Abstract
Masters athletes (MA), men and woman older than 35 years who participate in competitive athletics, is a rapidly growing population that is increasingly encountered in clinical cardiovascular practice. Although the high levels of exercise typically performed by MA confer numerous health advantages, no amont of exercise confers complete immunity from cardiovascular disease. The review was written to cover the clinical management of MA with cardiovascular disease. Focus is dedicated to four of the most common clinical scenarios including atrial fibrillation, myocardial fibrosis, coronary artery disease, and dilation of the ascending aorta.
Keywords: masters athlete, competitive athlete, atrial fibrillation, coronary artery disease, myocardial fibrosis, aortic dilation, sports cardiology
Introduction
The term masters athlete (MA) refers to men and woman older than 35 years who participate in competitive athletics. This is a rapidly growing population worldwide that is increasingly encountered in clinical cardiovascular practice.[1] Unlike younger competitive athletes who typically require cardiovascular care for underlying congenital or genetic heart disease, MA are more often afflicted by acquired disease processes of the heart muscle, electrical system, and coronary arteries. The most common clinical cardiovascular issues among MA include atrial tachyarrhythmias (particularly atrial fibrillation), fibrotic disorders of the myocardium, atherosclerotic coronary disease, and dilation of the ascending aorta. MA afflicted with these and other disease processes often present very differently than sedentary or more normally active people. Optimal care of MA simultaneously prioritizes guideline-based disease diagnosis and management with maintenance of exercise capacity and athletic performance goals. This review was designed to address pathogenesis, diagnosis, and management of the most common forms of cardiovascular disease among MA with a strong emphasis on strategies developed in a high-volume sports cardiology referral center.
Atrial Fibrillation (AF)
Atrial fibrillation/flutter (AF), the most common clinically relevant arrhythmia in the general population, may be even more prevalent among aging MA. A compelling and steadily growing body of literature suggests that MA are at increased risk for AF compared to age and gender-matched normally active people.[2–6] Risk appears to be the higher among male than among female athletes and arises most commonly in men that engage in endurance sporting disciplines. The pathogenesis of AF in MA is complex, multi-factorial, and remains incompletely understood.[7] Factors that have been suggested to contribute to pathogenesis include atrial remodeling characterized by dilation and fibrosis, enhanced vagal nerve activity resulting in marked resting bradycardia coupled with the intense surges in sympathetic nervous system activity that occur during exercise, periodic inflammation, exercise-induced surges in left atrial pressure, genetics, alcohol consumption, sleep apnea, and psychosocial stress.[8–10] Given the importance of atrial function during exercise, the majority of MA will experience a decline in exercise capacity while in atrial fibrillation, and MA are often symptomatic under resting conditions.
MA with new onset AF typically present in one of three fashions. Often, MA will have no sense of underlying arrhythmia but seek medical attention due to decrements in performance or exercise capacity. Consequently, it is of paramount importance that clinicians evaluating MA with performance decrement keep AF high on the differential diagnosis. Alternatively, some MA are exquisitely sensitive to bouts of AF and will report the sensation of irregular palpitations either during exercise or under quiet resting conditions. As detailed below, it is important to differentiate MA who develop AF under high heart rate conditions (i.e. exercise) from those that develop AF under low heart rate conditions (i.e. sleep or quiet rest). Finally, MA may seek evaluation based on concerns about data derived from commercial heart rate monitors. The increasing use of chest strap and wrist-based heart rate monitors that store data and enable subsequent downloads for computer or smart phone-based storage and review is leading to increasing numbers of athletes that “detect” arrhythmia using these devices. In such cases, MA may or may not recall symptoms that correlate with data derived from heart rate monitoring. While these devices can and do enable arrhythmia detection, false positive readings generated by inadequate skin-device contact are common, particularly during the first few minutes of exercise. Regardless of presentation type, we routinely utilize medical-grade ambulatory rhythm monitoring devices, most commonly adhesive patch technology, to confirm the presence and to define the burden of AF prior to initiating any therapeutic interventions.
An overview of our approach to the management of AF in MA is presented in Figure 1. Following definitive confirmation of atrial fibrillation, we evaluate for the presence of modifiable risk factors including uncontrolled hypertension both at rest and in response to maximal effort exercise, thyroid disease, obstructive sleep apnea, stimulant/supplement use, and excessive alcohol consumption. Identification and management of these issues often reduces or eliminates recurrent AF thereby obviating the need for addition therapy. In conjunction with risk factor modification, we consider the risks and benefits of anticoagulation to reduce the risk of thromboembolic disease in all MA with atrial fibrillation.[11,12] Many MA have lone AF or a sufficiently low thromboembolic risk profiles to avoid anticoagulation.[13] Among MA with elevated risk as defined by contemporary general population algorithms,[14] we encourage immediate initiation of a direct oral anticoagulant. MA meeting criteria for these agents should be counseled about the risks of bleeding in the setting of contact sports or sports with an inherent risk of trauma such as cycling or martial arts.
Figure 1.
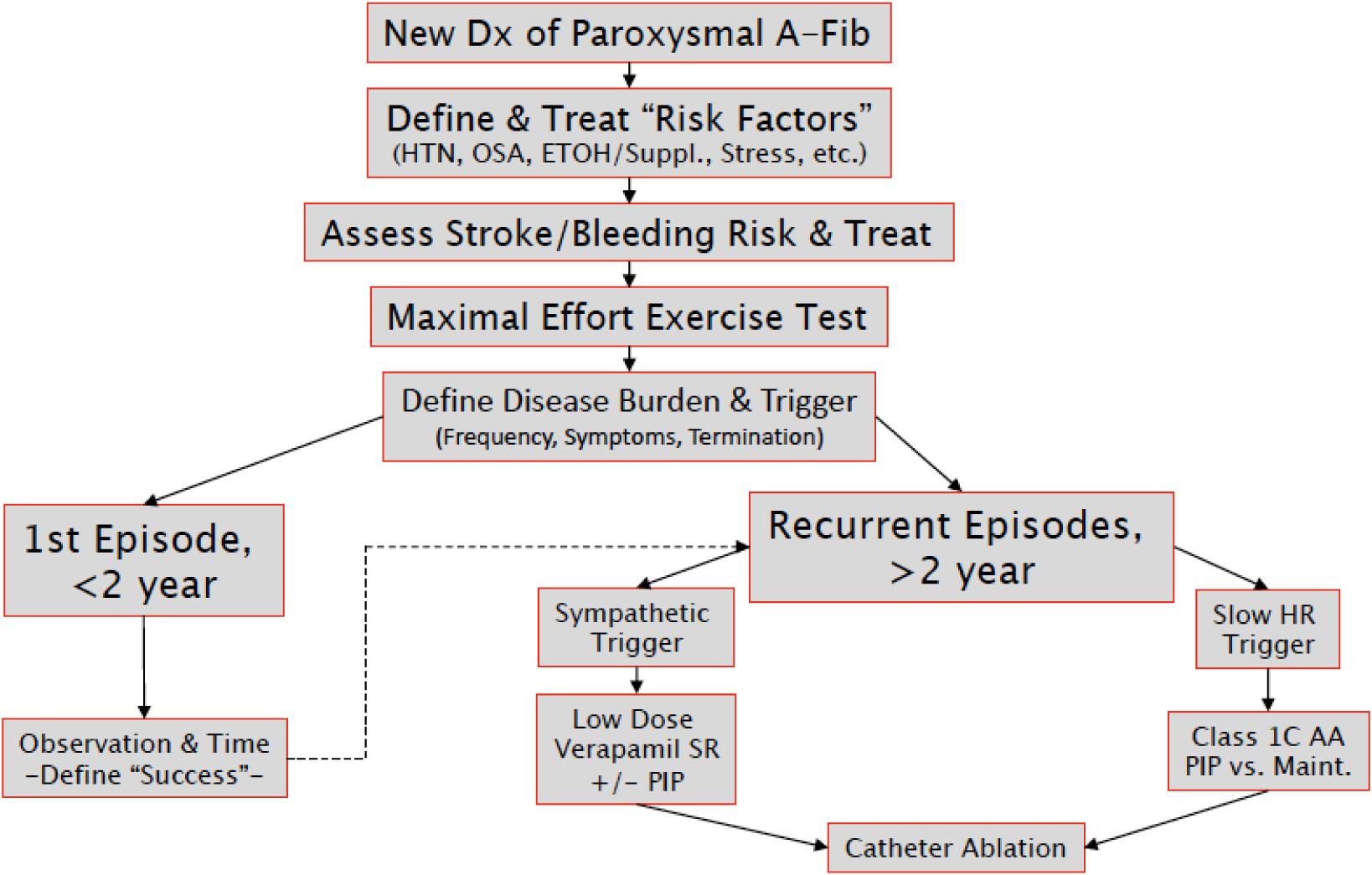
Overview of the clinical approach to masters athletes with paroxysmal atrial fibrillation
In our practice, all MA with newly diagnosed AF undergo a maximal effort limited exercise test with continuous gas exchange and 12-lead electrocardiography when they are in sinus rhythm. Data derived from this assessment serves several functions. First, the presence or absence of ischemic ECG changes, a surrogate for underlying obstructive coronary artery disease (CAD) plays an important role in determining the appropriateness of pharmacologic sinus rhythm maintenance therapy. Second, establishing a baseline level of peak exercise capacity permits meaningful reassessment after any subsequent intervention. We next work to define the burden of (i.e. duration and frequency) and symptoms associated with AF. Among MA with short (less than 24 hours), self-terminating, and very infrequent (<2 times year) bouts of AF, we favor conservative observation. In contrast, we routinely initiate a therapeutic strategy among MA with lengthier or more frequent bouts of AF and among MA that are highly symptomatic or those that require electrical cardioversion to restore sinus rhythm.
While contemporary clinical guidelines endorse a Class 2A recommendation for primary therapy with catheter-based ablation, we routinely start with a pharmacologic strategy. A “pill in the pocket” approach represent an appropriate first step.[15] Among MA that develop AF during exercise this can be accomplished using either an AV-nodal slowing agent (i.e. beta-blocker or non-dihydropyridine calcium channel blocker) or a class 1C antiarrhythmic agent (i.e. flecainide or propafenone). Among MA that develop AF during resting condition, class 1C antiarrhythmic agents represent the best option, and AV-nodal slowing agent should be avoided. The decision to escalate to maintenance medical therapy with a class 1 C agent must be made on an individual patient basis but is appropriate when bouts of AF demonstrate increasing frequency or a high burden of symptoms. While a rate control approach represents an acceptable option for sedentary or normally active patients, we avoid this option in MA as all medications used for this purpose have undesirable effects on exercise capacity. Failure of medical sinus maintenance therapy, as defined by frequent “breakthrough” AF episodes despite standing administration of a class 1 C agent or a patient intolerance of medication, justifies catheter-based ablation. We routinely opt for an initial ablation relying solely on pulmonary vein isolation without additional substrate modification to minimize detrimental impact on atrial mechanics.[16] In our opinion, we would rather accept the need for a second ablation than render an athlete cured of AF but with a stiff “porcelainized” left atrium as this situation is increasingly recognized as having adverse and permanent effects on exercise capacity. Following catheter ablation, we recommend at least 6 weeks of restriction to low intensity physical activity to facilitate complete healing of the intra-cardiac ablation lesions. Anticoagulation should be continued for some period of time following ablation, but data defining the optimal duration of therapy are lacking. In all cases, decisions about the duration of anticoagulation and the resumption of unrestricted sport participation should be made in conjunction with current guidelines and after a thorough shared decision-making process involving the patient, electrophysiologist, and sports cardiologist.
Myocardial Fibrosis (MF)
Repetitive participation in vigorous physical exercise stimulates adaptive changes in CV structure and function. This process, termed exercise-induced cardiac remodeling (EICR), varies considerably across individual MA based on factors including but not limited to sporting discipline, gender, ethnicity, genetic make-up, and duration of exercise exposure.[17] While EICR has historically been regarded as an adaptive phenomenon without adverse prognostic implications, recent data challenge this notion. Numerous studies examining the myocardial response to exercise document evidence of cardiac fatigue following prolonged bouts of exercise.[18–20] In most cases, complete resolution of fatigue patterns has been shown to occur within 1–2 weeks. In addition, elevated levels of biomarkers of cardiac injury have been documented among ostensibly healthy and asymptomatic athletes following prolonged bouts of exercise.[21] In aggregate, these data have generated interest in whether repeated bouts of exercise-induced cardiac fatigue may lead to permanent damage in the form of myocardial scar.
This topic has now been investigated by numerous groups with several studies reporting concerning rates of MF among MA. In a sentinel report, Breuckmann et al. used cardiac MRI to evaluate for MF among marathon runners and found scarring in 12/102 participants.[22] Subsequently, Wilson et al. used similar techniques to document patchy left ventricular MF among 6 of 12 studied MA with the majority of scar localized to the septum and right ventricular insertion sites,[23] and La Gerche et al found scarring of similar anatomic distribution among 13% of a sizeable cohort of accomplished MA.[24] The proposed mechanism underlying these findings, as presented in each of these papers, is that cardiac tissue damage may develop in response to the hemodynamic stresses inherent in decades of high level exercise. Some confirmatory evidence for this hypothesis was presented by Tahir et al who found an exaggerated blood pressure response to exercise to be a significant predictor of MF among 17 of 54 MA triathletes.[25]
Placing the above data in context may best be accomplished by consideration of the following issues. First, each of these studies examined healthy asymptomatic athletes raising uncertainty about the clinical relevance of these findings. Second, similar and in some cases higher rates of myocardial fibrosis have been documented among normally active age-matched populations raising question the causal role of exercise.[26,27] Third, numerous subsequent studies have failed to detect any appreciable fibrosis among MA.[28–30]
At present, we do not recommend screening for MF among asymptomatic MA as there is no established prognostic value to this information. However, we routinely use cardiac MR for the evaluation of myocardial substrate among symptomatic MA or among MA with other findings suggestive of myocardial pathology. In this context, we frequently observe scar as a component of diseases including acute or resolved myocarditis, hypertrophic cardiomyopathy, arrhythmogenic cardiomyopathy, and anabolic steroid-induced heart disease. In contrast, we very seldomly observe non-ischemic scar in the absence of explanatory pathology leading us to conclude that exercise in isolation is incapable of causing permanent cardiac injury. Instead, the development of patchy MF among MA likely represents a multiple hit pathogenesis with exercise possibly exacerbating other toxins or sources or injury. While this phenotype appears to be benign in asymptomatic MA, we do recommend evaluating for scar among MA presenting with otherwise unexplained malignant ventricular arrhythmias as this may inform therapeutic options including the use of antiarrhythmics and catheter-based ablation.[31]
Coronary Artery Disease (CAD)
Routine moderate intensity exercise has favorable effects on traditional cardiovascular risk factors and reduces incident CAD. However, no amount of exercise confers complete immunity from CAD. Recent survey data from a community-based population of competitive MA demonstrated a high prevalence of both CAD risk factors and established CAD.[32] Additionally, autopsy and clinical data from observational sudden death and cardiac arrest studies routinely identify CAD as the most common cause of sudden death in MA.[33,34] The development of clinically relevant CAD, defined as epicardial coronary stenosis sufficient to cause anginal symptoms or the rupture of unstable plaque leading to an acute coronary syndrome, among MA is driven primarily by traditional risk factors including genetics, dyslipidemia, hypertension, and active or prior tobacco use. Additional factors including dietary choices, performance enhancing drug or supplement use, and psychological stress may contribute to the development of CAD among MA.[35,36]
The presentation of CAD among MA is highly variable. In some cases, the index presentation may be cardiac arrest during training or competition. However, most MA with obstructive CAD present with symptoms ranging from typical angina to subtle performance declines without chest pain. Clinicians should keep CAD high the differential diagnosis among MA with any new chief complaint that includes a subjective decline in exercise performance or the presence of chest tightness or pressure during the first few minutes of exercise. Diagnostic assessment for MA with symptoms suggestive of CAD must include a comprehensive medical history with an emphasis on familial premature CAD patterns, the use of illicit drugs or performance enhancing agents, a resting ECG, measurement plasma lipids, and exercise testing according to contemporary guidelines.[37] Although common in clinical practice, exercise testing in MA should not be terminated at a predefined target heart rate but rather continued to maximal volitional exercise capacity in order to maximize the likelihood of symptom reproduction. Conventional exercise protocols such as the Standard Bruce treadmill test may not be sufficient to reproduce cardiac symptoms in MA. Customized testing designed to simulate conditions under which symptoms of suspected CAD develop should be considered on a case by case basis. Test customization often involves manipulation of exercise intensity, duration, modality, and climate and comprehensive exercise assessment in MA may best accomplished in referral centers that house advanced exercise testing laboratory designed for this purpose and staff familiar with the individualization of exercise protocols.[38] Inducible ischemia among MA often resolves very rapidly during exercise recovery thereby limiting the sensitivity of tests that require assessment of ischemia in recovery, such as treadmill-based exercise echocardiography.
Management of MA with CAD, including those with stable disease and those who manifest with acute coronary syndromes, should adhere to consensus recommendations for the general population in the complete absence of outcome data derived from athletic populations.[39,40] Sports cardiologists often elect for complete revascularization strategies in MA based on data documenting asymptomatic exertional ischemia in the setting of obstructive CAD as an important cause of sudden death.[34] All MA with CAD should be prescribed high dose statins and antiplatelet agents as dictated by presentation and coronary anatomy.[41] MA taking dual antiplatelet therapy are at increased risk of bleeding in the setting of trauma and should be counselled to avoid contact and potentially high impact sports while taking these agents. Duration of dual antiplatelet therapy should adhere to contemporary clinical guidelines with any deviation resulting from careful consideration of risks and benefits as discussed in the context of a shared decision-making process. Statin-related muscle side effects that occur during exercise are relatively frequent among athletes. Following an acute coronary syndrome, statin therapy, even if it requires reductions in exercise training and competition, should be prioritized for 1 to 2 years, as this critical early period of drug exposure appears to have the greatest benefit.[42] The role for novel lipid-lowering agents in MA who are intolerant of statins has not yet been described, but we have routinely begun to use PSK9-inhibitors in MA with CAD who fail to meet secondary prevention LDL targets on maximal dose statin. Guidelines for sport participation among MA with CAD have been presented both by United States and European expert writing panels.[43,44]
Coronary artery calcium (CAC) scoring using cardiac CT imaging provides important prognostic information in the sedentary general public and its use is now recommended by numerous clinical guidelines.[45] While there are no primary data to guide how to interpret and what to do with a CAC score among MA, it is being increasingly measured in this population. Several important studies have shown that MA harbor higher levels of CAC than more sedentary age-matched counterparts. The first study to examine CAC among MA compared 108 male marathon runners to age and Framingham Risk Score matched sedentary controls and found that MA were significantly more likely to have an Agatston CAC score > 100 than controls.[46] Subsequent reports confirm that MA are more likely to have CAC than less active people,[47] and that the amount of CAC is associated with the amount of prior exercise exposure.[48] However, studies documenting a high prevalence and burden of CAC among MA must be considered in the context of data which demonstrate increased longevity and reduced hospital utilization for ischemic heart disease among MA. Thus, CAC in MA may have a distinctly different pathogenesis and prognostic implications than among normally active aging men and women.[49] Indeed, preliminary outcomes data demonstrate a clear attenuation of the risk attributable to CAC by increasing cardiorespiratory fitness.[50]
We do not routinely utilize CAC scoring in our assessment of MA. However, we are increasingly asked to evaluate MA who harbor CAC. An outline of our approach to this clinical scenario is presented in Figure 2. We begin by confirming why a CT assessment of CAC was performed. In our experience, this test is commonly ordered during the assessment of MA with symptoms suggestive of CAD in lieu of exercise testing which is a more appropriate diagnostic test in this situation. All MA, including those with and without CAC, should undergo a comprehensive assessment for atherosclerotic risk factors and corollary intervention as dictated by current guidelines. Once CAC has been documented, a maximal effort exercise with protocol customization considerations discussed above is indicated. In the absence of inducible ischemia, we perform no further testing and do not impose any athletic restriction. All MA with inducible ischemia should undergo conventional coronary angiography to confirm the presence of obstructive CAD and to determine the need for revascularization.
Figure 2.
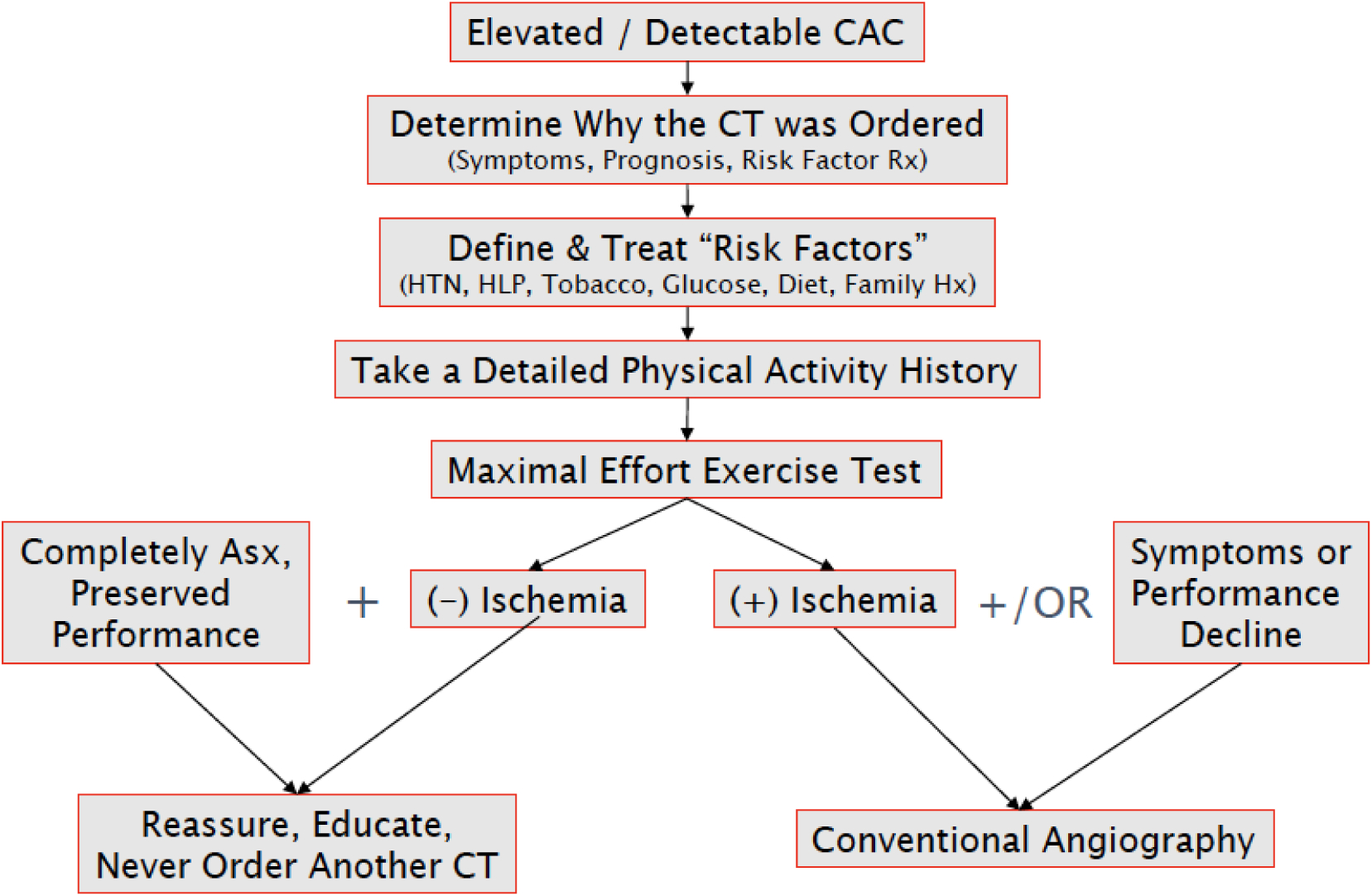
Overview of the clinical approach to masters athletes with coronary artery calcium detected during computed tomography imaging.
Aortic Dilation
Exercise responsiveness of the cardiovascular system was first documented in the late 19th century,[51,52] and numerous longitudinal studies have demonstrated a causal relationship between vigorous exercise and myocardial adaptation.[53] This process of EICR commonly results in 4-chamber cardiac dilation and mild ventricular wall thickening yielding a phenotype that may overlap with forms of cardiomyopathy. Remodeling of the electrical system has also been documented. [54] By contrast, the vascular system, specifically the aorta, has traditionally been viewed as a less plastic organ capable of comparatively less exercise-induced adaptation. To date, data characterizing aortic size in elite athletes have focused almost exclusively on athletes in the first 3 decades of life. Among young competitors, aortic dilation, most commonly often defined by dimensions of the aortic root or ascending aorta of ≥40mm in males and ≥34mm in females, is rare.[55–58] Until recently, data examining aortic dimensions in MA have been lacking.
Recent papers have begun to examine aortic dimensions in distinct populations of MA, exploring the hypothesis that decades of exercise exposure may be required to manifest a clinically relevant increase in aortic size. An important study came examined aortic size in a group of 206 former professional American-style football athletes and found that almost 30% had aortic dimensions >40mm.[59] However, these data are limited to a unique group of men with primarily early-life, strength-focused athletic exposure in a sport commonly associated with weight gain and early onset hypertension,[60] making their generalizability limited. The most comprehensive survey of aortic dimensions among MA competing in endurance disciplines evaluated aortic size among 442 male and female rowers and runners ages 50–75 and found an overall prevalence of aortic dimensions ≥ 40mm of 21%.[61] Among men, this figure increased to a prevalence of 31%, and aortic dilation was particularly enriched among the most elite competitors in the cohort (i.e. rowers who had competed in the World Championships or the Olympics). Comparison with age, sex, and body-size adjusted population nomograms revealed a rightward shift of the distribution of aortic sizes among MA as compared to individual ‘predicted’ sizes, and a total of 24% of subjects had a z-score ≥ 2, indicating a measurement greater than 2 standard deviations above the population mean. Participation in rowing as opposed to rowing was additionally associated with increased aortic size, suggesting a potential role from the combined pressure and volume stress in this sport as a greater driver of aortic adaptation.
The interpretation of these findings and the attendant clinical implications remain uncertain. Mild to moderate aortic dilation in the MA may represent an adaptive form of vascular remodeling in response to long-term endurance exercise and in this context may be viewed as a benign exercise-associated adaptation. Alternatively, it is also possible that this phenotype represents a form of overuse pathology with attendant increases in risk for acute aortic syndromes. At present, the incidence of aortic events among MA remains unknown and longitudinal follow-up will be needed to adjudicate this uncertainty between these two possible interpretations. In the meantime, we advise individualized assessment of MA found to have aortic dilation, including a careful personal and family history to exclude underlying genetic etiologies, blood pressure assessment both at rest and with exercise coupled with guideline-directed blood pressure control, and serial imaging to monitor for progressive increases in aortic size.
Conclusion
MA represent a unique and increasingly common population of patients encountered in clinical cardiovascular practice. Comprehensive care of MA requires an understanding of the physiology of sport coupled with the application of personalized medical strategies that seek to prevent and treat disease while simultaneously optimizing exercise capacity and competitive potential. Future work directed at resolving key areas of scientific and clinical uncertainty present numerous opportunities for critically need research.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Potential Conflict of Interest: Dr. Baggish has received funding from the National Institute of Health/National Heart, Lung, and Blood Institute (R01 HL125869), the National Football Players Association, and the American Heart Association for the study of cardiovascular adaptations to sport and preparticipation screening. He receives compensation for his role as team cardiologist from US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University. Dr. Churchill declares that he has no conflicts of interest.
Informed Consent / Ethics Approval: N/A
References
- 1.Baggish AL, Battle RW, Beckerman JG, Bove AA, Lampert RJ, Levine BD, et al. (2017). Sports Cardiology: Core Curriculum for Providing Cardiovascular Care to Competitive Athletes and Highly Active People. J Am Coll Cardiol, 70(15), 1902–1918, doi: 10.1016/j.jacc.2017.08.055. [DOI] [PubMed] [Google Scholar]
- 2.Abdulla J, & Nielsen JR (2009). Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace, 11(9), 1156–1159. [DOI] [PubMed] [Google Scholar]
- 3.Karjalainen J, Kujala UM, Kaprio J, Sarna S, & Viitasalo M (1998). Lone atrial fibrillation in vigorously exercising middle aged men: case-control study. BMJ, 316(7147), 1784–1785, doi: 10.1136/bmj.316.7147.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mont L, Sambola A, Brugada J, Vacca M, Marrugat J, Elosua R, et al. (2002). Long-lasting sport practice and lone atrial fibrillation. Eur Heart J, 23(6), 477–482. [DOI] [PubMed] [Google Scholar]
- 5.Baldesberger S, Bauersfeld U, Candinas R, Seifert B, Zuber M, Ritter M, et al. (2008). Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J, 29(1), 71–78, doi: 10.1093/eurheartj/ehm555. [DOI] [PubMed] [Google Scholar]
- 6.Andersen K, Farahmand B, Ahlbom A, Held C, Ljunghall S, Michaelsson K, et al. (2013). Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J, 34(47), 3624–3631, doi: 10.1093/eurheartj/eht188. [DOI] [PubMed] [Google Scholar]
- 7.Sorokin AV, Araujo CG, Zweibel S, & Thompson PD (2011). Atrial fibrillation in endurance-trained athletes. Br J Sports Med, 45(3), 185–188. [DOI] [PubMed] [Google Scholar]
- 8.Iskandar A, Mujtaba MT, & Thompson PD (2015). Left Atrium Size in Elite Athletes. JACC Cardiovasc Imaging, 8(7), 753–762, doi: 10.1016/j.jcmg.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Guasch E, Benito B, Qi X, Cifelli C, Naud P, Shi Y, et al. (2013). Atrial fibrillation promotion by endurance exercise: demonstration and mechanistic exploration in an animal model. J Am Coll Cardiol, 62(1), 68–77, doi: 10.1016/j.jacc.2013.01.091. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelm M, Roten L, Tanner H, Wilhelm I, Schmid JP, & Saner H (2011). Atrial remodeling, autonomic tone, and lifetime training hours in nonelite athletes. Am J Cardiol, 108(4), 580–585, doi: 10.1016/j.amjcard.2011.03.086. [DOI] [PubMed] [Google Scholar]
- 11.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr., et al. (2014). 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol, 64(21), e1–76, doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, & Lip GY (2010). A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest, 138(5), 1093–1100, doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 13.Friberg L, Skeppholm M, & Terent A (2015). Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2-VASc score of 1. J Am Coll Cardiol, 65(3), 225–232, doi: 10.1016/j.jacc.2014.10.052. [DOI] [PubMed] [Google Scholar]
- 14.Lip GY, Nieuwlaat R, Pisters R, Lane DA, & Crijns HJ (2010). Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest, 137(2), 263–272, doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 15.Alboni P, Botto GL, Baldi N, Luzi M, Russo V, Gianfranchi L, et al. (2004). Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med, 351(23), 2384–2391, doi: 10.1056/NEJMoa041233. [DOI] [PubMed] [Google Scholar]
- 16.Gibson DN, Di Biase L, Mohanty P, Patel JD, Bai R, Sanchez J, et al. (2011). Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm, 8(9), 1364–1371, doi: 10.1016/j.hrthm.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Weiner RB, & Baggish AL (2015). Cardiovascular Adaptation and Remodeling to Rigorous Athletic Training. Clin Sports Med, 34(3), 405–418, doi: 10.1016/j.csm.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Neilan TG, Januzzi JL, Lee-Lewandrowski E, Ton-Nu TT, Yoerger DM, Jassal DS, et al. (2006). Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston marathon. Circulation, 114(22), 2325–2333. [DOI] [PubMed] [Google Scholar]
- 19.Douglas PS, O’Toole ML, Hiller WD, Hackney K, & Reichek N (1987). Cardiac fatigue after prolonged exercise. Circulation, 76(6), 1206–1213. [DOI] [PubMed] [Google Scholar]
- 20.Oxborough D, Birch K, Shave R, & George K (2010). “Exercise-induced cardiac fatigue”--a review of the echocardiographic literature. Echocardiography, 27(9), 1130–1140, doi: 10.1111/j.1540-8175.2010.01251.x ECHO1251 [pii]. [DOI] [PubMed] [Google Scholar]
- 21.Shave R, Baggish A, George K, Wood M, Scharhag J, Whyte G, et al. (2010). Exercise-induced cardiac troponin elevation: evidence, mechanisms, and implications. [Review]. J Am Coll Cardiol, 56(3), 169–176, doi: 10.1016/j.jacc.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Breuckmann F, Mohlenkamp S, Nassenstein K, Lehmann N, Ladd S, Schmermund A, et al. (2009). Myocardial late gadolinium enhancement: prevalence, pattern, and prognostic relevance in marathon runners. Radiology, 251(1), 50–57, doi:251/1/50 [pii] 10.1148/radiol.2511081118. [DOI] [PubMed] [Google Scholar]
- 23.Wilson M, O’Hanlon R, Prasad S, Deighan A, Macmillan P, Oxborough D, et al. (2011). Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J Appl Physiol, 110(6), 1622–1626, doi:japplphysiol.01280.2010 [pii] 10.1152/japplphysiol.01280.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, et al. (2012). Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J, 33, 998–1006, doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 25.Tahir E (2017). Myocardial fibrosis in competitive triathletes detected by contrast enhanced CMR correlate with exercise-induced hypertension and competition history. JACC Cardiovasc Imaging, --(--), --. [DOI] [PubMed] [Google Scholar]
- 26.Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, et al. (2012). Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA, 308(9), 890–896, doi: 10.1001/2012.jama.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbier CE, Bjerner T, Johansson L, Lind L, & Ahlstrom H (2006). Myocardial scars more frequent than expected: magnetic resonance imaging detects potential risk group. J Am Coll Cardiol, 48(4), 765–771, doi: 10.1016/j.jacc.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 28.Trivax JE, Franklin BA, Goldstein JA, Chinnaiyan KM, Gallagher MJ, deJong AT, et al. (2010). Acute cardiac effects of marathon running. [Research Support, Non-U.S. Gov’t]. J Appl Physiol (1985), 108(5), 1148–1153, doi: 10.1152/japplphysiol.01151.2009. [DOI] [PubMed] [Google Scholar]
- 29.Scharhag J, Urhausen A, Schneider G, Herrmann M, Schumacher K, Haschke M, et al. (2006). Reproducibility and clinical significance of exercise-induced increases in cardiac troponins and N-terminal pro brain natriuretic peptide in endurance athletes. Eur J Cardiovasc Prev Rehabil, 13(3), 388–397, doi: 10.1097/01.hjr.0000219117.33038.90. [DOI] [PubMed] [Google Scholar]
- 30.Abdullah SM, Barkley KW, Bhella PS, Hastings JL, Matulevicius S, Fujimoto N, et al. (2016). Lifelong Physical Activity Regardless of Dose Is Not Associated With Myocardial Fibrosis. Circ Cardiovasc Imaging, 9(11), doi: 10.1161/CIRCIMAGING.116.005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahida S, Sacher F, Dubois R, Sermesant M, Bogun F, Haissaguerre M, et al. (2017). Cardiac Imaging in Patients With Ventricular Tachycardia. Circulation, 136(25), 24912507, doi: 10.1161/CIRCULATIONAHA.117.029349. [DOI] [PubMed] [Google Scholar]
- 32.Shapero K, Deluca J, Contursi M, Wasfy M, Weiner RB, Lewis GD, et al. (2015). Cardiovascular Risk and Disease Among Masters Endurance Athletes: Insights from the Boston MASTER (Masters Athletes Survey To Evaluate Risk) Initiative. Sports Med Open, 2, 29, doi: 10.1186/s40798-016-0053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckart RE, Shry EA, Burke AP, McNear JA, Appel DA, Castillo-Rojas LM, et al. (2011). Sudden death in young adults: an autopsy-based series of a population undergoing active surveillance. J Am Coll Cardiol, 58(12), 1254–1261, doi: 10.1016/j.jacc.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Malhotra R, Chiampas G, d’Hemecourt P, Troyanos C, Cianca J, et al. (2012). Cardiac arrest during long-distance running races. N Engl J Med, 366(2), 130–140, doi: 10.1056/NEJMoa1106468. [DOI] [PubMed] [Google Scholar]
- 35.Baggish AL, Weiner RB, Kanayama G, Hudson JI, Lu MT, Hoffmann U, et al. (2017). Cardiovascular Toxicity of Illicit Anabolic-Androgenic Steroid Use. Circulation, 135(21), 1991–2002, doi: 10.1161/CIRCULATIONAHA.116.026945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, et al. (2017). Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet, 389(10071), 834–845, doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson PD, Myerburg RJ, Levine BD, Udelson JE, & Kovacs RJ (2015). Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 8: Coronary Artery Disease: A Scientific Statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol, 66(21), 2406–2411, doi: 10.1016/j.jacc.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 38.Churchill TW, Disanto M, Singh TK, Groezinger E, Loomer G, Contursi M, et al. (2019). Diagnostic Yield of Customized Exercise Provocation Following Routine Testing. Am J Cardiol, 123(12), 2044–2050, doi: 10.1016/j.amjcard.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 39.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. (2012). 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol, 60(24), e44–e164, doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr., Ganiats TG, Holmes DR Jr., et al. (2014). 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 64(24), e139–e228, doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 41.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. (2016). 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation, 134(10), e123–155, doi: 10.1161/CIR.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 42.Zhao XQ, Dong L, Hatsukami T, Phan BA, Chu B, Moore A, et al. (2011). MR imaging of carotid plaque composition during lipid-lowering therapy a prospective assessment of effect and time course. JACC Cardiovasc Imaging, 4(9), 977–986, doi: 10.1016/j.jcmg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson PD, Myerburg RJ, Levine BD, Udelson JE, Kovacs RJ, American Heart Association E, et al. (2015). Eligibility and Disqualification Recommendations for Competitive Athletes with Cardiovascular Abnormalities: Task Force 8: Coronary Artery Disease: A Scientific Statement from the American Heart Association and American College of Cardiology. Circulation, 132(22), e310–314, doi: 10.1161/CIR.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 44.Borjesson M, Dellborg M, Niebauer J, LaGerche A, Schmied C, Solberg EE, et al. (2019). Brief recommendations for participation in leisure time or competitive sports in athletes-patients with coronary artery disease: Summary of a Position Statement from the Sports Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol, 2047487319876186, doi: 10.1177/2047487319876186. [DOI] [PubMed] [Google Scholar]
- 45.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. (2019). 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol, 74(10), e177–e232, doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohlenkamp S, Lehmann N, Breuckmann F, Brocker-Preuss M, Nassenstein K, Halle M, et al. (2008). Running: the risk of coronary events : Prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J, 29(15), 1903–1910, doi: 10.1093/eurheartj/ehn163. [DOI] [PubMed] [Google Scholar]
- 47.Merghani A, Maestrini V, Rosmini S, Cox AT, Dhutia H, Bastiaenan R, et al. (2017). Prevalence of Subclinical Coronary Artery Disease in Masters Endurance Athletes With a Low Atherosclerotic Risk Profile. Circulation, 136(2), 126–137, doi: 10.1161/CIRCULATIONAHA.116.026964. [DOI] [PubMed] [Google Scholar]
- 48.Aengevaeren VL, Mosterd A, Braber TL, Prakken NHJ, Doevendans PA, Grobbee DE, et al. (2017). Relationship Between Lifelong Exercise Volume and Coronary Atherosclerosis in Athletes. Circulation, 136(2), 138–148, doi: 10.1161/CIRCULATIONAHA.117.027834. [DOI] [PubMed] [Google Scholar]
- 49.Baggish AL, & Levine BD (2017). Coronary Artery Calcification Among Endurance Athletes: “Hearts of Stone”. Circulation, 136(2), 149–151, doi: 10.1161/CIRCULATIONAHA.117.028750. [DOI] [PubMed] [Google Scholar]
- 50.Radford NB, DeFina LF, Leonard D, Barlow CE, Willis BL, Gibbons LW, et al. (2018). Cardiorespiratory Fitness, Coronary Artery Calcium, and Cardiovascular Disease Events in a Cohort of Generally Healthy Middle-Age Men: Results From the Cooper Center Longitudinal Study. Circulation, 137(18), 1888–1895, doi: 10.1161/CIRCULATIONAHA.117.032708. [DOI] [PubMed] [Google Scholar]
- 51.DARLING EA (1899). The Effects of Training. The Boston Medical and Surgical Journal, 141(10), 229–233, doi:doi: 10.1056/NEJM189909071411001. [DOI] [Google Scholar]
- 52.Henschen S (1899). Skidlauf und skidwettlauf: eine medizinische sportstudie. Mitt Med Klin Upsala, 2. [Google Scholar]
- 53.Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, et al. (2008). Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol (1985), 104(4), 1121–1128, doi: 10.1152/japplphysiol.01170.2007. [DOI] [PubMed] [Google Scholar]
- 54.Kim JH, Noseworthy PA, McCarty D, Yared K, Weiner R, Wang F, et al. (2011). Significance of electrocardiographic right bundle branch block in trained athletes. Am J Cardiol, 107(7), 1083–1089, doi: 10.1016/j.amjcard.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 55.Pelliccia A, Di Paolo FM, De Blasiis E, Quattrini FM, Pisicchio C, Guerra E, et al. (2010). Prevalence and clinical significance of aortic root dilation in highly trained competitive athletes. Circulation, 122(7), 698–706, 693 p following 706, doi: 10.1161/CIRCULATIONAHA.109.901074. [DOI] [PubMed] [Google Scholar]
- 56.D’Andrea A, Cocchia R, Riegler L, Scarafile R, Salerno G, Gravino R, et al. (2010). Aortic root dimensions in elite athletes. Am J Cardiol, 105(11), 1629–1634, doi: 10.1016/j.amjcard.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 57.Boraita A, Heras ME, Morales F, Marina-Breysse M, Canda A, Rabadan M, et al. (2016). Reference Values of Aortic Root in Male and Female White Elite Athletes According to Sport. Circ Cardiovasc Imaging, 9(10), doi: 10.1161/CIRCIMAGING.116.005292. [DOI] [PubMed] [Google Scholar]
- 58.Gati S, Malhotra A, Sedgwick C, Papamichael N, Dhutia H, Sharma R, et al. (2019). Prevalence and progression of aortic root dilatation in highly trained young athletes. Heart, doi: 10.1136/heartjnl-2018-314288. [DOI] [PubMed] [Google Scholar]
- 59.Gentry JLC,D; Joshi PH; Maroules CD; Ayers CR; De Lemos JA; Aagaard P; Hachamovitch R; Desai MY; Roselli EE; Dunn RE; Alexander K; Lincoln AE; Tucker AM; Phelan DM (2017). Ascending Aortic Dimensions in Former National Football League Athletes. Circ Cardiovasc Imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin J, Wang F, Weiner RB, DeLuca JR, Wasfy MM, Berkstresser B, et al. (2016). Blood Pressure and LV Remodeling Among American-Style Football Players. JACC Cardiovasc Imaging, 9(12), 1367–1376, doi: 10.1016/j.jcmg.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Churchill T, Groezinger E, Kim JH, Loomer G, Guseh J, Wasfy M, et al. (2020). Association of Ascending Aortic Dilatation and Long-term Endurance Exercise. JAMA Cardiol, 5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]


