Abstract
Origanum majorana (L.) is an herb used in the treatment of diseases related to the nervous system in traditional medicine (e.g. as an anticonvulsant and sedative). The present study was conducted to investigate the antidepressant-like effects of Origanum majorana essential oil (OMEO) on mice in the forced swimming test (FST). The animals were intraperitoneally (i.p.) injected with OMEO (10–80 mg/kg) 1 h before the FST. To assess the involvement of the monoaminergic system in the antidepressant activity of OMEO, different pharmacological antagonists were administered 15 min before OMEO administration (80 mg/kg). The administration of OMEO (40 and 80 mg/kg, i.p.) decreased immobility time and increased swimming and climbing times significantly. OMEO did not cause any changes in spontaneous locomotor function in the open-field test (OFT). The pre-treatment of the animals with SCH23390, sulpiride, haloperidol, WAY100135, p-chlorophenylalanine (pCPA), ketanserin, prazosin, yohimbine, reserpine, but not propranolol, inhibited the anti-immobility effect of OMEO in the FST. A combination of sub-effective doses of fluoxetine (5 mg/kg, i.p.) or imipramine (5 mg/kg, i.p.) with OMEO (10 mg/kg, i.p.) increased the antidepressant-like effects. OMEO showed antidepressant-like effects through involvement with the dopaminergic (D1 and D2), serotonergic (5HT1A, 5-HT2A receptors) and noradrenergic (α1 and α2 adrenoceptors) systems.
Keywords: Antidepressant, Monoamines, Forced swimming test, Mice, Origanum majorana
Abbreviations: ANOVA, analysis of variance; DA, dopamine; FST, forced swimming test; GC-MS, gas chromatography-mass spectrometry analysis; 5-HT, 5-hydroxytryptamine; KI, Kovats index; NA, noradrenaline; OFT, open-field test; OMEO, Origanum majorana essential oil; p-CPA, p-chlorophenylalanine; RT, retention time
Chemical compounds studied in this article: WAY 100135 (PubChem CID: 14801905), Ketanserin (PubChem CID: 3822), SCH 23390 (PubChem CID: 5018), Sulpiride (PubChem CID: 5355), p-chlorophenylalanine [pCPA] (PubChem CID: 4652), Reserpine (PubChem CID: 5770)
Graphical abstract
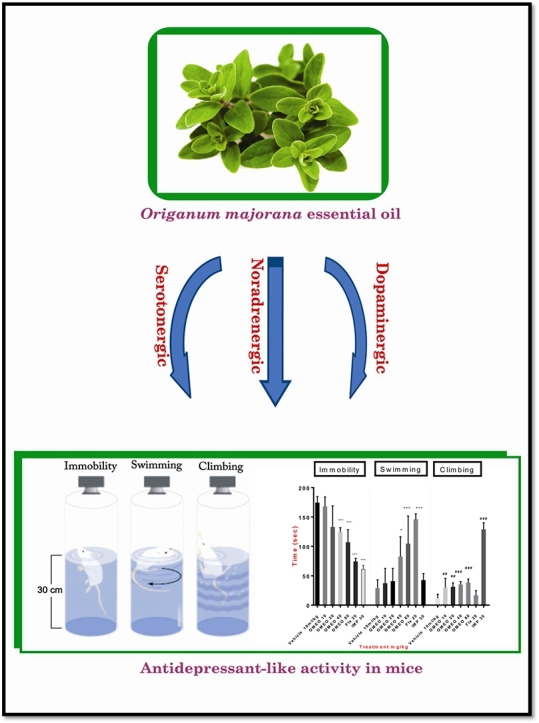
Highlights
-
•
Origanum majorana (L.) belongs to the mint family (Labiatae) and is used extensively in traditional medicine (in the treatment of diseases related to the nervous system) and the food and cosmetic industries.
-
•
The main monoterpene alcohols of OMEO (especially terpinene-4-ol) may be resposbile for their antidepressant-like activity.
-
•
The administration of Origanum majorana essential oil decreased immobility time and increased swimming and climbing times significantly in the FST.
-
•
Origanum majorana essential oil showed antidepressant-like effects through involvement with the dopaminergic (D1 and D2), serotonergic (5HT1A, 5-HT2A receptors) and noradrenergic (α1 and α2 adrenoceptors) systems.
1. Introduction
Depression is a common mood disorder. The WHO estimates that the highest disease burden will pertain to this disorder by 2030.1 The neurobiology of depression is not completely known, but the monoaminergic system plays a role in it.2 Studies usually report that conventional antidepressants promote synaptic activity in the three monoamines including serotonin (5-HT), noradrenaline (NA) and dopamine (DA) by blocking the neuronal reuptake of these monoamines.3 These antidepressants have disadvantages as well, including certain side-effects and the non-responsiveness of a large number of people to antidepressants.1 People suffering from depression need conventional drugs that have the least amount of side-effects. Medicinal herbs and their derivatives may be better options in terms of reduced side-effects and improved efficacy of the treatment.4
Origanum majorana (L.) belongs to the mint family (Labiatae) and is used extensively in traditional medicine and the food and cosmetic industries. Origanum majorana is an herb commonly growing in the Mediterranean region and used as a carminative, antispasmodic, diaphoretic and diuretic agent.5 Origanum majorana affects the nervous system6; however, there is still a lack of experimental evidence on these effects. Studies have documented anticonvulsant activities for Origanum majorana.7 Flavonoids are largely present in Origanum majorana and exert sedative effects on the nervous system.8
The forced swimming test (FST) is used extensively in pharmacological models for evaluating antidepressant effects. This test can determine antidepressant activity by detecting a decline in immobility time. Porsolt et al.9 showed that the FST is quite sensitive for studying rodent behaviors, especially in mice, and predicts the antidepressant potential by detecting a decline in immobility time.
Considering that the antidepressant action of OMEO has not yet been elucidated in literature and its involvement in the central nervous system requires further investigation, the present study was conducted to investigate the involvement of the monoaminergic system in the antidepressant-like effects of OMEO on mice using the FST.
2. Methodology
2.1. Preparation of OMEO
Fresh marjoram (Origanum majorana) was prepared and recognized by an experienced botanist in Adonis Gol Daru Co. (Tehran, Iran). The voucher specimen (AGD. 2219) was deposited at the herbarium of the same Pharmaceutical Co. The plant samples were subsequently dried at room temperature and then extracted for their essential oil using a Clevenger apparatus.
2.2. Gas chromatography-mass spectrometry analysis (GC-MS)
An Agilent 6890 GC apparatus equipped with an HP-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm) and an Agilent 5973 mass detector were used for the separation and detection of chemical compounds. The chemical compounds were identified as described by Adams10 and the data are presented in Table 1. The Kovats index and a homologous series of n-alkanes (C8-C25) were used for the identification. The mass spectra were ultimately compared to the samples in the Wiley library and the data obtained through literature.
Table 1.
The chemical compounds of Origanum majorana essential oil.
| Peak no. | Compound | RT (min) | KI | Concentration (%) |
|---|---|---|---|---|
| 1 | Thujene | 6.337 | 930 | 0.60 |
| 2 | α-Pinene | 6.568 | 939 | 0.77 |
| 3 | Sabinene | 8.011 | 975 | 6.21 |
| 4 | β- Pinene | 8.099 | 979 | 0.31 |
| 5 | β- Myrcene | 8.679 | 991 | 1.49 |
| 6 | α- Phellandrene | 9.172 | 1003 | 0.24 |
| 7 | α -Terpinene | 9.727 | 1017 | 7.98 |
| 8 | ρ-Cymene | 10.035 | 1025 | 2.32 |
| 9 | β - Phellandrene | 10.215 | 1030 | 2.64 |
| 10 | γ-Terpinene | 11.586 | 1060 | 12.88 |
| 11 | cis-Sabinene hydrate | 11.909 | 1070 | 2.92 |
| 12 | α-Terpinolene | 12.808 | 1089 | 3.36 |
| 13 | trans-Sabinene hydrate | 13.393 | 1098 | 8.47 |
| 14 | (E)-p-Menth-2-en-1-ol | 14.328 | 1140 | 2.25 |
| 15 | Terpinene-4-ol | 17.132 | 1177 | 32.69 |
| 16 | α-Terpineol | 17.599 | 1189 | 5.25 |
| 17 | trans- Piperitol | 18.333 | 1208 | 0.83 |
| 18 | Linalyl acetate | 20.881 | 1257 | 0.93 |
| 19 | Neryl acetate | 26.524 | 1362 | 0.18 |
| 20 | trans-Caryophyllene | 27.746 | 1418 | 2.31 |
| 21 | α- Humulene | 28.969 | 1455 | 0.12 |
| 22 | Bicyclogermacrene | 30.494 | 1494 | 1.60 |
| 23 | Spathulenol | 33.128 | 1578 | 0.16 |
| 24 | Caryophyllene oxide | 33.272 | 1583 | 0.31 |
| Total | 96.82 |
2.3. Chemicals
The chemicals used included SCH 23390 (Sigma-Aldrich, St. Louis, MO, USA), sulpiride (Sigma-Aldrich, St. Louis, MO, USA), haloperidol (Caspian Tamin, Iran), prazosin hydrochloride (Razak Pharmaceutical Co., Iran), yohimbine hydrochloride (Iran Daru Co., Iran), p-chlorophenylalanine (pCPA; Sigma-Aldrich, St. Louis, MO, USA), WAY100135 (Sigma-Aldrich, St. Louis, MO, USA), ketanserin (Sigma-Aldrich, St. Louis, MO, USA), propranolol (Abidi Pharmaceutical Co., Iran), reserpine (Sigma-Aldrich, St. Louis, MO, USA), fluoxetine (Arya Pharmaceutical Co., Iran), and imipramine (Amin Pharmaceutical Co., Iran). The chemicals and OMEO used in this study were dissolved in normal saline with 10% Tween-80, in respective order, and administered intraperitoneally (i.p.) at a constant volume of 10 ml/kg, with the exception of SCH 23390, which were injected subcutaneously (s.c.). The FST was conducted 1 h after the single injection of the chemicals and OMEO.
2.4. Laboratory animals
Six-week-old male Naval Medical Research Institute (NMRI) mice weighing 20–30 g were obtained from Tehran University of Medical Sciences (Tehran, Iran). The mice were kept in cages (n = 6) in a 12-h light/dark cycle. The ambient temperature and humidity were maintained at 23–25 °C and 50 ± 10%, respectively. The mice had access to standard commercial pellet food (supplied from Razi Vaccine and Serum Research Institute, Karaj, Iran) and water ad libitum. The researchers complied with all the ethical principles of research recommended by Tehran University of Medical Sciences (#IR.TUMS.REC. 3045.Mar 2016) and the US guidelines for the care and use of laboratory animals.
2.5. Acute toxicity test
After 24 h of fasting, 36 NMRI mice were divided into six groups (each group consisting of six mice) and received a vehicle (normal saline with 10% Tween-80; 10 ml/kg) intraperitoneally and OMEO (100, 200, 300, 400 and 500 mg/kg). The mice were monitored for behavioral and toxicity signs for five hours. In addition, the number of deaths in 24 h was also recorded and the mice were observed for any other signs during the next two weeks.
2.6. The forced swimming test (FST)
The FST is used extensively in pharmacological models for evaluating antidepressant activity. This method can rapidly identify the antidepressant properties of substances. In the present study, the FST was used in the same manner described by Porsolt et al.9 The mice were forced to swim in an open cylindrical container (10 cm diameter × 25 cm height) filled with 15 cm of water at 25 ± 1 °C. Fifty-six NMRI mice were divided into seven groups as follows: Group I = vehicle (with 10% Tween 80; 10 ml/kg, i.p.); Groups II-V= OMEO (10, 20, 40, and 80 mg/kg; i.p., respectively); and Groups VI-VII = fluoxetine (20 mg/kg, i.p.) or imipramine (30 mg/kg, i.p.). The medication and essential oil administration schedule was chosen based on the authors' recent studies and a review of literature.11, 12, 13, 14, 15, 16 A time-sampling procedure was used to score multiple behaviors in a single viewing. The evaluators (two people) were blinded to the treatment and scored the mice for their behavioral responses as follows: 1) Immobility: The mice were judged as immobile when they tried to float on water without struggling and only tried to maintain their head above the water; 2) Swimming: In this phase, the mice were judged on the basis of active swimming motions and whether or not they tried to maintain their head above the water level more than the necessary amount, i.e. moving around the cylinder; 3) Climbing: The mice were judged on the basis of climbing and whether or not they performed active movements with their forepaws on the container walls. The test was randomly performed several times. The total immobility, swimming and climbing times were recorded in the last four minutes in a six-minute test using a video camera positioned directly above the cylinder. A reduction in immobility time and an increase in active response times, i.e. climbing or swimming times, were considered behavioral profiles consistent with antidepressant-like activity.9
2.7. The potential mechanisms of OMEO's antidepressant-like effects in the FST
2.7.1. Dopaminergic involvement
To examine the potential involvement of the dopaminergic system in the antidepressant-like action of OMEO, the mice were pre-treated with SCH 23390 (0.05 mg/kg, s.c., a dopamine D1 receptor antagonist), sulpiride (50 mg/kg, i.p., a dopamine D2 receptor antagonist) and haloperidol (0.2 mg/kg, i.p., non-selective dopamine receptor antagonist) 15 min before the i.p. administration of OMEO (80 mg/kg, i.p.) or vehicle (10 ml/kg, i.p.); 1 h after the treatment, the mice were exposed to FST.11,12
2.7.2. Serotonergic involvement
For examining the potential involvement of the serotonergic system in the antidepressant-like action of OMEO in the FST, the mice were pre-treated with pCPA (150 mg/kg, i.p; an inhibitor of serotonin synthesis) or the vehicle daily for three consecutive days. The mice were then treated with OMEO (80 mg/kg, i.p.) 15 min after the last pCPA, and 1 h after the treatment, they were exposed to the FST. In addition, the involvement of the 5-HT1 receptor subtype in the antidepressant action of OMEO was evaluated in the FST. The mice were pre-treated with WAY100135 (10 mg/kg, i.p., a selective 5-HT1A receptor antagonist) and treated with OMEO (80 mg/kg, i.p.) 15 min later, and the FST was performed 1 h after the said treatment. Additionally, for investigating the contribution of the 5HT2 receptor in the antidepressant effect of OMEO in the FST, the mice were pre-treated with ketanserin (5 mg/kg, i.p., a selective 5HT2A/C receptor antagonist) or the vehicle (10 ml/kg, i.p.); 15 min later, OMEO (80 mg/kg, i.p.) was injected, and the FST was performed after 1 h.11,12
2.7.3. Noradrenergic involvement
For investigating the potential involvement of the noradrenergic system in the antidepressant-like action of OMEO, the mice were pre-treated with prazosin (1 mg/kg i.p., an α1-adrenoceptor antagonist), propranolol (a β-adrenoceptor receptor antagonist) and yohimbine (1 mg/kg, i.p., an α2-adrenoceptor antagonist) 15 min before the i.p. treatment with OMEO (80 mg/kg, i.p.) or the vehicle (10 ml/kg, i.p.); the mice were exposed to the FST 1 h after the treatment.11,12
2.7.4. Effects of reserpine on the antidepressant-like effect of OMEO in the FST
The animals were pre-treated with reserpine (2 mg/kg, i.p., vesicular monoamine depleter) 4 h before the i.p. injection of OMEO (80 mg/kg, i.p.); 1 h later, the mice were exposed to the FST.11
2.7.5. Interactions of OMEO with common antidepressants in the FST
Sub-therapeutic doses of imipramine and fluoxetine (5 mg/kg, i.p.) were administered 15 min before i.p. treatment with the sub-therapeutic dose of OMEO (10 mg/kg, i.p.) and after 1 h of the co-treatment; the mice were exposed to the FST.11,12
2.8. Open-field test
The open-field test (OFT) was applied for the evaluation of the psychomotor stimulant activity. For investigating the potential effects of OMEO on exploratory behaviors, the mice were exposed to the OFT just as described by Brown et al.17 The apparatus used for the OFT was made of Plexiglas and was 50 cm in length, 50 cm in width and 25 cm in height. The surface of the box was separated into 16 8-cm squares. One hour after the injection of OMEO (10, 20, 40 and 80 mg/kg; i.p.) in the mice (n = 6), they were placed in the center of the box and permitted to explore the apparatus freely for 5 min. After this period, the animals were moved back to their cages. The open-field apparatus was cleaned by 10% ethanol and dried in between the tests. The number of crossings (squares crossed with all the paws) and the number of rearings (raising the front paws) were calculated in a 5-min period. The count for the squares traveled by the mice with their four paws was recorded as a sign of locomotor activity, and the count for the rearings was taken as a sign of exploratory behavior.
2.9. Statistical analysis
The data are presented as mean ± standard deviation [S.D.] (n = 6) and were analyzed using the one-way (dose-response curves and time-course curves) or two-way (evaluating of the mechanism of action) ANOVA followed by Tukey's post-hoc test when appropriate. P < 0.05 was considered statistically significant. All the statistical analyses were performed in Prism-7 software (GraphPad Software, Inc., San Diego, CA, USA).
3. Results
3.1. GC-MS analysis data
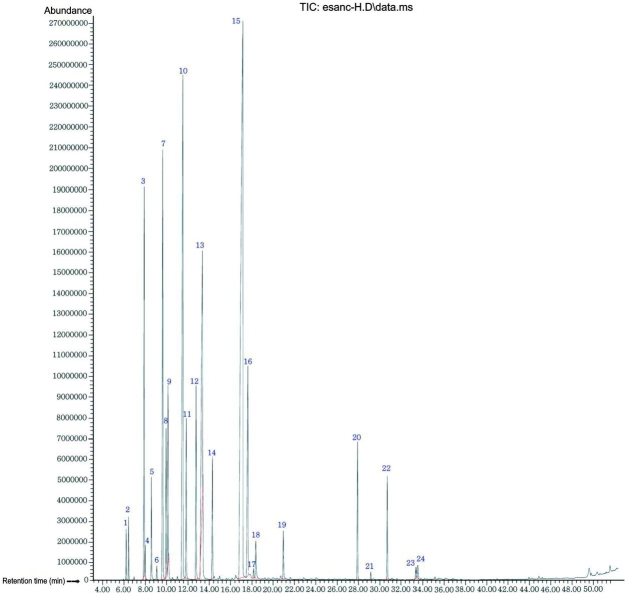
According to the GC-MS analysis, OMEO was made up of 24 compounds (representing 96.82%). The main component was terpinene-4-ol (32.69%), followed by γ-terpinene (12.88%), trans-sabinenehydrate (8.47%), α-terpinene (7.98%), sabinene (6.21%), α-terpineol (5.25%), α-terpinolene (3.36), cis-sabinenehydrate (2.92), β-phellandrene (2.64%), ρ-cymene (2.32%), trans-caryophyllene (2.31%), (E)-p-menth-2-en-1-ol (2.25), bicyclogermacrene (1.60) and β-myrcene (1.49%), as shown in Table 1. These components (14) accounted for 92.37% of the yield, while the other detected components represented <1% (Fig. 1).
Fig. 1.
GC-MS chromatogram of Origanum majorana essential oil.
3.2. Acute toxicity test
No mortality was observed with any of the doses (100–500 mg/kg, i.p.), but the relevant data are not shown.
3.3. Immobility, swimming and climbing behaviors
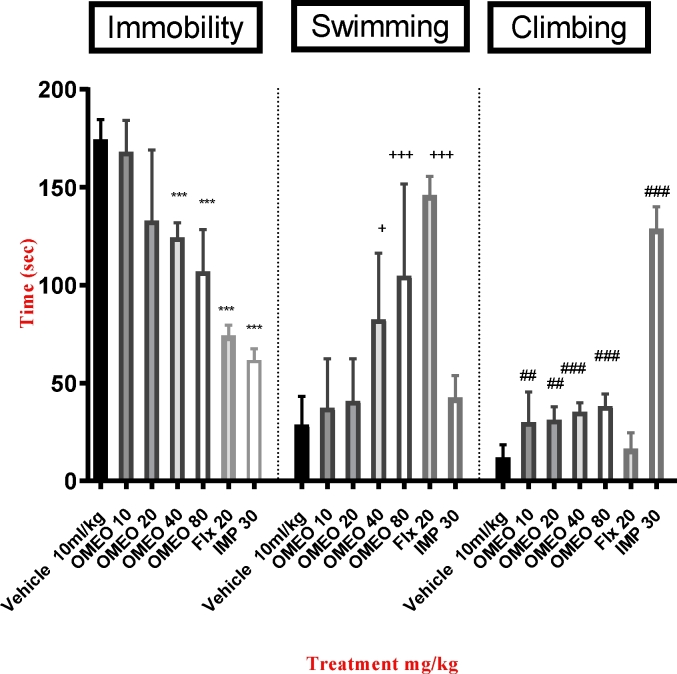
As shown in Fig. 2, the one-way ANOVA indicated a significant decline in immobility time [F (6, 35) = 46.03, P < 0.001] and a significant increase in swimming time [F (6, 35) = 16.54, P < 0.001] and climbing time [F (6, 35) = 113.4, P < 0.001] in the mice treated with OMEO (40 and 80 mg/kg, i.p.) compared to the vehicle group. There were no significant differences in the immobility time yielded with OMEO (40 and 80 mg/kg, i.p.) compared to that yielded with imipramine and fluoxetine. The one-way ANOVA did not show significant differences between the mice treated with OMEO (80 mg/kg, i.p.) and fluoxetine in swimming time (P > 0.05) and also between the mice treated with OMEO (80 mg/kg, i.p.) and imipramine in climbing time (P > 0.05). Since the best response was achieved with the 80-mg/kg dose, this amount was used for the subsequent tests.
Fig. 2.
The effects of the i.p. administration of Origanum Majorana essential oil (OMEO), fluoxetine and imipramine on the mean immobility, swimming and climbing times. Values are presented as mean ± S.D. (n = 6) mice/group. ***, +, ++++, and ### show significant differences between the vehicle (control) group at P < 0.001, P < 0.05, P < 0.001 and P < 0.001 in immobility time, swimming time, and climbing time, respectively. Results were analyzed by one-way ANOVA followed by Tukey's post-hoc test.
3.4. Monoaminergic involvement in the antidepressant-like effect of OMEO in the FST
3.4.1. Dopaminergic involvement
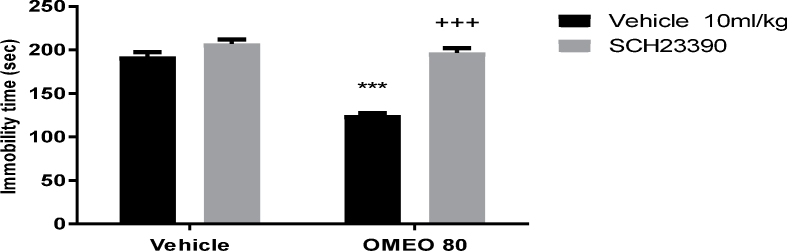
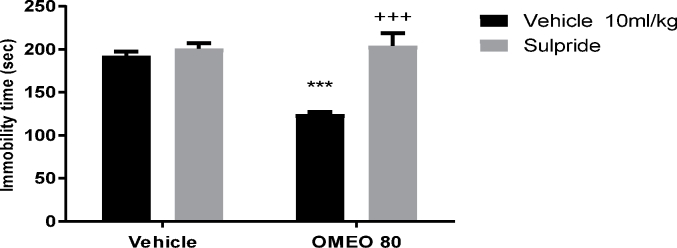
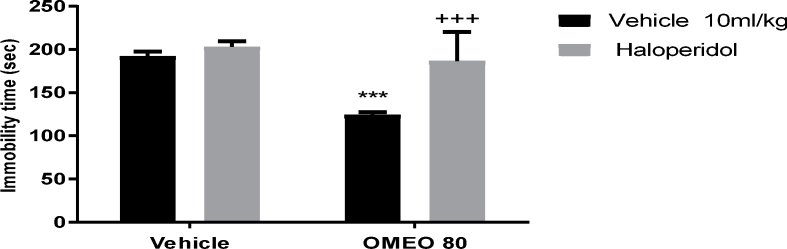
The results showed that the pre-treatment of the animals with SCH 23390 (a dopamine D1 receptor antagonist), sulpiride (a dopamine D2 receptor antagonist) and haloperidol (non-selective dopamine receptor antagonist) prevented the antidepressant-like effect of OMEO significantly (Fig. 3, Fig. 4, Fig. 5). The two-way ANOVA for DA receptor antagonist were performed as follows: For the SCH 23390 pre-treatment [F (1, 20) = 158.70, P < 0.0001], OMEO treatment [F (1, 20) = 127.70, P < 0.0001] and SCH 23390 pre-treatment × essential oil interaction [F(1,20) = 69.40, P < 0.0001], for sulpiride pre-treatment [F (1, 20) = 97.45, P < 0.0001], OMEO treatment [F (1, 20) = 51.90, P < 0.0001] and sulpiride pre-treatment × essential oil interaction [F(1,20) = 63.53, P < 0.001] and for haloperidol pre-treatment [F (1, 20) = 23.15, P = 0.0001], OMEO treatment [F (1, 20) = 30.34, P < 0.0001] and haloperidol pre-treatment × essential oil interaction [F(1,20) = 11.47, P = 0.0029].
Fig. 3.
The effects of the pre-treatment of the animals with SCH 23390 (0.05 mg/kg, s.c., a dopamine D1 receptor antagonist) on the antidepressant-like effect induced by OMEO in the FST. Values are presented as mean ± S.D. (n = 6) mice/group. *** shows significant differences compared to the vehicle (control) group at P < 0.001. +++ compared to the essential oil group. Results were analyzed by two-way ANOVA followed by Tukey's post-hoc test.
Fig. 4.
The effects of the pre-treatment of the animals with sulpiride (50 mg/kg, i.p., dopamine D2 receptor antagonist) on the antidepressant-like effect induced by OMEO in the FST. Values are presented as mean ± S.D. (n = 6) mice/group. *** shows significant differences compared to the vehicle (control) group at P < 0.001. +++ compared to the essential oil group. Results were analyzed by two-way ANOVA followed by Tukey's post-hoc test.
Fig. 5.
The effects of the pre-treatment of the mice with haloperidol (0.2 mg/kg, i.p., a dopamine D1 receptor antagonist) on the antidepressant-like effect induced by OMEO in the FST. Values are presented as mean ± S.D. (n = 6) mice/group. *** shows significant differences compared to the vehicle (control) group at P < 0.001. +++ compared to the essential oil group. Results were analyzed by two-way ANOVA followed by Tukey's post-hoc test.
3.4.2. Serotonergic involvement
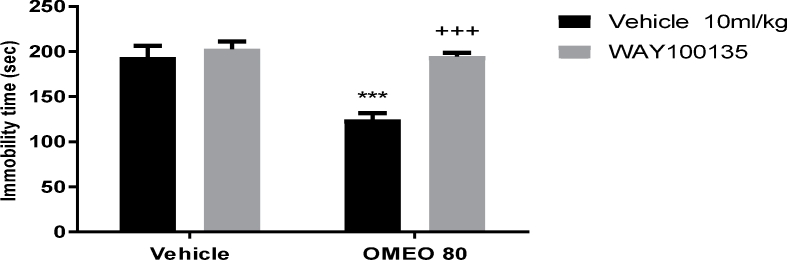
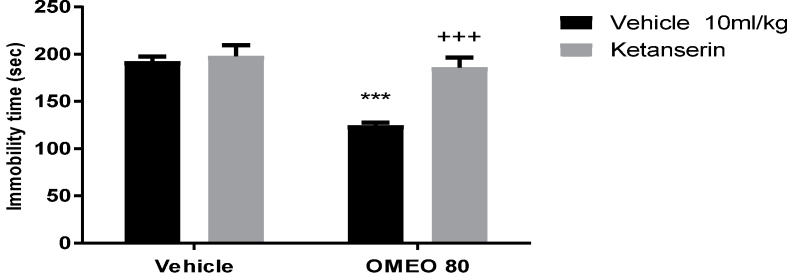
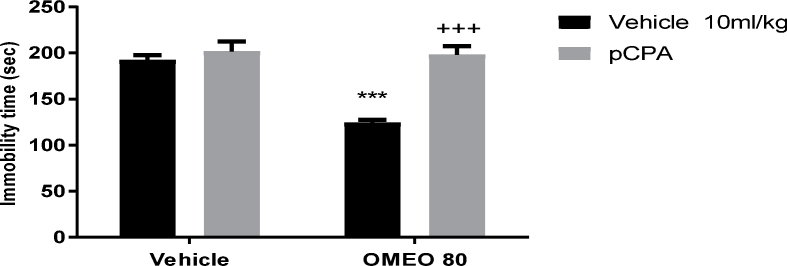
The results showed that the pre-treatment of the mice with WAY 100135 (5-HT1A receptor antagonist), ketanserin (preferential 5-HT2A receptor antagonist) and pCPA (an inhibitor of serotonin synthesis) inhibited the antidepressant-like effect of OMEO significantly (Fig. 6, Fig. 7, Fig. 8). The two-way ANOVA for 5-HT antagonists were performed as follows: For WAY 100135 pre-treatment [F (1, 20) = 123.4, P < 0.001], OMEO treatment [F (1, 20) = 116.8, P < 0.001] and WAY 100135 pre-treatment × essential oil interaction [F(1,20) = 73.83, P < 0.001], for ketanserin pre-treatment ketanserin [F (1, 20) = 57.84, P < 0.001], OMEO treatment [F (1, 20) = 81.57, P < 0.001] and ketanserin pre-treatment × essential oil interaction [F(1,20) = 39.47, P < 0.001] and for pCPA pre-treatment [pCPA [F (1, 20) = 94.70, P < 0.001], OM essential oil treatment [F (1, 20) = 68.80, P < 0.001] and pCPA pre-treatment × essential oil interaction [F(1,20) = 57.12, P < 0.001].
Fig. 6.
The effects of the pre-treatment of the animals with WAY 100135 (10 mg/kg, i.p., 5-HT1A receptor antagonist) on the antidepressant-like effect induced by OMEO in the FST. Values are presented as mean ± S.D. (n = 6) mice/group. *** shows significant differences compared to the vehicle (control) group at P < 0.001. +++ compared to the essential oil group. Results were analyzed by two-way ANOVA followed by Tukey's post-hoc test.
Fig. 7.
The effects of the pre-treatment of the animals with ketanserin (5 mg/kg, i.p., preferential 5-HT2A receptor antagonist) on the antidepressant-like effect induced by OMEO in the FST. Values are presented as mean ± S.D. (n = 6) mice/group. *** shows significant differences compared to the vehicle (control) group at P < 0.001. +++ compared to the essential oil group. Results were analyzed by two-way ANOVA followed by Tukey's post-hoc test.
Fig. 8.
The effects of the pre-treatment of the animals with pCPA (150 mg/kg, i.p., an inhibitor of serotonin synthesis) on the antidepressant-like effect induced by OMEO in the FST. Values are presented as mean ± S.D. (n = 6) mice/group. *** shows significant differences compared to the vehicle (control) group at P < 0.001. +++ compared to the essential oil group. Results were analyzed by two-way ANOVA followed by Tukey's post-hoc test.
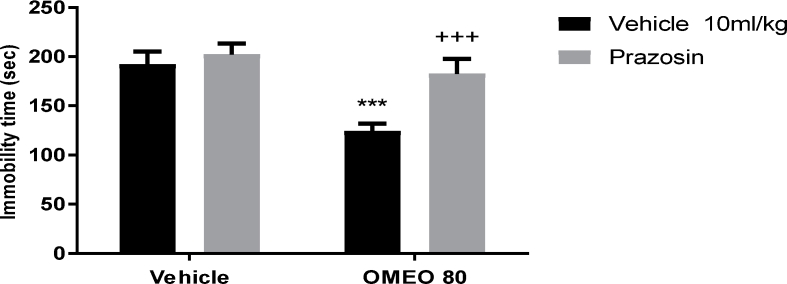
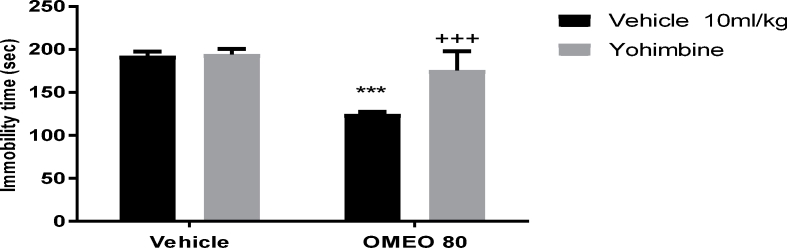
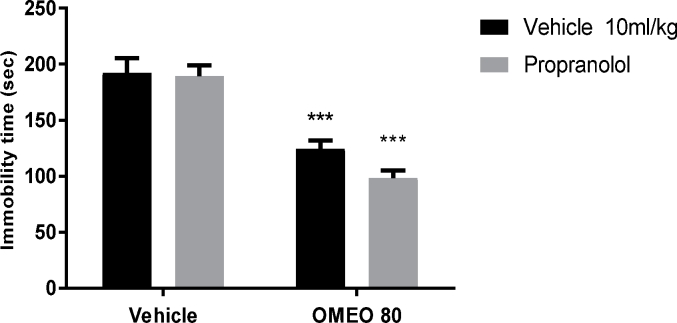
3.4.3. Noradrenergic involvement
The results showed that the pre-treatment of the animals with prazosin (an α1-adrenoceptor antagonist) and yohimbine (an α2-adrenoceptor antagonist) inhibited the antidepressant-like effect of OMEO significantly (Fig. 9, Fig. 10). Nonetheless, the pre-treatment of the animals with propranolol (a β-adrenoceptor receptor antagonist) could not prevent the antidepressant-like effect induced by OMEO (Fig. 11). The two-way ANOVA were carried out for the noted antagonists as follows: For prazosin pre-treatment [F (1, 20) = 47.69, P < 0.001], OMEO treatment [F (1, 20) = 78.23, P < 0.0001] and prazosin pre-treatment × essential oil interaction [F(1,20) = 23.91, P < 0.001], for yohimbine pre-treatment [F (1, 20) = 22.04, P < 0.001], OMEO treatment [F (1, 20) = 58.29, P < 0.001] and yohimbine pre-treatment × essential oil interaction [F(1,20) = 18.86, P = 0.0003] and for propranolol pre-treatment [F (1, 20) = 4.71, P = 0.0421], OMEO treatment [F (1, 20) = 144.9, P < 0.001] and propranolol pre-treatment × essential oil interaction [F(1,20) = 3.10, P = 0.0932].
Fig. 9.
The effects of the pre-treatment of the animals with prazosin (1 mg/kg, i.p., α1-adrenoceptor antagonist) on the antidepressant-like effect induced by OMEO in the FST. Values are presented as mean ± S.D. (n = 6) mice/group.*** shows significant differences compared to the vehicle (control) group at P < 0.001. +++ compared to the essential oil group. Results were analyzed by two-way ANOVA followed by Tukey's post-hoc test.
Fig. 10.
The effects of the pre-treatment of the animals with yohimbine (1 mg/kg, i.p., an α2-adrenoceptor antagonist) on the antidepressant-like effect induced by OMEO in the FST. Values are presented as mean ± S.D. (n = 6) mice/group. *** shows significant differences compared to the vehicle (control) group at P < 0.001. +++ compared to the essential oil group. Results were analyzed by two-way ANOVA followed by Tukey's post-hoc test.
Fig. 11.
The effects of the pre-treatment of the animals with propranolol (1 mg/kg, i.p., a β-adrenoceptor antagonist) on the antidepressant-like effect induced by OMEO in the FST. Values are presented as mean ± S.D. (n = 6) mice/group. *** shows significant differences compared to the vehicle (control) group at P < 0.001. Results were analyzed by two-way ANOVA followed by Tukey's post-hoc test.
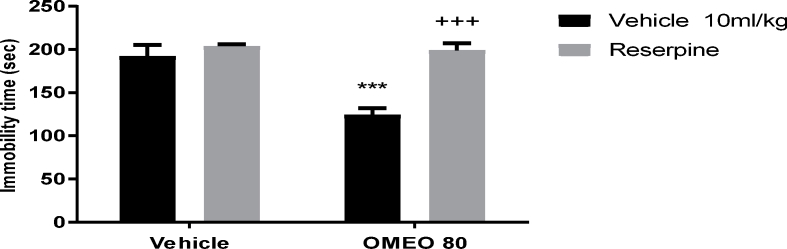
3.4.4. The effect of reserpine on the antidepressant-like effect of OMEO
The pre-treatment of the mice with reserpine (vesicular monoamine depleter) increased the immobility time in the FST (Fig. 12). The two-way ANOVA for reserpine were performed as follows: Reserpine pre-treatment [F (1, 20) = 69.76, P < 0.001], OMEO treatment [F (1, 20) = 49.23, P < 0.001] and reserpine pre-treatment × essential oil interaction [F (1, 20) = 37.16, P < 0.001].
Fig. 12.
The effects of the pre-treatment of the animals with reserpine (2 mg/kg, i.p., vesicular monoamine depleter) on the antidepressant-like effect induced by OMEO in the FST. Values are presented as mean ± S.D. (n = 6) mice/group. *** shows significant differences compared to the vehicle (control) group at P < 0.001. +++ compared to the extract group. Results were analyzed by two-way ANOVA followed by Tukey's post-hoc test.
3.4.5. The interaction between sub-effective doses of fluoxetine and imipramine and a sub-effective dose of OMEO
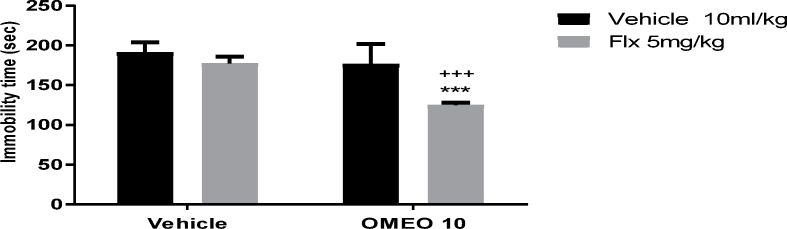
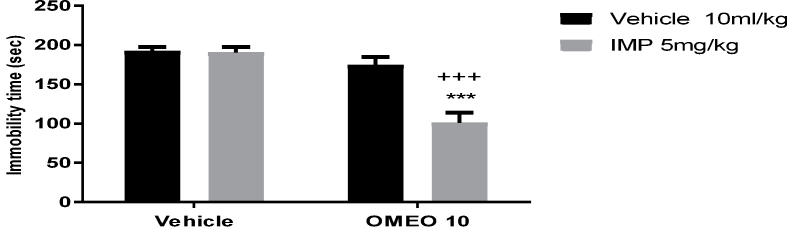
The findings revealed the potentiating effect of the sub-effective dose of OMEO (80 mg/kg, i.p.) when used in combination with sub-effective doses of fluoxetine (Fig. 13) and imipramine (Fig. 14). The two-way ANOVA for conventional antidepressants were performed as follows: For fluoxetine [F (1, 20) = 19.40, P = 0.0003], OMEO treatment [F (1, 20) = 20.10, P = 0.0002] and fluoxetine × essential oil interaction [F (1,20) = 6.24, P = 0.021] and for imipramine [F (1, 20) = 33.72, P < 0.001], OMEO treatment [F (1, 20) = 67.69, P < 0.001] and imipramine × essential oil interaction [F (1,20) = 30.33, P < 0.001].
Fig. 13.
The effects of the sub-effective doses of fluoxetine (5 mg/kg, i.p.) on the sub-effective dose of OMEO (10 mg/kg, i.p.) in the FST in the mice. ∗∗∗P < 0.001 compared to the vehicle (control) group. +++ compared to the essential oil group. Results were analyzed by two-way ANOVA followed by Tukey's post-hoc test.
Fig. 14.
The effects of the sub-effective doses of imipramine (5 mg/kg, i.p.) on the sub-effective dose of OMEO (10 mg/kg, i.p.) in the FST in the mice. ∗∗∗P < 0.001 compared to the vehicle (control) group. +++ compared to the essential oil group. Results were analyzed by two-way ANOVA followed by Tukey's post-hoc test.
3.5. The open-field test (OFT)
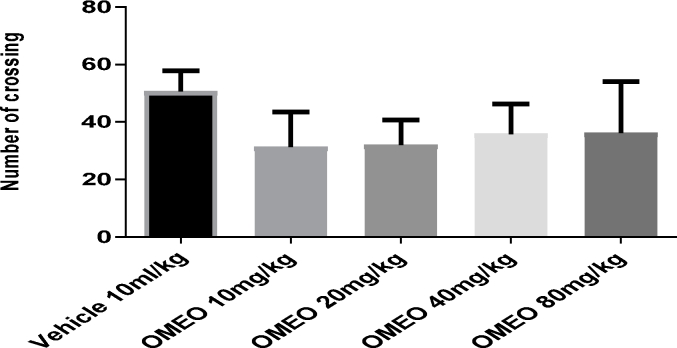
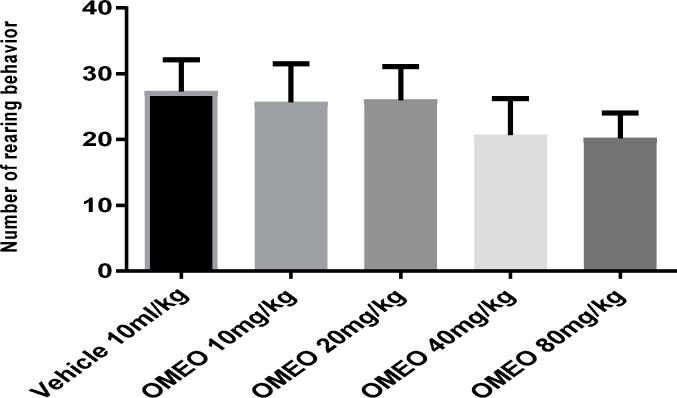
No significant changes (P > 0.05) were observed in the crossing (Fig. 15) and rearing (Fig. 16) counts in the animals treated with OMEO (10–80 mg/kg; i.p.) compared to the vehicle group.
Fig. 15.
The effects of OMEO in the open field test (crossing) on the mice. Values are presented as mean ± S.D. (n = 6) mice/group. P > 0.05 compared to the vehicle (control) group. Results were analyzed by one-way ANOVA followed by Tukey's post-hoc test.
Fig. 16.
The effects of OMEO in the open field test (rearing) on the mice. Values are presented as mean ± S.D. (n = 6) mice/group. P > 0.05compared to the vehicle (control) group. Results were analyzed by one-way ANOVA followed by Tukey's post-hoc test.
4. Discussion
The present findings revealed that acute doses of OMEO, i.e. 40 and 80 mg/kg, have significant antidepressant-like effects in the FST. In addition, OMEO (40 and 80 mg/kg) and fluoxetine increased swimming time compared to when the vehicle was administered. Climbing time also increased in the mice treated with OMEO and imipramine compared to those treated with the vehicle and fluoxetine. Studies have shown that swimming is affected by serotonergic agents such as fluoxetine, while climbing is affected by noradrenergic agents such as imipramine. In agreement with the present findings, some reports have shown that tricyclic antidepressants, i.e. imipramine, increase climbing time, but do not affect swimming time.18 Nevertheless, the behavioral responses caused by the administration of OMEO (40 and 80 mg/kg) in terms of swimming time were similar to those caused by fluoxetine administration, suggesting that serotonergic neurotransmission may modulate the antidepressant-like effect of OMEO. In addition, the behavioral responses caused by OMEO administration (at all doses) in terms of climbing time were similar to those caused by imipramine administration; as a result, OMEO exerts its antidepressant-like effects through the adrenergic system. There is no evidence to confirm the role of OMEO in the antidepressant-like effect of the monoaminergic system. Nonetheless, any active components of OMEO may produce the effects observed in this study. The GC/MS analysis data were in line with the data obtained in previous studies, which have shown that terpinene-4-ol, followed by other monoterpene alcohols such as γ-terpinene, trans-sabinene hydrate, α-terpinene and α-terpineol, are the main volatile components of OMEO.19 In line with the present findings, Guzman-Gutierrez et al. also revealed that linalool and β-pinene are two volatile monoterpenes with antidepressant-like activities through the modulation of the monoaminergic system.20 Similarly, studies have shown that the monoterpenes of citrus essential oil (e.g. R-limonene, γ-terpinene and citral) increase the release of monoamines from rat brain slices.21 Apart from monoterpenes, flavonoids are also reportedly responsible for the medicinal properties of Origanum majorana. In this regard, recent studies demonstrated the antidepressant-like activity of flavonoids.22 Nevertheless; the main monoterpene alcohols of OMEO (especially terpinene-4-ol) may be resposbile for their antidepressant-like activity.
The antioxidant properties of Origanum majorana have also been the subject of much research.5 Some studies have documented that antioxidants can prevent the reuptake of 5-HT,23 suggesting that 5-HT availability can be increased in synaptic clefts. One study showed the positive effects of Origanum extract administration on behavioral responses by causing an increase in monoamine compounds in humans.24 Moreover, locomotor activity was not altered in the OFT. This finding might have been false if OMEO increased the locomotor activity. Additionally, the decline in immobility time as a result of OMEO administration cannot be attributed to any psycho-stimulant effects,25 because no significant effects were reported on locomotor activity in the OFT, suggesting that OMEO can be used for the treatment of depression without dangerous side-effects.
The dopaminergic system is modulated to cause the antidepressant-like effect of OMEO since the DA receptor antagonists (SCH 23390, sulpiride, and haloperidol) prevent such effect. Delgado stated that, as a primary monoamine, the dopaminergic system may be involved in major depression.26 Animal studies have confirmed the role of the dopaminergic system in antidepressant-like effects.27 This section presents some other reasons to prove the role of DA in antidepressant-like activities. A deficiency in DA has been observed in the behavioral responses of individuals suffering from major depression, such as in their amount of motivation, enjoyment and attention.25 These researchers also reported that some DA receptor agonists can be used in the treatment of refractory and bipolar depression. People suffering from major depression do not have DA turnover because two DA metabolites (homovallinic acid and 3, 4-dihydroxyphenylacetic acid) are decreased in them.28 Nonetheless, the pre-treatment of the animals with D1 and D2 receptor antagonists inhibited the antidepressant-like effect of OMEO, suggesting that D1 and D2 are in fact involved in this activity.
The involvement of the serotonergic system in the antidepressant-like effect of OMEO is confirmed since 5-HT receptor antagonists inhibit this activity. The present findings, illustrated in Fig. 2, show that OMEO was sensitive to the serotonergic system since the swimming test is sensitive to this system. Due to the fact that 5-HT is involved in some of the factors causing major depression, such as memory and learning, it has been proposed as the main neurotransmitter involved in depression.29 The 5-HT1A receptors are directly involved in clinical antidepressant activity as they are positioned at the soma and dendrites of the 5-HT neurons in the dorsal raphe and prevent 5-HT release, and this prevention confirms their antidepressant activity.29 The 5-HT2 receptors are located in the brain and hippocampus, which are responsible for depression.30 In line with the present findings, other studies have reported that ketanserin inhibits the antidepressant activity of some medicinal plants in mice.21By studying the Origanum genus, Mombeini et al.,31 showed that oregano extract prevents the degradation of monoamine neurotransmitters and increases extracellular 5-HT levels in the brain in anxiolytic mice in a dose-dependent manner. OMEO, which is similar to lemon oil, may thus increase the turnover of 5-HT. This notion requires more thorough investigations.
In another series of experiments, the pre-treatment of animals with reserpine (a vesicular monoamine depleter) increased the immobility time with OMEO; this finding suggests that OMEO may regulate the brain monoamine neurotransmitters.
The pre-treatment of animals with prazosin and yohimbine similarly prevented the antidepressant-like effect of OMEO; nevertheless, pre-treatment with propranolol cannot inhibit this effect, suggesting that OMEO induces antidepressant-like effects through α-adrenoceptor but not β-adrenoceptor. As shown in Fig. 2, OMEO was sensitive to the climbing test in all doses, and can thus be said to act through the adrenergic system. The involvement of the noradrenergic system in the pathophysiology of depression is debatable. Elhwuegi32 stated that major depression is associated with the hypofunction of the noradrenergic system, and conventional antidepressants may thus increase the availability of NA in the synaptic clefts. Several studies have shown that the pre-treatment of animals with α-adrenoceptor antagonist prevents the antidepressant-like effect of herbal medicines.11,12 These studies confirm that the activation of α-adrenoceptor is necessary for this effect.
Other findings have demonstrated that sub-effective doses of OMEO accompanied with conventional antidepressants (imipramine and fluoxetine) increase the antidepressant-like response. Based on the present findings, since OMEO potentiates the effects of conventional antidepressants, both OMEO and conventional antidepressants can be used for the treatment of depression at sub-effective doses without causing harmful side-effects.
5. Conclusion
At high doses, i.e. 40 and 80 mg/kg i.p., OMEO reduced immobility time. In addition, the same levels increased swimming and climbing times. The prevention of the antidepressant activity of OMEO after pre-treatment with SCH 23390, sulpiride, haloperidol, WAY 100135, pCPA, ketanserin, prazosin, and yohimbine confirms the involvement of the D1, D2, 5-HT1A, 5-HT2A, α1 and α2 antagonist receptors in the antidepressant effect of OMEO. Since propranolol could not prevent this effect, β antagonist receptors were found to not affect this antidepressant-like activity. The potentiating effect of OMEO, when used with conventional antidepressants, warrants the usage of OMEO for the treatment of depression in traditional medicine. These findings propose antidepressant activities for OMEO and its main monoterpene alcohols (especially terpinene-4-ol) may be involved in this action. However, to clarify the role of other components and understanding the relationship between OMEO and antidepressant activity requires further research.
Conflicts of interest
We have no known conflicts of interest in the publication of this article.
Acknowledgments
This project was carried out through the cooperation of Urmia Branch, Islamic Azad University in Iran and was funded by Adonis Gol Darou Pharmaceutical Co. (Tehran, Iran) under Grant No. 52749 (24 Feb., 2016).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Cohn D.W., Kinoshita D., Palermo-Neto J. Antidepressants prevent hierarchy destabilization induced by lipopolysaccharide administration in mice: a neurobiological approach to depression. Ann NY Acad Sci. 2012;1262:67–73. doi: 10.1111/j.1749-6632.2012.06635.x. [DOI] [PubMed] [Google Scholar]
- 2.Nutt D.J. Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatr. 2008;69:4–7. 2008. [PubMed] [Google Scholar]
- 3.Lambert G., Johansson M., Agren H., Friberg P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Arch Gen Psychiatr. 2000;57:787–793. doi: 10.1001/archpsyc.57.8.787. [DOI] [PubMed] [Google Scholar]
- 4.Wang R., Xu Y., Wu H.L. The antidepressant effects of curcumin in the forced swimming test involve 5-HT1 and 5-HT2 receptors. Eur J Pharmacol. 2008;578:43–50. doi: 10.1016/j.ejphar.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 5.Vagi E., Rapvi E., Hadolin M. Phenolic and triterpenoid antioxidants from Origanum majorana L. herb and extracts obtained with different solvents. J Agric Food Chem. 2005;53:17–21. doi: 10.1021/jf048777p. [DOI] [PubMed] [Google Scholar]
- 6.Chevallier A. Andrew Chevallier and Dorling Kindersley Limited Publishers; London: 1996. The Encyclopedia of Medicinal Plants; pp. 9–78. [Google Scholar]
- 7.Deshmane D.N., Gadgoli C.H., Halade G.V. Anticonvulsant effect of Origanum majorana L. Pharmacology (online) 2007;1:64–68. [Google Scholar]
- 8.Medina J.H., Viola H., Wolfman C. Neuroactive flavonoids: new ligands for the benzodiazepine receptors. Phytomedicine. 1998;5:235–243. doi: 10.1016/S0944-7113(98)80034-2. 1998. [DOI] [PubMed] [Google Scholar]
- 9.Porsolt R.D., Bertin A., Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 10.Adams R.P. Allured Publishing Corporation; Carol Stream IL: 2007. Identification of Essential Oil Components by Gas chromatography/Mass Spectrometry. [Google Scholar]
- 11.Abbasi-Maleki S., Bakhtiarian A., Nikoui V. Involvement of the monoaminergic system in the antidepressant-like effect of the crude extract of Menthapiperita (Lamiaceae) in the forced swimming test in mice. Synergy. 2017;5:21–28. [Google Scholar]
- 12.Abbasi-Maleki S., Mousavi Z. Hydroethanolic extract of Carthamus tinctorius induces antidepressant-like effects: modulation by dopaminergic and serotonergic systems in tail suspension test in mice. Iran J Basic Med Sci. 2017;20:1063–1073. doi: 10.22038/IJBMS.2017.9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirshafa S., Azadbakht M., Ahangar N. Study of antidepressant and sedative-hypnotic activity of hydroalcoholic extract of Asperugo procumbens L. Aerial parts in mice. Iran J Pharm Res (IJPR) 2013;12:529–535. [PMC free article] [PubMed] [Google Scholar]
- 14.Sadighi S., Abbasi-Maleki S., Moradi-Kor N. 1st International Conference on Medicine, Public Health and Biological Sciences (MPHBS); Sep 9-19. 2016. Evaluation of the effect of Origanum majorana L. essential oil on morphine withdrawal signs in male mice. Tehran, Iran. Abstract 222. [Google Scholar]
- 15.Kazemi P., Jowhary H., Sharifi E., Zeraatpishe A. Androgenic effect of Origanum vulgarel L.spp viride extract on hormone level of pituitary-gonadal Axis in mature male vistar rats. J Arak Uni Med Sci. 2012;14:89–96. [Google Scholar]
- 16.Foroozandeh M., Bigdeli M., Rahnema M. The effect of hydroalcoholic extract of Origanum vulgare on blood brain barrier (BBB) permeability and neurologic deficits in rat stroke model. J Torbat Heydariyeh Uni Med Sci. 2014;2(3):1–9. [Google Scholar]
- 17.Brown R.E., Corey S.C., Moore A.K. Differences in measures of exploration and fearin MHC-congenic C57BL/6J and B6-H-2K mice. Behav Genet. 1999;26:263–271. [Google Scholar]
- 18.Detke M.J., Rickels M., Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacol. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 19.Vera R.R., Chane-Ming J. Chemical composition of the essential oil of marjoram (Origanum majorana L.) from Reunion Island. Food Chem. 1999;66:143–145. [Google Scholar]
- 20.Guzman-Gutierrez S.L., Bonilla-Jaim H., Gomez-Cansino R., Reyes-Chilpa R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015;1:24–29. doi: 10.1016/j.lfs.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Fukumoto S., Sawasaki E., Okuyama S., Miyake Y., Yokogoshi H. Flavor components of monoterpenes in citrus essential oils enhance the release of monoamines from rat brain slices. Nutr Neurosci. 2006;9:73–80. doi: 10.1080/10284150600573660. [DOI] [PubMed] [Google Scholar]
- 22.Guan L.P., Liu B.Y. Antidepressant-like effects and mechanisms of flavonoids and related analogues. Eur J Med Chem. 2016;121:47–57. doi: 10.1016/j.ejmech.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Khanzode S.D., Dakhale G.N., Khanzode S.S., Saoji A., Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003;8:365–370. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- 24.Mechan A.O., Fowler A., Seifert N. Monoamine reuptake inhibition and mood-enhancing potential of a specified oregano extract. Br J Nutr. 2011;105:1150–1163. doi: 10.1017/S0007114510004940. [DOI] [PubMed] [Google Scholar]
- 25.Ishola I.O., Agbaje E.O., Akinleye M.O., Ibeh C.O., Adeyemi O. Antidepressant-like effect of the hydroethanolic leaf extract of Alchorneacordifolia (Schumach. &Thonn.) Mull. Arg.(Euphorbiaceae) in mice: involvement of monoaminergic system. J Ethnopharmacol. 2014;158:364–372. doi: 10.1016/j.jep.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Delgado P.L. Depression: the case for a monoamine deficiency. J Clin Psychiatr. 2000;61:7–11. [PubMed] [Google Scholar]
- 27.Evans J., Sun Y., McGregor A., Connor B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology. 2012;63:1315–1326. doi: 10.1016/j.neuropharm.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Mitani H., Shirayama Y., Yamada T., Kawahara R. Plasma levels of homovanillicacid,5-hydroxyindoleacetic acid and cortisol, and serotonin turn over in depressed patients. Prog Neuropsychopharmacol Biol Psychiatr. 2006;30:531–534. doi: 10.1016/j.pnpbp.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Shrestha S., Hirvonen J., Hines C.S. Serotonin-1A receptors in major depression quantified using PET: controversies, confounds, and recommendations. Neuroimage. 2012;59:3243–3251. doi: 10.1016/j.neuroimage.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nestler E.J., Barrot M., DiLeone R.J., Eisch A.J., Stephen J.G., Monteggia L.M. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 31.Mombeini T., Mazloumi S., Shams T. Pharmacological effects of Origanum vulgare L. In the elevated plus-maze and open field tests in the rat. J Basic Clinic Patophysiol. 2015;3:29–37. [Google Scholar]
- 32.Elhwuegi A.S. Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatr. 2004;28:435–451. doi: 10.1016/j.pnpbp.2003.11.018. [DOI] [PubMed] [Google Scholar]