Abstract
Objective:
We evaluated trajectories of attention-deficit/hyperactivity (ADHD)-relevant behaviors in a sample of infants at high and low familial risk for ADHD who were prospectively evaluated at 12, 18, and 24 months of age.
Method:
Participants included 43 infants at risk for ADHD based on family history (i.e., diagnosed first-degree relative) and 40 low-risk infants (i.e., no family history of ADHD). Instances of inattention, out-of-seat, and grabbing behavior were coded from video; analogous constructs were rated by examiners unaware of familial risk status after completing structured standardized assessments with the infants/toddlers. At the end of each study visit, examiners solicited parents’ concerns about their child’s behavior. Differences in ADHD-related behaviors and parent concerns were examined between 12 to 24 months of age.
Results:
Infants with an older sibling or parent diagnosed with ADHD were distinguishable from infants with no family history of ADHD as early as 12 months of age based on directly-observed and examiner reports of behavior, particularly with respect to hyperactive-impulsive behavior. Parents of infants at familial risk for ADHD also reported significantly more behavior/temperament concerns as early as 12 months of age compared to parents of infants at low risk for ADHD.
Conclusions:
These findings highlight the ability to detect genetic liability for ADHD by the end of the first year of life, suggesting that well-designed family risk studies of ADHD are feasible and may be clinically valuable. They also suggest the potential for earlier detection of risk for ADHD than has previously been possible.
Keywords: ADHD, infancy, early detection
Attention-deficit/hyperactivity disorder (ADHD) is one of the most prevalent disorders of childhood, affecting approximately 8% of children in the United States (Danielson et al., 2018). There is ample evidence that elevated levels of core ADHD symptoms—inattention-disorganization and hyperactivity-impulsivity—in early childhood predict poor outcomes later in life. Indeed, in a sample of 4-6-year-old children with ADHD, a higher number of ADHD symptoms in early childhood predicted higher levels of ADHD symptoms as well as broad-based functional impairment in adolescence, with only 10% of individuals diagnosed with ADHD as young children being classified as functioning in the normative range by adolescence (Lahey et al., 2016). Given these long-term functional impairments, the ability to detect behaviors consistent with the development of ADHD as early as possible is imperative to identifying very young children at risk for ADHD. Such approaches could facilitate earlier behavioral intervention in hopes of preventing these well-documented poor outcomes (Sonuga-Barke & Halperin, 2010; Sonuga-Barke, Koerting, Smith, McCann, & Thompson, 2011).
The median age of diagnosis of ADHD is around 6 years (Visser et al., 2014). However, children with more severe ADHD symptoms tend to be diagnosed significantly earlier, at a median age of 4.4 years (Visser et al., 2014). Studies show that careful ADHD diagnoses made during the preschool years persist into school-age in 70-89% of cases, although movement between presentations (formerly subtypes) is common (Lahey, Pelham, Loney, Lee, & Willcutt, 2005; Law, Sideridis, Prock, & Sheridan, 2014; Riddle et al., 2013). Still, it has been difficult to conduct research on the very early ADHD phenotype given its protracted development compared to other prevalent childhood conditions like autism spectrum disorder (ASD). Additional barriers to investigating the early emergence of ADHD include that it is developmentally typical for young children to display high levels of inattention and hyperactive-impulsive behavior, and that there are extremely few (if any) tools available to validly measure such behaviors in very young children, particularly not via direct observation of behavior (versus parent report of more global constructs, such as temperament).
Most of the studies that do focus on this question have relied on community samples or retrospective chart reviews and highlight infant temperament traits such as effortful control, activity level, and negative affectivity (Arnett, Macdonald, & Pennington, 2013; Frick, Bohlin, Hedqvist, & Brocki, 2019; Frick, Forslund, & Brocki, 2019; Meeuwsen, Perra, van Goozen, & Hay, 2018; N. Miller, Degnan, Hane, Fox, & Chronis-Tuscano, 2019; N. Miller, Hane, Degnan, Fox, & Chronis-Tuscano, 2019; Rende, 1993; Sanson, Smart, Prior, & Oberklaid, 1993; Sullivan et al., 2015; Willoughby, Gottfredson, & Stifter, 2017) as well as early motor and language delays (Arnett et al., 2013; Gurevitz, Geva, Varon, & Leitner, 2014) as relatively consistent predictors of ADHD symptoms. Although these studies have identified key behavioral dimensions in infancy/early childhood that are predictive of later ADHD symptomatology, most tend to focus on non-specific factors (e.g., temperament) rather than extending the measurement of specific core ADHD features into earlier developmental periods. Extending the examination of core ADHD features to younger children is likely necessary in order to identify ADHD, as currently defined, at an earlier age with some specificity. Additionally, because most prior studies rely on general population or community samples, rather than those enriched for ADHD, they may not capture the full range of phenotypic variation in these behaviors.
In order to address these gaps in the literature, studies focused on samples of infants enriched for ADHD are necessary. Given the high heritability of ADHD (estimates ranging from .70-.80; Faraone et al., 2005), one approach is to enroll infants with a family history of ADHD. Very few studies have reported on samples of infants recruited based on familial risk for ADHD. In an initial innovative study, Auerbach and colleagues (2008) found that infants of fathers with elevated ADHD symptoms (n = 36) showed differences from low-risk infants (n = 22) spanning several parent-reported temperament domains including attention, activity level, and inhibitory control as early as 7 months of age, with the most striking difference being related to overactivity. A more recent study found that family history of ADHD symptoms in parents (n = 38) was associated with disrupted affective responses at 6 months of age during a laboratory-based task (Sullivan et al., 2015), but this study was limited by a small low-risk comparison sample (n = 10) and did not specifically evaluate the core symptoms of ADHD, instead focusing on the associated, but important, construct of emotion regulation. Critically, ascertaining diagnoses of adult parents—including evidence of childhood symptomatology—can be challenging; these two key studies did not require confirmation of a prior diagnosis in all parent probands, but rather focused on self-report of previous diagnosis and/or elevated dimensional levels of symptomatology. Moreover, only one of these studies examined developmental trajectories in a high-risk sample beyond the first year of life. Still, both demonstrate that investigations focused on samples at heightened risk for developing ADHD are needed in order to identify the earliest possible indicators of the disorder.
As a proof-of-concept for infant identification of school-age ADHD outcomes, we recently presented data from a small prospective sample of infants that included younger siblings of children with ASD, a population known to be at elevated risk for ADHD (Jokiranta-Olkoniemi et al., 2016; Miller et al., 2019). This particular investigation was unique in that it followed the sample through middle childhood, ascertaining formal DSM-5 diagnoses of ADHD between the ages of 8-11 years. Findings indicated that the infants who later developed ADHD (without ASD) demonstrated atypical longitudinal patterns of sustained visual attention beginning at 3-6 months of age; were more likely to demonstrate parent-reported behavior/temperament problems at 36 months of age; and were more likely to exhibit examiner-reported inattentive, hyperactive, and/or impulsive behaviors by 18 months of age (Miller, Iosif, Young, Hill, & Ozonoff, 2018). However, a major limitation was that the majority of infants who developed ADHD had a family history of ASD, potentially reducing generalizability of findings to infants without a history of ASD and necessitating similar investigations in samples at heightened risk for ADHD but not for ASD.
Thus, in the present investigation, we expand upon previous studies by examining ADHD-related behaviors in a unique cohort of infants specifically recruited based on family history of ADHD as well as infants at low familial risk for ADHD. In this study, we sought to determine whether infants at familial risk for ADHD exhibit more ADHD-related behaviors than those at low familial risk and, if so, how early such differences are evident. Using multi-method, multi-informant data, we examine trajectories of ratings of ADHD-related behavioral dimensions (attention, activity, impulsivity) completed by expert examiners and coded by highly-trained research assistants both unaware of familial risk status, as well as parental concerns about child behavior from 12 to 24 months of age. Based on the research reviewed above, we expected that trajectories of examiner ratings and coded ADHD-related behaviors between 12-24 months of age would distinguish infants at risk for ADHD from those at low risk. Given the relatively limited literature focused on infants at familial risk for ADHD, we did not make explicit predictions about specific ages at which the two groups would differ based on examiner ratings and coded behavior but anticipated that differences between the two groups would increase over time. We also predicted that, consistent with Miller et al. (2018), parents of children in the ADHD-risk group would report significantly more behavioral concerns by 18 months of age, with differences between the two groups increasing over time.
Method
Overview of Procedure
This study utilizes data from a prospective longitudinal investigation of infants at high (ADHD-risk) and low (low-risk) familial risk for ADHD and was conducted under the approval of the University of California, Davis Institutional Review Board. Informed consent was obtained from parents prior to conducting assessments; parents received monetary compensation for their time. Infants/toddlers were assessed by Masters- or Ph.D.-level examiners unaware of risk group membership. Stringent administration and scoring fidelity procedures were in place to ensure minimal cross-examiner differences. The primary measures of interest were obtained at the 12-, 18-, and 24-month assessments.
Participants
Infants were recruited from the Sacramento region, consisting of a mixture of urban, suburban, and rural communities into one of two familial risk groups: ADHD-risk or low-risk. At time of initial recruitment, for the ADHD-risk group, diagnoses of any ADHD subtype in affected older siblings or parents (probands) were confirmed via (1) an intake screener (DSM-5 checklist for ADHD, completed on the parent or sibling proband) and (2) diagnostic reports from a clinician, or clinician documentation of treatment for ADHD. When sibling proband records were not available, a diagnostic evaluation was performed by the study team (parent- and teacher-completed rating scales, behavioral observation during brief cognitive testing). When parent proband records were not available, eligibility was established by self-report of prior ADHD diagnosis and T-scores of ≥65 on the ADHD Index from the Conners Adult ADHD Rating Scale (CAARS; Conners, Erhardt, & Sparrow, 1998), as rated by the parent’s partner/spouse. Of the 44 infants originally recruited into the ADHD-risk group, 54.5% had an older sibling diagnosed with ADHD and 45.5% had a parent with ADHD (n = 14 fathers, 6 mothers). Exclusion criteria for the ADHD-risk group included birth before 32-weeks’ gestation; ASD in first-, second-, or third-degree relatives; or a known genetic disorder in the proband or infant. One participant in the ADHD-risk group was diagnosed with ASD by 24 months of age and was thus excluded from analyses.
Low-risk status was confirmed by an intake screener including DSM-5 symptoms of ADHD prior to initial enrollment. After enrollment, low-risk status was again confirmed by screening for ADHD symptoms in the parents using the CAARS and in siblings using the ADHD-Rating Scale or CASI-5 (depending on age) to ensure low levels of ADHD symptoms. If any low-risk first-degree relatives screened above the DSM-5 symptom count cutoff for ADHD, they were invited for further evaluation to determine potential eligibility for the ADHD-risk group or ineligibility for the study; this occurred extremely rarely. Exclusion criteria for the low-risk group included birth before 37-weeks’ gestation; developmental, learning, or medical conditions in any older siblings; and ADHD or ASD in any first-, second-, or third-degree relative.
The final analyzed sample included 43 participants in the ADHD-risk group and 40 in the low-risk group. All were enrolled by 18 months of age, with 88% of the sample having completed their first assessment by 9 months of age and 96% having completed their first assessment by 12 months of age. Table 1 displays characteristics of the sample by familial risk group.
Table 1.
Participant characteristics.
| Low-risk (n = 40) | ADHD-risk (n = 43) | p-value | |
|---|---|---|---|
| Male sex, n, % | 23 (57.5%) | 29 (67.4%) | 0.35 |
| Racial/ethnic minority, n (%)a | 11 (27.5%) | 12 (28.6%) | 0.91 |
| Family income, n (%) | 0.06 | ||
| ≤ $100,000 | 14 (35.0%) | 23 (53.5%) | |
| ≥ $100,001 | 21 (52.5%) | 14 (32.6%) | |
| Decline to report/missing | 5 (12.5%) | 6 (13.9%) | |
| Gestational age (weeks), mean (SD) | 39.2 (1.3) | 39.1 (2.0) | 0.77 |
| Nonverbal DQ, mean (SD)b | |||
| 12 months | 57.5 (7.5) | 55.2 (5.7) | 0.14 |
| 18 months | 52.0 (6.2) | 51.4 (5.3) | 0.66 |
| 24 months | 54.5 (8.2) | 48.8 (8.0) | < 0.01 |
| Verbal DQ, mean (SD)b | |||
| 12 months | 47.8 (8.0) | 45.5 (6.2) | 0.17 |
| 18 months | 48.5 (9.8) | 45.5 (13.5) | 0.27 |
| 24 months | 53.5 (8.6) | 50.1 (9.7) | 0.12 |
Note. ADHD = Attention-Deficit/Hyperactivity Disorder; DQ = Developmental Quotient. Group differences assessed using χ2 test for sex, racial/ethnic minority status, and family income, and two-sample t-test for remaining variables.
Missing for n = 1 ADHD-risk participant.
Nonverbal (averaged Visual Reception and Fine Motor subtest T-scores) and Verbal DQ (averaged Receptive and Expressive Language subtest T-scores) missing for n = 1 low-risk and n = 4 ADHD-risk participants at 12 months; n = 2 low-risk and n = 6 ADHD-risk participants at 18 months; and n = 4 low-risk group and n = 6 ADHD-risk participants at 24 months.
Measures
Sample characterization measures.
Child & Adolescent Symptom Inventory, 5th Ed. (CASI-5; Gadow & Sprafkin, 2013).
This caregiver-report checklist provides both DSM-5 symptom count and severity measurements of a variety of common childhood disorders, including ADHD. Parents completed the ADHD section of the CASI-5 on sibling probands in order to confirm risk group classification.
NICHQ Vanderbilt Teacher Rating Scale (American Academy of Pediatrics, 2002).
This is a teacher-report rating scale that is commonly used to assess inattention, hyperactivity, impulsivity, and other behavioral symptoms within the classroom context. It includes each of the 18 DSM symptoms of ADHD and therefore can serve as a DSM checklist for ADHD to inform assessment of cross-situational symptoms. We collected this rating scale on sibling probands when prior diagnostic reports were not available as part of a multi-informant diagnostic evaluation process.
Conners Adult ADHD Rating Scale – Observer Report: Long Version (CAARS-O:L; Conners et al., 1998).
This rating scale evaluates symptoms of ADHD in adults. Parents completed forms on each other to confirm parental symptoms when diagnosed parents served as probands. In instances of a single parent, another observer completed the rating scale about the parent (e.g., family member, close friend, co-worker) whenever possible.
Mullen Scales of Early Learning (MSEL; Mullen, 1995).
This standardized developmental test for children birth to 68 months was administered at each visit to evaluate cognitive functioning. Participants were seated at the table across from the examiner and in their parent’s lap during testing. MSEL subscales have excellent internal consistency (median 0.91) and test-retest reliability (median 0.84). Four subscales were administered to the infants/toddlers: Visual Reception, Fine Motor, Receptive Language, and Expressive Language, each resulting in separate T-scores. For sample characterization purposes, two composite scores were calculated: A nonverbal developmental quotient (DQ) score (average of Visual Reception and Fine Motor T-scores) and a verbal DQ score (average of Receptive and Expressive Language T-scores). In addition, for the purposes of this study, behavior was coded from video acquired during developmental testing (see below).
Measures to track emergence of ADHD-related behaviors.
ADHD-Related Behavior Codes.
Videos were coded in real time using BORIS behavioral observation software (Friard & Gamba, 2016) at each age (12, 18, and 24 months). Three behaviors were coded intending to map on to the core symptom dimensions of ADHD: Inattention, Out-of-Seat, and Grab (see Table 2 for abbreviated code definitions). The code development process was iterative and initially involved review of filmed developmental assessments from an independent sample of infants as well as review of the literature utilizing similar methods in older children (e.g., DeWolfe, Byrne, & Bawden, 2000). Codes were then applied to an additional independent sample and refined as needed before establishing reliability. Coders unaware of child history or familial risk status were initially trained to 70% agreement on all codes, as measured by intraclass correlation coefficients (ICCs). Average ICCs across all coders were in the good-to-excellent range for frequencies of all codes (Cicchetti, 1994; Mitchell, 1979): Inattention 0.82, Out-of-Seat 0.96, and Grab 0.84. Twenty percent of data were double-coded to maintain ongoing reliability.
Table 2.
Abbreviated descriptions of ADHD-related behaviors coded.
| Behavior | Description |
|---|---|
| Inattention | Any instance of inattentive/bored/distracted behavior. |
| Out-of-seat | Any instance in which, in a clear attempt to get free, contact between the child’s bottom is severed from the parent’s lap or chair, and/or clear attempts to get out of seat/parent’s lap or chair even if contact is not severed. |
| Grab | Attempts (successful or unsuccessful) to obtain objects intrusively and/or when it is inappropriate to do so. |
Behavior was coded during the first five minutes of the MSEL (Mullen, 1995) Fine Motor subtest of each visit. This context was selected because (1) it was assessed at each visit, (2) the tasks require attention and cooperation in the context of a structured assessment during which the infant/toddler is expected to remain seated, and (3) the tasks involve toys and objects thought to be of interest to infants/toddlers. If the Fine Motor subtest was completed in less than five minutes, the remaining time was coded from the Visual Reception subtest, resulting in a full five minutes of coded behavior for each participant at each of the three visit ages. In rare instances, parts or all of the Fine Motor and Visual Reception subscales could not be coded due to skipped or non-standard administration (e.g., on the floor and out of the view of the camera); in these cases, behavior was coded from video of any available MSEL subscale (i.e., Fine Motor, Visual Reception, Receptive Language, Expressive Language). Frequencies of each behavior were analyzed.
Examiner Ratings: Behavior Rating Inventory for Children (BRIC; Gopin, Healey, Castelli, Marks, & Halperin, 2010).
The BRIC is a clinician-rated measure originally developed to identify ADHD in preschoolers, and includes 5-point Likert scales ranging from 1 to 5 for each of three dimensions: Attention, Activity, and Impulsivity. Higher scores reflect more problematic behavior. We modified the anchors for each dimension to be appropriate for infants and toddlers by adjusting examples of the behavioral descriptors. For example, for the original BRIC Activity subscale score of “5”, we added the italicized text: “under table, constantly on the go, crawled across/climbed down from table, rapidly moved around room and/or handled objects. Cronbach’s alphas were 0.89 at 12 months, 0.90 at 18 months, and 0.91 at 24 months of age. Examiners had M.A.- or Ph.D.-level training and completed these ratings after table testing at each age (12, 18, and 24 months), which consisted of administration of the MSEL and several brief experimental tasks.
Parent concerns (Ozonoff et al., 2009).
At the end of each assessment at each age (12, 18, and 24 months), parents were asked whether they had any developmental or behavioral concerns about their child using a standardized probe. Parents’ verbal responses about current concerns were coded into pre-specified categories by examiners unaware of group membership or prior concerns: None, speech/language, social, stereotyped behavior, motor, medical/regulatory, behavior/temperament, unspecified autism, or general developmental concerns. Examiners were trained to 90% agreement on all categories. Here, we focused on behavior/temperament concerns. This category includes any reports by parents of high activity level, poor attention (e.g., inability to settle down, distractibility), behavioral dysregulation (e.g., intensity of response, aggression, impulsivity, non-compliance), and difficulties in mood/general disposition (e.g., irritable, cranky, anxious). This variable was dichotomized to reflect the presence (1) versus absence (0) of at least one behavior/temperament concern.
Statistical Analysis
Statistical analyses were conducted within a generalized linear mixed-effects models framework (McCulloch, Searle, & Neuhaus, 2008). This framework is appropriate for longitudinal designs, in which data for individuals are collected across multiple assessments, and can accommodate dependent variables that are normally distributed (e.g., BRIC subscales), counts (behavior code frequencies), or binary (dichotomized parent concerns). An advantage of this approach is the ability to account for the correlated structure of the data due to repeated assessments over time and to produce valid inference under the assumption that data were missing at random. We used identity link and a normal variance function for BRIC data, a logit link and binomial variance function for parent concerns data (i.e., whether the parent had behavior/temperament concerns or not), and a log link and negative binomial function to model the frequencies of coded behavior since there was evidence of overdispersion in the coded behaviors. The primary focus of our analyses was to describe the developmental trajectories for the dependent variables between 12 to 24 months of age and to test whether the ADHD-risk group had different baseline (12-month) values, or experienced changes over time that were different at 18 or 24 months than the low-risk group. Thus, for each of the dependent variables, we fitted a model with a main effect for group (ADHD-risk, low-risk), time (12, 18, or 24 months), and their interactions. The intercept in the model provides an estimate of the mean level in the reference group (low-risk) at baseline (12 months). In addition, the model estimates the average difference at baseline between the ADHD-risk and low-risk groups, as well as terms to test whether there is a significant change from baseline to 18 months (Time 1) or to 24 months (Time 2) in the reference group. Finally, the interaction term between group and Time 1 tests whether the change from baseline (12 months) to 18 months differs between the two groups; the interaction term between group and Time 2 tests whether the change from baseline to 24 months differs between the two groups. To account for the within-child dependence, all models included a random effect for the child-specific intercept. Hypothesis tests were two-sided; p-values < .05 were considered statistically significant. All analyses were implemented using PROC MIXED and GLIMMIX in SAS Version 9.4 (SAS Institute Inc., Cary, NC).
Results
Table 3 provides descriptive statistics for the three examiner-rated behaviors, the three coded ADHD-related behaviors, and parent concerns across time. Table 4 summarizes the results of the mixed-effects regression models used to analyze these variables. Results are presented below by behavioral domain evaluated.
Table 3.
Summary of examiner ratings, coded behavior, and parent concerns for ADHD-risk and low-risk groups at 12, 18, and 24 months of age.
| Variable | 12 months | 18 months | 24 months | |||
|---|---|---|---|---|---|---|
|
| ||||||
| ADHD-risk (n = 43) | Low-risk (n = 40) | ADHD-risk (n = 43) | Low-risk (n = 40) | ADHD-risk (n = 43) | Low-risk (n = 40) | |
| Examiner Ratings a | ||||||
| Attention, mean (SD) | 2.5 (0.9)* | 2.1 (1.0)* | 3.0 (1.0) | 2.7 (0.9) | 3.2 (1.1)* | 2.4 (1.0)* |
| Activity, mean (SD) | 3.0 (1.1)* | 2.4 (1.1)* | 3.3 (1.1) | 2.9 (1.1) | 3.3 (1.2)* | 2.6 (1.2)* |
| Impulsivity, mean (SD) | 2.7 (1.0)* | 1.8 (0.8)* | 3.2 (1.0)* | 2.7 (1.1)* | 3.4 (1.0)* | 2.8 (1.3)* |
| Coded Behavior b | ||||||
| Inattention | ||||||
| Mean (SD) | 2.5 (2.6) | 2.4 (1.8) | 4.3 (2.9) | 3.2 (2.5) | 3.2 (2.4) | 2.3 (2.1) |
| Median [Q1-Q3] | 2 [0 - 4] | 2 [1 - 3] | 5 [2 - 6] | 3 [2 - 6] | 3 [1 - 5] | 2 [0 - 4] |
| Out of Seat | ||||||
| Mean (SD) | 1.6 (2.6)* | 0.6 (1.1)* | 3.0 (3.2)* | 1.7 (1.9)* | 2.3 (2.9) | 1.4 (1.8) |
| Median [Q1-Q3] | 1 [0 - 2] | 0 [0 - 1] | 3 [0 - 5] | 1 [0 - 3] | 1 [0 - 3.5] | 1 [0 - 2] |
| Grab | ||||||
| Mean (SD) | 4.1 (2.7)* | 2.7 (2.3)* | 4.3 (3.0)* | 2.8 (3.0)* | 2.6 (2.5) | 1.9 (2.1) |
| Median [Q1-Q3] | 4 [2 - 6] | 2 [1 - 4] | 4 [1 - 6] | 3 [1 - 4] | 2 [1 - 3.5] | 1.5 [0 - 3] |
| Parent Concernsc, n (%) | 7 (18.9%)* | 2 (5.1%)* | 14 (34.2%)* | 1 (2.6%)* | 12 (32.4%)* | 4 (11.1%)* |
Data are missing for:
n = 6 ADHD-risk and 1 low-risk at 12 months, n = 2 ADHD-risk and 2 low-risk at 18 months, and n = 6 ADHD-risk and 4 low-risk at 24 months;
n = 6 ADHD-risk and 1 low-risk at 12 months, n = 2 ADHD-risk and 2 low-risk at 18 months, and n = 7 ADHD-risk and 4 low-risk at 24 months;
n = 6 ADHD-risk and 1 low-risk at 12 months, n = 2 ADHD-risk and 2 low-risk at 18 months, and n = 6 ADHD-risk and 4 low-risk at 24 months;
p < .05 at specified age for the linear contrast estimating visit-specific differences between the ADHD-risk and low-risk groups from mixed-effect linear regression models for examiner ratings, mixed-effect negative binomial regression models for behavior codes, and mixed-effect logistic regression model for parent concerns.
Table 4.
Parameter estimates (SE) for the mixed-effects regression models predicting ADHD behavior codes.
| Examiner Ratingsa | Behavior Codesa | Parent Concernsa | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Attention | Activity | Impulsivity | Inattention | Out-of-Seat | Grab | ||
| Estimated trajectory for Low-Risk group | |||||||
|
| |||||||
| Baseline (12mos) | 2.07 (0.16)*** | 2.40 (0.18)*** | 1.85 (0.17)*** | 0.84 (0.14)*** | −0.59 (0.27)* | 0.95 (0.14)*** | −4.23 (0.74)** |
| Change from 12-18mos | 0.68 (0.19)*** | 0.54 (0.23)* | 0.84 (0.23)*** | 0.31 (0.18)^ | 0.98 (0.32)** | 0.04 (0.19) | −0.82 (0.89) |
| Change from 12-24mos | 0.36 (0.20)^ | 0.14 (0.23) | 0.99 (0.24)*** | −0.04 (0.20) | 0.74 (0.33)* | −0.36 (0.21)^ | 1.28 (0.71)^ |
| Estimated difference between ADHD-Risk and Low-Risk group | |||||||
| Baseline (12mos) | 0.49 (0.23)* | 0.63 (0.26)* | 0.84 (0.24)*** | 0.05 (0.20) | 0.93 (0.35)** | 0.42 (0.20)* | 1.89 (0.89)* |
| Change from 12-18mos | −0.22 (0.28) | −0.25 (0.32) | −0.28 (0.33) | 0.26 (0.26) | −0.33 (0.42) | 0.02 (0.26) | 2.29 (0.99)* |
| Change from 12-24mos | 0.29 (0.28) | 0.09 (0.33) | −0.26 (0.33) | 0.33 (0.29) | −0.37 (0.44) | −0.08 (0.28) | −0.10 (0.84) |
Note:
p < .05,
p < .01,
p < .001,
p < .10.
SE = standard error
From mixed-effect linear regression (for examiner ratings), negative binomial (for coded behavior), or logistic (for parent concerns) models with fixed effects for group (ADHD-risk, low-risk), time (12 [baseline], 18, and 24 months), and their interaction and person as a random effect.
Inattention
Infants with an older sibling or parent diagnosed with ADHD were distinguishable from infants with no family history of ADHD as early as 12 months of age based on examiner ratings (i.e., BRIC) of inattentive behavior (Table 4, Figure 1A). Specifically, the ADHD-risk group had significantly higher examiner-rated scores than the low-risk group at 12 months. The interactions between time and group did not reach statistical significance; the ADHD-risk group trajectory generally paralleled that of the low-risk group between 12 to 24 months of age. Table 4 presents the results (on the log scale) of the negative binomial mixed-effects models fitted to the behaviors measured via behavioral coding. To ease interpretation, we calculated estimates and 95% confidence intervals (CI) on the original scale (see Figure 2A). With respect to the frequency of inattentive behavior measured via second-by-second behavioral coding, there were no differences between the two groups; the low-risk group demonstrated a marginally significant 36% increase in the frequency of inattention from 12 to 18 months. The interaction terms between time and group were not significant.
Figure 1.
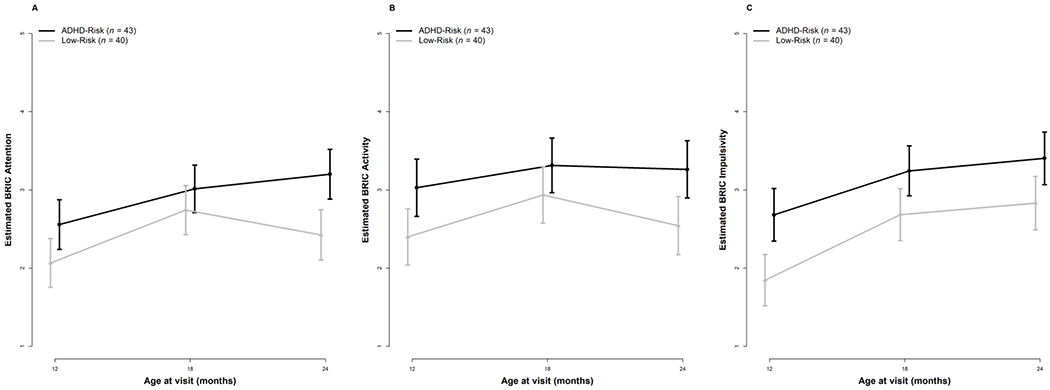
Examiner ratings of ADHD-related behaviors for the low-risk and ADHD-risk groups from 12-24 months of age. Error bars represent 95% Confidence Intervals.
Figure 2.
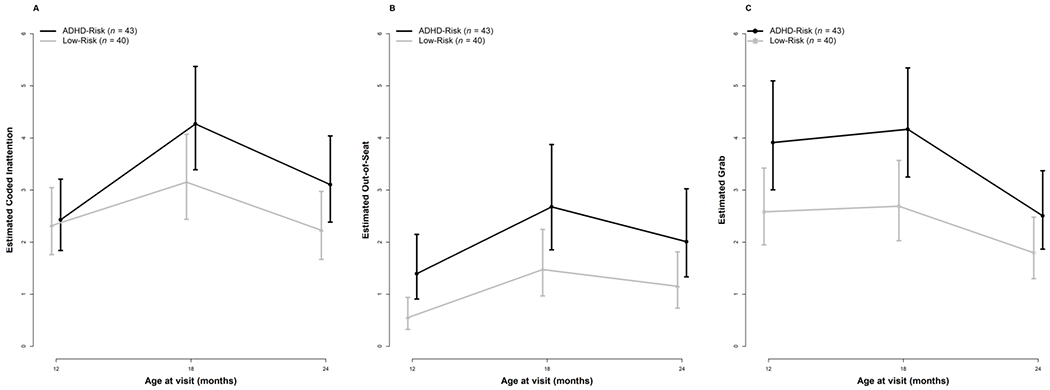
Second-by-second behavioral coding of inattentive and hyperactive-impulsive behaviors for the low-risk and ADHD-risk groups between 12-24 months of age. Error bars represent 95% Confidence Intervals.
Hyperactivity
As shown in Table 4, infants in the ADHD-risk group were rated by examiners significantly higher on the activity level subscale of the BRIC than the low-risk group at 12 months of age. The interaction between time and group did not reach statistical significance; as with examiner ratings of inattention, the ADHD-risk group trajectory generally paralleled that of the low-risk group between 12 to 24 months of age (Table 4, Figure 1B). With respect to the frequency of Out-of-Seat behavior measured via second-by-second behavioral coding, the ADHD-risk group differed from the low-risk group at 12 months of age, exhibiting 153% more Out-of-Seat behavior than the low-risk group (Table 4, Figure 2B). In terms of change over time, the low-risk group demonstrated a significant 167% increase in Out-of-Seat behavior between 12 to 18 months and maintained these levels at 24 months. The interactions between group and time did not reach statistical significance, again suggesting similar patterns of change between the two groups over time.
Impulsivity
Examiner ratings of impulsive behavior on the BRIC revealed significantly higher scores among infants with an older sibling or parent diagnosed with ADHD compared to infants with no family history of ADHD as early as 12 months of age (see Table 4, Figure 1C). As with all other models, the interactions between time and group were not statistically significant. Impulsivity was measured via second-by-second behavioral coding by specifically quantifying the frequency of grabbing behavior. Reflecting a consistent pattern, the ADHD-risk group differed from the low-risk group at 12 months of age, exhibiting 52% more Grabbing behavior than the low-risk group. The interactions between group and time were not statistically significant, reflecting similar patterns of change between the two groups between 12-24 months of age (Table 4, Figure 2C).
General behavior/temperament concerns
As shown in Table 3, 7 (18.9%) parents of children in the ADHD-risk vs. only 2 (5.1%) in the low-risk group expressed behavior/temperament concerns at 12 months, and this difference reached statistical significance (see Table 4 and Figure 3). The interaction between Time 1 and group was also significant (p = .02). While the proportion of parents of low-risk children expressing such concerns remained relatively stable from 12 to 24 months, the number of parents of children at risk for ADHD expressing concerns increased from 12 to 18 months, with more than a third of these parents reporting at least one behavior/temperament concern by 18 months of age. Parents of children at risk for ADHD continued to be more likely to express behavior/temperament concerns at 24 months of age.
Figure 3.
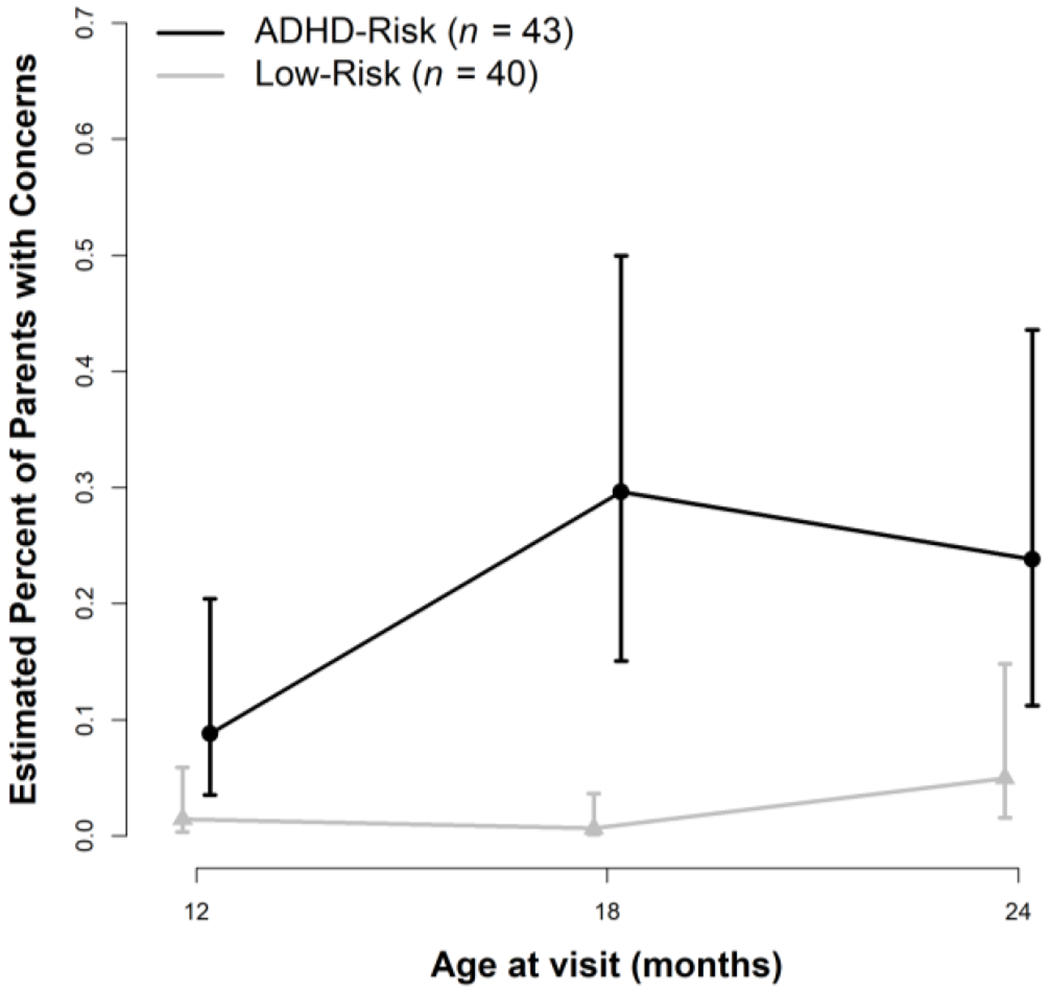
Parent-reported behavior/temperament concerns for the low-risk and ADHD-risk groups from 12-24 months of age. Error bars represent 95% Confidence Intervals.
Because this variable is less specific than the other measures examined (i.e., can include ADHD-relevant concerns as well as broader disruptive and temperament-related concerns), we also recoded parent behavior/temperament concerns to focus only on ADHD-relevant behaviors. In the ADHD-risk group, 75% of parent behavior/temperament concerns were ADHD-related at 12 months of age, 56% at 18 months of age, and 50% at 24 months of age. In the low-risk group, 0% of parent behavior/temperament concerns were ADHD-related at each age (12, 18, and 24 months).
Correlations between behavioral coding and examiner-rated behaviors
To examine the relationship between the ADHD-related behaviors coded from a 5-minute video segment and examiner-rated behaviors (summed across the three dimensions rated), Spearman rank correlation coefficients were computed (Table 5). Starting with the 12-month visit, the frequency of Inattention and Out-of-Seat behaviors were moderately positively associated with examiner-rated behaviors, which persisted through 24 months of age. A trend-level correlation between examiner ratings and the Grab behavior code was evident at 12 months of age, which became significant at 18 months of age and remained so at 24 months of age. By 24 months of age, correlations were moderate-to-large.
Table 5.
Spearman correlation coefficients between examiner BRIC ratings (total) and ADHD-related behavior code frequencies.
| Inattention | Out-of-Seat | Grab | |
|---|---|---|---|
| 12 months | 0.29* | 0.39*** | 0.22^ |
| 18 months | 0.24* | 0.37*** | 0.23* |
| 24 months | 0.39*** | 0.51*** | 0.37** |
p < .05;
p < .01;
p < .001;
p < .10
Discussion
Using a multi-method, multi-informant approach, we found that familial risk for ADHD is evident as early as 12 months of age based on examiner observation, parent report, and behavior coded by individuals unaware of child history or familial risk status. Correlations between metrics obtained via second-by-second behavioral coding during a 5-minute behavior sample and more global examiner ratings of attention, activity level, and impulsivity were significant and of moderate magnitude, suggesting both the potential utility of our novel coding system in infants and toddlers and the possibility of measuring ADHD-relevant behaviors at very young ages.
Prior research in older children has suggested that overactivity may be a core feature of ADHD. Specifically, using an objective measurement of activity level (actigraphy), one study measured hyperactivity in a large sample of children with ADHD, their siblings, and low-risk siblings and found differences in hyperactivity-related metrics based on familial ADHD status (e.g., non-diagnosed siblings of children with ADHD vs. low-risk siblings) (Wood, Asherson, Rijsdijk, & Kuntsi, 2009). In our study, the most consistent differences between the ADHD-risk and low-risk groups were in the hyperactive-impulsive dimension. Examiner ratings of activity level and impulsivity distinguished the groups as early as 12 months of age, as did second-by-second behavioral coding of directly-observed hyperactive-impulsive behavior, consistent with findings from Auerbach et al. (2008) who reported elevated parent-reported activity level at 7, 12, and 25 months among infants at familial risk for ADHD, and in line with studies examining associations between parent-rated hyperactive behavior in infancy and dimensionally-measured ADHD symptoms in normative samples (Frick, Bohlin, et al., 2019). Combined, this pattern of findings suggests that the underlying symptomology of ADHD may already be emerging in infants and toddlers. Moreover, this set of findings demonstrating group differences in hyperactivity based on familial risk status suggests that this behavior may represent an endophenotype for ADHD that is apparent within the first two years of life (Gottesman & Gould, 2003).
A key challenge in identifying markers of ADHD in infancy and early childhood is that it is developmentally typical for young children to display high levels of inattention, hyperactivity, and impulsivity. This makes measurement of, and determination of clinically meaningful differences in, such constructs at young ages difficult. Indeed, some ADHD-related behaviors are elevated during infancy and toddlerhood (e.g., high activity level, diminished inhibitory control, short attention spans) in many young children and will only persist to an impairing degree in a small subset. Consequently, in our study, only some infants in the high-risk group will receive an eventual clinical diagnosis of ADHD. Thus, it will be important to follow such cohorts into middle childhood, when formal diagnoses can be ascertained. In the present study, although we hypothesized that differences between the two risk groups would increase over time, we generally did not find evidence of this (with the exception of parent concerns). However, we suspect that such interaction effects are most likely to be apparent once we have childhood outcomes on this sample and can address related questions based on diagnostic classification rather than familial risk status.
As discussed at the outset, various methods have been employed to examine potential early predictors of ADHD symptoms and diagnostic outcomes. The majority of prior studies of infant/toddler predictors have relied on parent-reported temperament ratings that index non-specific dimensions such as emotion regulation and effortful control, as well as more ADHD-specific constructs like activity level and attention regulation. To the extent that it was possible, we took a different and novel approach in line with the initial studies of infant markers of ASD (see, for example, Ozonoff et al., 2010), seeking to map the core DSM-5 ADHD symptom dimensions onto specific observable behaviors, measurable at earlier ages. This decision was made in an attempt to increase the likelihood that specific risk for ADHD was captured, versus non-specific risk for emotional and behavioral challenges. Whether this approach will allow for greater sensitivity and specificity in predicting full diagnostic outcomes as these children enter middle childhood remains an open question. It may be that combinations of broadband and ADHD-specific variables will best identify infants with the greatest propensity for developing the full ADHD phenotype.
By the time ADHD is typically detected, it is already difficult to treat. The economic burden—resulting from elevated healthcare costs, costs to families, and costs associated with lost work—is massive (Chorozoglou et al., 2015; Doshi et al., 2012; Matza, Paramore, & Prasad, 2005). As noted previously, although preschool diagnoses of ADHD have been shown to be quite stable (Lahey et al., 2005), the median age of diagnosis is 7 years (Visser et al., 2014), with prevalence rates increasing with age (2.1% of 2-5 year-olds, 8.9% of 6-11 year-olds, and 11.9% of 12-17-year-olds) (Danielson et al., 2018). As a result, many families and providers must wait to access services until the child with attention and behavior regulation problems experiences substantial, sustained failures. Indeed, because of the significant impairments that accrue from ADHD over time (Dalsgaard, Østergaard, Leckman, Mortensen, & Pedersen, 2015), and because of its high prevalence, it is critically important to be able to identify ADHD early in life and to leverage what is learned from early detection studies to develop effective early behavioral interventions and psychosocial prevention programs for this population. The hope is that the present study helps lay the groundwork for future investigations in this area. We note that we are not advocating for an approach that would result in earlier medication for ADHD; rather, our hope is that the opportunity to intervene on a behavioral level earlier in life may reduce the future need for medication in some cases.
This study is not without limitations. Because of the young age of enrolled participants, categorical diagnostic outcomes (i.e., ADHD vs. typically developing) are not available. This makes it impossible to distinguish predictors of the development of ADHD from markers of familial risk. That is, the prognostic value of these findings, as yet, is unclear. Ongoing data collection at later ages will allow for the eventual examination of prediction of dimensional symptom levels and formal DSM-5 ADHD diagnoses. Another limitation of this study is that the behavioral observation of ADHD-related behaviors was conducted in a highly structured, cognitively demanding context of standardized developmental testing, which is not an environment in which most parents or clinicians observe infants until concerns have already been raised. Thus, it may be useful for future studies to investigate whether these findings can be replicated in less structured, more naturalistic situations that resemble an infant’s home environment, a daycare setting, or a routine doctor’s appointment. We developed a behavioral coding system to measure ADHD-relevant behaviors in infants and toddlers which adds to the novelty of our study, but it has not been previously utilized in young children. In this study, parents reported on their own concerns, but concerns were compiled into a single indicator of ‘behavior/temperament concerns’ which reflects both ADHD-related concerns as well as broader temperament-related concerns. Although we attempted to recode these data to focus specifically on ADHD-relevant behaviors, the frequency of such behaviors in the low-risk group was low (0) making it impossible to reliably analyze group differences in trajectories of ADHD-relevant parent concerns. However, the fact that such concerns were present in the ADHD-risk group at a rate of ≥50% at each age suggests that this may be worth examining in future, larger studies. In addition, although we confirmed proband diagnoses of ADHD for all infants in the ADHD-risk group, we did not conduct comprehensive evaluations of low-risk probands, instead screening for ADHD symptoms in this group using standardized questionnaires. However, the lack of extensive low-risk proband evaluations is most likely to introduce a conservative bias, if any, in that there could be undetected ADHD in a family member of an infant in the low-risk group. Finally, although our sample is relatively diverse (~25% identifying as racial/ethnic minority), it is small and exclusively comprised of infants at high and low risk based on family history of ADHD; the extent to which findings from such a sample generalize to the larger population is unknown.
In summary, the present study builds on the few prior investigations of infants at elevated familial risk for ADHD. In particular, we address some of the methodological weaknesses in the literature by requiring formal ADHD diagnoses in probands, by focusing on directly-observed core behaviors related to ADHD, and by evaluating infants multiple times over the second year of life allowing for examination of trajectories of behavior. The findings suggest that risk for ADHD may be detectable earlier than previously thought, particularly in the hyperactive-impulsive dimension, with potential implications for screening and monitoring and raising the possibility for psychosocial prevention and earlier behavioral intervention.
Acknowledgments
This study was supported by grants from the National Institute of Mental Health R00 MH106642 (Miller) and R01 MH068398 (Ozonoff), and the National Institute of Child Health and Human Development Intellectual and Developmental Disabilities Research Center U54 HD079125 (Abbeduto).
We gratefully acknowledge the families who have participated in our ongoing longitudinal investigation and the undergraduate behavioral coders who contributed to the project: Anne Donegan, Caitlin Ferguson, Summer Mostafa, Tiffany Nguyen, and Makayla Soller.
Footnotes
One parent proband with a self-reported prior diagnosis of ADHD had a T-score of 59 on the CAARS ADHD Index, but moderately elevated T-scores of 61 and 63 on the Inattention/Memory Problems and Hyperactivity/Restlessness subscales, respectively. We chose to include this family in analyses; results remained the same when this family was excluded.
The authors report no conflicts of interest.
References
- Arnett AB, Macdonald B, & Pennington BF (2013). Cognitive and behavioral indicators of ADHD symptoms prior to school age. Journal of Child Psychology and Psychiatry and Allied Disciplines, 54(12), 1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach JG, Berger A, Atzaba-Poria N, Arbelle S, Cypin N, Friedman A, & Landau R (2008). Temperament at 7, 12, and 25 months in children at familial risk for ADHD. Infant and Child Development, 17(4), 321–338. [Google Scholar]
- Chorozoglou M, Smith E, Koerting J, Thompson MJ, Sayal K, & Sonuga-Barke EJS (2015). Preschool hyperactivity is associated with long-term economic burden: Evidence from a longitudinal health economic analysis of costs incurred across childhood, adolescence and young adulthood. Journal of Child Psychology and Psychiatry and Allied Disciplines, 56(9), 966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D (1994). Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment, 6(4), 284–290. [Google Scholar]
- Conners KC, Erhardt D, & Sparrow E (1998). The Conners adult ADHD rating scale (CAARS). Toronto: Multi-Health Systems Inc. [Google Scholar]
- Dalsgaard S, Østergaard SD, Leckman JF, Mortensen PB, & Pedersen MG (2015). Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. The Lancet, 385(9983), 2190–2196. [DOI] [PubMed] [Google Scholar]
- Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, & Blumberg SJ (2018). Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. Journal of Clinical Child & Adolescent Psychology, 47(2), 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWolfe NA, Byrne JM, & Bawden HN (2000). Preschool inattention and impulsivity-hyperactivity: Development of a clinic-based assessment protocol. Journal of Attention Disorders, 4(2), 80–90. [Google Scholar]
- Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ, Setyawan J, … Neumann PJ (2012). Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 990–1002. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, & Sklar P (2005). Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. [DOI] [PubMed] [Google Scholar]
- Friard O, & Gamba M (2016). BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology & Evolution, 7, 1325–1330. [Google Scholar]
- Frick MA, Bohlin G, Hedqvist M, & Brocki KC (2019). Temperament and cognitive regulation during the first 3 years of life as predictors of inattention and hyperactivity/impulsivity at 6 years. Journal of Attention Disorders, 23(11), 1291–1302. [DOI] [PubMed] [Google Scholar]
- Frick MA, Forslund T, & Brocki KC (2019). Can reactivity and regulation in infancy predict inattentive and hyperactive/impulsive behavior in 3-year-olds? Development and Psychopathology, 31(2), 619–629. [DOI] [PubMed] [Google Scholar]
- Gadow K, & Sprafkin J (2013). Child & Adolescent Symptom Inventory - 5th Edition. Stony Brook: Checkmate Plus. [Google Scholar]
- Gopin C, Healey D, Castelli K, Marks D, & Halperin JM (2010). Usefulness of a clinician rating scale in identifying preschool children with ADHD. Journal of Attention Disorders, 13(5), 479–488. [DOI] [PubMed] [Google Scholar]
- Gottesman II, & Gould TD (2003). The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry, 160, 636–645. [DOI] [PubMed] [Google Scholar]
- Gurevitz M, Geva R, Varon M, & Leitner Y (2014). Early markers in infants and toddlers for development of ADHD. Journal of Attention Disorders, 18(1), 14–22. [DOI] [PubMed] [Google Scholar]
- Jokiranta-Olkoniemi E, Cheslack-Postava K, Sucksdorff D, Suominen A, Gyllenberg D, Chudal R, … Sourander A (2016). Risk of psychiatric and neurodevelopmental disorders among siblings of probands with autism spectrum disorders. JAMA Psychiatry, 73(6), 622–629. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Lee SS, & Willcutt E (2005). Instability of the DSM-IV Subtypes of ADHD from preschool through elementary school. Archives of General Psychiatry, 62(8), 896–902. [DOI] [PubMed] [Google Scholar]
- Lahey, Benjamin B, Lee SS, Sibley MH, Applegate B, Molina BSG, & Pelham WE (2016). Predictors of adolescent outcomes among 4–6-year-old children with attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology, 125(2), 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law EC, Sideridis GD, Prock LA, & Sheridan MA (2014). Attention-deficit/hyperactivity disorder in young children: Predictors of diagnostic stability. Pediatrics, 133(4), 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matza LS, Paramore C, & Prasad M (2005). A review of the economic burden of ADHD. Cost Effectiveness and Resource Allocation, 3, 5. 10.1186/1478-7547-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch C, Searle S, & Neuhaus J (2008). Generalized, Linear, and Mixed Models. Hoboken, NJ: Wiley. [Google Scholar]
- Meeuwsen M, Perra O, van Goozen SHM, & Hay DF (2018). Informants’ ratings of activity level in infancy predict ADHD symptoms and diagnoses in childhood. Development and Psychopathology, 15, 1–15. [DOI] [PubMed] [Google Scholar]
- Miller M, Iosif AM, Young GS, Hill MM, & Ozonoff S (2018). Early detection of ADHD: Insights from infant siblings of children with autism. Journal of Clinical Child and Adolescent Psychology, 47, 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Musser E, Young G, Olson B, Steiner R, & Nigg J (2019). Sibling recurrence risk and cross-aggregation of attention-deficit/hyperactivity disorder and autism spectrum disorder. JAMA Pediatrics, 173(2), 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N, Degnan K, Hane A, Fox N, & Chronis-Tuscano A (2019). Infant temperament reactivity and early maternal caregiving: independent and interactive links to later childhood attention-deficit/hyperactivity disorder symptoms. Journal of Child Psychology and Psychiatry, 60(1), 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N, Hane A, Degnan K, Fox N, & Chronis-Tuscano A (2019). Investigation of a developmental pathway from infant anger reactivity to childhood inhibitory control and ADHD symptoms: interactive effects of early maternal caregiving. Journal of Child Psychology and Psychiatry, 60(7), 762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SK (1979). Interobserver agreement, reliability, and generalizability of data collected in observational studies. Psychological Bulletin, 86(2), 376–390. [Google Scholar]
- Mullen EM (1995). Mullen Scales of Early Learning, AGS Edition: Manual and Item Administrative Books. American Guidance Services, Inc., 1–92. [Google Scholar]
- Ozonoff S, Iosif A-M, Baguio F, Cook IC, Hill MM, Hutman T, … Young GS (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry, 49(3), 256–266e2. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Steinfeld MB, Hill MM, Cook I, Hutman T, … Sigman M (2009). How early do parent concerns predict later autism diagnosis? Journal of Developmental and Behavioral Pediatrics, 30(5), 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediatrics AA of. (2002). Caring for children with ADHD: A resource toolkit for clinicians. Chicago, IL: Centers for Education and Research on Therapeutics. [Google Scholar]
- Rende RD (1993). Longitudinal relations between temperament traits and behavioral syndromes in middle childhood. Journal of the American Academy of Child & Adolescent Psychiatry, 32(2), 287–290. [DOI] [PubMed] [Google Scholar]
- Riddle MA, Yershova K, Lazzaretto D, Paykina N, Yenokyan G, Greenhill L, … Posner K (2013). The Preschool Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS) 6-year follow-up. Journal of the American Academy of Child and Adolescent Psychiatry, 52(3), 264–278.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson A, Smart D, Prior M, & Oberklaid F (1993). Precursors of hyperactivity and aggression. Journal of the American Academy of Child & Adolescent Psychiatry, 32(6), 1207–1216. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, & Halperin JM (2010). Developmental phenotypes and causal pathways in attention deficit/hyperactivity disorder: potential targets for early intervention? Journal of Child Psychology and Psychiatry, and Allied Disciplines. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Koerting J, Smith E, McCann DC, & Thompson M (2011). Early detection and intervention for attention-deficit/hyperactivity disorder. Expert Review of Neurotherapeutics, 11(4), 557–563. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Holton KF, Nousen EK, Barling AN, Sullivan CA, Propper CB, & Nigg JT (2015). Early identification of ADHD risk via infant temperament and emotion regulation: A pilot study. Journal of Child Psychology and Psychiatry, 56(9), 949–957. [DOI] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, … Blumberg SJ (2014). Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. Journal of the American Academy of Child and Adolescent Psychiatry, 53(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby MT, Gottfredson NC, & Stifter CA (2017). Observed temperament from ages 6 to 36 months predicts parent- and teacher-reported attention-deficit/hyperactivity disorder symptoms in first grade. Development and Psychopathology, 29(1), 107–120. [DOI] [PubMed] [Google Scholar]
- Wood AC, Asherson P, Rijsdijk F, & Kuntsi J (2009). Is overactivity a core feature in ADHD? Familial and receiver operating characteristic curve analysis of mechanically assessed activity level. Journal of the American Academy of Child and Adolescent Psychiatry, 48(10), 1023–1030. [DOI] [PubMed] [Google Scholar]


