Abstract
PURPOSE
Pembrolizumab is a humanized monoclonal antibody that blocks interaction between programmed death receptor-1 (PD-1) and its ligands (PD-L1, PD-L2). Although pembrolizumab is approved for recurrent/metastatic head and neck squamous cell carcinoma (HNSCC), its role in the management of locally advanced (LA) disease is not defined. We report a phase IB study evaluating the safety and efficacy of adding pembrolizumab to cisplatin-based chemoradiotherapy in patients with LA HNSCC.
PATIENTS AND METHODS
Eligible patients included those with oral cavity (excluding lip), oropharyngeal, hypopharyngeal, or laryngeal stage III to IVB HNSCC (according to American Joint Committee on Cancer, 7th edition, staging system) eligible for cisplatin-based, standard-dose (70 Gy) chemoradiotherapy. Pembrolizumab was administered concurrently with and after chemoradiotherapy with weekly cisplatin. Safety was the primary end point and was determined by incidence of chemoradiotherapy adverse events (AEs) and immune-related AEs (irAEs). Efficacy was defined as complete response (CR) rate on end-of-treatment (EOT) imaging or with pathologic confirmation at 100 days postradiotherapy completion. Key secondary end points included overall (OS) and progression-free survival (PFS).
RESULTS
The study accrued 59 patients (human papillomavirus [HPV] positive, n = 34; HPV negative, n = 25) from November 2015 to October 2018. Five patients (8.8%) required discontinuation of pembrolizumab because of irAEs, all of which occurred during concurrent chemoradiotherapy; 98.3% of patients completed the full planned treatment dose (70 Gy) of radiotherapy without any delays ≥ 5 days; 88.1% of patients completed the goal cisplatin dose of ≥ 200 mg/m2. EOT CR rates were 85.3% and 78.3% for those with HPV-positive and -negative HNSCC, respectively.
CONCLUSION
Pembrolizumab in combination with weekly cisplatin-based chemoradiotherapy is safe and does not impair delivery of curative radiotherapy or chemotherapy in HNSCC. Early efficacy data support further investigation of this approach.
INTRODUCTION
Patients with locally advanced (LA) head and neck squamous cell carcinoma (HNSCC) have a 5-year survival of only 50% with the current standard treatment of concurrent chemoradiotherapy.1 Although those with human papillomavirus (HPV)–associated HNSCC have better outcomes, patients with significant tobacco use or advanced tumor or nodal stage still have 3-year survival rates of 70%.2 Strategies to improve survival with intensified therapy for patients with high-risk disease have been limited by excessive toxicity.3,4 Recent understanding of the immune response during chemoradiotherapy has offered new therapeutic possibilities.
The interaction between programmed death receptor-1 (PD-1) and its ligands (PD-L1, PD-L2) may contribute to immune escape in HNSCC.5 PD-L1 upregulation and resulting immune exhaustion have been seen in preclinical models with both radiotherapy6,7 and cisplatin.8 Analysis of circulating immune cells during concurrent chemoradiotherapy in HNSCC demonstrated an exhausted immunophenotype, in part because of increased PD-1 expression on CD4+ T cells.9
Pembrolizumab is a high-affinity immunoglobulin G4 monoclonal antibody against the interaction between PD-1 and PD-L1/PD-L2. It was granted accelerated approval for platinum-refractory, recurrent/metastatic (R/M) HNSCC on August 5, 2016, based on response and survival data from KEYNOTE-012 and supported by KEYNOTE-040 trials.10,11 In these studies, 13% to 17% of patients experienced grade ≥ 3 treatment-related adverse events (AEs), a majority of which required discontinuation of monotherapy. KEYNOTE-048 evaluated pembrolizumab in combination with platinum and fluorouracil chemotherapy for first-line treatment of R/M HNSCC; however, the all-cause grade ≥ 3 toxicity rate was 85.1%.12 Because the acute grade ≥ 3 toxicity rates already exceed 77% for standard concurrent chemoradiotherapy in HNSCC, the safety of adding pembrolizumab warrants prudent investigation.13,14
With these data in mind, we performed a clinical trial adding pembrolizumab to definitive chemoradiotherapy in LA HNSCC to explore safety and efficacy. We used weekly cisplatin at a dose of 40 mg/m2 rather than standard high-dose cisplatin (100 mg/m2 every 3 weeks) as our chemotherapy backbone because of its decreased potential for causing severe (grade 3-4) myelosuppression15,16 and its improved tolerability in other studies.17,18 This was combined with radiotherapy at a total dose of 70 Gy.
PATIENTS AND METHODS
Study Design and Participants
This phase IB study took place at 3 National Cancer Institute (NCI) Community Oncology Research Program Centers (Sanford Health) and an NCI Comprehensive Cancer Center (UCSD). The study enrolled an initial lead-in cohort to evaluate safety and efficacy regardless of HPV status. After a planned interim analysis, the study expanded into 2 cohorts, evaluating efficacy in HPV-positive and -negative disease. Enrolled patients were age ≥ 18 years with TNM clinical stage III, IVA, or IVB (by American Joint Committee on Cancer, 7th edition, staging system) histologically confirmed HNSCC of the oral cavity (excluding lip), oropharynx, hypopharynx, or larynx who were candidates for curative, definitive chemoradiotherapy. Participants were required to have adequate organ function and performance status (Eastern Cooperative Oncology Group performance status 0 or 1) to receive cisplatin-based chemoradiotherapy. Measurable disease by RECIST (version 1.1) using computed tomography (CT) or magnetic resonance imaging was required.19 HPV status by p16 immunohistochemistry or HPV16 in situ hybridization (ISH) was required for enrollment regardless of disease site.20 Exclusion criteria included prior radiotherapy or systemic cancer therapy, known immunosuppression, active autoimmune disease, prior noninfectious pneumonitis, or any condition that could interfere with trial participation. The study was approved by the institutional review board at each participating institution, and all patients provided written informed consent.
Study Treatment
Treatment included a loading dose of pembrolizumab 200 mg administered intravenously (IV) 7 days before chemoradiotherapy (day −7), 2 additional doses on days 15 and 36 during chemoradiotherapy (concurrent), and after completion (consolidation) every 3 weeks on days 57, 78, 99, 120, and 141. PD-1 blockade was used during both these periods based on previously reported circulating immunophenotype findings,9 high rates of viable nodal disease on neck dissection after radiotherapy,21 and positive survival data with consolidation PD-L1 blockade for non–small-cell lung cancer.22 Chemoradiotherapy was started on day 1 with cisplatin 40 mg/m2 IV weekly for a total of 6 planned doses (240 mg/m2 maximum) with concomitant head and neck irradiation (intensity-modulated radiotherapy) at 70 Gy fractionated at 2 Gy once daily over 35 fractions. Corticosteroid antinausea prophylaxis was allowed but not required.
Safety Evaluation During Treatment and Response Assessment
Participants were seen weekly for adverse event (AE) assessment and laboratory studies during chemoradiotherapy treatment (days −7 to 50). During consolidation treatment (starting day 57), participants were seen every 3 weeks for AE assessment and laboratory studies. Safety follow-up with examinations, laboratory studies, and AE assessments then continued every 3 months for the first year, every 6 months for the second year, and then annually through year 5.
Response was determined using a composite end point of overall end-of-treatment (EOT) complete response (CR) at day 150 (approximately 12 weeks after completion of chemoradiotherapy) by using CT of the neck. Optional positron emission tomography (PET) imaging was allowed rather than neck dissection if CT could not confirm CR.23 Complete metabolic response was assessed using Hopkins score of 1, 2, or 3 on PET imaging.24 Assessments were performed by a radiology subinvestigator at each center and confirmed by the site principal investigator. For those without an imaging CR, pathologic confirmation was recommend (but not required) by selective neck dissection and/or directed biopsy of the suspected active disease site. If pathologic evaluation of the potential disease site confirmed no residual invasive or in situ cancer, the patient was determined to have a pathologic CR. In cases with both an imaging CR and pathologic response assessment, the pathologic response defined final overall response. Therefore, patients with a final EOT CR included those with either an imaging (CT or PET) or pathologic CR. Recurrence assessment with CT scan of the neck occurred every 3 months for year 1, every 6 months for year 2, and then annually until year 5. Key secondary efficacy end points included progression-free (PFS) and overall survival (OS).
Tumor Assessments
HPV status was determined based on immunohistochemical (IHC) staining for p16. Positivity was based on > 70% of tumor cell staining positive for p16 expression. For cases in which p16 testing could not be accurately performed, HPV positivity could be evaluated by high-risk HPV DNA ISH testing as previously described.20
Retrospective analysis of PD-L1 expression was performed on all accessible baseline tumor specimens after completion of enrollment. Testing was performed centrally by QualTek Laboratories (Santa Barbara, CA) using the PD-L1 IHC 22C3 pharmDx (Dako North America, Carpinteria, CA) IHC assay as previously described.25 PD-L1 evaluation was performed using the combined proportional score (CPS). This includes the tumor cells and any tumor-infiltrating mononuclear inflammatory cells with adjacent supporting stroma, which demonstrate membrane staining PD-L1 > 1+ intensity divided by the total number of tumor cells × 100 (range, 0-100).
Statistical Analysis
The primary objective of this trial was to characterize the safety and tolerability of pembrolizumab in combination with chemoradiotherapy in patients with LA HNSCC. Safety was assessed by reported AEs and serious AEs (SAEs) using Common Terminology Criteria for Adverse Events (version 4.0). Immune-related adverse events (irAEs) were collected, and serious irAEs were designated as events of clinical interest (ECIs).26 Toxicities and treatment compliance to chemotherapy and radiotherapy were assessed. After an initial safety lead-in of 8 patients, ECIs and SAEs were reviewed and compared with data from a prior study in this setting27 by a data safety monitoring board (DSMB) as part of a planned analysis. If deemed safe, 27 total patients would then be enrolled for interim efficacy analysis. Treatment would be determined futile if ≤ 13 overall CRs were seen in the 27 patients. After this analysis, the study moved to expansion cohorts to estimate efficacy in patients with HPV-positive or -negative disease. The primary efficacy end point was EOT CR rate. Sample size calculations for the expansion cohorts were based on EOT response data from a similar study using standard high-dose cisplatin in the same treatment groups.27 Sample size calculations were based on a 1-arm binomial design with a type I error rate of 5% and power of 80%. For the HPV-positive cohort, 34 patients were required to show an improvement in EOT CR rate from 74% to 90%. For the HPV-negative cohort, 23 patients were required to show an improvement in EOT CR rate from 50% to 75%.
Safety and tolerability were assessed by clinical review of all relevant parameters, including AEs, laboratory tests, treatment compliance, and vital signs. Survival outcomes were estimated by the Kaplan-Meier method. Patients with missing PFS or OS data were censored to the time of their last visit. Treatment group comparisons of mean radiotherapy and chemotherapy doses were evaluated by Welch’s t test. Pembrolizumab discontinuation because of irAEs and completion of all doses were compared using Barnard’s and χ2 tests, respectively.
RESULTS
Patient Characteristics
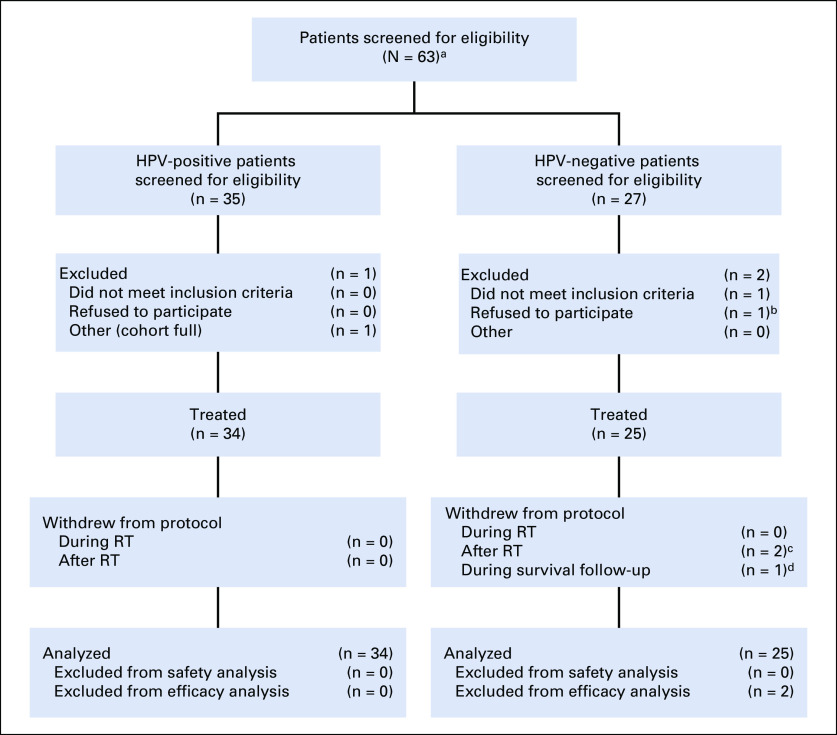
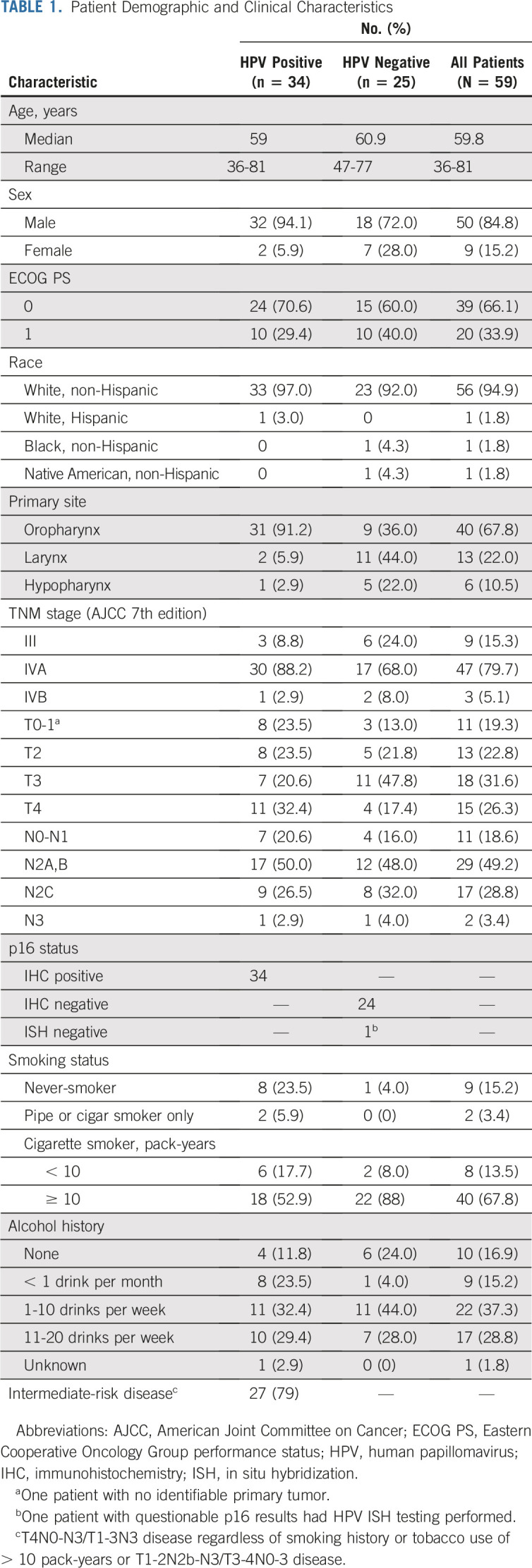
Patients were enrolled from November 2015 to October 2018. The DSMB reviewed safety lead-in data on March 8, 2016, and determined that there were no SAEs or grade ≥ 3 irAEs and the study could continue enrollment to 27 patients. The study was paused on August 23, 2016, for planned efficacy analysis.28 Expansion cohorts reopened on January 12, 2017, and concluded enrollment in October 2018. Of 63 total patients screened, 59 were evaluable (HPV positive, n = 34; HPV negative, n = 23) for the primary safety end point and 57 were evaluable for the efficacy end points. As outlined in Figure 1, 4 participants were excluded before treatment and 2 withdrew during active treatment and were replaced per study protocol criteria. Demographic and baseline disease characteristics of the evaluable patients are listed in Table 1. PD-L1 status was high in both groups based on both the CPS ≥ 1 and ≥ 20 cutoffs, as summarized in Appendix Table A1 (online only).
FIG 1.
Patient screening and disposition. RT, radiotherapy. (a) One screening failure before determination of human papillomavirus (HPV) status. (b) Was enrolled but withdrew before any study treatment because of insurance reasons. (c) Two patients withdrew before day-150 efficacy analysis and replaced per the protocol replacement strategy. (d) One patient was withdrawn after day 150 at physician’s discretion because of incarceration and was not replaced.
TABLE 1.
Patient Demographic and Clinical Characteristics
Treatment Delivery and Compliance
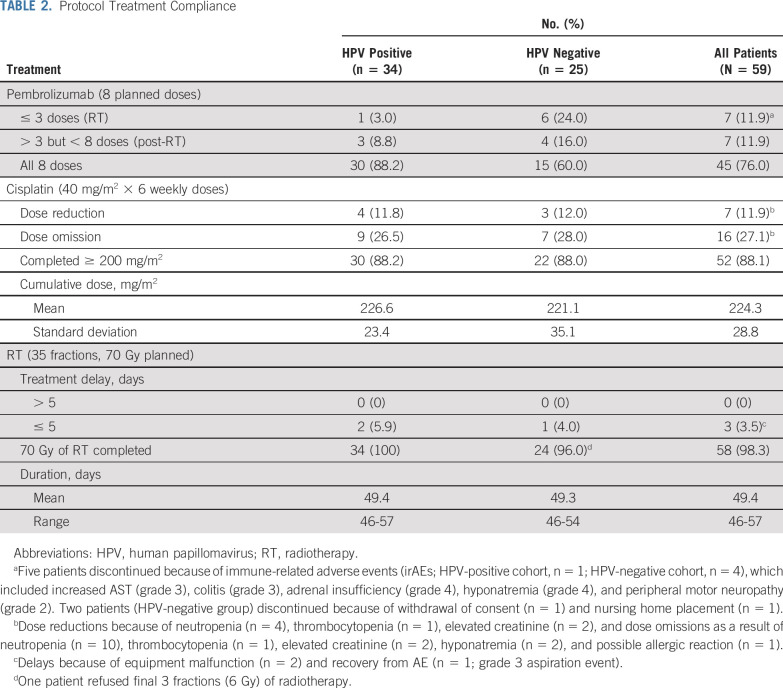
Detailed treatment compliance and toxicity events are listed in Table 2. A total of 59 patients started study therapy; 2 withdrew consent before treatment completion (both patients declined consolidation treatment because of travel concerns and inability to comply with protocol requirements). As a result, 59 patients were evaluated for safety, and 57 were included for response assessments; 76% of participants completed all 8 of the planned pembrolizumab doses. Pembrolizumab discontinuation because of irAEs during chemoradiotherapy occurred in 5 patients (8.8%). Discontinuation because of irAEs was higher in the HPV-negative cohort (16%) compared with the HPV-positive cohort (2.9%), but this was not statistically significant (P = .0841). These irAEs were reversible with drug discontinuation and corticosteroid therapy, if indicated. One patient discontinued before starting consolidation treatment because of nursing home placement and did not continue therapy per protocol. Another patient discontinued because of withdrawal of consent. After chemoradiotherapy, 11.9% of patients discontinued therapy. None of these discontinuations were because of irAEs; rather, they were related to nonprotocol-related procedures (early neck dissection, n = 2; prolonged hospitalization, n = 1; acute kidney injury, n = 1; patient declined, n = 1; withdrawal of consent, n = 1); however, in 1 patient, pembrolizumab was held at the investigator’s discretion because of concern for worsening postradiotherapy Lhermitte syndrome–like symptoms.
TABLE 2.
Protocol Treatment Compliance
Cisplatin dose reductions and omissions predominantly resulting from myelosuppression and elevated creatinine. All but 1 of the participants received the full planned dose of radiotherapy, without any treatment delays > 5 days. Evaluating treatment compliance between the HPV-negative and -positive groups, the only significant difference was in those completing all 8 doses of pembrolizumab (HPV positive, 88.2% v HPV negative, 60%; P = .0118).
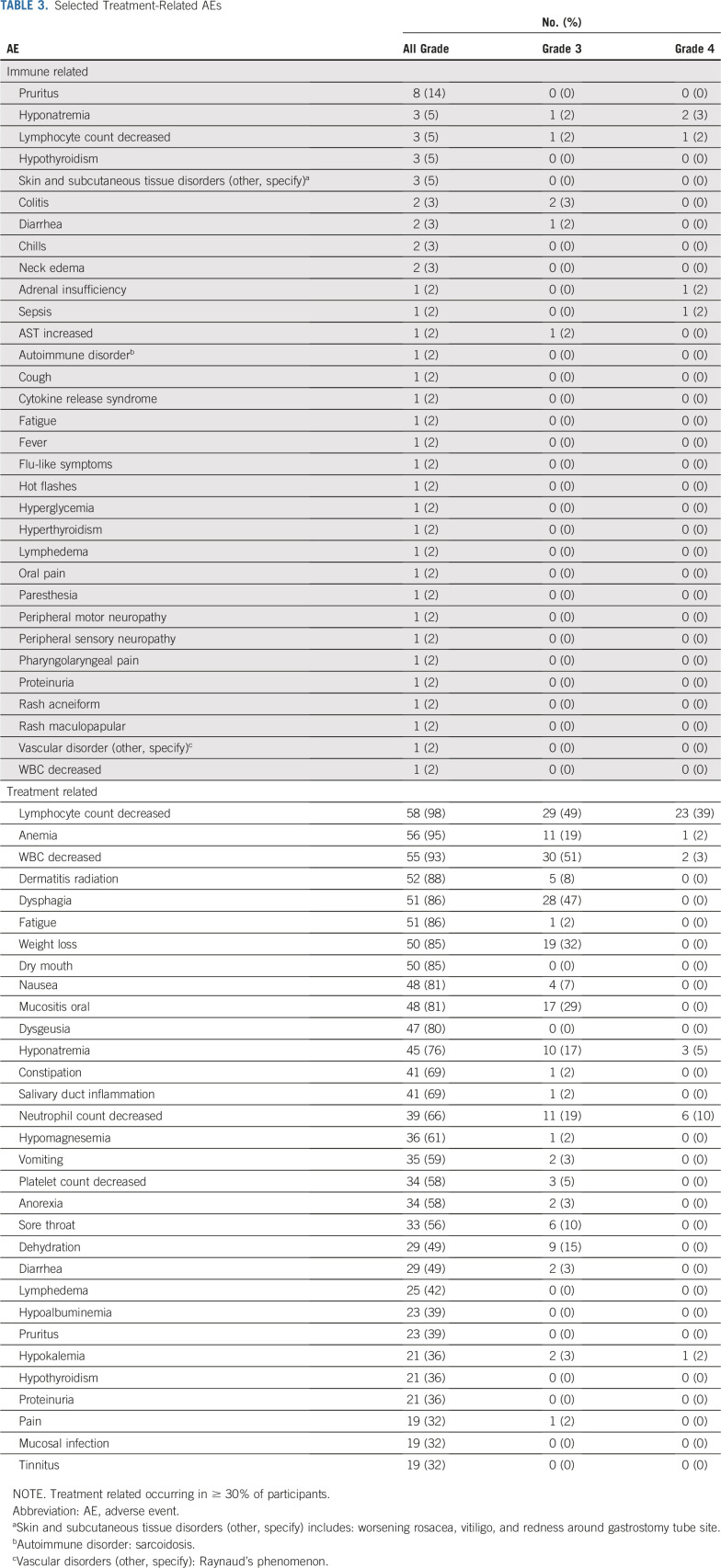
Toxicity
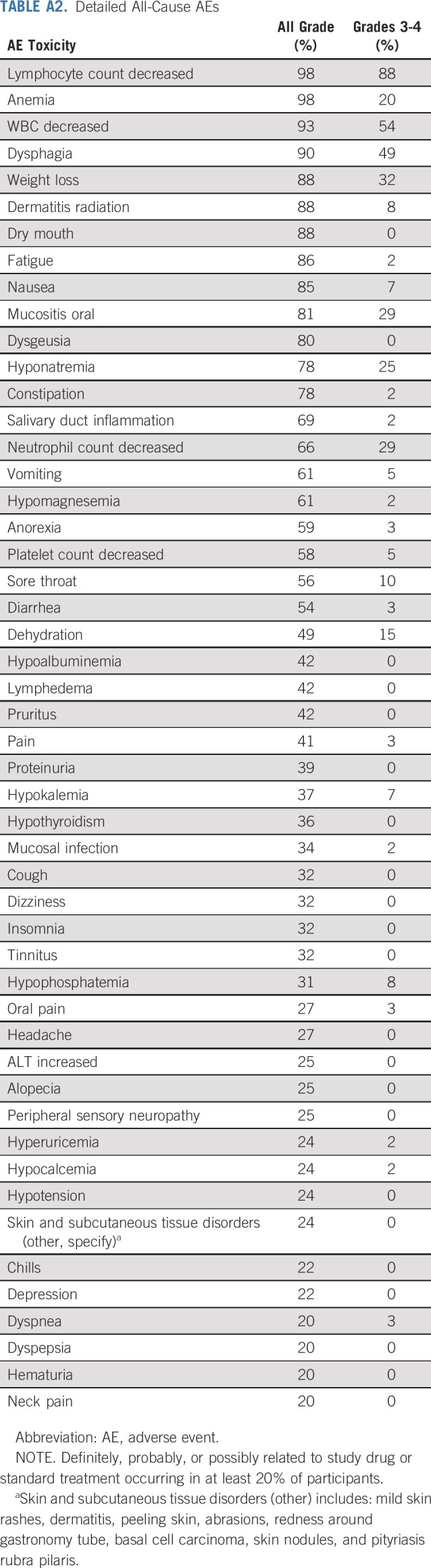
Pooled treatment-related (irAEs and any treatment-related AEs) and all-cause toxicities are listed in Table 3 and Appendix Table A2 (online only), respectively. No significant difference in toxicity was seen between the 2 cohorts. There was 1 patient death resulting from cardiac arrest without prior progression in the HPV-negative cohort before response assessment but after completion of chemoradiotherapy. This was felt to be unrelated to study therapy; the patient had discontinued pembrolizumab after 2 doses because of increased AST (grade 3), which had resolved with corticosteroid treatment 15 weeks before death. Hematologic and electrolyte abnormalities were the only grade 4 toxicities experienced. Beyond these, common grade 3 chemoradiotherapy-related toxicities occurring at least once included dysphagia, weight loss, oral mucositis, dehydration, sore throat, radiation dermatitis, nausea, vomiting, and anorexia.
TABLE 3.
Selected Treatment-Related AEs
Response
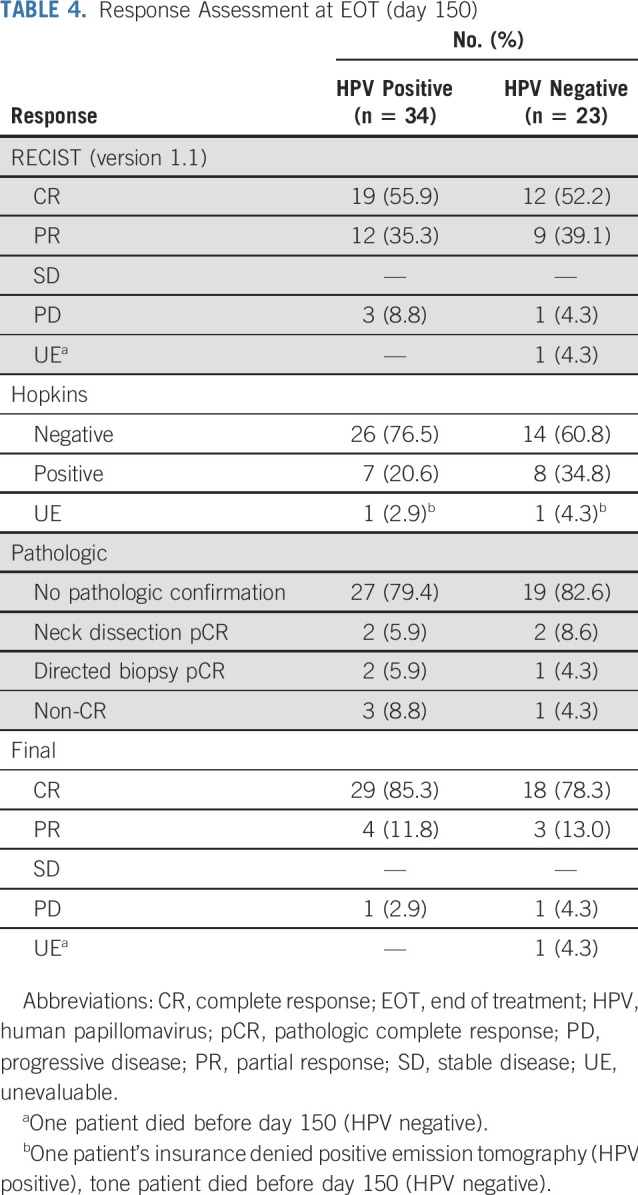
Of the 57 per-protocol population, all were evaluable except for the patient who died during study treatment. Detailed response data for the HPV-positive and -negative cohorts are listed in Table 4. In the HPV-positive group, 19 (55.9%) achieved a CR based on RECIST (version 1.1) criteria. Of the 12 (35.3%) with a RECIST (version 1.1) partial response (PR), an additional 8 patients (23.5%) were determined to have a CR based on PET Hopkins criteria. Of the remaining patients without an imaging CR, an additional 2 (5.9%) were determined to have a pathologic CR (neck dissection, n = 1; biopsy of distant site, n = 1). As a result, the final overall CR rate for the HPV-positive group at EOT was 85.3%. Two additional patients with an imaging PR did not undergo confirmatory surgery or biopsy, because there was no apparent clinical disease to biopsy. These patients both achieved a CR on 12-month imaging. In the HPV-negative cohort, 12 (52.2%) achieved a CR based on RECIST (version 1.1) criteria. Of the 9 (39.1%) with a RECIST (version 1.1) PR, an additional 5 (21.7%) were determined to have a CR based on PET Hopkins criteria. One additional patient (4.3%) with an imaging PR underwent neck dissection confirming pathologic CR. As a result, the final CR rate for the HPV-negative cohort at EOT was 78.3%.
TABLE 4.
Response Assessment at EOT (day 150)
Survival
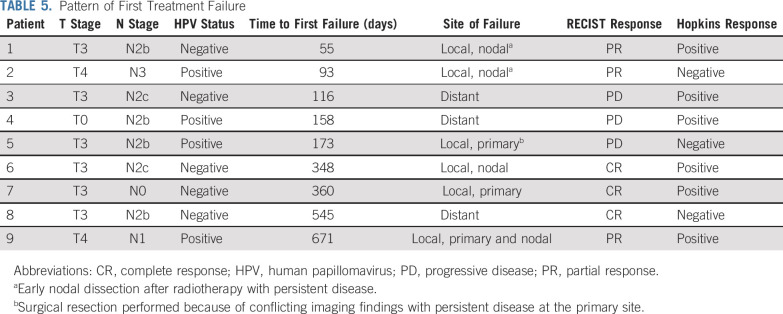
Median follow-up time was 28.4 months (range, 9.5-40.1 months) and 17.5 months (range, 5.3-38.5 months) for the HPV-positive and -negative cohorts, respectively. In addition to 1 patient death (HPV-negative cohort) during study treatment, 8 deaths occurred during survival follow-up (HPV positive, n = 2; HPV negative, n = 6), with 4 resulting from disease recurrence and 4 from other causes (2nd primary cancer, motor vehicle accident, GI bleed, respiratory failure resulting from pneumonia). On the basis of current follow-up, 1- and 2-year OS and PFS estimates for both cohorts are shown in Figure 2. For the HPV-positive cohort, 2-year OS was 97.1% (95% CI, 80.9% to 99.6%) and PFS was 92.8% (95% CI, 73.7% to 98.2%). In the HPV-positive cohort, 1 patient experienced distant failure, 2 had persistent locoregional disease, and 1 had late locoregional relapse. Follow-up time limited estimation of 2-year OS and PFS for the HPV-negative cohort, but 1-year OS was 86.5% (95% CI, 63.8% to 95.5%) and PFS was 72.6% (95% CI, 48.7% to 86.7%). In this group, 2 patients experienced distant failure and 3 experienced locoregional failure (persistent disease, n = 1; recurrent disease, n = 2). Detailed information on patterns of first failure by HPV status is listed in Table 5.
FIG 2.
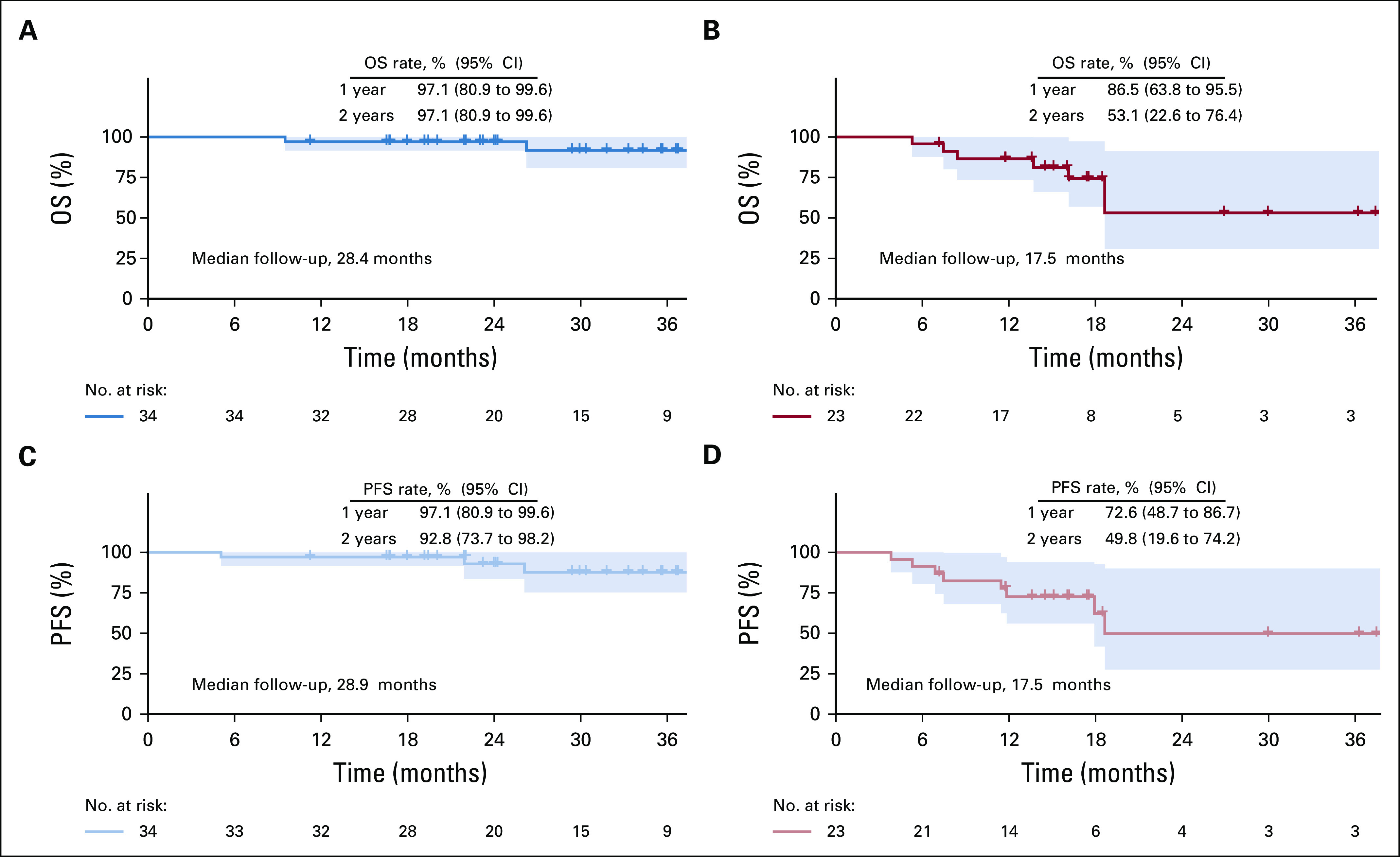
Kaplan-Meier estimates of survival. Overall survival (OS) for (A) human papillomavirus (HPV)–positive and (B) HPV-negative cohorts. Progression-free survival (PFS) for (C) HPV-positive and (D) HPV-negative cohorts.
TABLE 5.
Pattern of First Treatment Failure
DISCUSSION
This prospective phase IB study combining pembrolizumab with standard chemoradiotherapy demonstrates that this approach is safe in LA HNSCC. Pembrolizumab discontinuations because of irAEs were in line with monotherapy, and no new safety signals were identified.10,26 Furthermore, the addition of pembrolizumab did not impair delivery of curative doses of standard therapy. Although optimal cisplatin dosing during definitive chemoradiotherapy is not clearly defined, cumulative doses ≥ 200 mg/m2 are generally recommended.16,29 In pivotal studies using high-dose cisplatin, 84% to 93% of patients have achieved this dose.13,30 In our study, 88.1% of patients achieved this target dose, with dose reductions and omissions as a result of anticipated toxicities. Radiotherapy delivery was also not compromised, with related toxicities (mucositis, radiation dermatitis, and dysphagia) limited to grade 3 and comparable to those in large randomized trials in this setting.4 In addition to an acceptable safety profile, early efficacy data are promising.
Despite not meeting the final EOT response end point in the HPV-positive group, the 2-year OS rate of 97.1% was encouraging, because 79% of participants had intermediate-risk disease. Only 1 distant failure occurred in this group; however, follow-up was limited, and late recurrences can occur in HPV-positive disease. For the HPV-negative cohort, although the follow-up period was too short to draw major conclusions, the EOT CR rate exceeded the primary end point goal, and 1-year survival data are supportive of further investigation. Treatment failures did occur in both groups; however, recurrence rates were consistent with historical disease control rates.
Although other chemoradiotherapy combination trials have used response end points,31 our use of a PET imaging end point posed a challenge in this study. Despite not being widely evaluated in large, prospective trials, we chose this modality because of increasing clinical use and supporting data demonstrating a strong concordance of EOT PET response with recurrence and survival.32,33 In this study, use of PET led to a high false-positive rate, which in turn may have contributed to the failure to meet the final efficacy end point for the HPV-positive group. However, with surgical confirmation and continued imaging surveillance, CR was confirmed in an additional 4 patients (11.8%). Variability in PET imaging and interpretation across centers could pose challenges in accurate response assessment despite the use of established scoring criteria. This highlights the importance of confirmation of response as this treatment approach moves forward.
It is also important to note that although our overall compliance rates were similar to those seen with other combinations,34 significantly fewer HPV-negative participants were able to complete all planned pembrolizumab doses compared with HPV-positive participants (60% v 88.2%; P = .0118). Although there was a nonsignificant increase in irAEs leading to pembrolizumab discontinuation in the HPV-negative group, a majority did not result directly from drug toxicity. This is important to consider, because this group historically has a higher rate of comorbidities and overall mortality.35 Despite these discontinuations, chemoradiotherapy delivery was similar between the groups.
Our study has several limitations. First, on the basis of the single-arm design, we cannot make direct toxicity comparisons with standard-of-care chemoradiotherapy. Second, follow-up time was limited, so we cannot draw any conclusions about late toxicities or long-term survival at this point. The small sample size limits the use of PD-L1 as a predictive biomarker and additional subgroup analyses. Despite these limitations, early toxicity and outcome data from this study have been provocative enough to support the development of several phase III trials using a similar approach.36,37
In conclusion, this study demonstrates an acceptable safety profile and efficacy signal from the addition of pembrolizumab to definitive chemoradiotherapy in HNSCC. Confirmation of the safety and efficacy of this approach awaits the completion of larger randomized studies.
ACKNOWLEDGMENT
We thank the patients who participated in this study and our clinical research coordinators: Staci Vogel, Jenna Hove, Cindy Horst, Corliss Miller, Mark Weichel, and Amanda Natsuhara. We also thank the Sanford Research Biostatistics Core Members, Valerie Bares, PhD, and Solomon Adu, MS.
APPENDIX
TABLE A1.
PD-L1 Expression by HPV Status
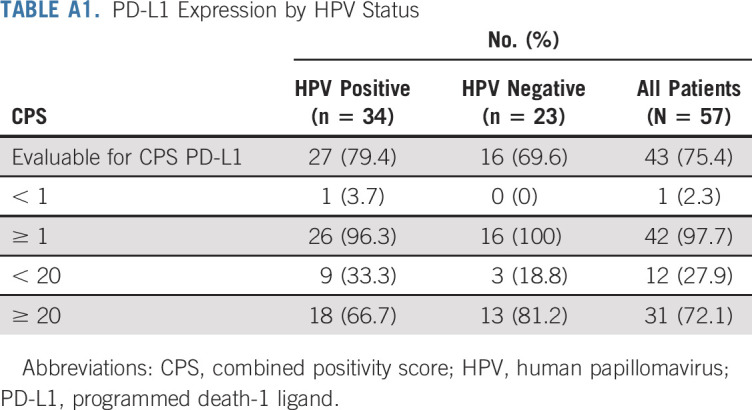
TABLE A2.
Detailed All-Cause AEs
PRIOR PRESENTATION
Presented at the 52nd ASCO Annual Meeting, Chicago, IL, June 3-7, 2016; 31st Annual Meeting and Associated Programs of the Society for Immunotherapy of Cancer (SITC), National Harbor, MD, November 9-13, 2016; 53rd ASCO Annual Meeting, Chicago, IL, June 2-6, 2017; and 33rd Annual Meeting and Pre-Conference Programs of SITC, Washington, DC, November 7-11, 2018.
SUPPORT
Supported by the Merck Investigator Studies Program and by Center of Biology Research Excellence Award No. 5P20-GM103548-09, sponsored by the National Institute of General Medical Science.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Steven F. Powell, Michele M. Lohr, Steven C. McGraw, Ryan K. Nowak, Miran J. Blanchard, Lora J. Black, Paul A. Thompson, John H. Lee, Ezra E. W. Cohen, William C. Spanos
Administrative support: Christie A. Ellison, John H. Lee
Provision of study material or patients: Kathryn A. Gold, Christopher J. Sumey, Michele M. Lohr, Ryan K. Nowak, Ashley W. Jensen, Miran J. Blanchard, Ezra E. W. Cohen, William C. Spanos
Collection and assembly of data: Steven F. Powell, Kathryn A. Gold, Mark M. Gitau, Michele M. Lohr, Ashley W. Jensen, Miran J. Blanchard, Christopher D. Fischer, Julie Bykowski, Christie A. Ellison, Lora J. Black, Paul A. Thompson, Juan L. Callejas-Valera, Ezra E. W. Cohen, William C. Spanos
Data analysis and interpretation: Steven F. Powell, Kathryn A. Gold, Christopher J. Sumey, Michele M. Lohr, Miran J. Blanchard, Julie Bykowski, Christie A. Ellison, Paul A. Thompson, Juan L. Callejas-Valera, John H. Lee, Ezra E. W. Cohen, William C. Spanos
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Safety and Efficacy of Pembrolizumab With Chemoradiotherapy in Locally Advanced Head and Neck Squamous Cell Carcinoma: A Phase IB Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Steven F. Powell
Consulting or Advisory Role: Bristol Myers Squibb (Inst)
Research Funding: Merck Sharp & Dohme (Inst), Novartis (Inst), Genentech/Roche (Inst), Incyte (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Vyriad (Inst), Actuate Therapeutics (Inst), AstraZeneca/MedImmune (Inst)
Kathryn A. Gold
Consulting or Advisory Role: Takeda, AstraZeneca, Regeneron, Rakuten
Speakers’ Bureau: Takeda
Research Funding: Pharmacyclics, Bristol Myers Squibb, AstraZeneca, ARIAD, Roche/Genentech, AbbVie
Christopher J. Sumey
Consulting or Advisory Role: Bristol Myers Squibb
Miran J. Blanchard
Stock and Other Ownership Interests: Celgene, Gilead Sciences
Christopher D. Fischer
Travel, Accommodations, Expenses: GE Healthcare
Julie Bykowski
Consulting or Advisory Role: Spiral Therapeutics
Travel, Accommodations, Expenses: Spiral Therapeutics
Lora J. Black
Travel, Accommodations, Expenses: Clinical Research As a Care Option (Inst)
Travel, Accommodations, Expenses: ASCO (Inst)
John H. Lee
Employment: NantKwest
Leadership: NantKwest
Stock and Other Ownership Interests: NantKwest
Ezra E. W. Cohen
Consulting or Advisory Role: ALX Oncology, Ascendis, Bayer, Bioline Rx, Bristol Myers Squibb, Debiopharm Group, Dynavax, Merck Sharp & Dohme, Merck, Regeneron, Sanofi
William C. Spanos
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Ambu, Regeneron
Travel, Accommodations, Expenses: NantKwest, Regeneron, Ambu
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pignon J-P, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gau M, Karabajakian A, Reverdy T, et al. Induction chemotherapy in head and neck cancers: Results and controversies. Oral Oncol. 2019;95:164–169. doi: 10.1016/j.oraloncology.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dovedi S, Adlard A, Lipowska-Bhalla G, et al. The anti-tumor immune response generated by radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD-L1 blockade. J Immunother Cancer. 2014 ;2(suppl 3):O9. [Google Scholar]
- 7.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran L, Allen CT, Xiao R, et al. Cisplatin alters antitumor immunity and synergizes with PD-1/PD-L1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol Res. 2017;5:1141–1151. doi: 10.1158/2326-6066.CIR-17-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh F, Duluc D, Imai N, et al. Chemoradiotherapy-induced upregulation of PD-1 antagonizes immunity to HPV-related oropharyngeal cancer. Cancer Res. 2014;74:7205–7216. doi: 10.1158/0008-5472.CAN-14-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 11. Cohen EEW, Soulières D, Le Tourneau C, et al: Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 393:156-167, 2019 [Erratum: Lancet 393:132, 2019] [DOI] [PubMed]
- 12. Rischin D, Harrington KJ, Greil R, et al: Protocol-specified final analysis of the phase 3 KEYNOTE-048 trial of pembrolizumab (pembro) as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol 37, 2019 (suppl; abstr 6000) [Google Scholar]
- 13.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 14.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed A, Twardy B, Zordok MA, et al. Concurrent chemoradiotherapy with weekly versus triweekly cisplatin in locally advanced squamous cell carcinoma of the head and neck: Comparative analysis. Head Neck. 2019;41:1490–1498. doi: 10.1002/hed.25379. [DOI] [PubMed] [Google Scholar]
- 16.Szturz P, Wouters K, Kiyota N, et al. Weekly low-dose versus three-weekly high-dose cisplatin for concurrent chemoradiation in locoregionally advanced non-nasopharyngeal head and neck cancer: A systematic review and meta-analysis of aggregate data. Oncologist. 2017;22:1056–1066. doi: 10.1634/theoncologist.2017-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar A, Chakravarty N, Bhatnagar S, et al. Efficacy and safety of concurrent chemoradiotherapy with or without nimotuzumab in unresectable locally advanced squamous cell carcinoma of head and neck: Prospective comparative study—ESCORT-N study. South Asian J Cancer. 2019;8:108–111. doi: 10.4103/sajc.sajc_38_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fury MG, Lee NY, Sherman E, et al. A phase 2 study of bevacizumab with cisplatin plus intensity-modulated radiation therapy for stage III/IVB head and neck squamous cell cancer. Cancer. 2012;118:5008–5014. doi: 10.1002/cncr.27498. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenson KM, Haraf DJ, Pelzer H, et al. The role of cervical lymphadenectomy after aggressive concomitant chemoradiotherapy: The feasibility of selective neck dissection. Arch Otolaryngol Head Neck Surg. 2000;126:950–956. doi: 10.1001/archotol.126.8.950. [DOI] [PubMed] [Google Scholar]
- 22.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 23.Mehanna H, Wong W-L, McConkey CC, et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med. 2016;374:1444–1454. doi: 10.1056/NEJMoa1514493. [DOI] [PubMed] [Google Scholar]
- 24.Marcus C, Ciarallo A, Tahari AK, et al. Head and neck PET/CT: Therapy response interpretation criteria (Hopkins Criteria)—Interreader reliability, accuracy, and survival outcomes. J Nucl Med. 2014;55:1411–1416. doi: 10.2967/jnumed.113.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24:392–397. doi: 10.1097/PAI.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: Results from a single-arm, phase II study. J Clin Oncol. 2017;35:1542–1549. doi: 10.1200/JCO.2016.70.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Powell SF, Spanos WC, Black LJ, et al: Phase II study of dichloroacetate (DCA) in combination with chemoradiation (CRT) for unresected, locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN). J Clin Oncol 33, 2015 (suppl; abstr e17089) [Google Scholar]
- 28. Powell SF, Gitau MM, Sumey CJ, et al: Safety of pembrolizumab with chemoradiation (CRT) in locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN). J Clin Oncol 35, 2017 (suppl; abstr 6011) [Google Scholar]
- 29.Ang KK. Concurrent radiation chemotherapy for locally advanced head and neck carcinoma: Are we addressing burning subjects? J Clin Oncol. 2004;22:4657–4659. doi: 10.1200/JCO.2004.07.962. [DOI] [PubMed] [Google Scholar]
- 30. Ang K, Zhang Q, Wheeler RH, et al: A phase III trial (RTOG 0129) of two radiation-cisplatin regimens for head and neck carcinomas (HNC): Impact of radiation and cisplatin intensity on outcome. J Clin Oncol 28, 2010 (suppl; abstr 5507) [Google Scholar]
- 31.Pfister DG, Su YB, Kraus DH, et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: A pilot phase II study of a new combined-modality paradigm. J Clin Oncol. 2006;24:1072–1078. doi: 10.1200/JCO.2004.00.1792. [DOI] [PubMed] [Google Scholar]
- 32.Paidpally V, Tahari AK, Lam S, et al. Addition of 18F-FDG PET/CT to clinical assessment predicts overall survival in HNSCC: A retrospective analysis with follow-up for 12 years. J Nucl Med. 2013;54:2039–2045. doi: 10.2967/jnumed.113.121285. [DOI] [PubMed] [Google Scholar]
- 33.Gupta T, Master Z, Kannan S, et al. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: A systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38:2083–2095. doi: 10.1007/s00259-011-1893-y. [DOI] [PubMed] [Google Scholar]
- 34.Egloff AM, Lee J-W, Langer CJ, et al. Phase II study of cetuximab in combination with cisplatin and radiation in unresectable, locally advanced head and neck squamous cell carcinoma: Eastern Cooperative Oncology Group trial E3303. Clin Cancer Res. 2014;20:5041–5051. doi: 10.1158/1078-0432.CCR-14-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skillington SA, Kallogjeri D, Lewis JS, Jr, et al. Prognostic importance of comorbidity and the association between comorbidity and p16 in oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:568–575. doi: 10.1001/jamaoto.2016.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Machiels J-PH, Licitra L, Rischin D, et al: KEYNOTE-412: Pembrolizumab (pembro) in combination with chemoradiation versus chemoradiation alone in locally advanced head and neck squamous cell carcinoma (LA-HNSCC). J Clin Oncol 35, 2017 (suppl; abstr TPS6090) [Google Scholar]
- 37. Lee NY, Ferris RL, Beck JT, et al: JAVELIN head and neck 100: A phase 3 trial of avelumab in combination with chemoradiotherapy (CRT) vs CRT for 1st-line treatment of locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). J Clin Oncol 35, 2017 (suppl; abstr TPS6093) [Google Scholar]