Abstract
Background and aim
Lignosus rhinocerus (LR) is an edible mushroom with a variety of medicinal properties such as neurostimulation, immunomodulation, anti-inflammation, anti-oxidation, anti-proliferation, anti-diabetes and especially antiviral activity. Human immunodeficiency virus type-1 (HIV-1) needs the HIV-1 protease (PR) and reverse transcriptase (RT) for its replication. Therefore, both HIV-1 PR and RT are important targets for antiretroviral drug development.
Experimental procedure
The crude hexane (LRH), ethanol (LRE) and water (LRW) extracts of LR were in vitro screened for inhibitory activity against HIV-1 PR and RT, then anti-HIV-1 activity on the infected MOLT-4 cells were determined. Chemical constituents of the extracts were identified by gas chromatography-mass spectrometry (GC-MS) and liquid chromatography (LC)-MS. The identified compounds were in silico analysed for drug-likeness property and molecular modelling.
Results and conclusion
According to our screening assays, LRE and LRW significantly inhibited both enzymes (25–55%), while LRH suppressed only the HIV-1 PR activity (88.97%). At 0.5 mg/ml of LRW showed significant inhibition of HIV-1 induced syncytial formation and p24 production in the infected MOLT-4 cells. Investigation of chemical analysis revealed that major groups of identified constituents found in the extracts were fatty acids, peptides and terpenoids. In silico analysis showed that heliantriol F and 6 alpha-fluoroprogesterone displayed great binding energies with HIV-1 PR and HIV-1 RT, respectively. These findings suggest that LR could be a potential source of compounds to inhibit HIV-1 PR and/or RT activities in vitro. Furthermore, our results provide beneficial data for the development of novel HIV-1 PR and RT inhibitors.
Keywords: Lignosus rhinocerus, Tiger milk mushroom, HIV-1 protease inhibitor, HIV-1 reverse transcriptase inhibitor, Human immunodeficiency virus
Taxonomy (classification by EVISE): HIV/AIDS, HIV Protease Inhibitor, Reverse Transcriptase, Natural Product Analysis, Computational Analytical Methods, Molecular Docking
Graphical abstract
Highlights of the findings and novelties
-
•
The hexane extract of L. rhinocerus strongly inhibited HIV-1 PR activity.
-
•
The ethanol and water extracts of L. rhinocerus showed HIV-1 PR and RT inhibitions.
-
•
Chemical constituents of L. rhinocerus could block HIV-1 PR and RT in silico studies.
Abbreviations
- HIV-1
Human immunodeficiency virus type 1
- LR
Lignosus rhinoceros
- PR
Protease
- RT
Reverse transcriptase
- LRH
L. rhinocerus crude hexane extract
- LRE
L. rhinocerus crude ethanol extract
- LRW
L. rhinocerus crude water extract
- GC
Gas chromatography
- MS
Mass spectrometry
- LC
Liquid chromatography
- NVP
Nevirapine
- APV
Amprenavir
- BE
Binding energy
1. Introduction
Lignosus rhinocerus (LR), known as the tiger milk mushroom, is traditionally used as folk medicine in Southeast Asia and China. The medicinally beneficial part of LR is the sclerotium, an underground hardened part of the mushroom1 that has been reported to have several medicinal properties such as neurostimulation,2 immunomodulation, anti-inflammation,3 anti-oxidation, anti-proliferation,4,5 anti-diabetes6 and especially antiviral activity.7
Human immunodeficiency virus (HIV) can cause acquired immunodeficiency syndrome (AIDS), a worldwide serious health issue. It is classified into the two major types of type-1 (HIV-1) and type-2 (HIV-2). According to previous reports, HIV-1 is spread worldwide and has a higher severity of infection and progression of the disease in infected patients than HIV-2.8 Currently, plenty of antiretroviral drugs are available and have been designed to interfere with processes in the viral life cycle, such as reverse transcription and virion maturation. The reverse transcription is the step where retroviruses convert viral RNA to complementary DNA using HIV-1 reverse transcriptase (RT).9 For HIV-1 maturation, the immature viruses transform to mature viruses by cleavage of Gag and Gag-Pol polyproteins using HIV-1 protease (PR).10,11 Thus, these two enzymes have generally been used as targets in antiretroviral drug development.
The discovery of natural products exerting antiretroviral activities by blocking both HIV-1 RT and PR is of great interest. According to previous studies on the antiviral activity of LR,7 we hypothesized that LR could have antiviral activity against other viruses, especially HIV-1. As an initial investigation into the anti-HIV-1 activity of LR, crude extracts from LR were determined for their inhibitory activities against HIV-1 PR and RT. Moreover, phytochemical compounds in the extracts were identified using gas chromatography-mass spectrometry (GC-MS) and liquid chromatography (LC)-MS. The identified compounds were then in silico analysed for their drug-likeness property and affinity to bind both the active sites of the enzymes using ADMET online server and AutoDock 4.0 molecular docking program, respectively. Herein, we suggest that active compounds from LR extracts could inhibit HIV-1 PR and RT activities. This report provides useful data for anti-HIV-1 drugs development and a novel knowledge of the anti-HIV-1 property of LR.
2. Material and methods
2.1. Chemicals and reagents
Hexane and ethanol were purchased from Merck (Darmstadt, Germany). Dimethyl sulfoxide (DMSO) was purchased from RCI Labscan (Bangkok, Thailand). Roswell Park Memorial Institute (RPMI)-1640 medium, fetal bovine serum (FBS), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and phosphate buffered saline were purchased from Thermo Scientific HyClone (Logan, UT, USA). Phorbolmyristate acetate (PMA) was purchased from Sigma-Aldrich (St. Louis, MO, USA). 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) was purchased from Promega (Madison, WI, USA). Darunavir (DRV) and Nevirapine (NVP) were obtained from the NIH AIDS Research and Reference Program. The HIV-1 protease inhibitor screening kit (Fluorometric), HIV-1 reverse transcriptase assay kit and HIV-1 p24 SimpleStep ELISA kit were purchased from Biovision Incorporated (Milpitas, CA, USA), Roche Diagnostics (Mannheim, Germany) and Abcam (Cambridge, UK), respectively.
2.2. Mushroom extraction
Cultivated sclerotia powder of LR strain TM02 was obtained from LiGNO Biotech Sdn Bhd, Selangor, Malaysia. The mushroom material was then extracted by the sequential maceration method. Briefly, the powder (100 g) was macerated with hexane (1 L) in a shaker incubator at 225 rpm for 72 h at room temperature. The hexane extract was harvested by filtration through Whatman® No.2 filter paper and the hexane was removed by rotary evaporation (Heidolph, Laborota 4011) to yield the crude hexane extract (LRH; 0.28 g). The residual hexane-insoluble LR part was then dried and extracted as above but in ethanol (1 L), with subsequent filtration and evaporation as above to yield the crude ethanol extract (LRE; 0.62 g). Finally, the LR residue from the ethanol extraction was dried and then extracted in autoclaved water as above except at 4 °C, harvested by filtration and the water was removed in a freeze-dry lyophilizer (Modulyod freeze dryer, Thermo) to give the crude water extract (LRW; 12.18 g). The flow diagram of the sequential extraction of the LR sclerotia is shown in the Supplementary data 1.
2.3. Screening for HIV-1 PR inhibitor activity
The extracts (1 mg/mL, dissolved in 1% DMSO) were screened for HIV-1 PR inhibitory activity using an HIV-1 protease inhibitor screening kit (Fluorometric) (Biovision; Milpitas, CA, USA) according to the manufacturer's instruction as previously described.12 Briefly, each sample was incubated with the HIV-1 PR enzyme at room temperature for 15 min. Then, the fluorescent substrate was added and the fluorescence (excitation/emission = 330/450 nm) was measured in a kinetic mode for 90 min at 37 °C using PerkinElmer EnSpire plate reader. Pepstatin (1 mM) which was provided in the kit and DMSO (1%, v/v) were used as a positive and vehicle control, respectively.
2.4. Screening for HIV-1 RT inhibitor activity
1 mg/mL of the extracts were dissolved in 1% DMSO then screened for HIV-1 RT inhibitory activity using an HIV-1 reverse transcriptase assay, colorimetric kit (Roche, Germany) following the manufacturer's instruction. Each sample was incubated with HIV-1 RT and reaction mixture, including poly-A template, biotin-conjugated dUTP and DIG-conjugated dUTP for an hour at 37 °C. The incubated mixtures were then transferred to a pre-coated streptavidin microplate module and incubated at 37 °C for 1 h. The reaction wells were then washed with washing buffer to remove the unbound products and the POD-conjugated anti-DIG were added into the reaction wells and incubated for 1 h at 37 °C. The reaction wells were then washed with washing buffer before the ABTS substrate was added and incubated at room temperature for 15 min on a shaking incubator at 250 rpm. The final products were measured at an absorbance of 405 nm and a reference wavelength at 490 nm. In addition, nevirapine (NVP; 200 μM) and DMSO (1%, v/v) were used as an inhibitor (positive control) and vehicle control, respectively.
2.5. Cell culture
The MOLT-4 cells, a human T lymphoblast cell line, were cultured in RPMI-1640 supplemented with 10% (v/v) FBS at 37 °C in a humidified incubator with 5% (v/v) of CO2. The ACH-2 cells, an HIV-1 latent human T-cell line were maintained in RPMI-1640 supplemented with 10 mM HEPES and 10% (v/v) FBS at the same condition as the MOLT-4 cells.
2.6. Cytotoxicity assay
The crude LR extracts (LRH, LRE, and LRW) were tested for their cytotoxicity against MOLT-4 cells using the surrogate MTS assay. The extracts were dissolved in DMSO and tested at final concentrations of 0.50, 0.25, 0.13, 0.06 and 0.03 mg/mL for LRH and LRE, or 4.00, 2.00, 1.00, 0.50 and 0.25 mg/ml for LRW, with the carrier DMSO at a final concentration of 0.1% (v/v). The MOLT-4 cells (10 × 103 cells/well) were plated into 96-well plates and incubated with the respective crude extract for 24, 48 and 72 h, while DMSO at 0.1% (v/v) was used as vehicle control and untreated cells as the reference normal control. At the end of each incubation period, MTS reagent was added into each well and incubated for 4 h before the absorbance at 490 nm was measured. The relative number of viable cells was determined in comparison to the untreated cell control (set as 100% viability).
2.7. Virus expression
Active HIV-1 was produced following the previous study 13, the virus stock was determined a quantitative of p24 by using HIV-1 p24 ELISA kit (Abcam, UK). The stock was either used immediately or kept at −80 °C for further use.
2.8. Syncytial formation assay
MOLT-4 cells (2 × 105 cells/well) were infected with HIV-1 viruses (2 × 104 pg of HIV-1 p24) for 2 h at 37 °C and 5% of CO2. The cells were washed three times with PBS to eliminate unbounded viruses. The infected cells were treated with the treatments then cultured on 48-well plate for 24, 48 and 72 h. HIV-1 induced syncytial formation was observed by an inverted light microscope. Untreated cells (no treatment) was used as a control, DRV and NVP at 2 μM were used as drug inhibitor controls. The results were reported in percentage of syncytia cell compared to the untreated cell control.
2.9. Determination of HIV-1 p24 level
The MOLT-4 cells (2 × 105 cells) were infected with HIV-1 viruses (2 × 104 pg of HIV-1 p24) for 2 h at 37 °C and 5% of CO2. The cells were washed three times with PBS to eliminate unbounded viruses. The infected cells were re-suspended in complete culture media (200 μl) and treated with LRW (200 μl) to make final concentrations at 0.5 mg/ml. The treated cells were cultured on a 48-well plate for 72 h. Then the culture media were collected in 1.5 ml microcentrifuge tube and centrifuged at 2000 rpm for 10 min. The clear supernatants were determined HIV-1 p24 levels by using HIV-1 p24 ELISA kit (Abcam, UK). The assay was performed according to the manufacturer's instruction. The final color of the reactions was measured absorbance at 450 nm. The quantitative of HIV1 p24 was calculated by comparing to the standard curve. Untreated cell condition (no treatment) was used as a control, DRV and NVP at 2 μM were used as drug controls.
2.10. Statistical analysis
All experiments were performed in triplicate and repeated at least three times. The results are presented as the mean of three independent experiments ± one standard error of the mean (SEM). Statistical analysis was studied by one-way ANOVA following the post hock Dunnett's test using the SPSS version 16.0 software. Comparisons with P values less than 0.05 were considered as statistically significant.
2.11. Qualitative phytochemical screening
All extracts were submitted to Institute of Systems Biology (Universiti Kebangsaan Malaysia, Malaysia) for phytochemical profile screening using GC-MS for LRH and LC-MS for both LRE and LRW.
2.11.1. Analysis of the LRH extract by GC-MS
The GC-MS analyses were performed using a Clarus 600 GC-MS (PerkinElmer), run on fused silica capillary columns coated with a 5% diphenyl/95% dimethyl polysiloxane stationary phase (30 m × 250 mm x id 0.25). The samples were analysed using an injector temperature of 250 °C, split ratio 50:1, in a helium flow with an oven temperature of 70 °C (1 min), then increased to 76 °C at 1 °C/min and held for 1 min, and then heated to 300 °C at 6 °C/min and held for 5 min. The compounds were identified using the National Institute of Standards and Technology (NIST) library.14
2.11.2. Analysis of the LRE and LRW extracts by LC-MS
The LC-MS analysis was performed using a Dionex™ UltiMate 3000 UHPLC system (Thermo Scientific) equipped with an Acclaim™ Polar Advantage II C18 column (3 × 150 mm, 3 μm particle size) (Thermo Scientific) coupled with a MicrOTOF-Q III (Bruker Daltonik GmbH, Bremen, Germany) in positive electrospray ionization mode. The compounds were identified by comparing the obtained m/z values to the METLIN databases,15 with a tolerance value lower than 15 parts-per-million (ppm). The relative amount of each compound (%) was calculated from the percentage of the respective peak area relative to the total area in the chromatogram.
2.12. Evaluation of Lipinski's rule of five and ADMET parameters
Lipinski's rule of five, adsorption, distribution and toxicity parameters of identified compounds were determined using SwissADME (http://www.swissadme.ch) and preADMET (https://preadmet.bmdrc.kr/toxicity) online servers.16 Drug-likeness was indicated by Lipinski's rule of five parameters: molecular weight (MW) ≤ 500; the number of rotatable bonds (n-ROTB) ≤ 10; the number of hydrogen bond acceptor (HBA) ≤ 10; the number of hydrogen bond acceptor (HBD) ≤ 5 and MlogP ≤ 4.15).17 The compounds which passed the selection criteria were used for molecular docking study.
2.13. Molecular docking
Molecular docking studies were performed using AutoDock 4.0 software. The X-ray crystal structure of HIV-1 PR (PDB ID: 5KR0)18 and HIV-1 RT (PDB ID: 3QIP)19 were downloaded from RCSB Protein Data Bank. The target proteins were prepared by removing ligands and water molecules and adding missing hydrogens. All ligands were drawn and minimized using BIOVIA Draw 2018. The grid-based approach was used in this study, the positions of grid boxes were set following the original inhibitors of those proteins (amprenavir, APV for HIV-1 PR and nevirapine, NVP for HIV-1 RT). For HIV-1 PR, the size of the grid box was set to 60 × 60 x 60 xyz points with a grid spacing of 0.375 Å at the center of the protein. While the grid box of HIV-1 RT at polymerase active site was set to 60 × 60 x 60 xyz points with a grid spacing of 0.375 Å, the xyz center of grid box at 11.172, 13.44 and 17.661, respectively. The docking simulations were performed using the Lamarckian genetic algorithm with default parameters. The docking results provided 10 different conformations, but the conformation with the lowest binding energy (BE; ΔG) was selected for further studying the protein-ligand interactions using the Discovery studio visualizer. The results were compared to APV and NVP, the original ligands of HIV-1 PR and RT protein complexes, respectively.
3. Results
3.1. Extraction of the LR sclerotia
The LR sclerotia powder was sequentially extracted with hexane (LRH), ethanol (LRE), and cold water (LRW) with extractive values of 0.28, 0.62, and 12.18 g/g of the dry weight of LR sclerotia powder, respectively.
3.2. Inhibitory effect of the LR extracts on HIV-1 PR
The LR extracts were screened for their inhibitory activity against HIV-1 PR. All extracts at 1 mg/mL significantly inhibited the HIV-1 PR activity when compared to the vehicle control, 1% (v/v) DMSO. LRH showed 88.97 ± 1.57% inhibition which was slightly greater than those of 1 mM pepstatin (positive control; 81.48 ± 0.76%). On the contrary, LRE and LRW displayed less inhibition with 37.43 ± 1.08 and 25.72 ± 3.41%, respectively. DMSO slightly suppressed HIV-1 PR activity with 8.07 ± 0.13% inhibition. We found that the 50% inhibitory concentration (IC50) of LRH is approximately 0.53 ± 0.02 mg/mL (Table 1). Since the highest concentration of LRE and LRW were tested in the in vitro assay and showed only 25–37% inhibition against HIV-1 PR activity, therefore we did not determine the IC50 since the higher concentrations might toxic to the cells.
Table 1.
; Percent relative inhibition and half-maximal inhibition concentration (IC50) on HIV-1 PR and RT activities of LR crude extracts, pepstatin, NVP and DMSO.
| Tested compounds | HIV-1 PR |
HIV-1 RT |
||
|---|---|---|---|---|
| % Inhibition | IC50 | % Inhibition | IC50 | |
| LRH (1 mg/ml) | 88.97 ± 1.57∗ | 0.53 ± 0.02 mg/mL | 9.94 ± 3.12 | ND |
| LRE (1 mg/ml) | 37.43 ± 1.08∗ | ND | 53.03 ± 3.32∗ | ND |
| LRW (1 mg/ml) | 25.72 ± 3.41∗ | ND | 55.56 ± 3.51∗ | ND |
| Pepstatin (1 mM) | 81.48 ± 0.76∗ | 0.32 ± 0.05 mM | ND | ND |
| NVP (200 μM) | ND | ND | 99.02 ± 0.98∗ | 0.16 ± 0.00 μM |
| DMSO (1%, v/v) | 8.07 ± 0.13 | ND | 2.34 ± 0.59 | ND |
*P < 0.05 versus the vehicle control. ND, Not determine.
3.3. Inhibitory effect of the LR extracts on HIV-1 RT
The screening test revealed that at 1 mg/mL of LRE and LRW significantly inhibited HIV-1 RT activity with 53.03 ± 3.32% and 55.56 ± 3.51%, respectively. Additionally, 200 μM NVP which is the positive control induced 99.02 ± 0.98% inhibition. On the other hand, there was no significant effect of LRH and DMSO on HIV-1 RT (Table 1). Due to the inhibition caused by LRE and LRW was only slightly higher than 50%, we decided to not further test these extracts at vary concentrations to determine the actual IC50.
3.4. In vitro cytotoxicity of LR extracts on MOLT-4 cells
The cytotoxicity of the crude LR extracts against MOLT-4 cells, a human T lymphoblast cell line, was performed using the MTS assay. The data showed that IC50 of LRH, LRE and LRW were 0.09 ± 0.01, 0.14 ± 0.01 and 2.04 ± 0.03 mg/mL, respectively. The LRH and LRE showed marked cytotoxicity at all tested concentrations (Fig. 1A and 1B). On the contrary, the LRW extract showed no significant cytotoxicity against MOLT-4 cells tested at 0.5 mg/ml (Fig. 1C). Thus, only LRW at 0.5 mg/ml was selected for anti-HIV-1 in infected MOLT-4 cells.
Fig. 1.
;Cytotoxicity of the LR extracts:
(A) LRH, (B) LRE and (C) LRW against MOLT-4 cells. Cell viability of MOLT-4 cells was determined using the MTS assay. Anti-HIV-1 effect of LRW on HIV-1 infected MOLT-4 cells: (D) Syncytial formation and (E) HIV-1 p24 production inhibitions of LRW (0.5 mg/ml), DMSO (0.1%, v/v), DRV (2 μM) and NVP (2 μM). Data are shown as the mean ± SEM of triplicate values. *P < 0.05 versus the cell control.
3.5. Inhibitory effects of LRW on HIV-1 induced syncytial formation of MOLT-4 cells
To determine the antiviral activity of LRW in cell culture, cytopathic effect (CPE) was observed following infection. Syncytia cell or multinucleated giant cell is one of CPE induced by HIV-1 infection. Herein, LRW at 0.5 mg/ml significantly decreased the syncytial formation of infected MOLT-4 cells when treated for 24, 48 and 72 h, compared to 0.1% (v/v) DMSO, a vehicle control. The percentages of syncytia cell were decreased to approximately 57–65%. In addition, drug positive controls which are 2 μM of DRV and NVP displayed 34–55% of syncytial formation whereas no decrease of syncytial formation was detected after the vehicle control treatment (Fig. 1D). The reduction in the percentage of syncytial formation suggests that the LRW could inhibit HIV-1 replication in the infected MOLT-4 cells.
3.6. Inhibitory effect of LRW on p24 production of infected MOLT-4 cells
LRW at 0.50 mg/ml significantly inhibited the p24 production of HIV-1 infected MOLT-4 cells, in agreement with DRV and NVP (2 μM), drug positive controls. While 0.1% DMSO, the vehicle control showed insignificant in the p24 level when compared with untreated cell control (Fig. 1E).
3.7. Phytochemical profile of LR extracts
According to the in vitro assays, all LR extracts exhibited interesting activity on both HIV-1 PR and RT inhibitions. Although LRH and LRE showed toxicity against MOLT-4 cells, these extracts might compose of active compound(s) with high effective but low toxicity. Furthermore, the LRH, LRE and LRW were analysed the chemical profile by using GC-MS or LC-MS to determine the active component presented in the LR extracts.
The chemical constituents of LRH were analysed by GC-MS, then the chromatographic peaks were identified by comparison to the NIST library. The chromatogram of LRH exhibited about 50 isolated peaks, the identified compounds that provided a match factor and/or a reverse match factor greater than 80020 are shown in the supplementary data 2. The identified compounds could be classified into four major groups which were alkanes (32.6%), fatty acids (29.8%), terpenes (21.8%), and ketones (15.8%).
The LC-MS analysis revealed that LRE composed of more than 100 isolated peaks on the chromatogram. The m/z values of each chromatographic peak were tentatively identified base on the METLIN database with mass error values less than 15 ppm. Herein, 42 identified compounds were reported in the supplementary data 3, included phospholipids, peptides and other organic compounds.
The chemical constituents of LRW were examined using LC-MS, revealing more than 70 peaks in the chromatogram and 37 compounds were identified. In the supplementary data 4, the data showed that LRW comprised of triacylglycerols, fatty acids, and terpenes.
3.8. Lipinski's rule of five and ADMET parameters
Drug-likeness of identified compounds was evaluated using Lipinski's rule of five. Physicochemical parameters of the compounds were calculated by SwissADME online server. All LRH identified compounds were acceptable for the Lipinski's rule, while 34 out of 42 compounds from LRE fitted with the Lipinski's rule. Moreover, only 31 compounds from 36 candidate compounds of LRW reached the criteria (Supplementary data 5).
In silico ADMET profile of candidate compounds was shown in the supplementary data 5. Human intestine absorption (HIA), blood-brain barrier (BBB) distribution and toxicity were considered. We found that 10 compounds have high absorption and non-mutagen, these compounds were selected for molecular docking study.
3.9. Molecular docking
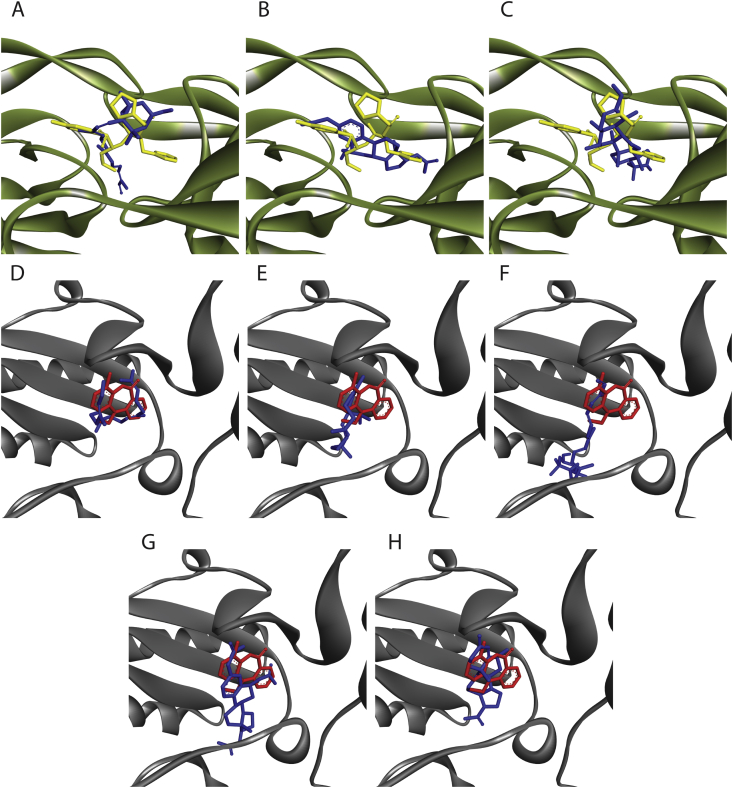
To predict the active compound(s) from LR extracts that block the viral enzymes activity, the computational simulation was performed using AutoDock 4.0 molecular docking software. A comparison of BE between candidate ligands and original ligands was considered, where, theoretically, a low BE represents a good prediction in molecular docking. For HIV-1 PR (PDB ID: 5KR0) analysis by re-docking determination, the BE of APV with HIV-1 PR was −9.73 kcal/mol. Ligand-protein interaction showed that APV bonded to the ASN25, ILE50 and ASP30 residues of the protein with hydrogen bonds (H-bond) (Table 2). Among the candidate compounds, heliantriol F, apo-12′-violaxanthal and estra-1,3,5(10)-triene-3,6 beta,17 beta-triol triacetate showed the BE at −12.57, −9.98 and −9.96 kcal/mol, respectively. These BEs were lower than that of APV, a reference inhibitor for HIV-1 PR (Table 2). Receptor-ligand interaction exhibited that heliantriol F, apo-12′-violaxanthal and estra-1,3,5(10)-triene-3,6 beta,17 beta-triol triacetate could possibly be docked into the HIV-1 PR binding pocket similar to APV (Fig. 2A–C).
Table 2.
; Molecular docking results of candidate ligands from LR extracts at the active site of HIV-1 PR.
| Ligand | Binding energy (kcal/mol) | Inhibition constant (Kpi) | Number of H-bond | Amino acid interaction (Bond length) | |
|---|---|---|---|---|---|
| APV | −9.73 | 73.84 nM | 3 | B:ASN25 (3.39168), B:ILE50 (2.77165), B:ASP30 (3.24437) | |
| LRH | |||||
| Geranylgeraniol | −7.28 | 4.60 μM | 1 | A:ASP29 (2.67949) | |
| Thunbergol | −8.93 | 286.98 nM | 1 | B:GLY49 (3.04361) | |
| LRE | |||||
| 2-Heptoxyethanethiol | −3.25 | 4.12 mM | 1 | A:GLY27 (1.98476) | |
| Apo-12′-violaxanthal | −9.98 | 48.59 nM | 3 | B:ARG8 (2.72933), A:THR80 (1.68262), B:ARG8 (3.63695) | |
| Estra-1,3,5(10)-triene-3,6beta,17beta-triol triacetate | −9.96 | 50.25 nM | 1 | A:ILE50 (2.99369) | |
| Methyl 10-gingerol | −5.74 | 61.60 μM | 2 | A:ASP30 (1.67391), A:GLY49 (2.99561) | |
| LRW | |||||
| 2-Heptoxyethanethiol | −3.25 | 4.12 mM | 1 | A:GLY27 (1.98476) | |
| 6 alpha-Fluoroprogesterone | −9.64 | 85.85 nM | 3 | A:ASP29 (3.24964), A:ASP30 (3.07318), B:THR80 (2.4667) | |
| 1,2-Dioctanoyl-sn-glycerol | −4.71 | 349.87 μM | 2 | A:GLY48 (3.32212), A:GLY48 (2.01816) | |
| Heliantriol F | −12.57 | 607.42 pM | 2 | B:ASN25 (3.06036), B:GLY49 (2.96205) | |
| 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl) hexadecan-3-one | −6.06 | 35.85 μM | 6 | A:ASN25 (2.83581), A:ASN25 (2.90096), B:ASN25 (2.15681), A:ASP30 (1.78535), A:ASP29 (3.64172), A:ASP30 (3.03094) | |
Fig. 2.
;Three-dimensional schematics present ligand orientations of candidate compounds compared to original inhibitors: APV and NVP in HIV-1 PR and RT binding pockets, respectively.
(A) apo-12′-violaxanthal, (B) estra-1,3,5(10)-triene-3,6beta,17beta-triol triacetate and (C) heliantriol F were docked into the active site of HIV-1 PR. (D) geranylgeraniol, (E) thunbergol, (F) apo-12′-violaxanthal, (G) estra-1,3,5(10)-triene-3,6beta,17beta-triol triacetate and (H) 6 alpha-fluoroprogesterone were docked into DNA polymerase active site of HIV-1 RT. Green and gray macromolecules represent HIV-1 PR and HIV-1 RT, respectively. Yellow, red and blue small molecules represent APV, NVP and candidate ligands, respectively.
At polymerase active site of HIV-1 RT (PDB ID: 3QIP), the re-docking analysis demonstrated that the BE of NVP was −9.36 kcal/mol. The NPV, a reference inhibitor of this enzyme interacted with LEU100, LYS101, LYS103, VAL106, TYR188 and LEU234 residues of the enzyme (Table 3). Interestingly, five candidate compounds which are 6 alpha-fluoroprogesterone, estra-1,3,5(10)-triene-3,6beta,17beta-triol triacetate, geranylgeraniol, thunbergol and apo-12′-violaxanthal displayed BE with HIV-1 RT at polymerase active site at −11.12, −10.11, −10.09, −10.01 and −9.78 kcal/mol, respectively. These BEs were less than that of NVP, suggesting that these candidate compounds might block polymerase activity of HIV-1 RT by bind to the active site of the enzyme stronger than NVP (Table 3). Besides, these compounds interacted with HIV-1 RT in the same orientation as NVP (Fig. 2D–H).
Table 3.
; Molecular docking results of candidate ligands from LR extracts at polymerase active site of HIV-1 RT.
| Ligand | Binding energy (kcal/mol) | Inhibition constant (Kpi) | Number of H-bond | Amino acid interaction (Bond length) |
|---|---|---|---|---|
| NPV | −9.36 | 137.68 nM | 1 | A:LYS101 (3.29016) |
| LRH | ||||
| Geranylgeraniol | −10.09 | 40.33 nM | 1 | A:TYR188 (3.54739) |
| Thunbergol | −10.01 | 45.80 nM | 0 | – |
| LRE | ||||
| 2-Heptoxyethanethiol | −4.78 | 314.72 μM | 4 | A:LYS101 (2.08296), A:PRO236 (3.18057), A:TYR318 (3.24429), A:PRO236 (3.10392) |
| Apo-12′-violaxanthal | −9.78 | 67.32 nM | 3 | A:ASN175 (2.80294), A:PRO176 (2.75443), A:ILE178 (2.06618) |
| Estra-1,3,5(10)-triene-3,6beta,17beta-triol triacetate | −10.11 | 38.92 nM | 2 | A:LYS101 (3.31447), A:PRO236 (3.08095) |
| Methyl 10-gingerol | −8.08 | 1.20 μM | 2 | A:LYS101 (1.85019), A:GLY190 (3.37988) |
| LRW | ||||
| 2-Heptoxyethanethiol | −4.78 | 314.72 μM | 4 | A:LYS101 (2.08296), A:PRO236 (3.18057), A:TYR318 (3.24429), A:PRO236 (3.10392) |
| 6 alpha-Fluoroprogesterone | −11.12 | 7.12 nM | 0 | – |
| 1,2-Dioctanoyl-sn-glycerol | −6.59 | 14.79 μM | 0 | – |
| Heliantriol F | −6.45 | 18.6 μM | 3 | A:TRP383 (2.65415), A:ARG172 (2.69148), A:ILE180 (3.05751) |
| 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl) hexadecan-3-one | −7.28 | 4.61 μM | 2 | A:TYR181 (3.33568), A:TYR318 (2.44427) |
4. Discussion
At present, HIV-1 infected patients cannot completely eradicate the virus from their body therefore antiretroviral drug treatment is necessary to control the virus number. Although a diverse array of effective antiretroviral drugs is currently available, increasing drug resistance21 and the adverse effects of the antiretroviral drugs are of limitations. Many side effects from antiretroviral drugs have been reported, such as mitochondrial toxicity of long-term treatment with nucleotide reverse transcriptase inhibitors22 and hepatotoxicity of NVP administration.23 Therefore, the discovery of antiretroviral drugs with better efficacy and lower side effect has attracted much attention. Researchers have found several active compounds exhibiting an anti-HIV-1 property isolated from mushrooms, such as Pleurotus nebrodensis,24 Velleratretraol from Lactarius vellereus25 and melanin-glucan complex from Fomes fomentarius.26 Although LR has been studied for several medicinal properties, information on its anti-HIV-1 activity is not available. In this study, we were interested in the inhibitory activity of LR against the PR and RT HIV-1 enzymes.
LRE and LRW extracts at 1 mg/mL exhibited significant inhibition of both HIV-1 PR and RT activities. Moreover, among the three different LR extracts, LRH displayed the highest inhibition of HIV-1 PR activity, which was comparable to pepstatin, a known inhibitor of HIV-1 PR.
Based on the phytochemical profile data of LRH obtained from the GC-MS analysis, four major groups of components (alkanes, fatty acids, terpenes, and ketones) were revealed. Eicosane, heneicosane, and palmitic acid found in LRH have also been reported in previous studies.14,27 In addition, this is the first study to report the existence of certain identified compounds (Supplementary data 2) found in LRH that have previously been found in other natural sources, such as 3,7,11,15-tetramethyl-2-hexadecen-1-ol in the leaf extract of Erythrina variegate,28 geranylgerniol from the fruiting body extract of Boletinus cavipes,29 thunbergol from the leaf extract of Pistacia lentiscus,30 pine needle extract of Pinus sylvestris L,31 and 2-nonadecanone from an Alhagi maurorum leaf extract.32
The chemical constituents in LRE and LRW were preliminarily analysed by LC-MS, revealing abundant levels of phospholipids, peptides and other organic compounds. With respect to the phospholipids in LRE, they were derived from fatty acids, such as linoleic, oleic, and palmitic acids, in accord with a previous finding.27 Furthermore, the high content of triacylglycerols and terpenoids were found in LRW. Several fatty acids have been evaluated for anti-HIV-1 activity, for example, linoleic and oleic acids isolated from brown algae (Sargassum fusiforme) could inhibit HIV-1 RT,33 while palmitic acid was reported to inhibit viral entry by directly blocking gp120-CD4 complex formation.34 Additionally, the detection of all essential amino acids in LR powder35 is consistent with the presence of amino acids found in the LRE and LRW extracts. These data suggest that LRE and LRW are composed of active components that have been ascribed for anti-HIV-1 activity.
In this study, anti-HIV-1 replication was investigated in MOT-4 cell, a human T lymphoblast which is generally used for HIV-1 research such as cytotoxicity assay as well as a target for HIV-1 infection.36,37 Cytotoxicity investigation exhibited that LRH and LRE were toxic to MOLT-4 cells in a dose-dependent manner with IC50 of 0.03 and 0.14 mg/ml, respectively, which were lower than the IC50 of LRH (0.53 mg/mL) and LRE (>1 mg/ml) on HIV-1 PR inhibition. While LRW extract showed the toxicity against MOLT-4 cells with IC50 of 2.04 mg/ml, this extract could potentially be safely used in vitro cell-based studies.
In vitro cell-based assays demonstrated that LRW could inhibit HIV-1 replication by the significantly reduced syncytial formation and p24 production. From the chemical constituent analysis, LRW was composed of betulonic acid and oleanolic acid which have been reported for HIV-1 effects.38,39 These data suggest that belulonic acid and oleanolic acid might be the active compounds that could inhibit HIV-1 replication in the MOLT-4 infected cells.
In search of potential newly active compounds, molecular docking was performed. This in silico virtual analysis revealed that heliantriol F and 6 alpha-fluoroprogesterone found in LRW provided the lowest BE to HIV-1 PR and HIV-1 RT, respectively, compared to the other candidate compounds. Moreover, Lipinski's rule of five and ADMET parameters indicated that these compounds could be good drug-likeness with non-toxicity. These results suggest that heliantriol F and 6 alpha-fluoroprogesterone could have the potential to be the active compound with HIV-1 PR or RT inhibitory activity. According to previous studies, heliantriol F derived from edible flower extract of Chrysanthemum morifolium exhibited an antitumor activity.40,41 However, HIV-1 inhibitory activity of these proposed compounds have not previously been reported, making this finding the first-time discovery.
This molecular docking study is a useful tool for describing the interaction of a single compound with a target protein. However, a synergistic effect of active compounds should be concerned. Kametani S et al. determined the synergistic effect of chemical constituents from dichloromethane crude extract of Aloe ferox Miller for Ehrlich ascites tumor cell growth inhibition, they suggested that the inhibitory effect of crude extract did not depend on an isolated compound alone, but on the synergistic effect from the combination of the compounds.42 To the best of our knowledge, the HIV-1 PR and RT inhibitions might affect from single active compounds or the synergistic effect of different compounds. Therefore, the identified compounds should be further isolated and evaluated the synergistic effect on HIV-1 PR and RT inhibitory activities.
Previous studies reported that 8-O-methylingol-3,12-diacetate-7-benzoate, 3-(2-naphthoyl) ingeno, euphorneroid D, and ent-3-oxoatisan-16a,17-acetonide, known as diterpenoids, exhibited anti-HIV-1 activity.43, 44, 45 Additionally, the inhibition of HIV-1 replication by triterpenoids has also been investigated. For example, oleanolic acid, uvaol, ursolic acid, maslinic acid, and 2α, 19α-dihydroxy-3-oxo-12-ursen-28-oic acid all displayed anti-HIV-1 activity by targeting HIV-1 PR.39,46,47 Moreover, 1β-hydroxymaprounic 3-p-hydroxybenzoate, 2α-hydroxymaprounic acid 2,3-bis-p-hydroxybenzoate, 1β-hydroxymaprounic, 2α-hydroxymaprounic acid, garciosaterpenes A, garciosaterpenes C, and betulinic inhibited HIV-1 RT activity.48, 49, 50 Herein, we discovered that LR extracts contain several diterpenoids and triterpenoids such as geranylgeraniol, thunbergol, estra-1,3,5(10)-triene-3,6beta,17beta-triol triacetate, 6 alpha-fluoroprogesterone and heliantriol F. Moreover, these compounds presented good binding capacity with HIV-1 enzymes. Therefore, we suggest that terpenoids are interesting active compounds responsible for HIV-1 enzymes inhibition. Furthermore, these proposed terpenoids should be deeply studied for anti-HIV-1 activity.
5. Conclusions
Our work is the first to demonstrate the in vitro anti-HIV-1 PR and RT of the LR sclerotia extracts, as well as their metabolite profiles. The crude LRH extract showed a high in vitro inhibitory activity against HIV-1 PR. Moreover, the LRE and LRW extracts could suppress both HIV-1 PR and RT activities in vitro. Cell-based assays demonstrated that at 0.5 mg/ml of LRW significantly inhibited HIV-1 replication. The metabolite profile and in silico analysis supported the hypothesis that the LR extracts were composed of active compounds that could have anti-HIV-1 PR and/or RT activities. These results suggested that LR could be a potential source of compounds to inhibit HIV-1 replication. Furthermore, our results provided the preliminary data for the development of further novel HIV-1 PR and RT inhibitors.
Conflicts of interest
The author has declared no conflict of interest.
Funding
This research was financially supported by Innovation Center for Research and Development of Medical Diagnostic Technology Project under Chulalongkorn University Centenary Academic Development Project and Chulalongkorn University Grant for Special Task Force for Activating Research (STAR), both to SC. This work was also partly supported by the 90th anniversary Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund: GCUGR1125602097 M) to CS.
Acknowledgements
The scholarship from the Graduate School, Chulalongkorn University to commemorate the 72nd anniversary of his Majesty King Bhumibol Aduladeja is gratefully acknowledged. The authors sincerely thank Ms. Szu Ting Ng and Dr. Chon Seng Tan from Ligno Biotech™Sdn. Bhd for the LR sclerotial powder. We acknowledge the NIH AIDS Research and Reference Program for providing ACH-2 cell line, DRV and NVP (antiretroviral drugs). We would like to thank Associated Professor Songyot Anuchapreeda for a kind gift of MOLT-4 cells.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2019.08.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lau B.F., Abdullah N., Aminudin N., Lee H.B. Chemical composition and cellular toxicity of ethnobotanical-based hot and cold aqueous preparations of the tiger' s milk mushroom (Lignosus rhinocerotis) J Ethnopharmacol. 2013;150(1):252–262. doi: 10.1016/j.jep.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 2.Eik L.-F., Naidu M., David P., Wong K.-H., Tan Y.-S., Sabaratnam V. 2012. Lignosus Rhinocerus (Cooke) Ryvarden: A Medicinal Mushroom that Stimulates Neurite Outgrowth in PC-12 Cells. Evidence-Based Complementary and Alternative Medicine. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S.S., Tan N.H., Fung S.Y., Sim S.M., Tan C.S., Ng S.T. Anti-inflammatory effect of the sclerotium of Lignosus rhinocerotis (Cooke) Ryvarden, the tiger milk mushroom. BMC Complement Altern Med. 2014;14(1):359. doi: 10.1186/1472-6882-14-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap Y.H., Tan N., Fung S., Aziz A.A., Tan C., Ng S. Nutrient composition, antioxidant properties, and anti-proliferative activity of Lignosus rhinocerus Cooke sclerotium. J Sci Food Agric. 2013;93(12):2945–2952. doi: 10.1002/jsfa.6121. [DOI] [PubMed] [Google Scholar]
- 5.Yap H.Y.Y., Tan N.H., Ng S.T., Tan C.S., Fung S.Y. Molecular attributes and apoptosis-inducing activities of a putative serine protease isolated from Tiger Milk mushroom (Lignosus rhinocerus) sclerotium against breast cancer cells in vitro. PeerJ. 2018;6 doi: 10.7717/peerj.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yap H.-Y.Y., Tan N.-H., Ng S.-T., Tan C.-S., Fung S.-Y. Inhibition of protein glycation by tiger milk mushroom [Lignosus rhinocerus (Cooke) Ryvarden] and search for potential anti-diabetic activity-Related metabolic pathways by genomic and transcriptomic data mining. Front Pharmacol. 2018;9:103. doi: 10.3389/fphar.2018.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau B.F., Abdullah N., Aminudin N., Lee H.B., Tan P.J. Ethnomedicinal uses, pharmacological activities, and cultivation of Lignosus spp.(tiger׳ s milk mushrooms) in Malaysia–A review. J Ethnopharmacol. 2015;169:441–458. doi: 10.1016/j.jep.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 8.Nyamweya S., Hegedus A., Jaye A., Rowland-Jones S., Flanagan K.L., Macallan D.C. Comparing HIV-1 and HIV-2 infection: lessons for viral immunopathogenesis. Rev Med Virol. 2013;23(4):221–240. doi: 10.1002/rmv.1739. [DOI] [PubMed] [Google Scholar]
- 9.Sarafianos S.G., Marchand B., Das K. Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J Mol Biol. 2009;385(3):693–713. doi: 10.1016/j.jmb.2008.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lv Z., Chu Y., Wang Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV/AIDS (Auckland, NZ) 2015;7:95. doi: 10.2147/HIV.S79956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman A., Cherepanov P. The structural biology of HIV-1: mechanistic and therapeutic insights. Nat Rev Microbiol. 2012;10(4):279. doi: 10.1038/nrmicro2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad R., Sahidin I., Taher M. Polygonumins A, a newly isolated compound from the stem of Polygonum minus Huds with potential medicinal activities. Sci Rep. 2018;8(1):4202. doi: 10.1038/s41598-018-22485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiser K., Mathys L., Curbo S. The cellular thioredoxin-1/thioredoxin Reductase-1 driven oxidoreduction represents a Chemotherapeutic target for HIV-1 entry inhibition. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnathan M., Gan S., Ezumi M.W., Faezahtul A., Nurul A. Phytochemical profiles and inhibitory effects of Tiger Milk mushroom (Lignosus rhinocerus) extract on ovalbumin-induced airway inflammation in a rodent model of asthma. BMC Complement Altern Med. 2016;16(1):167. doi: 10.1186/s12906-016-1141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasansuklab A., Meemon K., Sobhon P., Tencomnao T. Ethanolic extract of Streblus asper leaves protects against glutamate-induced toxicity in HT22 hippocampal neuronal cells and extends lifespan of Caenorhabditis elegans. BMC Complement Altern Med. 2017;17(1):551. doi: 10.1186/s12906-017-2050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razzaghi-Asl N., Mirzayi S., Mahnam K., Sepehri S. Modelling. Identification of COX-2 inhibitors via structure-based virtual screening and molecular dynamics simulation. J Mol Graph Model. 2018;83:138–152. doi: 10.1016/j.jmgm.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Lipinski C.A., Lombardo F., Dominy B.W., PJJAddr Feeney. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23(1-3):3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z., Huang X., Hu L. Effects of hinge Region natural polymorphisms on human immunodeficiency virus-1 protease structure, dynamics and drug-pressure evolution. J Biol Chem. 2016;291(43):22741–22756. doi: 10.1074/jbc.M116.747568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lansdon E.B., Liu Q., Leavitt S.A. Structural and binding analysis of pyrimidinol carboxylic acid and N-hydroxy quinazolinedione HIV-1 RNase H inhibitors. Antimicrob Agents Chemother. 2011;55(6):2905–2915. doi: 10.1128/AAC.01594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duangjan C., Rangsinth P., Gu X., Wink M., Tencomnao T. Caenorhabditis elegans. Oxidative Medicine and Cellular Longevity. 2019. 2019. Lifespan extending and oxidative stress resistance properties of a leaf extracts from anacardium occidentale L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apisarnthanarak A., Jirayasethpong T., Sa-nguansilp C. Antiretroviral drug resistance among antiretroviral-naïve persons with recent HIV infection in Thailand. HIV Med. 2008;9(5):322–325. doi: 10.1111/j.1468-1293.2008.00562.x. [DOI] [PubMed] [Google Scholar]
- 22.Brinkman K., ter Hofstede H.J., Burger D.M., Smeitink J.A., Koopmans P.P. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS. 1998;12(14):1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Wongtrakul J., Paemanee A., Wintachai P. Nevirapine induces apoptosis in liver (HepG2) cells. Asian Pacific journal of tropical medicine. 2016;9(6):547–553. doi: 10.1016/j.apjtm.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Lv H., Kong Y., Yao Q. Nebrodeolysin, a novel hemolytic protein from mushroom Pleurotus nebrodensis with apoptosis-inducing and anti-HIV-1 effects. Phytomedicine. 2009;16(2-3):198–205. doi: 10.1016/j.phymed.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Luo D.-Q., Zhao L.-Y., Shi Y.-L. Velleratretraol, an unusual highly functionalized lactarane sesquiterpene from Lactarius vellereus. J Antibiot. 2009;62(3):129. doi: 10.1038/ja.2008.25. [DOI] [PubMed] [Google Scholar]
- 26.Seniuk O.F., Gorovoj L.F., Beketova G.V. Anti-infective properties of the melanin-glucan complex obtained from medicinal tinder bracket mushroom, Fomes fomentarius (L.: Fr.) Fr.(Aphyllophoromycetideae) Int J Med Mushrooms. 2011;13(1):7–18. doi: 10.1615/intjmedmushr.v13.i1.20. [DOI] [PubMed] [Google Scholar]
- 27.Lau B.F., Abdullah N., Aminudin N. Chemical composition of the tiger's milk mushroom, Lignosus rhinocerotis (Cooke) Ryvarden, from different developmental stages. J Agric Food Chem. 2013;61(20):4890–4897. doi: 10.1021/jf4002507. [DOI] [PubMed] [Google Scholar]
- 28.Muthukrishnan S., Palanisamy S., Subramanian S., Selvaraj S., Mari K.R., Kuppulingam R. Phytochemical profile of Erythrina variegata by using high-performance liquid chromatography and gas chromatography-mass spectroscopy analyses. Journal of acupuncture and meridian studies. 2016;9(4):207–212. doi: 10.1016/j.jams.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Kamo T., Sato K., Sen K., Shibata H., Hirota M. Geranylgeraniol-type diterpenoids, boletinins A− J, from Boletinus c avipes as inhibitors of superoxide anion generation in macrophage cells. J Nat Prod. 2004;67(6):958–963. doi: 10.1021/np030535g. [DOI] [PubMed] [Google Scholar]
- 30.Mharti F.Z., Lyoussi B., Abdellaoui A. Antibacterial activity of the essential oils of Pistacia lentiscus used in Moroccan folkloric medicine. Natural product communications. 2011;6(10):1505–1506. [PubMed] [Google Scholar]
- 31.Ka M.-H., Choi E.H., Chun H.-S., Lee K.-G. Antioxidative activity of volatile extracts isolated from Angelica tenuissimae roots, peppermint leaves, pine needles, and sweet flag leaves. J Agric Food Chem. 2005;53(10):4124–4129. doi: 10.1021/jf047932x. [DOI] [PubMed] [Google Scholar]
- 32.Samejo M., Memon S., Bhanger M., Khan K. Chemical composition of essential oils from Alhagi maurorum. Chem Nat Comp. 2012;48(5):898–900. [Google Scholar]
- 33.Lee D.Y.-W., Canki M. 2010. Methods and Compositions for the Use of Sargassum Fusiforme for the Inhibition of Hiv-1 Infection. in: Google Patents. [Google Scholar]
- 34.Lee D.Y.-W., Lin X., Paskaleva E.E. Palmitic acid is a novel CD4 fusion inhibitor that blocks HIV entry and infection. AIDS Res Hum Retrovir. 2009;25(12):1231–1241. doi: 10.1089/aid.2009.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong B.-H., Tan N.-H., Fung S.-Y., Pailoor J., Tan C.-S., Ng S.-T. Nutritional composition, antioxidant properties, and toxicology evaluation of the sclerotium of Tiger Milk Mushroom Lignosus tigris cultivar. E. Nutrition Research. 2016;36(2):174–183. doi: 10.1016/j.nutres.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Harada S. The broad anti-viral agent glycyrrhizin directly modulates the fluidity of plasma membrane and HIV-1 envelope. Biochem J. 2005;392(1):191–199. doi: 10.1042/BJ20051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Widiyanti P., Prajogo B., Widodo A. Effect of varying incubation periods on cytotoxicity and virucidal activities of Justicia gendarussa Burm. f. leaf extract on HIV-infected MOLT-4 cells. African journal of infectious diseases. 2018;12(1S):133–139. doi: 10.2101/Ajid.12v1S.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu Q., Wang R.-R., Zhang X.-M. A new benzofuranone and anti-HIV constituents from the stems of Rhus chinensis. Planta Med. 2007;73(03):279–282. doi: 10.1055/s-2007-967113. [DOI] [PubMed] [Google Scholar]
- 39.Mengoni F., Lichtner M., Battinelli L. In vitro anti-HIV activity of oleanolic acid on infected human mononuclear cells. Planta Med. 2002;68(02):111–114. doi: 10.1055/s-2002-20256. [DOI] [PubMed] [Google Scholar]
- 40.Ukiya M., Ohkubo C., Kurita M., Fukatsu M., Suzuki T., Akihisa T. Cytotoxic and apoptosis-inducing activities of taraxastane-type triterpenoid derivatives in human cancer cell lines. Chem Biodivers. 2016;13(8):1018–1029. doi: 10.1002/cbdv.201500356. [DOI] [PubMed] [Google Scholar]
- 41.Ukiya M., Akihisa T., Yasukawa K. Constituents of compositae plants. 2. Triterpene diols, triols, and their 3-o-fatty acid esters from edible chrysanthemum flower extract and their anti-inflammatory effects. J Agric Food Chem. 2001;49(7):3187–3197. doi: 10.1021/jf010164e. [DOI] [PubMed] [Google Scholar]
- 42.Kametani S., Kojima-Yuasa A., Kikuzaki H., Kennedy D.O., Honzawa M., Matsui-Yuasa I. Chemical constituents of cape aloe and their synergistic growth-inhibiting effect on Ehrlich ascites tumor cells. Biosc Biotech Biochem. 2007;71(5):1220–1229. doi: 10.1271/bbb.60659. 0704050365-0704050365. [DOI] [PubMed] [Google Scholar]
- 43.Dong M., Chen X.Q., Chen C.H., Li R.T. Terpenes from Euphorbia antiquorum and their in vitro anti-HIV activity. Chem Biodivers. 2018;15(5) doi: 10.1002/cbdv.201700560. [DOI] [PubMed] [Google Scholar]
- 44.Yan S.-L., Li Y.-H., Chen X.-Q., Liu D., Chen C.-H., Li R.-T. Diterpenes from the stem bark of Euphorbia neriifolia and their in vitro anti-HIV activity. Phytochemistry. 2018;145:40–47. doi: 10.1016/j.phytochem.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Liu Q., Li W., Huang L. Identification, structural modification, and dichotomous effects on human immunodeficiency virus type 1 (HIV-1) replication of ingenane esters from Euphorbia kansui. Eur J Med Chem. 2018;156:618–627. doi: 10.1016/j.ejmech.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Min B.S., Jung H.J., Lee J.S. Inhibitory effect of triterpenes from Crataegus pinatifida on HIV-I protease. Planta Med. 1999;65(04):374–375. doi: 10.1055/s-2006-960792. [DOI] [PubMed] [Google Scholar]
- 47.Xu H.-X., Zeng F.-Q., Wan M., Sim K.-Y. Anti-HIV triterpene acids from Geum japonicum. J Nat Prod. 1996;59(7):643–645. doi: 10.1021/np960165e. [DOI] [PubMed] [Google Scholar]
- 48.Pengsuparp T., Cai L., Fong H.H. Pentacyclic triterpenes derived from Maprounea africana are potent inhibitors of HIV-1 reverse transcriptase. J Nat Prod. 1994;57(3):415–418. doi: 10.1021/np50105a017. [DOI] [PubMed] [Google Scholar]
- 49.Rukachaisirikul V., Pailee P., Hiranrat A. Anti-HIV-1 protostane triterpenes and digeranylbenzophenone from trunk bark and stems of Garcinia speciosa. Planta Med. 2003;69(12):1141–1146. doi: 10.1055/s-2003-818006. [DOI] [PubMed] [Google Scholar]
- 50.Esposito F., Sanna C., Del Vecchio C. Hypericum hircinum L. components as new single-molecule inhibitors of both HIV-1 reverse transcriptase-associated DNA polymerase and ribonuclease H activities. Pathogens and disease. 2013;68(3):116–124. doi: 10.1111/2049-632X.12051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.