Abstract
Background:
Intraoperative burst-suppression is associated with postoperative delirium. Whether this association is causal remains unclear. Therefore, we investigated whether burst-suppression during cardiopulmonary bypass (CPB) mediates the effects of known delirium risk factors on postoperative delirium.
Methods:
This was a retrospective cohort observational sub-study of the Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep trial. We analyzed data from patients over 60 years old undergoing cardiac surgery (n = 159). Univariate and multivariable regression analyses were performed to assess for associations and enable causal inference. Delirium risk factors were evaluated using the abbreviated Montreal Cognitive Assessment, and Patient-Reported Outcomes Measurement Information System questionnaires for applied cognition, physical function, global health, sleep, and pain. We also analyzed electroencephalogram data (n = 141).
Results:
The incidence of delirium in patients with CPB burst-suppression was 25% (15/60) compared to 6% (5/81) in patients without CPB burst-suppression. In univariate analyses, age (OR:1.08 [95% CI: 1.03, 1.14], P=0.002), lowest CPB temperature (OR:0.79 [0.66, 0.94], P=0.010), alpha power (OR:0.65 [0.54, 0.80], P<0.001), and physical function (OR:0.95 [0.91, 0.98], P=0.007) were associated with CPB burst-suppression. In separate univariate analyses, age (OR:1.09 [1.02, 1.16], P=0.009), abbreviated Montreal Cognitive Assessment (OR:0.80 [0.66, 0.97], P=0.024), alpha power (OR:0.75 [0.59, 0.96], P=0.025), and CPB burst-suppression (OR:3.79 [1.5, 9.6], P=0.005) were associated with delirium. However, only physical function (OR:0.96 [0.91, 0.99], P=0.044), lowest CPB temperature (OR:0.73 [0.58, 0.88], P=0.003), and electroencephalogram alpha power (OR:0.61 [0.47, 0.76], P<0.001) were retained as predictors in the burst-suppression multivariable model. Burst-suppression (OR:4.1 [1.5, 13.7], P=0.012) and age (OR:1.07 [0.99, 1.15], P=0.090) were retained as predictors in the delirium multivariable model. Delirium was associated with decreased electroencephalogram power from 6.8 to 24.4 Hertz.
Conclusions:
The inference from our study is that CPB burst-suppression mediates the effects of physical function, lowest CPB temperature, and electroencephalogram alpha power on delirium.
Keywords: electroencephalography, cardiac surgery, burst-suppression, delirium, physical function, cognitive status
Introduction
Delirium is an acute brain dysfunction characterized by disturbances in attention, awareness, and cognition.1 Normal aging2, poor physical function3,4, pre-existing cognitive impairment5,6, sedative drugs7, sleep disturbance8, and inflammation9 are risk factors that predispose patients to delirium. Although previously reported associations between delirium and increased mortality may not be causal,10 delirium remains a leading cause of preventable morbidity in hospitalized elderly patients.10,11 Thus, strategies to reduce the incidence of delirium and identify patients at risk of delirium are needed.
Burst-suppression during general anesthesia is associated with postoperative delirium.12–14 Burst-suppression consists of alternations between isoelectricity and brief bursts of electrical activity.15 Burst-suppression can be induced by anesthetic drugs that significantly modulate γ-aminobutyric acid A (GABAA) receptors. Although sometimes induced intentionally for therapeutic purposes to treat refractory status epilepticus or increased intracranial pressure,16,17 burst-suppression is generally considered potentially harmful and to be avoided. Whether burst-suppression is a modifiable risk factor for delirium versus merely an epiphenomenon or downstream “readout” for other factors that cause delirium is an open question.
If burst-suppression contributes causally to delirium, this argues for anesthetic protocols to reduce the incidence of intraoperative burst-suppression. Conversely, a non-causal association would argue for anesthetic protocols to identify patients with intra-operative burst-suppression for pre-emptive geriatric consultation. In a recent investigation, we found that patients with intraoperative burst-suppression during cardiopulmonary bypass (CPB) exhibited decreased alpha and beta oscillation power compared to age-matched control patients.18 This finding suggests that patients exhibiting burst-suppression at age-adjusted anesthetic concentrations are neurobiologically distinct. Consistent with this finding, an electroencephalogram guided anesthetic protocol reduced the incidence of intraoperative burst-suppression but not postoperative delirium.19 Thus, the association between drug induced intraoperative electroencephalogram burst-suppression and delirium in non-critically ill patients may not be entirely causal.
In this study we investigated associations between delirium risk factors, electroencephalogram burst-suppression during CPB and postoperative delirium. We analyzed the electroencephalogram for burst-suppression during CPB, a period with stable and controlled anesthetic and physiologic management. Decreased alpha power has been associated with burst-suppression during CPB18 and cognitive impairment.20,21 Cognitive impairment has also been associated with postoperative delirium.5,6 We hypothesized that pre-existing cognitive impairment accounts for electroencephalogram burst-suppression during CPB. We also hypothesized that electroencephalogram burst-suppression during CPB mediates the effect of cognitive impairment on delirium.
Methods
Patient Selection and Data Collection
Ethics statement
The Partners Human Research Committee approved this research study (Institutional Review Board 20168000742). This is a sub-study of the ongoing Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep (MINDDS) trial.22 The MINDDS trial is a 370-patient block-randomized, placebo-controlled, double-blinded, single-site, parallel-arm superiority trial of a sleep-inducing dose of dexmedetomidine for delirium prevention in elderly patients undergoing major cardiac surgery. For this sub-study, data from eligible MINDDS trial patients that underwent pre-operative assessments and had intraoperative electroencephalogram recordings were analyzed. All participants provided written informed consent.
Study Population
Study details for the MINDDS trial including inclusion and exclusion criteria have previously been published.22 Study eligibility criteria were age ≥ 60, scheduled for a cardiac surgical procedure with CPB, planned postoperative admission to the intensive care unit for ≥ 24 hours, and scheduled same day surgical admission. Study exclusion criteria were blindness, deafness, inability to speak English, greater than 2 days of ICU admission in the month preceding the current surgical procedure, renal and liver failure requiring dialysis or Child-Pugh score > 5, anticipated follow-up difficulties, previous cardiac surgery within 1 year of surgical procedure, allergy to dexmedetomidine, chronic therapy with benzodiazepines or antipsychotics, severe neurological deficit, and surgical procedure requiring total circulatory arrest. Patients scheduled for a second surgical procedure during their hospital stay or postoperative intubation >12 hours, were dropped from the study. Data from 159 patients were analyzed in this pre-specified sub-study: 117 patients were followed up in the MINDDS trial, 7 patients withdrew consent for MINDDS trial long term follow-up post-surgery, 18 patients met objective drop criteria for MINDDS trial long term follow-up, and 17 patients did not consent to be randomized into the MINDDS trial.
Data Collection
Patients underwent a baseline pre-randomization assessment for study inclusion and exclusion criteria. Subjects were recruited and data collected between March 2017 and February 2019. We evaluated baseline cognitive function using the abbreviated Montreal Cognitive Assessment (aMOCA), physical function with the Patient-Reported Outcomes Measurement Information System (PROMIS) SF v2.0-Physical function 8b, general health with the PROMIS SF v1.2-Global Health, pain with the PROMIS SF v1.0-Pain Interference 8a, applied cognition with the PROMIS v2.0-Applied Cognition Abilities 8a, sleep quality with the PROMIS v1.0-Sleep Disturbance 4A. We also screened for delirium during the pre-randomization assessment using the 3-minute Confusion Assessment Method.
PROMIS measures were normalized to a standardized T-distribution (https://www.assessmentcenter.net/ac_scoringservice). The T-score mean for PROMIS questionnaires is 50 for the population, with standard deviation of 10. Higher scores indicate more of the concept being measured. This could be a desirable or undesirable outcome, depending on the concept being measured (i.e., higher scores for physical function is desirable while higher scores for pain or sleep disturbance is undesirable). None of the study patients screened positive for delirium during the baseline assessments. Comorbid conditions were extracted from the history and physical notes that were documented during the pre-surgical planning visit.
We recorded electroencephalogram data using Sedline monitor (Masimo Inc, Irvine, CA). Sedtrace electrode arrays were placed on the forehead at approximately Fp1, Fp2, F7, and F8, the ground electrode at approximately Fpz, and the reference electrode approximately 1 centimeter above Fpz. Data were recorded with a pre-amplifier bandwidth of 0.5 to 92 Hertz, a sampling rate of 250 Hertz, with 16-bit, 29 nano Volts resolution. Electrode impedance was maintained at less than 5kΩ in each channel. General anesthesia was induced with an intravenous induction agent, followed by maintenance with isoflurane. We selected electroencephalogram data segments using information from the electronic medical record and spectral analysis of the electroencephalogram. For each patient, we carefully selected 2-minute electroencephalogram segments that represented the maintenance phase of general anesthesia during surgery. The data were selected from a period at least 15 minutes after the initial induction bolus of the intravenous hypnotic, while the expired concentration of isoflurane was stable and before the onset of CPB. We visually inspected the selected segments in both the time and spectral domains to ensure data quality. These data have not been reported in any previous publication.
Burst-suppression Analysis
We manually identified patients that exhibited burst-suppression during CPB by analyzing electroencephalogram data in the spectral and time-series domain. Two independent anesthesiologists (J.P., O.A.) identified periods of burst-suppression defined as the presence of at least three consecutive suppression events within 60 seconds periods during CPB. Only cases that both evaluators agreed upon were formally coded as burst suppression events. We used complete cases analysis (n = 141).
Postoperative Delirium Analysis
Patients were screened for postoperative delirium twice daily (before midday and past midday with at least 6 hours between tests) beginning on postoperative day 1 using the long version of the Confusion Assessment Method, until postoperative day 3. Delirium was also assessed with a structured chart review beginning on postoperative day 1 until postoperative day 3 by performing a text search for the diagnosis of “delirium” or “delirious” in the medical record.
Spectral Analysis
We computed multitaper spectral estimates using the Chronux Matlab toolbox with the following parameters: window length T = 2 seconds without overlap, time-bandwidth product TW = 3, number of tapers K = 5. We equally weighted the signals from Fp1, Fp2, F7, and F8 channels.
Bias
Selection bias was managed by analyzing data from all patients that were sequentially enrolled in the MINDDS study until the sample size for this sub-study was reached. Misclassification was reduced by clearly defining exposures (burst-suppression during CPB) and outcomes (delirium). Data collection with the aid of standardized clinical tools (aMOCA, PROMIS Health Measures, and Confusion Assessment Method) helped to minimize recall bias. However, the nature of our does not preclude bias introduced by unknown or unmeasured confounders.
Statistical Methods
Data and statistical analyses plans were defined and written after the data were accessed. Continuous variables are presented as median [Q1 (25th percentile), Q3 (75th percentile)] and categorical variables as frequency (percentage). We used the Mann-Whitney U test for associations between continuous and categorical variables, and the Fisher’s Exact test for associations among categorical variables. All p-values were computed based on the two-sided tests at significance level of 0.05. In some cases, multiple testing was corrected using false discovery rate (FDR). Significance was declared if FDR < 0.05.
Power analysis:
A primary objective of this study was to detect a difference in mean preoperative cognitive scores between burst-suppression and no-burst-suppression patient groups. We assumed a sampling ratio (burst-suppression/no burst-suppression) during CPB in major cardiac surgery of 50%18, a reduction in abbreviated MOCA score of 1.5 in the burst-suppression group, and an abbreviated MOCA standard deviation of 2.5. Based on type I error of 0.05, and power of 0.90, a total of 132 patients was expected to enable detection of this difference using a two-sample t test. We assumed approximately 20% data was loss due to electroencephalogram poor quality and incomplete recordings and thus assumed our n of 159 to be adequate.
Electroencephalogram analysis:
We manually matched patients by actual age (± 2 years) and aMOCA (± 3 points) score using a one to one matching criteria. An empirical bootstrap approach was used to enable statistical inferences. First, we bootstrapped the estimates of each non-overlapping window. Next, we computed a median of the bootstrapped estimates at the subject level and then computed the group median of this estimate. We computed the median difference between groups and then iterated the above procedure 5,000 times to obtain a distribution of the median difference between groups. We computed the 99% confidence interval of this distribution. We defined our threshold for statistical significance as when the upper and lower confidence intervals of the median difference distribution did not border zero over a contiguous frequency range greater than 2 bandwidths (2W).
Univariate linear regression analysis:
In separate linear regression analyses, we estimated the association between aMOCA (using continuity correction) and the following delirium risk factors: age, American Society of Anesthesiologists (ASA) Physical Status (using continuity correction), education (> High-school (HS) education categorized), applied cognition (PROMIS Cognition), physical function (PROMIS Physical Function), global health (PROMIS Global Health Physical and Mental), pain (PROMIS Pain), sleep (PROMIS Sleep), alpha power, and burst-suppression during CPB. Regression models were constructed in R (RStudio Inc, Boston, MA, Version 1.1.453).
Univariate logistic regression analysis:
In separate logistic regression analyses, we estimated the association between burst-suppression during CPB and the following delirium risk factors: age, ASA Physical Status, abbreviated MOCA, applied cognition (PROMIS Cognition), physical function (PROMIS Physical Function), global health (PROMIS Global Health Physical and Mental), pain (PROMIS Pain), sleep (PROMIS Sleep), alpha power, length of CPB, and lowest temperature during CPB. We also estimated the association between delirium and the same predictors as above including an analysis for the predictor burst-suppression during CPB. Regression models were constructed in R (RStudio Inc, Boston, MA, Version 1.1.453).
Causal/Mediational inference analysis:
The analysis of potential underlying causal mechanisms suggested fitting separate multivariable logistic regression models for the dependent variables: burst-suppression during CPB and delirium. In the model for burst-suppression, the predictors were: age, ASA Physical Status, aMOCA, PROMIS Physical, PROMIS Global Mental Health, PROMIS Pain, PROMIS Cognition, PROMIS Sleep, alpha power, CPB length, and lowest temperature during CPB. In the model for delirium, the same predictors were analyzed with the addition of burst-suppression. In both models, a backward elimination algorithm was applied to the predictors. Only predictor terms that remained after backward elimination using a P < 0.1 significance threshold were included in the final model. These analyses were performed with SAS statistical software (SAS Institute Inc, Cary, NC, Version 9.4). The hypothetical underlying causal model that guided our data analysis strategy is illustrated in Figure 1. This figure makes clear that we are testing the hypothesis that burst-suppression partly mediates the hypothetical causal effects of the exogenous variables (age, ASA, aMOCA, PROMIS, alpha power, CPB measures) on delirium. However, we are also positing the possibility of additional direct effects of the exogenous variables on delirium additive to their indirect effects via burst-suppression, as indicated by the direct arrows from exogenous variables to delirium. The age variable was the only exogenous variable that had largely the role of a potential confounding covariate rather than being of direct substantive interest in this study as the other predictors were. No “modifier” effects were tested, i.e., no interactions among predictors.
Figure 1.
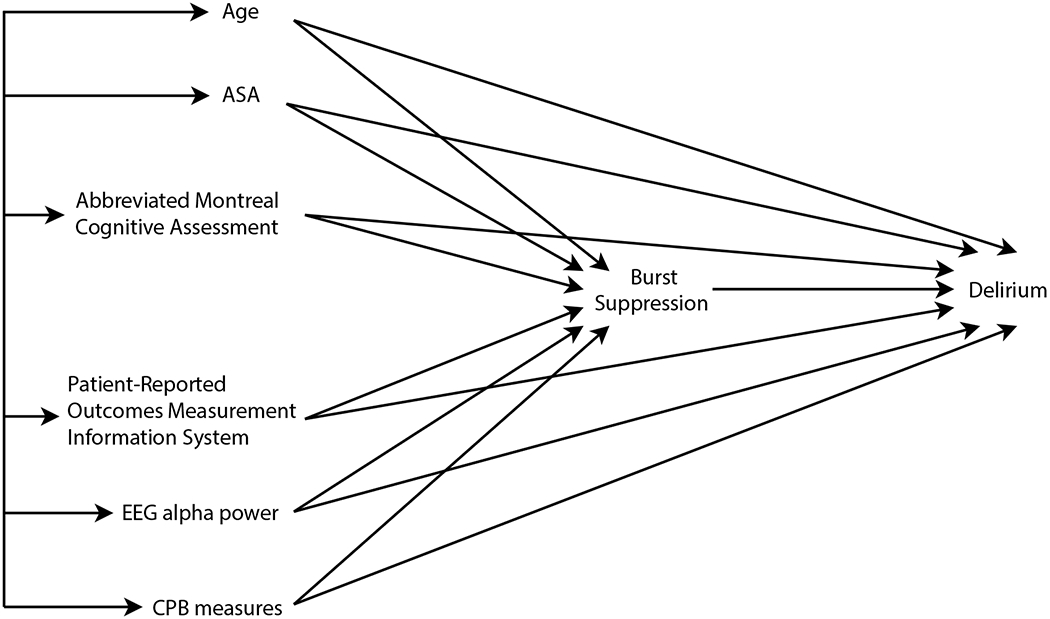
Initial Hypothetical Causal Model. Burst-suppression during cardiopulmonary bypass was hypothesized to mediate the association between known delirium risk factors and delirium. However, the initial model also allowed for the possibility of direct effects of the risk factors on delirium, in addition to, or instead of the indirect, mediational effect of burst-suppression. PROMIS measures included applied cognition, physical function, global health, pain, and sleep. CPB measures included duration of CPB and lowest temperature during CPB. Straight arrows indicate causal effects; double-headed arrows connecting exogenous variables on the left indicate correlations not explicated in the model.
ASA, American Society of Anesthesiologists Physical Status; aMOCA, abbreviated Montreal Cognitive Assessment; PROMIS, Patient-Reported Outcomes Measurement Information System; EEG, electroencephalogram; CPB, cardiopulmonary bypass measures
Results
Patient Characteristics Stratified by Burst-suppression and Delirium
Data from 159 patients were analyzed in this manuscript. Patient characteristics are summarized in supplementary material (See Supplemental Table 1). Electroencephalogram data of 18 subjects could not be analyzed for burst-suppression because of poor quality or incomplete data capture throughout CPB. There were 23 patients that screened positive for delirium in our study cohort: 18 of the 117 MINDDS trial patients (16 from assessments, 2 chart review), 1 of the 7 patients that withdrew consent for MINDDS trial long term follow-up (1 from assessments, none from chart review), 3 of the 18 patients that met objective drop criteria for MINDDS trial long term follow-up (none from assessments, 3 from chart review), 1 of the 17 patients that did not consent to be randomized into the MINDDS trial (none from assessments, 1 from chart review). These data are summarized in Supplemental Table 2. The characteristics of patients with complete electroencephalogram data stratified by burst-suppression and delirium are summarized in Table 1 and Supplemental Table 3, respectively. Patient comorbidities are summarized in supplemental material (Supplemental Table 4 and Supplemental Table 5).
Table 1.
Patients characteristics with complete electroencephalogram data, stratified by burst-suppression.
| Burst Supression | No Burst Suppression (n = 81) |
Burst Suppression (n = 60) |
p value | False Discovery Rate p value |
|---|---|---|---|---|
| Age, (median [Q1,Q3]) | 67 [64, 73] | 73 [68, 78] | 0.001 | 0.007 |
| ASA Physical Status, n/total (%) | 0.667 | 0.788 | ||
| 2 | 4/81 (5) | 2/60 (3) | ||
| 3 | 57/81 (70) | 39/60 (65) | ||
| 4 | 20/81 (25) | 19/60 (32) | ||
| Abbreviated Montreal Cognitive Assessment, (median [Q1,Q3]) | 19 [17, 20] | 19[17, 20] | 0.965 | 0.965 |
| Patient-Reported Outcomes Measurement Information System Physical, (median [Q1,Q3]) | 48 [41, 60] | 43 [39, 49] | 0.007 | 0.018 |
| Patient-Reported Outcomes Measurement Information System Global Health Physical, (median [Q1,Q3]) | 50 [43, 55] | 46 [38, 51] | 0.009 | 0.020 |
| Patient-Reported Outcomes Measurement Information System Global Health Mental, (median [Q1,Q3]) | 56 [50, 62] | 54 [49, 61] | 0.541 | 0.703 |
| Patient-Reported Outcomes Measurement Information System Pain, (median [Q1,Q3]) | 41 [41, 52] | 41 [41, 55] | 0.158 | 0.228 |
| Patient-Reported Outcomes Measurement Information System Cognition, (median [Q1,Q3]) | 61 [51, 61] | 51 [51, 61] | 0.120 | 0.195 |
| Patient-Reported Outcomes Measurement Information System Sleep, (median [Q1,Q3]) | 50 [44, 56] | 50 [44, 57] | 0.750 | 0.813 |
| Alpha power, (median [Q1,Q3]) | 2.84 [1.85, 6.54] | 1.06 [0.68, 2.22] | < 0.001 | < 0.001 |
| CPB length, (median [Q1,Q3]) | 113 [93, 148] | 133[105, 188] | 0.028 | 0.052 |
| Lowest CPB temperature, (median [Q1,Q3]) | 34.1 [33.7, 34.5] | 33.8 [31.2, 34.3] | 0.005 | 0.016 |
| Delirium, n/total (%) | 5/81 (25) | 15/60 (75) | 0.003 | 0.013 |
ASA, American Society of Anesthesiologists; CPB, Cardiopulmonary Bypass.
Univariate Analyses of Independent Associations
Age, Education and Alpha Power were Independently Associated with the Abbreviated Montreal Cognitive Assessment
We found significant independent associations with aMOCA for age, education and intraoperative alpha power (Supplemental Table 6). The patient’s predicted aMOCA score decreased by 0.087 points for each year increase in age (FDR P = 0.008). Predicted aMOCA score increased by 1.096 points if patients were formally educated beyond high school education (FDR P = 0.014). HS education was coded a 1 for ≥ high school education and 0 for < high school education. Similarly, aMOCA score increased 0.155 points for each decibel increase in intraoperative alpha power (FDR P = 0.033).
Age, Physical Function Scores, Alpha Power and Lowest Temperature during Cardiopulmonary Bypass were Independently Associated with Burst-suppression during CPB
We found significant independent associations with the incidence of intraoperative burst-suppression during CPB for age, PROMIS Physical Function, PROMIS Global Health Physical, intraoperative alpha power, and lowest temperature during CPB. The odds of burst-suppression during CPB increased by 8% (OR:1.08 [1.03, 1.14], FDR P = 0.006) for each year increase in age. The odds of burst-suppression during CPB decreased by 5% (OR:0.95 [0.91, 0.98], FDR P = 0.020) for every T-score increase in PROMIS Physical Function. Similarly, the odds of burst-suppression during CPB decreased by 5% (OR:0.95 [0.92, 0.99], FDR P = 0.021) for every T-score increase in PROMIS Global Health Physical Function. The odds of burst-suppression during CPB decreased by 35% (OR:0.65 [0.54, 0.80], FDR P <0.001) for each decibel increase in electroencephalogram alpha power. Finally, the odds of burst-suppression during CPB decreased by 21% (OR:0.79 [0.66, 0.94], FDR P = 0.024) for each degree increase in lowest temperature during CPB. These data are summarized in Supplemental Table 7.
Age, Abbreviated Montreal Cognitive Assessment, Alpha Power and Burst-suppression during Cardiopulmonary Bypass were Independently Associated with Delirium
We found significant independent associations for age, aMOCA, alpha power, and burst-suppression during CPB with delirium. The odds of delirium increased by 9% (OR:1.09 [1.02, 1.16], uncorrected P = 0.009) for each year increase in age. The odds of delirium decreased by 20% (OR:0.80 [0.66, 0.97], uncorrected P = 0.024) for each point increase in aMOCA score. The odds of delirium decreased by 25% (OR:0.75 [0.59, 0.96], uncorrected P = 0.025) for each decibel increase in electroencephalogram alpha power. The odds of delirium increased by 279% (OR:3.79 [1.50, 9.60], uncorrected P = 0.005) in patients with burst-suppression during CPB. These findings did not meet our threshold for statistical significance after correction for multiple comparisons (Supplemental Table 8).
Multivariable Logistic Regression Models
Alpha Power, Lowest temperature during Cardiopulmonary Bypass, and PROMIS Physical Scores Predicted Electroencephalogram Burst-suppression.
After backward elimination, only alpha power, lowest temperature during CPB, and PROMIS Physical were retained as significant predictors. (These predictors had near zero correlations with each other in our sample; thus, multicollinearity was not of concern). The overall model of all 3 was also significant (Likelihood Ratio: 46.4, P <0.001): alpha power (OR:0.61 [0.47, 0.76], P <0.001), lowest temperature during CPB (OR:0.73 [0.58, 0.88], P = 0.003), and PROMIS Physical (OR:0.96 [0.91, 0.99], P = 0.044) were retained as significant predictors (Figure 2). The area under the receiver operating curve for this model was 0.84. Incidentally, the 3 significant predictors that we found after backward elimination were also individually significant, and no others were, in the initial model before backward elimination. Further, our finding was conserved when we ran a limited backward elimination using only predictors that were significant in univariate analyses (alpha power, p < 0.0001; lowest temperature during CPB, p = 0.003; PROMIS Physical, p = 0.044). This suggests that our findings were not chance artifacts resulting from the iterative backward elimination procedure.
Figure 2.
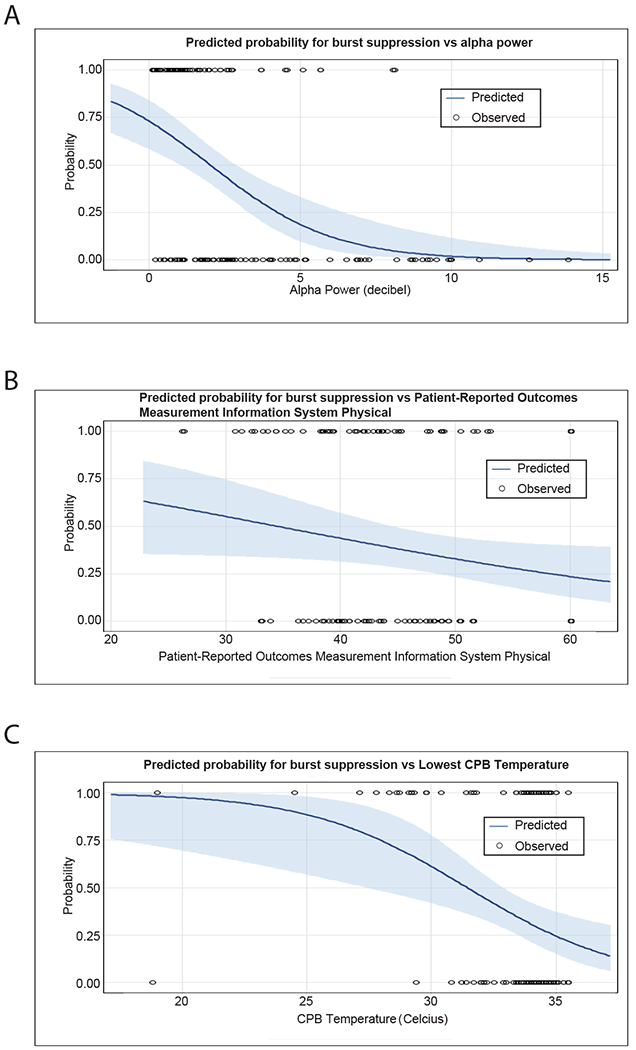
Predicted Probability for Burst-suppression during CPB from Multivariable Backward Logistic Regression Model. A. Relationship between alpha power and probability of burst-suppression during CPB. Physical function and lowest temperature during CPB were held constant at their grand means of 46.5 and 33.2° C, respectively. B. Relationship between physical function and probability of burst-suppression during CPB. Alpha power and lowest temperature during CPB were held constant at their grand means of 3.1 dB and 33.2° C, respectively. C. Relationship between lowest temperature during CPB and probability of burst-suppression during CPB. Alpha power and physical function were held constant at their grand means of 3.1 dB and 46.5, respectively.
Age and Burst-Suppression during Cardiopulmonary Bypass Predicted Postoperative Delirium
After backward elimination, only burst-suppression during CPB and age were retained as relevant predictors. The overall model of both predictors was also significant (Likelihood Ratio: 13.1, P = 0.002): age (OR:1.07 [0.99, 1.15], P = 0.090), and burst-suppression (OR:4.1 [1.5, 13.7], P = 0.012) (Supplemental Figure 1). The area under the receiver operating curve for this model was 0.74. (The point biserial correlation of burst-suppression and age was r=0.27, p=0.001, which was significant given the large sample size but well below the level of concerns associated with multicollinearity). As was the case for the retained predictors of burst-suppression, the significant predictor that we found after backward elimination was also individually significant, and no others were, in the initial model before backward elimination. This indicates that our findings were not chance artifacts resulting from the iterative backward elimination procedure. Further, our finding was conserved when we ran a limited backward elimination using only predictors that were significant in univariate analyses (burst-suppression, p = 0.0032).
Based on our two-step multivariable logistic regression approach, our final estimated causal model is illustrated in Figure 3.
Figure 3.
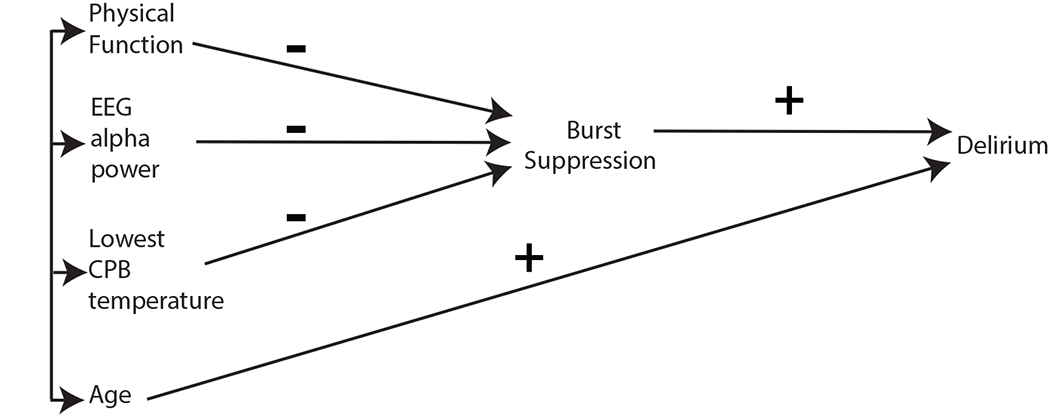
Final Estimated Causal Model. Physical function, electroencephalogram alpha power, and lowest temperature during CPB have effects on delirium mediated through their impact on burst-suppression during CPB. None of these predictors were found to have a separate direct effect on delirium outside of indirect effects through burst-suppression during CPB. Age had a direct positive effect on delirium. This model also suggests that the significant univariate association of age with burst-suppression during CPB (see results) may partly be mediated through one or more of the exogenous predictors on the left.
EEG, electroencephalogram; CPB, cardiopulmonary bypass
Electroencephalogram Analyses
Decreased but distinct patterns of broadband electroencephalogram power were associated with physical function, cognitive status, and delirium
Physical Function
We compared electroencephalogram spectral estimates of age-matched patients (n = 34 in each group) with low physical function scores (PROMIS Physical ≤ 45; PROMIS Global Health Physical ≤ 45, mean age, 71 ± 6.4) to patients with high physical function scores (PROMIS Physical > 45; PROMIS Global Health Physical > 45; mean age, 70 ± 6.1). The isoflurane concentrations for the electroencephalogram epochs analyzed were 0.8 ± 0.14% and 0.8 ± 0.11% for the low physical function group and high physical function group, respectively (p=0.849). Representative spectrograms and time series data of two age-matched and aMOCA-matched patients with high and low physical function are shown in Supplemental Figure 2. We observed decreased power in the low physical function group when compared to high physical function group. This difference met our threshold for statistical significance between 7.3 to 19.0 Hz (Figure 4A).
Figure 4.
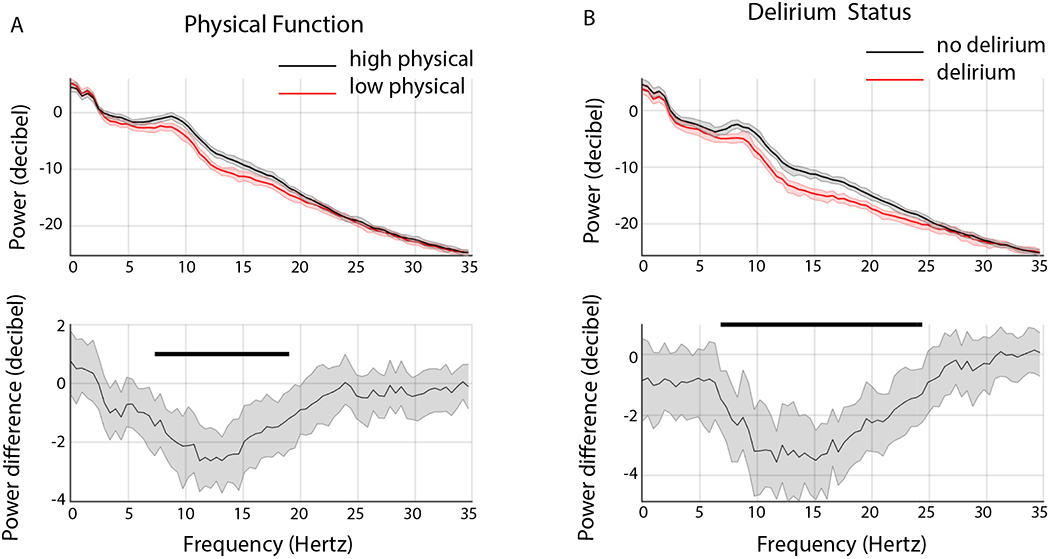
Group level spectra. A. Power spectra of high physical function (black) versus low physical function (red) groups (top panel). Electroencephalogram power was significantly greater in the high physical function group between 7.3 to 19 Hz (bottom panel, bootstrap difference of mean). B. Power spectra of no delirium (black) versus delirium (red) groups (top panel). Electroencephalogram power was significantly greater in the no delirium group between 6.8 to 24.4 Hz (bottom panel, bootstrap difference of mean).
Median bootstrapped spectra presented with 99% confidence intervals. Horizontal solid black lines represent significantly different frequencies.
Delirium
We compared electroencephalogram spectral estimates of age-matched patients (n = 23 in each group) with no delirium (aMOCA 18 ± 3, PROMIS Physical 45 ± 8, mean age 74 ± 6.8) to patients with delirium (aMOCA 17 ± 3, PROMIS Physical 44 ± 9, mean age 74 ± 6.7). The isoflurane concentrations for the electroencephalogram epochs analyzed were 0.8 ± 0.12% and 0.8 ± 0.15% for the no delirium group and delirium group, respectively (p=0.249).
We observed decreased power in the delirium group when compared to no delirium group. This difference met our threshold for statistical significance between 6.84 to 24.41 Hz (Figure 4B).
Cognitive Status
The abbreviated MOCA ranges from 0 to 22 points, and scores are categorized as “positive” for cognitive impairment if they are ≤17 (mild cognitive impairment, 13-17; mild dementia, 7-12; moderate dementia, ≤6).23 We computed and compared electroencephalogram spectral estimates of age matched patients (n = 48 in each group) that screened positive for cognitive impairment (aMOCA ≤17; mean age, 73 ± 7.3; mean aMOCA, 15.7 ± 2.5) to age-matched control patients (aMOCA >18; mean age, 72 ± 6.7; mean abbreviated MOCA, 20.4±3.0). The isoflurane concentrations for the electroencephalogram epochs analyzed were 0.8 ± 0.13% and 0.8 ± 0.13% for the cognitive impairment group and cognitively normal group, respectively (p=0.310). We observed decreased power in the cognitive impairment group (Low aMOCA) when compared to control patients (High aMOCA). This difference met our threshold for statistical significance between 4.88 to 9.77 Hz (Supplemental Figure 3).
Discussion
In this study, we investigated whether burst-suppression during cardiopulmonary bypass mediates the effects of known delirium risk factors on postoperative delirium. Based on a two-step multivariable logistic regression approach, a causal model consistent with the results of our analyses is that burst-suppression during CPB mediates the effects of physical function, lowest temperature during CPB, and alpha power on delirium. Age exhibited a direct effect on delirium in our final estimated model. However, our model also suggests that age may have an indirect effect on burst-suppression during CPB mediated through physical function and alpha power. This is because there was a significant univariate association between age and burst-suppression during CPB. Also, age was significantly correlated with physical function (r = −0.16, p=0.039) and electroencephalogram alpha power (r = −0.33, p<0.001). Thus, age has an additional indirect effect on delirium through burst-suppression (Fig. 3). Taken together, our results suggest that electroencephalogram burst-suppression during cardiopulmonary bypass in elderly patients is a mediator of postoperative delirium.
Intraoperative burst-suppression has been associated with postoperative delirium.12–14 Our finding that burst-suppression during cardiopulmonary bypass is associated with increased odds of delirium in our univariate analyses and our multivariable model is consistent with these reports.12–14 However, we note that the probability of postoperative delirium in elderly patients with burst-suppression during cardiopulmonary bypass was less than 0.5 across a range of ages (Supplemental Figure 1). Thus, the use of burst-suppression during cardiopulmonary bypass for the identification of patients at high risk for postoperative delirium may not benefit clinical decision making. Future studies are necessary to make clear whether other electroencephalogram dynamics–from burst-suppression (e.g., burst amplitude) and no burst-suppression epochs (e.g., cross-frequency coupling)–may benefit delirium prediction models.
Although pathophysiological mechanisms to explain delirium are not clear, there is strong biological plausibility to suggest that burst-suppression mediates the association between physical function and delirium. Physical activity is associated with increased cerebral blood flow,24 neurogenesis,25,26 cell proliferation,27,28 and synaptic plasticity29 in laboratory models. In humans, physical activity is associated with increased hippocampal volume,30,31 improved cognitive function,32,33 and a decreased incidence of dementia.30,34,35 These data are consistent with our finding that patients with low physical function scores exhibited a broadband decrease in power between 7.3 – 19 Hz. We note that poor physical function has been associated with delirium after major cardiac surgery.3 Our finding that patients who subsequently developed postoperative delirium exhibited a broadband decrease in power between 6.8 – 24.4 Hz is consistent with this notion. Thus, decreased broadband electroencephalogram power during isoflurane general anesthesia may reflect deliriogenic structural, and perhaps functional brain dynamics.
The underlying mechanism underpinning the decreased broadband power in patients that subsequently screened for delirium is an open question. Anesthetic drugs that significantly modulate GABAA receptors (i.e., isoflurane, sevoflurane) are associated with highly structured oscillations.36,37 The power of these oscillations exhibit a linear decrease as a function of age to suggest that they arise from intrinsic cellular properties such as synaptic integrity.38 Holschneider et al., demonstrated that a thiopental challenge unmasked an abnormality (decreased beta power during sedation) in frontal electroencephalogram oscillations of patients with Alzheimer’s disease that was not discernible at baseline.39 This abnormality was postulated to result from cortical deafferentation.39
Sun et al. recently conceptualized “brain age”–different from chronological age–from the electroencephalogram of sleep. They proposed the brain age index (brain age minus chronological age) to reflect the degree of deviation from normal aging.40 Using an interpretable machine learning model based on spectral, entropy, time-series features, patients with neurological or psychiatric diseases were found to exhibit increased brain age indices compared to healthy controls. Although the concept of brain age has not been applied to intraoperative electroencephalogram data, we conjecture that deviations from chronological aging may have peri-operative clinical implications. This is because; 1) anesthetic drugs may accentuate differences in electroencephalogram data from pathological brain regions;39 and, 2) we found significant differences in electroencephalogram power of patients with poor physical function and delirium.
Our study has several important limitations. First, we did not measure objective measures of physical function such as gait or grip strength. Second, we studied patients who presented for elective cardiac surgery without clinically diagnosed dementia. Thus, we cannot make inferences on whether cognitive status is associated with intraoperative burst-suppression during CPB in other patient populations (e.g., such as those with a clinical diagnosis of Alzheimer’s disease). We note that the aMOCA is not a substitute for a formal neuropsychological battery. Third, this study was powered to analyze the association between abbreviated Montreal Cognitive Assessment scores and burst-suppression during CPB. Fourth, anesthetic adjuncts may affect electroencephalogram power. Fifth, we did not analyze spectral characteristics of bursts or the duration burst-suppression. Sixth, our sample size was modest relative to the number of predictors initially considered our multivariable models. Therefore, replication of our findings is recommended in future research. Finally, this was a pre-specified sub-study of the MINDDS trial where patients were randomized to placebo or dexmedetomidine intervention, an adrenergic sedative medication41,42 that may affect the incidence of delirium. Thus, the incidence of delirium may have been underestimated in the MINDDS trial cohort.
In conclusion, the present study provides evidence that burst-suppression during CPB in patients older than 60 years who present for elective cardiac surgery mediates the effect of physical function, alpha power and lowest temperature during CPB, on delirium. We also conclude that patients with postoperative delirium in this cohort possessed a pre-existing susceptibility to delirium that was reflected in the intraoperative electroencephalogram as decreased broadband power. A clinical implication of our study is that physical function may be a modifiable risk factor for postoperative delirium. This concept is based on a growing body of evidence that has related cognitive 43,44, morbidity 45–48 and mortality 49,50 benefits to physical activity.
Supplementary Material
Supplemental Figure 2. Representative individual data obtained during isoflurane general anesthesia of low versus high physical function subjects. A. Spectrogram (top panel) and time-series data (bottom panel) of a 62-year-old male patient with aMOCA score of 20, physical function score of 49, and isoflurane concentration of 0.8% who did not develop burst-suppression during cardiopulmonary bypass. B. Spectrogram (top panel) and time-series data (bottom panel) of a 62-year-old male patient with aMOCA score of 20, physical function score of 40, and isoflurane concentration of 0.8% who developed burst-suppression during cardiopulmonary bypass.
Supplemental Figure 3. Group level spectra aMOCA. Power spectra of high aMOCA (black) versus low aMOCA (red) groups (top panel). Electroencephalogram power was significantly greater in the high physical function group between 4.9 to 9.8 Hz (bottom panel, bootstrap difference of mean).
Median bootstrapped spectra presented with 99% confidence intervals. Horizontal solid black lines represent significantly different frequencies.
Supplemental Figure 1. Predicted Probability of Delirium in no burst-suppression group (continuous blue line) and burst-suppression group (dashed red line) as a function of age. The predicted probability of delirium was less than 0.5 across all age ranges.
Acknowledgements:
Not Applicable
Funding Statement: NIH NIA RO1AG053582 to OA; NIH NINDS K23NS090900, R01NS102190, R01NS102574, and R01NS107291 to MBW; funds from División de Anestesiología, Escuela de Medicina, Pontificia Universidad Católica de Chile to JP; and, funds from the Department of Anesthesia, Critical Care, and Pain Medicine, MGH.
Footnotes
Clinical Trial Number: NCT02856594
Prior Presentations: Not Applicable
Summary Statement: Not Applicable
Conflicts of Interest: OA has received speaker’s honoraria from Masimo Corporation, and is listed as an inventor on pending patents on EEG monitoring and sleep that are assigned to Massachusetts General Hospital. All other authors declare that no competing interests exist.
Bibliography
- 1.American Psychiatric Association., American Psychiatric Association DSM-5 Task Force.: Diagnostic and statistical manual of mental disorders : DSM-5, 5th edition Washington, D.C., American Psychiatric Association, 2013 [Google Scholar]
- 2.Inouye SK: Delirium in older persons. N Engl J Med 2006; 354: 1157–65 [DOI] [PubMed] [Google Scholar]
- 3.Brown CHt, Max L, LaFlam A, Kirk L, Gross A, Arora R, Neufeld K, Hogue CW, Walston J, Pustavoitau A: The Association Between Preoperative Frailty and Postoperative Delirium After Cardiac Surgery. Anesth Analg 2016; 123: 430–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa M, Izawa KP, Satomi-Kobayashi S, Kitamura A, Tsuboi Y, Komaki K, Ono R, Sakai Y, Tanaka H, Okita Y: Preoperative exercise capacity is associated with the prevalence of postoperative delirium in elective cardiac surgery. Aging Clin Exp Res 2018; 30: 27–34 [DOI] [PubMed] [Google Scholar]
- 5.Culley DJ, Flaherty D, Fahey MC, Rudolph JL, Javedan H, Huang CC, Wright J, Bader AM, Hyman BT, Blacker D, Crosby G: Poor Performance on a Preoperative Cognitive Screening Test Predicts Postoperative Complications in Older Orthopedic Surgical Patients. Anesthesiology 2017; 127: 765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inouye SK, Marcantonio ER, Kosar CM, Tommet D, Schmitt EM, Travison TG, Saczynski JS, Ngo LH, Alsop DC, Jones RN: The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimer’s & Dementia 2016; 12: 766–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inouye SK, Westendorp RGJ, Saczynski JS: Delirium in elderly people Lancet (London, England: ) 2014; 383: 911–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadayomi AB, Ibala R, Bilotta F, Westover MB, Akeju O: A Systematic Review and Meta-Analysis Examining the Impact of Sleep Disturbance on Postoperative Delirium. Crit Care Med 2018; 46: e1204–e1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasunilashorn SM, Dillon ST, Inouye SK, Ngo LH, Fong TG, Jones RN, Travison TG, Schmitt EM, Alsop DC, Freedman SD, Arnold SE, Metzger ED, Libermann TA, Marcantonio ER: High C-Reactive Protein Predicts Delirium Incidence, Duration, and Feature Severity After Major Noncardiac Surgery. J Am Geriatr Soc 2017; 65: e109–e116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton GM, Wheeler K, Di Michele J, Lalu MM, McIsaac DI: A Systematic Review and Meta-analysis Examining the Impact of Incident Postoperative Delirium on Mortality. Anesthesiology 2017; 127: 78–88 [DOI] [PubMed] [Google Scholar]
- 11.Maldonado JR: Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry 2013; 21: 1190–222 [DOI] [PubMed] [Google Scholar]
- 12.Fritz BA, Kalarickal PL, Maybrier HR, Muench MR, Dearth D, Chen Y, Escallier KE, Ben Abdallah A, Lin N, Avidan MS: Intraoperative Electroencephalogram Suppression Predicts Postoperative Delirium. Anesth Analg 2016; 122: 234–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritz BA, Maybrier HR, Avidan MS: Intraoperative electroencephalogram suppression at lower volatile anaesthetic concentrations predicts postoperative delirium occurring in the intensive care unit. Br J Anaesth 2018; 121: 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soehle M, Dittmann A, Ellerkmann RK, Baumgarten G, Putensen C, Guenther U: Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiology 2015; 15: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akeju O, Brown EN: Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr Opin Neurobiol 2017; 44: 178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claassen J, Hirsch LJ, Emerson RG, Mayer SA: Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 2002; 43: 146–53 [DOI] [PubMed] [Google Scholar]
- 17.Bergey GK: Refractory status epilepticus: is EEG burst suppression an appropriate treatment target during drug-induced coma? What is the Holy Grail? Epilepsy Curr 2006; 6: 119–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plummer GS, Ibala R, Hahm E, An J, Gitlin J, Deng H, Shelton KT, Solt K, Qu JZ, Akeju O: Electroencephalogram dynamics during general anesthesia predict the later incidence and duration of burst-suppression during cardiopulmonary bypass. Clin Neurophysiol 2019; 130: 55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wildes TS, Mickle AM, Ben Abdallah A, Maybrier HR, Oberhaus J, Budelier TP, Kronzer A, McKinnon SL, Park D, Torres BA, Graetz TJ, Emmert DA, Palanca BJ, Goswami S, Jordan K, Lin N, Fritz BA, Stevens TW, Jacobsohn E, Schmitt EM, Inouye SK, Stark S, Lenze EJ, Avidan MS, Group ER: Effect of Electroencephalography-Guided Anesthetic Administration on Postoperative Delirium Among Older Adults Undergoing Major Surgery: The ENGAGES Randomized Clinical Trial. JAMA 2019; 321: 473–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giattino CM, Gardner JE, Sbahi FM, Roberts KC, Cooter M, Moretti E, Browndyke JN, Mathew JP, Woldorff MG, Berger M, Investigators M- P: Intraoperative Frontal Alpha-Band Power Correlates with Preoperative Neurocognitive Function in Older Adults. Front Syst Neurosci 2017; 11: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holschneider DP, Leuchter AF: Attenuation of brain high frequency electrocortical response after thiopental in early stages of Alzheimer’s dementia. Psychopharmacology 2000; 149: 6–11 [DOI] [PubMed] [Google Scholar]
- 22.Shelton KT, Qu J, Bilotta F, Brown EN, Cudemus G, D’Alessandro DA, Deng H, DiBiasio A, Gitlin JA, Hahm EY, Hobbs LE, Houle TT, Ibala R, Loggia ML, Pavone KJ, Shaefi S, Tolis G, Westover MB, Akeju O: Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep (MINDDS): protocol for a randomised, double-blind, parallel-arm, placebo-controlled trial. BMJ Open 2018; 8: e020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pendlebury ST, Welch SJ, Cuthbertson FC, Mariz J, Mehta Z, Rothwell PM: Telephone assessment of cognition after transient ischemic attack and stroke: modified telephone interview of cognitive status and telephone Montreal Cognitive Assessment versus face-to-face Montreal Cognitive Assessment and neuropsychological battery. Stroke 2013; 44: 227–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Zhang L, Yang X, Wan Y, Jia J: The Effects of Exercise Preconditioning on Cerebral Blood Flow Change and Endothelin-1 Expression after Cerebral Ischemia in Rats. Journal of Stroke and Cerebrovascular Diseases 2014; 23: 1696–1702 [DOI] [PubMed] [Google Scholar]
- 25.Han T- K, Leem Y- H, Kim H- S: Treadmill exercise restores high fat diet-induced disturbance of hippocampal neurogenesis through β2-adrenergic receptor-dependent induction of thioredoxin-1 and brain-derived neurotrophic factor. Brain Research 2019; 1707: 154–163 [DOI] [PubMed] [Google Scholar]
- 26.Klein C, Jonas W, Wiedmer P, Schreyer S, Akyüz L, Spranger J, Hellweg R, Steiner B: High-fat Diet and Physical Exercise Differentially Modulate Adult Neurogenesis in the Mouse Hypothalamus. Neuroscience 2019; 400: 146–156 [DOI] [PubMed] [Google Scholar]
- 27.Trejo JL, Carro E, Torres-Alemán I: Circulating Insulin-Like Growth Factor I Mediates Exercise-Induced Increases in the Number of New Neurons in the Adult Hippocampus. The Journal of Neuroscience 2001; 21: 1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C- W, Chang Y- T, Yu L, Chen H-i, Jen CJ, Wu S-Y, Lo C-P, Kuo Y-M: Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. Journal of Applied Physiology 2008; 105: 1585–1594 [DOI] [PubMed] [Google Scholar]
- 29.Shih P- C, Yang Y- R, Wang R- Y: Effects of exercise intensity on spatial memory performance and hippocampal synaptic plasticity in transient brain ischemic rats, PloS one, 2013, pp e78163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan ZS, Seshadri S, Spartano NL, Vasan RS, Auerbach SH, Beiser AS, DeCarli C: Physical Activity, Brain Volume, and Dementia Risk: The Framingham Study. The Journals of Gerontology: Series A 2016; 72: 789–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ten Brinke LF, Bolandzadeh N, Nagamatsu LS, Hsu CL, Davis JC, Miran-Khan K, Liu-Ambrose T: Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. British Journal of Sports Medicine 2015; 49: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schubert CR, Fischer ME, Pinto AA, Chen Y, Klein BEK, Klein R, Tsai MY, Tweed TS, Cruickshanks KJ: Brain Aging in Midlife: The Beaver Dam Offspring Study. Journal of the American Geriatrics Society 2019; 67: 1610–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stern Y, MacKay-Brandt A, Lee S, McKinley P, McIntyre K, Razlighi Q, Agarunov E, Bartels M, Sloan RP: Effect of aerobic exercise on cognition in younger adults. Neurology 2019; 92: e905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groot C, Hooghiemstra AM, Raijmakers PGHM, van Berckel BNM, Scheltens P, Scherder EJA, van der Flier WM, Ossenkoppele R: The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Research Reviews 2016; 25: 13–23 [DOI] [PubMed] [Google Scholar]
- 35.Neerland BE, Krogseth M, Juliebø V, Hylen Ranhoff A, Engedal K, Frihagen F, Ræder J, Bruun Wyller T, Watne LO: Perioperative hemodynamics and risk for delirium and new onset dementia in hip fracture patients; A prospective follow-up study. PLOS ONE 2017; 12: e0180641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akeju O, Hamilos AE, Song AH, Pavone KJ, Purdon PL, Brown EN: GABAA circuit mechanisms are associated with ether anesthesia-induced unconsciousness. Clin Neurophysiol 2016; 127: 2472–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavone KJ, Su L, Gao L, Eromo E, Vazquez R, Rhee J, Hobbs LE, Ibala R, Demircioglu G, Purdon PL, Brown EN, Akeju O: Lack of Responsiveness during the Onset and Offset of Sevoflurane Anesthesia Is Associated with Decreased Awake-Alpha Oscillation Power. Front Syst Neurosci 2017; 11: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JM, Akeju O, Terzakis K, Pavone KJ, Deng H, Houle TT, Firth PG, Shank ES, Brown EN, Purdon PL: A Prospective Study of Age-dependent Changes in Propofol-induced Electroencephalogram Oscillations in Children. Anesthesiology 2017; 127: 293–306 [DOI] [PubMed] [Google Scholar]
- 39.Holschneider DP, Leuchter AF, Uijtdehaage SHJ, Abrams M, Rosenberg-Thompson S: Loss of high-frequency brain electrical response to thiopental administration in alzheimer’s-type dementia. Neuropsychopharmacology 1997; 16: 269–275 [DOI] [PubMed] [Google Scholar]
- 40.Sun H, Paixao L, Oliva JT, Goparaju B, Carvalho DZ, van Leeuwen KG, Akeju O, Thomas RJ, Cash SS, Bianchi MT, Westover MB: Brain age from the electroencephalogram of sleep. Neurobiol Aging 2019; 74: 112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashmi JA, Loggia ML, Khan S, Gao L, Kim J, Napadow V, Brown EN, Akeju O: Dexmedetomidine Disrupts the Local and Global Efficiencies of Large-scale Brain Networks. Anesthesiology 2017; 126: 419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song AH, Kucyi A, Napadow V, Brown EN, Loggia ML, Akeju O: Pharmacological Modulation of Noradrenergic Arousal Circuitry Disrupts Functional Connectivity of the Locus Ceruleus in Humans. J Neurosci 2017; 37: 6938–6945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karssemeijer EGA, Aaronson JA, Bossers WJ, Smits T, Olde Rikkert MGM, Kessels RPC: Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Research Reviews 2017; 40: 75–83 [DOI] [PubMed] [Google Scholar]
- 44.Brasure M, Desai P, Davila H, Nelson VA, Calvert C, Jutkowitz E, Butler M, Fink HA, Ratner E, Hemmy LS, McCarten JR, Barclay TR, Kane RL: Physical Activity Interventions in Preventing Cognitive Decline and Alzheimer-Type Dementia: A Systematic ReviewPhysical Activity to Prevent Cognitive Decline and Dementia. Annals of Internal Medicine 2018; 168: 30–38 [DOI] [PubMed] [Google Scholar]
- 45.Whibley J, Peters CJ, Halliday LJ, Chaudry AM, Allum WH: Poor performance in incremental shuttle walk and cardiopulmonary exercise testing predicts poor overall survival for patients undergoing esophago-gastric resection. European Journal of Surgical Oncology 2018; 44: 594–599 [DOI] [PubMed] [Google Scholar]
- 46.Marmelo F, Rocha V, Moreira-Gonçalves D: The impact of prehabilitation on post-surgical complications in patients undergoing non-urgent cardiovascular surgical intervention: Systematic review and meta-analysis. European Journal of Preventive Cardiology 2018; 25: 404–417 [DOI] [PubMed] [Google Scholar]
- 47.Hughes MJ, Hackney RJ, Lamb PJ, Wigmore SJ, Christopher Deans DA, Skipworth RJE: Prehabilitation Before Major Abdominal Surgery: A Systematic Review and Meta-analysis. World Journal of Surgery 2019 [DOI] [PubMed] [Google Scholar]
- 48.Saxton A, Velanovich V: Preoperative frailty and quality of life as predictors of postoperative complications. Annals of surgery 2011; 253: 1223–1229 [DOI] [PubMed] [Google Scholar]
- 49.Dronkers JJ, Chorus AMJ, van Meeteren NLU, Hopman-Rock M: The association of pre-operative physical fitness and physical activity with outcome after scheduled major abdominal surgery. Anaesthesia 2013; 68: 67–73 [DOI] [PubMed] [Google Scholar]
- 50.Wilson RJT, Davies S, Yates D, Redman J, Stone M: Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. BJA: British Journal of Anaesthesia 2010; 105: 297–303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 2. Representative individual data obtained during isoflurane general anesthesia of low versus high physical function subjects. A. Spectrogram (top panel) and time-series data (bottom panel) of a 62-year-old male patient with aMOCA score of 20, physical function score of 49, and isoflurane concentration of 0.8% who did not develop burst-suppression during cardiopulmonary bypass. B. Spectrogram (top panel) and time-series data (bottom panel) of a 62-year-old male patient with aMOCA score of 20, physical function score of 40, and isoflurane concentration of 0.8% who developed burst-suppression during cardiopulmonary bypass.
Supplemental Figure 3. Group level spectra aMOCA. Power spectra of high aMOCA (black) versus low aMOCA (red) groups (top panel). Electroencephalogram power was significantly greater in the high physical function group between 4.9 to 9.8 Hz (bottom panel, bootstrap difference of mean).
Median bootstrapped spectra presented with 99% confidence intervals. Horizontal solid black lines represent significantly different frequencies.
Supplemental Figure 1. Predicted Probability of Delirium in no burst-suppression group (continuous blue line) and burst-suppression group (dashed red line) as a function of age. The predicted probability of delirium was less than 0.5 across all age ranges.


