
In 21 patients with baseline cortisol >5 µg/dL (13 nmol/L) and stimulated cortisol levels >22 µg/dL (600 nmol/L), PSH was ruled out and steroid substitutive therapy was withdrawn, with none of these patients having symptoms suggesting PSH. Thirty-four patients with baseline cortisol <5 µg/dL (138 nmol/L) and/or stimulated cortisol levels <16 µg/dL (441 nmol/L) were considered to be affected with PSH. Five patients with baseline cortisol >5 µg/dL (138 nmol/L) and LDCT stimulated cortisol levels between 16 µg/dL (441 nmol/L) and 22 µg/dL (600 nmol/L) underwent an insulin tolerance test (ITT) [27, 28]. The latter subjects showed a reduced cortisol response after an ITT (ie, stimulated cortisol levels <18 µg/dL [496 nmol/L], at any time during the test in the presence of symptomatic hypoglycemia and plasma glucose <40 mg/dL [2.22 mmol/L]) [27, 28] and were, therefore, diagnosed as having PSH.
We decided to use a higher cut-off (ie, 22 µg/dL, 600 nmol/L, rather than 18 µg/dL, 500 nmol/L) in order to improve sensitivity. Indeed, a higher cut-off may be needed in patients after withdrawal of inhalatory steroid therapy, who might be comparable to those with endogenous mild hypercortisolism [29].
Overall, 39 patients were found to have PSH and were retested by LDCT every 6 months. The PSH lasted 6, 12, 18, 24, and 36 months in 6, 15, 9, 7, and 2 patients, respectively.
All subjects gave their witnessed informed consent before entering the study, which was approved by local ethics committees and in accordance with the Helsinki Declaration II.
B. Methods
All samples were stored at –20°C until assayed. The assays used were the same in all centers. In all patients plasma ACTH levels (mean of 3 determinations at 20-minute intervals) were measured by immunoradiometric assay (BRAHMS Diagnostica GmbH, Berlin, Germany) and serum cortisol and UFC levels (after dichloromethane extraction) were determined immunofluorimetrically using TDX-FLX Abbott, GmbH, Diagnostika kits (Wiesbaden-Delkenheim, Germany). The intra- and interassay coefficients of variation for all assays were <5 and 10%, respectively. Low-dose corticotropin stimulation and ITT tests were performed as described elsewhere [22]
We recorded the following data, weight, height, body mass index (BMI), presence of arterial hypertension, obesity, dyslipidemia, diabetes mellitus, and metabolic syndrome, following the World Health Organization criteria published in 2001 [30], as at the time of data collection these were the commonly used criteria for diagnosing diabetes mellitus. However, the criteria to diagnose diabetes have changed during the last 10 years. Therefore, in this analysis, we reassessed these diagnoses on the basis of the new criteria [31] and did not find differences in the diagnostic classification.
C. Study design and statistical analysis
We postulated that the presence of PSH is an indirect sign of presurgical hypercortisolism. We firstly compared patients with and without PSH. Then we searched for the lowest value of F-1mgDST, UFC, and MSC and for the highest value for ACTH in patients with PSH. Since, by definition, in our cohort no patients with PSH, and therefore with presurgical hypercortisolism, had F-1mgDST, UFC, and MSC below and ACTH above those values, we considered these cut-offs as those that could possibly individuate AI patients without hypercortisolism. Then we assessed the sensitivity (SN), specificity (SP), positive and negative predictive values of these parameters for identifying the absence of PSH occurrence and considered them as possible indexes of the absence of hypercortisolism. Furthermore, we compared the clinical characteristics of patients grouped on the basis of the HPA axis parameters and their cut-off that resulted in being able to significantly rule out the PSH occurrence.
Statistical analysis was performed by SPSS version 26.0 statistical package (IBM SPSS, Milan, Italy). The results are expressed as mean ± standard deviation, unless specified otherwise. The normality of data distribution was tested by the Kolmogorov–Smirnov test.
The comparison of the continuous variables were performed using the Student t-test or Mann–Whitney U-test as appropriate, while the categorical variables were compared using the chi-square test or Fisher exact test, as appropriate. The bivariate associations were tested by the Spearman rho or Pearson correlation as appropriate.
P-values <.05 were considered statistically significant.
2. Results
The clinical characteristics of patients with or without PSH occurrence are summarized in Table 1. The 2 groups were comparable for age, BMI, diameter of adenoma, duration of presurgical follow-up, mean hydrocortisone equivalent daily dose, indication for which surgery was chosen (ie, size of adenoma or suspected subclinical hypercortisolism), and prevalence of hypertension, type 2 diabetes, obesity, and metabolic syndrome. Among the HPA axis function’s parameters, MSC levels were significantly higher in patients with PSH than in those without, whereas UFC, 1mg-DST, and ACTH levels were comparable between the two groups. The lowest value of F-1mgDST, UFC, and MSC and the highest value for ACTH in patients with PSH were 1.2 µg/dL (33 nmol/L), 10.4 µg/24 hours (29 nmol/24 hours), 1.2 µg/dL (33 nmol/L), and 26.9 pg/mL (6 pmol/L), respectively. The prevalence of postsurgical hypocortisolism was not different between patients selected for surgery by size criteria (18/31, 58%) or because of hypercortisolism (21/29, 72.4%, P = .287).
Table 1.
Presurgical clinical and biochemical features of patients with adrenal incidentalomas and without (PSH–) or with postsurgical hypocortisolism (PSH+)
| Group PSH+n = 39 | Group PSH-n = 21 | P value | |
|---|---|---|---|
| Females | 31 | 15 | .532 |
| Age (years) | 55.3 ± 12.0 (24-74) | 57.2 ± 8.3 (33-75) | .505 |
| BMI (kg/m2) | 27.4 ± 5.6 (17.4-41) | 29.6 ± 5.4 (21.5-41) | .163 |
| Reason for surgery (size of the mass/SH) | 18/21 | 13/8 | .287 |
| HC doses (mg/day) | 20.9 ± 2.6 [20, 30] | 20.9 ± 2.3 [20, 30] | .932 |
| ACTH (pg/mL) | 9.6 ± 5.7 (1.9-26.9) | 9.0 ± 4.4 (5.0-23.8) | .665 |
| ACTH >26.9 pg/mL (%) | 0 (0.0) | 1 (1.7) | .650 |
| F-1mg-DST (µg/dL) | 4.6 ± 3.5 (1.2-13.7) | 3.2 ± 2.1 (0.9-9.0) | .098 |
| F-1mg-DST <1.2 µg/dL (%) | 0 (0.0) | 9 (42.9) | <.0001 |
| UFC (µg/24 hours) | 68.2 ± 45.2 (10.4-189.6) | 51.3 ± 32.4 (10.0-120.0) | .136 |
| UFC <10.4 µg/24 hours | 0 (0.0) | 2 (9.5) | .119 |
| MSC (µg/dL) | 6.0 ± 3.1 (1.2-12.5) | 3.4 ± 2.0 (1.0-8.0) | .001 |
| MSC <1.2 µg/dL | 0 (0.0) | 1 (4.8) | .350 |
| Diameter of adenoma (cm) | 3.5 ± 1.3 (1.2-9.0) | 3.2 ± 1.0 (1.5-5.7) | .340 |
| Pts with type 2 diabetes mellitus (%) | 9 (23.1) | 5 (23.8) | 1.000 |
| Pts with arterial hypertension (%) | 18 (46.2) | 15 (71.4) | .102 |
| Pts with obesity (%) | 15 (38.5) | 10 (47.6) | .587 |
| Pts with dyslipidemia (%) | 12 (30.8) | 10 (47.6) | .263 |
| Pts with metabolic syndrome (%) | 5 (12.8) | 6 (28.6) | .169 |
For plasma cortisol multiply × 27.56 to convert from µg/dL to nmol/L. For 24 hour urinary cortisol multiply × 2.756 to convert from µg/24 hours to nmol/24 hours. For ACTH multiply × 0.22 to convert from pg/mL to pmol/L.
Abbreviations: ACTH, adrenocorticotropin; Pts, patients; PSH, postsurgical hypercortisolism; PSH–, absence of PSH; PSH+, presence of PSH; MSC, midnight serum cortisol; UFC, urinary free cortisol; F-1mgDST, cortisol after 1 mg overnight dexamethasone suppression test; HC, hydrocortisone dose during substitutive therapy period.
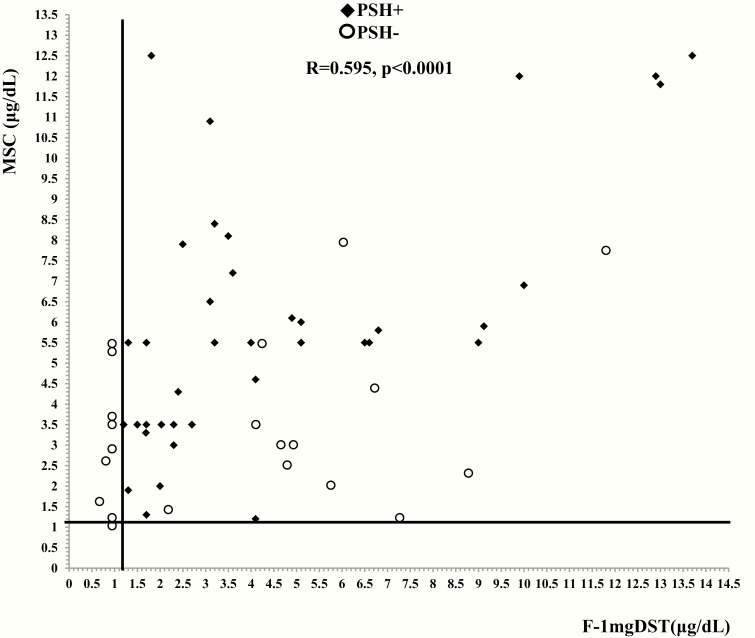
The number of subjects with F-1mgDST <1.2 µg/dL (33 nmol/L), UFC <10.4 µg/24 hours (29 nmol/24 hours), MSC <1.2 µg/dL (33 nmol/L), and ACTH >26.9 pg/mL (6 pmol/L) among patients with PSH and without PSH are also reported in Table 1. Among patients without PSH, 9, 2, 1, and 1 individuals showed F-1mgDST F-1mgDST <1.2 µg/dL (33 nmol/L), UFC <10.4 µg/24 hours (29 nmol/24 hours), MSC <1.2 µg/dL (33 nmol/L), and ACTH >26.9 pg/mL (6 pmol/L), respectively (Fig. 2). As noted, statistical significance was obtained only for F-1mgDST, probably due to the low number of patients with MSC levels <1.2 µg/dL (33 nmol/L), UFC levels <10.4 µg/24 hours (29 nmol/24 hours), and ACTH values >26.9 pg/mL (6 pmol/L). Therefore, in this cohort we considered the F-1mgDST test as the only test with enough reliability to be investigated as a possible marker of the absence of PSH occurrence after surgery.
Figure 2.
Presurgical levels of cortisol after 1-mg overnight dexamethasone suppression test (F-1mgDST) and midnight serum cortisol (MSC) in 60 patients with adrenal incidentalomas (AI) who underwent adrenalectomy. All AI patients with a postsurgical hypercortisolism (PSH) occurrence (black diamonds) showed F-1mgDST ≥1.2 µg/dL (33 nmol/L, vertical line) or MSC ≥1.2 µg/dL (33 nmol/L, horizontal line). In our cohort, 9 and 1 patients without PSH (empty circles) showed F-1mgDST or MSC <1.2 µg/dL (33 nmol/L).
By using a F-1mgDST cut-off set at 1.2 µg/dL (33 nmol/L, the lowest value in patients with PSH occurrence), we obtained 100% SN and positive predictive values (as expected) and 42.9% SP and 76.5% negative predictive value (accuracy 66.6%, P < .0001). The comparison between patients grouped on the basis of a F-1mgDST cut-off ≥1.2 µg/dL or <1.2 µg/dL (33 nmol/L) is reported in Table 2. Age, BMI, duration of presurgical follow-up, mean hydrocortisone equivalent daily doses, ACTH levels, and diameter of the adenoma were comparable between the 2 groups, while F-1mgDST (by definition), UFC, and MSC levels were higher in patients with F-1mgDST ≥1.2 µg/dL (33 nmol/L) than in those with F-1mgDST <1.2 µg/dL (33 nmol/L). This latter group showed a lower prevalence of dyslipidemia and tended to have a lower prevalence of diabetes mellitus than the former. Interestingly, among the 9 patients with F-1mgDST <1.2 µg/dL (33 nmol/L), no subjects were affected with diabetes mellitus and/or dyslipidemia and/or metabolic syndrome.
Table 2.
Presurgical clinical and biochemical features of patients with adrenal incidentalomas and with cortisol after 1 mg overnight dexamethasone suppression test <1.2 µg/dL or ≥1.2 µg/dL
| F-1mgDST <1.2 µg/dLn = 9 | F-1mgDST ≥1.2 µg/dLn = 51 | P value | |
|---|---|---|---|
| Females | 6 (66.7) | 40 (78.4) | .44 |
| Age (years) | 56.6 ± 4.5 48-63 | 55.9 ± 11.6 24-75 | .86 |
| BMI (kg/m2) | 26.6 ± 2.9 21.5-31.3 | 28.4 ± 6.0 17.4-41.0 | .38 |
| Duration of presurgical follow-up (months) | 8.4 ± 7.4 1.0 − 24.0 | 5.5 ± 5.0 0.5 − 19.6 | .142 |
| HC doses (mg/day) | 20.0 ± 0.0 [20] | 21.1 ± 2.5 [20, 30] | .21 |
| ACTH (pg/mL) | 10.1 ± 6.0 5.0-23.8 | 9.3 ± 5.2 1.9-26.9 | .69 |
| F-1mg-DST (µg/dL) | 1.1 ± 0.1 0.9-1.1 | 4.6 ± 3.2 1.2-13.7 | .001 |
| UFC (µg/24 hours) | 36.6 ± 17.7 10.0-59.0 | 66.8 ± 43.2 10.4-189.6 | .04 |
| MSC (µg/dL) | 3.0 ± 1.6 1.0-5.5 | 5.4 ± 3.1 1.2-12.5 | .03 |
| Diameter of adenoma (cm) | 3.4 ± 1.2 1.5-5.7 | 3.5 ± 1.3 1.2-9.0 | .85 |
| Pts with type 2 diabetes mellitus (%) | 0 (0.0) | 14 (27.5) | .07 |
| Pts with arterial hypertension (%) | 3 (33.3) | 30 (58.8) | .15 |
| Pts with obesity (%) | 3 (33.3) | 22 (43.1) | .72 |
| Pts with dyslipidemia (%) | 0 (0.0) | 22 (43.1) | .02 |
| Pts with metabolic syndrome (%) | 0 (0.0) | 11 (21.6) | .19 |
For plasma cortisol multiply × 27.56 to convert from µg/dL to nmol/L. For 24 hour urinary cortisol multiply × 2.756 to convert from µg/24 hours to nmol/24 hours. For ACTH multiply × 0.22 to convert from pg/mL to pmol/L.
Abbreviations: ACTH, adrenocorticotropin; Pts, patients; MSC, midnight serum cortisol; UFC, urinary free cortisol; F-1mgDST, cortisol after 1 mg overnight dexamethasone suppression test. HC, hydrocortisone dose during substitutive therapy period.
3. Discussion
In AI patients who underwent unilateral adrenalectomy, PSH occurrence has been suggested to be an indirect proof of the presence of hypercortisolism before surgery [5, 22, 23]. Therefore, the aim of the present study was to define how to exclude hypercortisolism in AI on the basis of the cut-off of F-1mgDST, MSC, UFC, and ACTH so that the absence of hypocortisolism after surgery could be predicted in AI patients before surgery. In our cohort, only F-1mgDST levels measured before surgery with a cut-off set at 1.2 µg/dL (33 nmol/L) could predict the PSH absence. Indeed, no AI patients with F-1mgDST <1.2 µg/dL (33 nmol/L) experienced PSH occurrence. Moreover, AI patients with F-1mgDST <1.2 µg/dL (33 nmol/L) tended to show a better metabolic profile than those with F-1mgDST ≥1.2 µg/dL (33 nmol/L). These findings could suggest that in AI patients the F-1mgDST cut-off for defining the absence of hypercortisolism should be lowered to 1.2 µg/dL (33 nmol/L).
The present findings are in line with some previous data. Indeed, in AI patients cortisol after 2 days of a low-dose (ie, 2 mg/day) dexamethasone suppression test with a cut-off set at 1.4 µg/dL (39 nmol/L) and 1.1 µg/dL (30 nmol/L) had higher accuracy in predicting cardiovascular risk and insulin resistance, respectively [11] and the F-1mgDST cut-off with the best conciliation between SN and SP for predicting cardiovascular events was found to be as low as 1.5 µg/dL (41 nmol/L) [21]. Moreover, previous studies showed that in patients with NFAI (F-1mgDST < 1.8 µg/dL, 50 nmol/L) the incidence of diabetes mellitus was increased compared with patients without AI [12] and the removal of an NFAI can lead to an amelioration of blood pressure, diabetes mellitus, and dyslipidemia [6, 21]. Finally, a recent meta-analysis showed that mortality was not increased differently in AI patients with autonomous cortisol secretion compared with NFAI patients [13].
The finding in the present cohort of AI patients with F-1mgDST <1.2 µg/dL (33 nmol/L) being less frequently affected with dyslipidemia and having a better metabolic profile is in agreement with the idea that at least some of the currently so-called nonfunctioning adrenal adenomas, are, in fact, responsible for a subtle but significant degree of cortisol hypersecretion. The hypothesis that some AI patients, who are not considered as affected with hypercortisolism on the basis of F-1mgDST level <1.8 µg/dL (50 nmol/L), may instead be hypercortisolemic, has already been proposed by other authors [19], but no suggestion on lowering the cut-off for F-1mgDST was given. Importantly, a lower cut-off of F-1mgDST has recently been proposed on the basis of data coming from subjects without AI and eucortisolemic (ie, with F-1mgDST <1.8 µg/dL, 50 nmol/L). Indeed, we have recently reported that in postmenopausal women without hypercortisolism abnormal cortisol secretion is associated with hypertension [32] and the cut-off of F-1mgDST set at 0.9 µg/dL (25 nmol/L) may be used for predicting the presence of at least 2 among hypertension, diabetes mellitus, and fragility fractures [33]. The role of cortisol secretion, even within the “normal” range, has been recently suggested as an index of cardiovascular risk by 3 independent research groups [34-36].
It must be observed that the F-1mgDST cut-off set at 1.2 µg/dL (33 nmol/L), while possibly useful for ruling out PSH and, therefore, for predicting the absence of hypercortisolism in AI, cannot be used for predicting certain PSH occurrence. Even in our cohort, many patients with F-1mgDST ≥1.2 µg/dL (33 nmol/L) did not develop PSH [22], as already reported even in patients with clinically overt ACTH-independent hypercortisolism [37]. The different duration of cortisol hypersecretion, the different sensitivity to glucocorticoids, and the different peripheral activation of cortisol may be all possible explanations of this apparently contradictory finding.
This study has several limits. Firstly, the low number of patients, in particular among those who did not experience PSH, suggests that the present findings should be taken with extreme caution. Secondly, we cannot completely exclude that some patients were affected with a cyclic form of hypercortisolism, thus rendering the F-1mgDST values less reliable. Thirdly, we should consider the possibility that in patients with F-1mgDST <1.2 µg/dL (33 nmol/L) the lack of PSH could be due to a short disease duration that could not have been sufficient to induce the HPA axis impairment, rather than being an index of a normal cortisol secretion. However, the fact that the presurgical follow-up duration was not shorter in AI patients with F-mgDST <1.2 µg/dL (33 nmol/L) than in those with F-1mgDST ≥1.2 µg/dL (33 nmol/L) is against this possibility. We are aware that, nowadays, the recommended hydrocortisone-equivalent doses for glucocorticoid substitutive therapy are slightly lower (ie, hydrocortisone 15-20 mg/day or cortisone acetate 20-25 mg/day) than those used at the time of the study [38]. This is because immediately after resolution of adrenal hypercortisolism a substitutive dose of hydrocortisone of 12 to 15 mg/m2/day has been recommended because it is enough to prevent adrenal crisis and to allow recovery of the HPA axis [39, 40]. Moreover, our protocol did not allow a step-by-step decrease in the dose of glucocorticoid substitution. Therefore, we cannot exclude that the high and not modifiable doses of glucocorticoid substitutive therapy might have influenced the present rate of postoperative hypocortisolism (ie, 70.9%), which, however, was in keeping with that (ie, 50-90%) reported in the literature [39]. We cannot exclude also that the long PSH duration in some patients could have been influenced by the relatively high and not-modifiable dose of glucocorticoid substitutive therapy. However, PSH duration in patients operated on for adrenal adenomas is reported to vary between 1 month and 50 months [39], figures that are in keeping with the present findings. In addition, we did not measure dexamethasone levels in blood, and, therefore, we cannot be sure that the HPA axis activity suppression was adequate in all patients. Finally, these results are derived from a cohort of patients operated on for adrenal adenomas and, therefore, the generalizability of these findings is limited
However, notwithstanding these limits, the present study suggests for the first time that the F-1mgDST cut-off <1.2 µg/dL (33 nmol/L) is able to rule out the possible occurrence of hypocortisolism in AI patients undergoing the removal of the adrenal adenoma. A first consequence of the present findings is that in AI patients with F-1mgDST <1.2 µg/dL before surgery, postoperative glucocorticoid substitutive therapy is not needed. In addition, this F-1mgDST cut-off has the highest sensitivity to exclude hypercortisolism and, at least in AI patients, could define a normal cortisol secretion. Further studies with a thorough and prolonged endocrine workup before surgery in AI patients are needed to confirm the need of lowering the currently used F-1mgDST cut-off for defining normal cortisol secretion.
Acknowledgments
Financial Support: The present study has been partially supported by Istituto Auxologico Italiano (Grant PRECOR Study 2019_01_29_06).
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- AI
adrenal incidentaloma
- BMI
body mass index
- F-1mgDST
1 mg overnight dexamethasone suppression test
- HPA
hypothalamic–pituitary–adrenal
- ITT
insulin tolerance test
- LDCT
low-dose corticotropin stimulation test
- PSH
postsurgical hypercortisolism
- MSC
midnight serum cortisol
- NFAI
nonfunctioning adrenal incidentaloma
- SN
sensitivity
- SP
specificity
- UFC
urinary free cortisol
Additional information
Disclosure Summary: All authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Morelli V, Arosio M, Chiodini I. Cardiovascular mortality in patients with subclinical Cushing. Ann Endocrinol (Paris). 2018;79(3):149-152. [DOI] [PubMed] [Google Scholar]
- 2. Di Dalmazi G, Vicennati V, Garelli S, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2(5):396-405. [DOI] [PubMed] [Google Scholar]
- 3. Patrova J, Kjellman M, Wahrenberg H, Falhammar H. Increased mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: a 13-year retrospective study from one center. Endocrine. 2017;58(2):267-275. [DOI] [PubMed] [Google Scholar]
- 4. Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. 2014;99(12):4462-4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiodini I. Clinical review: Diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab. 2011;96(5):1223-1236. [DOI] [PubMed] [Google Scholar]
- 6. Bancos I, Alahdab F, Crowley RK, et al. Therapy of endocrine disease: improvement of cardiovascular risk factors after adrenalectomy in patients with adrenal tumors and subclinical Cushing’s syndrome: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(6):R283-R295. [DOI] [PubMed] [Google Scholar]
- 7. Ermetici F, Dall’Asta C, Malavazos AE, et al. Echocardiographic alterations in patients with non-functioning adrenal incidentaloma. J Endocrinol Invest. 2008;31(6):573-577. [DOI] [PubMed] [Google Scholar]
- 8. Yener S, Comlekci A, Akinci B, et al. Non-functioning adrenal incidentalomas are associated with elevated D-dimer levels. J Endocrinol Invest. 2009;32(4):338-343. [DOI] [PubMed] [Google Scholar]
- 9. Sereg M, Szappanos A, Toke J, et al. Atherosclerotic risk factors and complications in patients with non-functioning adrenal adenomas treated with or without adrenalectomy: a long-term follow-up study. Eur J Endocrinol. 2009;160(4):647-655. [DOI] [PubMed] [Google Scholar]
- 10. Tuna MM, Imga NN, Doğan BA, et al. Non-functioning adrenal incidentalomas are associated with higher hypertension prevalence and higher risk of atherosclerosis. J Endocrinol Invest. 2014;37(8):765-768. [DOI] [PubMed] [Google Scholar]
- 11. Androulakis II, Kaltsas GA, Kollias GE, et al. Patients with apparently nonfunctioning adrenal incidentalomas may be at increased cardiovascular risk due to excessive cortisol secretion. J Clin Endocrinol Metab. 2014;99(8):2754-2762. [DOI] [PubMed] [Google Scholar]
- 12. Lopez D, Luque-Fernandez MA, Steele A, Adler GK, Turchin A, Vaidya A. “Nonfunctional” adrenal tumors and the risk for incident diabetes and cardiovascular outcomes: a cohort study. Ann Intern Med. 2016;165(8):533-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elhassan YS, Alahdab F, Prete A, et al. Natural history of adrenal incidentalomas with and without mild autonomous cortisol excess: a systematic review and meta-analysis. Ann Intern Med. 2019;171(2):107-116. [DOI] [PubMed] [Google Scholar]
- 14. Sadoul JL, Kézachian B, Altare S, Hadjali Y, Canivet B. Apparent activities of 21-hydroxylase, 17alpha-hydroxylase and 17,20-lyase are impaired in adrenal incidentalomas. Eur J Endocrinol. 1999;141(3):238-245. [DOI] [PubMed] [Google Scholar]
- 15. Del Monte P, Bernasconi D, Bertolazzi L, et al. Increased 17 alpha-hydroxyprogesterone response to ACTH in silent adrenal adenoma: cause or effect? Clin Endocrinol (Oxf). 1995;42(3):273-277. [DOI] [PubMed] [Google Scholar]
- 16. Reincke M, Peter M, Sippell WG, Allolio B. Impairment of 11 beta-hydroxylase but not 21-hydroxylase in adrenal ‘incidentalomas’. Eur J Endocrinol. 1997;136(2):196-200. [DOI] [PubMed] [Google Scholar]
- 17. Giorgi RB, Correa MV, Costa-Barbosa FA, Kater CE. Cyclic subclinical hypercortisolism: a previously unidentified hypersecretory form of adrenal incidentalomas. J Endocr Soc. 2019;3(3):678-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morelli V, Donadio F, Eller-Vainicher C, et al. Role of glucocorticoid receptor polymorphism in adrenal incidentalomas. Eur J Clin Invest. 2010;40(9):803-811. [DOI] [PubMed] [Google Scholar]
- 19. Olsen H. Cortisol secretion from incidentally discovered adrenal adenomas has been described as autonomous. J Clin Endocrinol Metab. 2014;99(11):L1-L2. [DOI] [PubMed] [Google Scholar]
- 20. Androulakis II, Kaltsas G. Letter to the editor response. J Clin Endocrinol Metab. 2014;99(11):L3. [DOI] [PubMed] [Google Scholar]
- 21. Morelli V, Reimondo G, Giordano R, et al. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab. 2014;99(3):827-834. [DOI] [PubMed] [Google Scholar]
- 22. Eller-Vainicher C, Morelli V, Salcuni AS, et al. Post-surgical hypocortisolism after removal of an adrenal incidentaloma: is it predictable by an accurate endocrinological work-up before surgery? Eur J Endocrinol. 2010;162(1):91-99. [DOI] [PubMed] [Google Scholar]
- 23. Lee SH, Song KH, Kim J, et al. New diagnostic criteria for subclinical hypercortisolism using postsurgical hypocortisolism: the Co-work of Adrenal Research study. Clin Endocrinol (Oxf). 2017;86(1):10-18. [DOI] [PubMed] [Google Scholar]
- 24. Terzolo M, Stigliano A, Chiodini I, et al. ; Italian Association of Clinical Endocrinologists AME position statement on adrenal incidentaloma. Eur J Endocrinol. 2011;164(6):851-870. [DOI] [PubMed] [Google Scholar]
- 25. Dickstein G, Shechner C, Nicholson WE, et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab. 1991;72(4):773-778. [DOI] [PubMed] [Google Scholar]
- 26. Kazlauskaite R, Evans AT, Villabona CV, et al. ; Consortium for Evaluation of Corticotropin Test in Hypothalamic-Pituitary Adrenal Insufficiency Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008;93(11):4245-4253. [DOI] [PubMed] [Google Scholar]
- 27. Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med. 2009;360(22):2328-2339. [DOI] [PubMed] [Google Scholar]
- 28. Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79(4):923-931. [DOI] [PubMed] [Google Scholar]
- 29. Broide J, Soferman R, Kivity S, et al. Low-dose adrenocorticotropin test reveals impaired adrenal function in patients taking inhaled corticosteroids. J Clin Endocrinol Metab. 1995;80(4):1243-1246. [DOI] [PubMed] [Google Scholar]
- 30. Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) J Am Med Ass. 2001; 285:2486-2497. [DOI] [PubMed] [Google Scholar]
- 31. American Diabetes Association, 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13-S28 [DOI] [PubMed] [Google Scholar]
- 32. Chiodini I, Gaudio A, Eller-Vainicher C, et al. Cortisol secretion, sensitivity, and activity are associated with hypertension in postmenopausal eucortisolemic women. J Clin Endocrinol Metab. 2019;104(10):4441-4448. [DOI] [PubMed] [Google Scholar]
- 33. Morelli V, Aresta C, Gaudio A, et al. Prediction of hypertension, diabetes and fractures in eucortisolemic women by measuring parameters of cortisol milieu. Endocrine. 2020;68(2):411-419. [DOI] [PubMed] [Google Scholar]
- 34. Mazgelytė E, Karčiauskaitė D, Linkevičiūtė A, et al. Association of hair cortisol concentration with prevalence of major cardiovascular risk factors and allostatic load. Med Sci Monit. 2019;25:3573-3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haas AV, Hopkins PN, Brown NJ, et al. Higher urinary cortisol levels associate with increased cardiovascular risk. Endocr Connect. 2019;8(6):634-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crawford AA, Soderberg S, Kirschbaum C, et al. Morning plasma cortisol as a cardiovascular risk factor: findings from prospective cohort and Mendelian randomization studies. Eur J Endocrinol. 2019;181(4):429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morelli V, Minelli L, Eller-Vainicher C, et al. Predictability of hypoadrenalism occurrence and duration after adrenalectomy for ACTH-independent hypercortisolism. J Endocrinol Invest. 2018;41(4):485-493. [DOI] [PubMed] [Google Scholar]
- 38. Isidori AM, Arnaldi G, Boscaro M, et al. ; Italian Society of Endocrinology Towards the tailoring of glucocorticoid replacement in adrenal insufficiency: the Italian Society of Endocrinology Expert Opinion. J Endocrinol Invest. 2020;43(5):683-696. [DOI] [PubMed] [Google Scholar]
- 39. Di Dalmazi G, Berr CM, Fassnacht M, Beuschlein F, Reincke M. Adrenal function after adrenalectomy for subclinical hypercortisolism and Cushing’s syndrome: a systematic review of the literature. J Clin Endocrinol Metab. 2014;99(8):2637-2645. [DOI] [PubMed] [Google Scholar]
- 40. Doherty GM, Nieman LK, Cutler GB Jr, Chrousos GP, Norton JA. Time to recovery of the hypothalamic-pituitary-adrenal axis after curative resection of adrenal tumors in patients with Cushing’s syndrome. Surgery. 1990;108(6):1085-1090. [PubMed] [Google Scholar]