Abstract
Foxtail millet (Setaria italica (L.) P. Beauv.) and adlay (Coix lachryma-jobi L. var. ma-yuen Stapf.) seeds have substantial benefits possesses remarkable edible and nutritive values, and ease of processing and food manufacturing. They have nutraceutical properties in the form of antioxidants which prevent deterioration of human health and have long been used in traditional Chinese medicine as a remedy for many diseases. The present study is designed to investigate the gastroprotective effect of foxtail millet and adlay processing product (APP) diet on water immersion restraint stress (WIRS) induced ulceration in rats. We examined the effects of intake of AIN-93G diet containing either foxtail millet (10, 20 and 40%, 4 weeks) or APP (15 and 30%, 5 weeks) on macroscopic ulcer index (UI), plasma calcium level, lipid peroxidation products (estimated by the thiobarbituric acid reactive substances; TBARS), non-protein sulfhydryl (NPSH), digestive enzyme activities, and histopathology were determined. The results showed that pretreatment with millet and adlay diets significantly prevented the gastric mucosal lesion development. In addition, ulcerated rats showed depletion of NPSH levels whereas treatment with millet and adlay reverted this decline in stress-induced rats. Histological studies confirmed the results. The finding suggests that millet and adlay diets promote ulcer protection by the decrease in ulcer index, TBARS values and increase NPSH concentrations. Millet and adlay diets retain the advantage of being a natural product which may protect the gastric mucosa against ulceration.
Keywords: Foxtail millet, Adlay, Water immersion restraint stress, Peptic ulcer disease, Gastroprotective effect
Graphical abstract
Highlights
-
•
Millet and adlay exerted the anti-ulcer response and extensive antioxidant effect.
-
•
They promote ulcer protection by the decrease in ulcer index and TBARS values.
-
•
Also, millet and adlay diets increased NPSH concentrations in stressed rats.
-
•
Foxtail millet and adlay diets prove a promising protective role in gastric ulcer.
List of abbreviations
- TCM
Traditional Chinese medicine
- APP
Adlay processing product
- WRIS
Water immersion restraint stress
- UI
Ulcer index
- TBARS
Thiobarbituric acid reactive substances
- NPSH
Non-protein sulfhydryl
- PUD
Peptic ulcer disease
- NSAIDs
Non-steroidal anti-inflammatory drugs
- ROS
Reactive oxygen species
- LPO
Lipid peroxidation
- MDA
Malondialdehyde
- LAP
Leucine aminopeptidase
- SD
Standard deviation
1. Introduction
Peptic ulcer disease (PUD) is a common disorder of the gastrointestinal system also known as peptic ulcer or gastric ulcers, follows gastric mucosal injuries as a result of imbalance between the defensive and the aggressive factors affecting the mucous.1 The leading cause of PUD include Helicobacter pylori infection,2 and the use of non-steroidal anti-inflammatory drugs (NSAIDs, such as aspirin, Advil or Motrin (ibuprofen), Aleve (naproxen), and others), are the major risk factors for PUD, and also the genetic, pepsin, smoking, alcohol, bile-acids, steroids and stress and comorbidity increase the risk of PUD occurrence.1 Stress is an acute (single or short exposure) threat to homeostasis that evokes an allostatic or adaptive response, affects the function of the gastrointestinal tract either in short-or-long term impacts.3
Typically, primary mucosal erosions are referred to as stress-related injury and, namely, stress ulcers represent focal deep mucosal damages with a high risk for bleeding. The pathophysiology of these disorders focus on the aggressive stress and gastric defense mechanism, such as hydrochloric acid (HCl) production, mucus secretion, non-protein sulfhydryl (NPSH) groups from the stomach and liver, and blood flow.2 Insufficient blood microcirculation in the upper gastrointestinal tissues is considered as the major cause of mucosal defense reduction leading to the ulcer formation. Reactive oxygen species (ROS), such as superoxide anion (O2ˉ), hydrogen peroxide (H2O2), and hydroxyl radical (OH•), accompany ischemic tissue and are suggested as mediators of gastrointestinal injures of different etiology including stress-induced lesions.4 In addition, ROS trigger lipid peroxidation (LPO) with subsequent loss of membrane fluidity, weakened ion transport and membrane integrity, and finally cell death.5
Several drug treatment approaches are available of PUD, but compliance is often poor and frequently associated with adverse effects thus limiting their use. Hence, the search for alternative products continues due to their perceived relative lower side effects, ease of accessibility and affordability, as well as natural phytochemicals isolated from plants used as traditional medicines are considered as good alternatives.
Foxtail millet (Setaria italica (L.) P. Beauv.) is one of the most important drought-resistant whole grain, has long been used to treat vacuity heat of spleen and stomach, stomach reflux vomiting, reduced food intake with abdominal distention, and diabetes mellitus in traditional Chinese medicine (TCM).6 In particular, millets have nutraceutical properties in the form of antioxidants which prevent deterioration of human health such as lowering blood pressure, risk of heart disease, prevention of cancer and cardiovascular diseases, diabetes, decreasing tumor cases etc.7 Previous studies have suggested the anti-diabetic effect of finger millet8 and barnyard millet.9 An animal experiment showed that foxtail millet feeding improved insulin sensitivity and cholesterol metabolism in genetically type 2 diabetic mice.10 A recent study indicated that bound polyphenols of inner shell from foxtail millet bran can display anti-inflammatory effects in LPS-induced HT-29 cells and in nude mice,11 another clinical trial have shown the blood glucose lowering of foxtail millet.12
In addition, adlay (Coix lachryma-jobi L. var. ma-yuen Stapf.) seeds that has been used as a TCM for edema, beriberi, inhibited urination, damp impediment and hypertonicity, spleen vacuity diarrhea, pulmonary welling abscess, intestinal welling abscess and warts, is known for its nutritional benefits.7 Numerous studies have indicated that adlay extract exhibits antioxidative,13, 14, 15 anti-inflammatory,16 anti-tumor,16, 17, 18 anti-allergic,19 hypoglycemic,20 hypolipidemic,21 antiulcer22 properties and xanthine oxidase inhibition.14 Several phenolic antioxidants were isolated from adlay seeds that possess gastroprotective activity against indomethacin-induced gastric ulcer in rats.22
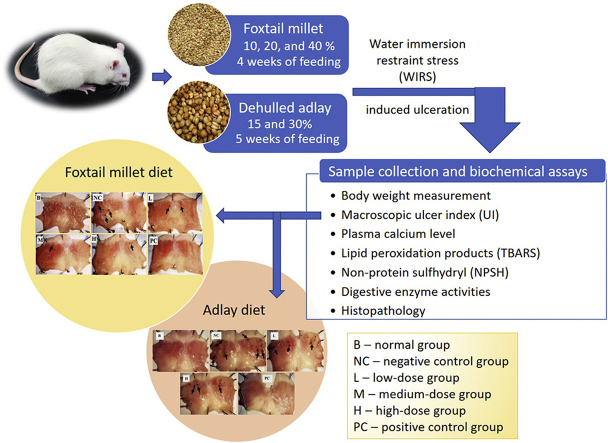
In this preventive study, the protective effect of modified feeds containing foxtail millet and adlay processing product (APP, by using dehulled adlay as the main raw material with burdock, licorice root and ganoderma to become health food by extrusion) at least four weeks feeding was investigated, and ulceration progress in rats subjected to water immersion restraint as a stress conditions model.
2. Materials and methods
2.1. Materials
Millet employed in this study is the Taitung No.7 millet (Setaria italica (L.) Beauv.), a foxtail millet, which is bred by the Taitung District Agricultural Research and Extension Station (Taitung, Taiwan), while APP was kindly provided by Kuang Ta Foods Ltd. (Taichung, Taiwan). Corn starch, dextrinized corn starch, casein, alphacel non-nutritive bulk, AIN-93 M vitamin, AIN-93 M mineral, l-cystine were commercially available ICN Biochemicals Inc. (Costa Mesa, CA, US). Sucrose and soybean oil were from Taiwan Sugar Corp., (Tainan, Taiwan). Other chemicals were of at least analytical reagent grade and were used as obtained, e.g., choline bitartrate, 2-thiobarbituric acid (TBA), cimetidine, butylated hydroxytoluene (BHT), potassium chloride, ethylenediaminetetraacetic acid disodium salt (EDTA-Na2), trichloroacetic acid (TCA), 1,1,3,3,-tetraethoxypropane (TEP), 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), Tris base, l-cysteine, maleic acid, iodoacetic acid, phenylmethylsulfonyl fluoride (PMSF), bovine serum albumin (BSA), D-(+)-maltose monohydrate, α-lactose monohydrate, sucrose, sodium phosphotungstate, sodium phosphate monobasic dihydrate, disodium phosphate dodecahydrate, potassium chloride, sodium hydroxide, sodium chloride, n-butanol, isobutanol, formaldehyde, hydrochloric acid, phosphoric acid, and sulfuric acid, etc. were from Sigma-aldrich (St. Louis, MO, US). Amylase (AY-891), lipase (LI-188), and leucine aminopeptidase (LA-561) activities were determined using commercially available kit (Randox Laboratories Ltd., Antrim, UK).
2.2. Feed preparation
This study designed the feed recipe with reference to the AIN-93G diet23 and modified the ratio of protein, fat, and starch in accordance with the composition of the foxtail millet and APP. The AIN-93G feed was used as the base feed for the normal, negative control, and positive control groups. Foxtail millet-containing experimental diets contained ash (1.0%), crude protein (12.3%), crude fat (2.4%), total dietary fiber (3.9%), moisture content (8.0%) and nitrogen-free extract (72.4%) (as presented in Table 1), was divided into low, medium, and high dosages with 10%, 20%, and 40% of the corn starch, casein, and soybean oil replaced by foxtail millet flour. APP-containing experimental diets contained ash (2.0%), crude protein (13.5%), crude fat (2.9%), total dietary fiber (12.6%), moisture content (2.3%) and nitrogen-free extract (66.7%), was divided into low and high dosages with 15% and 30% of the corn starch, casein, and soybean oil replaced by the APP (Table 2).
Table 1.
Foxtail millet-containing experimental diets.
| Diet constituents (%) | B | NC | L | M | H | PC |
|---|---|---|---|---|---|---|
| Corn starch | 39.749 | 39.749 | 31.436 | 23.124 | 6.499 | 39.749 |
| Casein | 20.000 | 20.000 | 18.588 | 17.176 | 14.351 | 20.000 |
| Dextrinized corn starch | 13.200 | 13.200 | 13.200 | 13.200 | 13.200 | 13.200 |
| Sucrose | 10.000 | 10.000 | 10.000 | 10.000 | 10.000 | 10.000 |
| Soybean oil | 7.000 | 7.000 | 6.724 | 6.449 | 5.898 | 7.000 |
| Alphacel | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 |
| AIN-93M-mineral mix | 3.500 | 3.500 | 3.500 | 3.500 | 3.500 | 3.500 |
| l-Cystine | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| AIN-93M-vitamin mix | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 |
| Choline bitartrate | 0.250 | 0.250 | 0.250 | 0.250 | 0.250 | 0.250 |
| tert-Butylhydroquinone | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Foxtail millet |
– |
– |
10.000 |
20.000 |
40.000 |
– |
| Total | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 |
B, normal group; NC, negative control group; L, low-dose group; M, medium-dose group; H, high-dose group; and PC, positive control group.
Table 2.
Adlay processing product (APP)-containing experimental diets.
| Diet constituents (%) | B | NC | L | H | PC |
|---|---|---|---|---|---|
| Corn starch | 39.749 | 39.749 | 27.829 | 15.909 | 39.749 |
| Casein | 20.000 | 20.000 | 17.467 | 14.933 | 20.000 |
| Dextrinized corn starch | 13.200 | 13.200 | 13.200 | 13.200 | 13.200 |
| Sucrose | 10.000 | 10.000 | 10.000 | 10.000 | 10.000 |
| Soybean oil | 7.000 | 7.000 | 6.453 | 5.906 | 7.000 |
| Alphacel | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 |
| AIN-93M-mineral mix | 3.500 | 3.500 | 3.500 | 3.500 | 3.500 |
| l-Cystine | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| AIN-93M-vitamin mix | 0.300 | 0.300 | 0.300 | 0.300 | 0.300 |
| Choline bitartrate | 0.250 | 0.250 | 0.250 | 0.250 | 0.250 |
| tert-Butylhydroquinone | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| APP |
– |
– |
15.000 |
30.000 |
– |
| Total | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 |
B, normal group; NC, negative control group; L, low-dose group; H, high-dose group; and PC, positive control group.
2.3. Induction of gastric ulceration
The experimental protocols for the animal study were approved by the Institutional Animal Care and Use Committees of National Taiwan University (95-EL-104). Wistar rats (3 weeks old) from the Laboratory Animal Center of the College of Medicine of National Taiwan University were used. All experiments were carried out in accordance with the International Council for Laboratory Animal Science guidelines for the care and use of laboratory animals. They were given ad libitum access to food and water and allowed to adapt to laboratory conditions for one week prior to the beginning of experiments. The room temperature was maintained at 24 ± 2 °C and the relative humidity was set at 40–70%. An automatic timer was set to control the light cycle; a light period and dark period were each set to account for 12 h. During the feeding period, the amount of food intake and body weight (body-wt.) of the rats were measured twice a week. The animals were randomly assigned to one of the five or six groups of 10 animals each according to weight: normal (B, without the WIRS process); negative control (NC); low-dose (L); medium-dose (M); high-dose (H); and positive control (PC, with 100 mg/kg cimetidine medication process). Injuries (ulcers, erosion, and hemorrhages) in gastric mucosa were caused by water immersion restraint stress (WIRS).24 The rats that had fasted for 24 h were restrained in firmly fitted restraint cages (6 × 7 × 20 cm3) and vertically immersed in water maintained at 20 ± 1 °C to the level of the xiphoid process for 4 h to induce gastric mucosal lesions.
2.4. Sample collection and biochemical assays
Two dietary feeding periods were used, foxtail millet (4 weeks) and APP (5 weeks), in this study. The 9-week-old rats were subjected to a 24-h fast the day before application of WIRS, and they were force-fed a saline solution or cimetidine 30 min before a 4-h induction of WIRS. Rats was sacrificed under CO2 anesthesia after treatment and the stomachs were quickly removed. Gastric mucosal lesions were examined under a dissecting microscope ( × 10), and the values of the ulcer index (UI) was expressed by measuring the total length (mm) of all gastric mucosal lesions in the stomachs induced during the stress.25 Also, rats were eviscerated for collection of livers, and small intestines as well as blood samples were separated and stored at −80 °C for later analyses. Histologic assessments were made with photomicroscope. Specimens from normal and abnormal gastric tissues were fixed in 10% formaldehyde, routinely dehydrated, cleaned, infiltrated with wax, embedded and made into serial 4-μm thick sections. The sections were dewaxed, stained with haematoxylin and eosin technique.
2.4.1. Appreciation of UI
Mucosal lesions were evaluated by macroscopic analysis and the score systems reported previously.25 Briefly, the stomach was dissected out and opened along the greater curvature and rinsed with 0.1 mol/L ice-cold phosphate-buffered saline. The stomach was then examined with a 10 × magnifier to observe erosions and make scores as 1–5: 1 point for small round hemorrhagic erosion, 2 points when the length of hemorrhagic erosion was less than 1 mm, 3 points when the length was 1–2 mm, 4 points when the length was 2–3 mm, 5 points when the length was longer than 4 mm. The score was multiplied by 2 when the width of erosion was larger than 1 mm.
2.4.2. Determination of plasma MDA concentration
The amount of produced malondialdehyde (MDA) was used as an index of lipid peroxidation, which was estimated by the thiobarbituric acid reactive substances assay (TBARS).26 To 20 μl of plasma was taken in centrifuge tube, 4 ml of N/12H2SO4 was added and the mixture was shaken gently. Then 0.5 ml of 10% phosphotungstic acid was added and mixed after standing at room temperature for 5 min. The mixture was centrifuged at 1570×g for 10 min. The supernatant was discarded and the sediment was suspended in 4 ml double-distilled (dd) water and 1 ml of 0.67% TBA reagent was added. The reaction mixture was heated for 60 min at 95 °C in boiling water bath. After cooling 5 ml of n-butanol was added and mixture was shaken vigorously. After the centrifugation at 1570×g for 15 min. The supernatants (n-butanol layer) were measured by a fluorophotometer spectrometer (F-4500, Hitachi, Japan) (with excitation at 515 nm and emission at 553 nm), and the results were recorded as absorbance units (AU).22
2.4.3. Determination of gastric mucosal MDA concentration
Gastric mucus homogenate (0.2 g) was mixed with 0.2 ml of 1.15% KCl/3 mM EDTA-Na2, 3 ml of 1% H3PO4, 0.3 ml of 0.3% BHT/EtOH, and 1 ml of a 0.6% TBA solution. Test samples were placed in a water bath at 100 °C for 60 min after being vortex-mixed. Isobutanol (2 ml) was added to cooled samples, and the absorbance of the BuOH fraction was measured at 532 nm. The calibration curve was established using various concentrations of TEP solutions. Results were recorded as μmol/g of tissue.22
2.4.4. Determination of gastric mucus and liver NPSH
A previously described method was used to examine the NPSH in the gastric mucus and liver.27 Briefly, 1 ml of gastric mucus or liver homogenate was mixed with 0.8 ml of dd water and 0.5 ml of a 50% TCA solution. After vortex mixing for 2 min, the solutions were centrifuged at 1570×g for 20 min. Supernatants (0.5 ml) were mixed with 1 ml of 0.4 M Tris-buffer (pH 8.9) and colored by 25 μl of a 0.01 M DTNB solution. The absorbance at 412 nm was recorded, and the results were presented as μg/g of tissue.22
2.4.5. Determination of digestive enzymes in small intestine
A segment of the small intestine was removed, washed in 0.9% NaCl solution, dried on filter paper, weighed, trimmed, and homogenized (1570×g) with 0.9% NaCl containing protease inhibitors (1 μM PMSF and 2.2 mM iodoacetic acid) for 10 min at 4 °C. The supernatant was used for the measurement of in vitro maltase, sucrase, and lactase activities and protein determination. Maltase (EC 3.2.1.20), lactase (EC 3.2.1.23), and sucrase (EC 3.2.1.48) activities (in mmol/mg protein), were determined using a glucose diagnosis kit based on the glucose oxidase reagent by the method described in work.28 The protein content was determined by Lowry’s method with bovine serum albumin as the standard.29 The assays for amylase (EC 3.2.1.1), lipase (EC 3.1.1.3), leucine aminopeptidase (LAP; EC 3.4.11.2) activity (in U/mg protein) have been described elsewhere with minor modification.30, 31, 32
2.5. Statistical analysis
The data are presented as the mean ± standard error of mean (SEM). Statistical analyses were performed using one-way ANOVA followed by the Duncan’s multiple-range test. ∗P < 0.1 and ∗∗P < 0.05 were considered statistically significant. All statistical tests were performed using SPSS v.10.0 (IBM Corp., Armonk, NY, USA).
3. Results and discussion
3.1. Changes in body weight
The foxtail millet and APP-containing experimental diets and the effects on body weight gain in rats, food intake, feed efficiency, as shown in Table 3(a) and Table 3(b), respectively. After 4 weeks (foxtail millet diet) and 5 weeks (APP diet) of the experimental period, the final body weights, average food intake levels, feed efficiencies did not differ significantly among all groups. The results demonstrated that foxtail millet and APP did not retard normal growth of the animals.
Table 3a.
Effect of foxtail millet on body weight, food intake and feed efficiency in rats for 4 weeks.
| Group | Initial body-wt. (g/rat) | Final body-wt. (g/rat) | Food intake (g/day/rat) | Feed efficiency (%) |
|---|---|---|---|---|
| B | 135 ± 15a | 297 ± 30a | 18.6 ± 1.3b | 36.3 ± 4.0a |
| NC | 135 ± 11a | 306 ± 16ab | 18.6 ± 0.9b | 38.3 ± 2.3a |
| L | 136 ± 10a | 320 ± 14b | 20.1 ± 1.3a | 38.2 ± 2.7a |
| M | 135 ± 11a | 312 ± 27ab | 19.4 ± 1.1ab | 38.1 ± 5.0a |
| H | 134 ± 12a | 309 ± 12ab | 18.9 ± 0.7b | 38.6 ± 2.1a |
| PC | 136 ± 12a | 304 ± 15ab | 18.9 ± 0.6b | 36.9 ± 3.0a |
Table 3b.
Effect of APP on body weight, food intake and feed efficiency in rats for 5 weeks.
| Group | Initial body-wt. (g/rat) | Final body-wt. (g/rat) | Food intake (g/day/rat) | Feed efficiency (%) |
|---|---|---|---|---|
| B | 93 ± 11a | 294 ± 44a | 15.4 ± 2.1a | 37.1 ± 3.4a |
| NC | 93 ± 9a | 292 ± 41a | 15.3 ± 1.9a | 37.0 ± 4.7a |
| L | 93 ± 8a | 323 ± 24a | 16.5 ± 1.1a | 40.0 ± 2.8a |
| H | 93 ± 8a | 316 ± 16a | 15.9 ± 0.9a | 40.0 ± 2.6a |
| PC | 94 ± 7a | 291 ± 51a | 15.4 ± 2.0a | 36.4 ± 6.7a |
Results are expressed as mean ± SEM (n = 10).
Feed efficiency = (Body weight gained/Total feed intake) × 100.
Values in the same column sharing common superscript small letter showed no significant difference (ANOVA, p > 0.05).
B, normal group; NC, negative control group; L, low-dose group; H, high-dose group; and PC, positive control group.
3.2. Changes in lipid peroxides
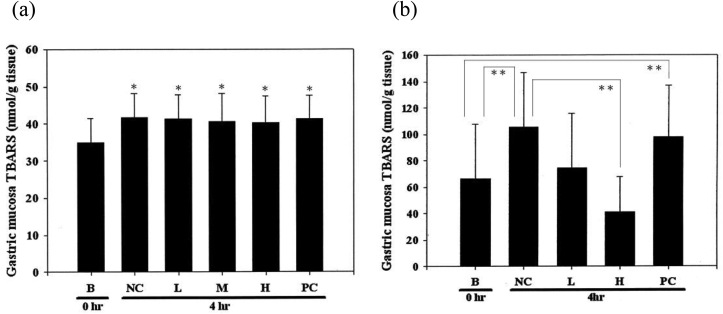
Fig. 1 indicates the plasma lipid peroxide levels, the lipid peroxidation products (TBARS) showed similar contents in all groups of rats administrated foxtail millet (Fig. 1a) and APP (Fig. 1b) subjected to 4 h of stress, no statistically significant difference between groups (p > 0.05). Fig. 2 shows the influence on the mucosal TBARS groups of stress-loaded rats administrated foxtail millet and APP diet. Mucosal TBARS was elevated in all foxtail millet groups compared to the normal group (p > 0.1) and did not show significant differences between groups (Fig. 2a). Mucosal TBARS was significant increased in the NC and PC group compared to the normal group (p > 0.05) but was lower in the high-dose APP group compared to the NC group (p > 0.05), while that in the low-dose group was not significant (Fig. 2b).
Fig. 1.
Effect on the plasma TBARS of WIRS-treated rats administrated (a) foxtail millet and (b) APP. Each bar represents the mean ± SEM (n = 10). There is no significant difference between groups (p > 0.05). B, normal group; NC, negative control group; L, low-dose group; M, medium-dose group; H, high-dose group; and PC, positive control group.
Fig. 2.
Effect on the mucosal TBARS of WIRS-treated rats administrated (a) foxtail millet and (b) APP. Each bar represents the mean ± SEM (n = 10). ∗p < 0.1 compared to the normal group without the WIRS process, ∗∗p < 0.05 compared to the normal and negative control group. B, normal group; NC, negative control group; L, low-dose group; M, medium-dose group; H, high-dose group; and PC, positive control group.
3.3. Changes in NPSH
The effects of foxtail millet and APP feeding followed by the onset of WIRS (4 h) and changes in the gastric mucosal NPSH concentrations in the rats are shown in Fig. 3. The gastric NPSH was significantly lower in the NC, medium-dose and high-dose group compared to the normal rats without WIRS (p < 0.05), while low-dose foxtail millet raised the NPSH to a normal status compared to the normal rats (Fig. 3a). Also, after 5 weeks prior feeding of APP diet, the gastric NPSH showed significant differences among the NC, low-dose and high-dose group compared to the normal group (p < 0.05, Fig. 3b). The hepatic NPSH decreased with administration of foxtail millet and APP diet, which was significantly suppressed compared to the normal group (p < 0.05, Fig. 4a and b).
Fig. 3.
Effect on the gastric mucosal NPSH of WIRS-treated rats administrated (a) foxtail millet and (b) APP. Each bar represents the mean ± SEM (n = 10). ∗∗p < 0.05 compared the normal group. B, normal group; NC, negative control group; L, low-dose group; M, medium-dose group; H, high-dose group; and PC, positive control group.
Fig. 4.
Effect on the hepatic NPSH of WIRS-treated rats administrated (a) foxtail millet and (b) APP. Each bar represents the mean ± SEM (n = 10). ∗∗p < 0.05 compared to the normal group. B, normal group; NC, negative control group; L, low-dose group; M, medium-dose group; H, high-dose group; and PC, positive control group.
3.4. Changes in UI
When rats were subjected to WIRS for 4 h, gastric mucosal lesions with a diameter of >2 mm were observed in the glandular regions of the stomach. These mucosal lesions appeared to be black and dark red with elongated bands. The values of the UI increased dramatically after stress as compared with the normal group. Fig. 5 shows the influence on the UI of intake foxtail millet (Fig. 5a) and APP (Fig. 5b) in the stress-loaded rats. A gastroprotective effect was observed in rats administrated foxtail millet/APP after the development of stress-induced gastric ulcers, which exhibited a statistically significant reduction in the severity and number of gastric lesions compared to the NC group (p < 0.05).
Fig. 5.
Effect on the UI of WIRS-treated rats administrated (a) foxtail millet and (b) APP. Each bar represents the mean ± SEM (n = 10). ∗∗p < 0.05 compared to the normal and negative control groups. B, normal group; NC, negative control group; L, low-dose group; M, medium-dose group; H, high-dose group; and PC, positive control group.
3.5. Pathological changes in gastric mucosa
The pathological examination of gastric mucosa in our ulcer model indicated obvious ulcer injury. There was no gastric mucosal lesion in normal group. Scattered spot or lineal erosions, hemorrhage and ulcers were observed in gastric mucosa in stress group. These observed changes were consistent with the significant elevation of the values of the ulcer index in NC rats subjected to WIRS (Fig. 6). Numbers of bleeding clots on the surface of the stomach of the low-/medium-/high-dose foxtail millet (Fig. 6a) and low-/high-dose APP (Fig. 6b) groups were fewer than those of the NC group with marked blood coagulum, while no macroscopic lesion was observed in the PC group.
Fig. 6.
Appearance of gastric ulcers induced by WIRS with administration of (a) foxtail millet and (b) APP. Blood coagulum at the bases of ulcers are identified by the arrows. B, normal group; NC, negative control group; L, low-dose group; M, medium-dose group; H, high-dose group; and PC, positive control group.
3.6. Histology
Fig. 7 shows the histopathological observations of H&E stained sections in the stomach of rats. The results showed that the erosion was severe in the NC group, while it was inhibited when low-/medium-dose foxtail millet (Fig. 7a) and low-/high-dose APP (Fig. 7b) were given. The present histopathological findings supported the protective effects of cimetidine and foxtail millet/APP diet and revealed relatively normal mucosa in rats pretreated with cimetidine and erosion and gastric healing in rats pretreated with foxtail millet/APP diet.
Fig. 7.
The partial histological appearances of H&E-stained sections in the gastric mucosa of rats subjected to WIRS with administration of (a) foxtail millet and (b) APP. B, normal group; NC, negative control group; L, low-dose group; M, medium-dose group; H, high-dose group; and PC, positive control group.
3.7. Changes in plasma calcium
Plasma calcium was measured using calorimetric assay by a Johnson & Johnson (Ortho-Clinical) Vitros 250 Chemistry Analyzer (Raritan, NJ, US). The plasma calcium concentrations in the rats with 4 h of WIRS were significantly lower than that in the normal rats without WIRS. No significant differences were observed between the treatment groups subjected to stress in the foxtail millet diet group.
In contrast to the plasma calcium concentrations in the APP diet group, low-dose APP elevated significantly higher than NC and PC group, but was no difference compared with those of the high-dose APP group. Moreover, there was no statistically significant difference in concentrations of plasma calcium between the NC group subjected to stress and the normal rats without WIRS.
3.8. Changes in small intestinal digestive enzyme activity
Table 4(a) shows that foxtail millet diet group had no statistical difference in the lipase, amylase, and ALP activities among the treatments. Maltase and sucrase in low-dose foxtail millet group were significantly higher than that in normal rats without WIRS, were similar to the positive control group. Both lactase and sucrose activities showed significantly higher increases than NC group subjected to stress fed with low-dose diet. Table 4(b) shows that APP diet group had no statistical difference in the lipase activity among the treatments. Both amylase and LAP activities in the rats with 4 h of WIRS (NC group) were significantly lower than that in the normal rats without WIRS, while the amylase and LAP activities in low/high-dose APP group were slightly higher as compared to the negative controls.
Table 4a.
Effect of foxtail millet on digestive enzymes in small intestine of rats subjected to WIRS.
| Group | Lipase |
Amylase |
LAP |
Lactase |
Maltase |
Sucrase |
|---|---|---|---|---|---|---|
| (U/mg protein) | (mmol/mg protein) | |||||
| B | 0.32 ± 0.18ab | 0.7 ± 0.5a | 0.05 ± 0.02a | 5.9 ± 2.2ab | 102 ± 35b | 19 ± 5c |
| NC | 0.54 ± 0.33ab | 1.9 ± 1.5a | 0.06 ± 0.03a | 3.9 ± 1.2b | 153 ± 52ab | 22 ± 9bc |
| L | 0.73 ± 0.52a | 1.0 ± 0.7a | 0.07 ± 0.02a | 7.9 ± 3.5a | 241 ± 144a | 36 ± 15a |
| M | 0.61 ± 0.40a | 2.8 ± 3.1a | 0.13 ± 0.14a | 5.3 ± 2.5ab | 179 ± 38ab | 25 ± 11abc |
| H | 0.55 ± 0.09ab | 0.7 ± 0.4a | 0.04 ± 0.02a | 5.0 ± 1.6ab | 257 ± 41ab | 26 ± 7abc |
| PC | 0.22 ± 0.20b | 1.2 ± 0.4a | 0.06 ± 0.02a | 7.9 ± 2.3a | 266 ± 118a | 33 ± 10ab |
Table 4b.
Effect of APP on digestive enzymes in small intestine of rats subjected to WIRS.
| Group | Lipase |
Amylase |
LAP |
Lactase |
Maltase |
Sucrase |
|---|---|---|---|---|---|---|
| (U/mg protein) | (mmol/mg protein) | |||||
| B | 0.30 ± 0.12a | 0.40 ± 0.16a | 0.07 ± 0.02a | 8.0 ± 1.2a | 63 ± 6b | 22 ± 2bc |
| NC | 0.21 ± 0.11a | 0.17 ± 0.09b | 0.03 ± 0.01b | 3.2 ± 0.4b | 59 ± 9b | 12 ± 3d |
| L | 0.21 ± 0.11a | 0.23 ± 0.12b | 0.04 ± 0.01b | 5.6 ± 1.4a | 89 ± 32b | 19 ± 6c |
| H | 0.29 ± 0.15a | 0.30 ± 0.18ab | 0.04 ± 0.01b | 7.8 ± 2.2a | 136 ± 60a | 27 ± 10ab |
| PC | 0.23 ± 0.12a | 0.25 ± 0.09ab | 0.03 ± 0.02b | 7.9 ± 4.1a | 128 ± 25a | 30 ± 9a |
Results are expressed as mean ± SEM (n = 10). Values with different superscript letters in a column are significantly different (ANOVA, p < 0.05).
B, normal group; NC, negative control group; L, low-dose group; H, high-dose group; and PC, positive control group.
In comparison to the disaccharidase activities in APP diet presented in Table 4(b), both lactase and sucrase activities in NC group were significantly lower than that in normal rats without WIRS. In contrast, the reverse was observed when assessing maltase and sucrase activities of the rats pretreated with cimetidine were significantly higher than that in normal rats without WIRS. Otherwise, maltase activity showed significantly higher increases than normal rats without WIRS and gave sucrose activity similar to that of the normal rats fed with high-dose APP diet.
WIRS mimics the clinical acute gastric ulcerations caused by trauma, surgery or sepsis and has been widely accepted for studying stress ulcers.33 Previous studies revealed a positive correlation between free radical-induced oxidative stress both in gastric and duodenal ulceration, and excessive stress leads to consumption of the internal antioxidative barrier.34,35 Further, the elimination of free radicals by an anti-ulcer agent that has antioxidant and free-radical scavenging activities may contribute to reduce the severity of ulcer recurrence.36,37
Our results showed that foxtail millet and APP diet suppressed levels of plasma and mucosal TBARS, while it increased gastric NPSH, which means that the antioxidative capacity of foxtail millet or APP is mainly responsible for its antiulcer activity. Millets are rich sources of phytochemicals such as phenolics (bound phenolic acid-ferulic acid, free phenolic acid-protocatechuic acid), lignans, β-glucan, inulin, phytates, sterols, tocopherol, and carotenoids.38 The main polyphenols are phenolic acids and tannins, while flavonoids are present in small quantities; they act as antioxidant and play many roles in the body immune system.7,39 Adlay seeds contains many bioactive products like lignans, flavonoids, and phenolic acids, were reported to possess antiproliferative and antitumor activities.15,16,40 A previous study showed the that caffeic acid in dehulled adlay was one of the compounds indicative of a gastroprotective agent.22 Also, the ethyl acetate fraction of adlay bran ethanolic extract was elucidated retard carcinogenesis through an anti-inflammatory pathway, and potential active component was ferulic acid.41 Free and bound phenolics of adlay bran, such as chlorogenic acid, p-coumaric acid, and ferulic acid exhibited significant 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activities, oxygen radical absorbance capacities, and superoxide radical scavenging activities,13,14 thus the antioxidative capacity of adlay seeds is mainly responsible for its antiulcer activity.
4. Conclusions
Since an antioxidant defense mechanism may be critically important in protecting against the development of acute gastric mucosal injury, the anti-ulcer response and extensive antioxidant effect of foxtail millet and APP diet may be valuable in prevention, which possesses preventive and gastroprotective effects on experimental gastric mucosal lesions in rats. Consumption of whole grains has been associated with reduced risk of developing major chronic diseases. Millet and adlay diet retain the advantage of being a natural product with no reported side effects which may prove a promising protective role in gastric ulcer.
Declaration of competing interest
All authors declare no conflicts of interest.
Acknowledgement
This study was supported by the grants from National Science Council (Taipei, Taiwan) (NSC 95-2313-B-002-086).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2020.01.003.
Contributor Information
Hui-Ching Lin, Email: r02629011@ntu.edu.tw.
Shi-Yuan Sheu, Email: ssyuan@ms68.hinet.net.
Lee-Yan Sheen, Email: lysheen@ntu.edu.tw.
Pei-Wen Sheu, Email: d10531920@gapps.fg.tp.edu.tw.
Wenchang Chiang, Email: chiang@ntu.edu.tw.
Tzong-Fu Kuo, Email: tzongfu@asia.edu.tw.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Asali A.M., Alghamdi M.A., Fallatah S.A., Alholaily W.A., Aldandan R.G., Alnosair A.H. Risk factors leading to peptic ulcer disease: systematic review in literature. Int J Community Med Public Health. 2018;5:4617–4624. [Google Scholar]
- 2.Nneli R.O., Nwafia W.C., Orji J.O. Diets/dietary habits and certain gastrointestinal disorders in the tropics: a review. Niger J Physiol Sci. 2007;22:1–13. doi: 10.4314/njps.v22i1-2.54878. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia V., Tandon R.K. Stress and the gastrointestinal tract. J Gastroenterol Hepatol. 2005;20:332–339. doi: 10.1111/j.1440-1746.2004.03508.x. [DOI] [PubMed] [Google Scholar]
- 4.Das D., Bandyopadhyay D., Bhattacharjee M., Banerjee R.K. Hydroxyl radical is the major causative factor in stress-induced gastric ulceration. Free Radic Biol Med. 1997;23:8–18. doi: 10.1016/s0891-5849(96)00547-3. [DOI] [PubMed] [Google Scholar]
- 5.Kudryavtsev K.V., Markevich A.O., Virchenko O.V. Pharmacological correction of stress-induced gastric ulceration by novel small-molecule agents with antioxidant profile. Sci World J. 2014;2014:217039. doi: 10.1155/2014/217039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan X.J., Zhou J.J., Xie G.R., Milne G.W.A. second ed. Routledge; New York, NY: 2003. Traditional Chinese Medicines: Molecular Structures, Natural Sources and Applications; p. 989. [Google Scholar]
- 7.Jadaun S., Singh E. Potential of millets: nutrients composition and health benefits. J Sci Innov Res. 2016;5:46–50. [Google Scholar]
- 8.Lakshmi Kumari P., Sumathi S. Effect of consumption of finger millet on hyperglycemia in non-insulin dependent diabetes mellitus (NIDDM) subjects. Plant Foods Hum Nutr. 2002;57:205–213. doi: 10.1023/a:1021805028738. [DOI] [PubMed] [Google Scholar]
- 9.Ugare R., Chimmad B., Naik R., Bharati P., Itagi S. Glycemic index and significance of barnyard millet (Echinochloa frumentacae) in type II diabetics. J Food Sci Technol. 2014;51:392–395. doi: 10.1007/s13197-011-0516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi Y.Y., Osada K., Ito Y., Nagasawa T., Choi M.R., Nishizawa N. Effects of dietary protein of Korean foxtail millet on plasma adiponectin, HDL-cholesterol, and insulin levels in genetically type 2 diabetic mice. Biosci Biotechnol Biochem. 2005;69:31–37. doi: 10.1271/bbb.69.31. [DOI] [PubMed] [Google Scholar]
- 11.Shi J., Shan S., Li H., Song G., Li Z. Anti-inflammatory effects of millet bran derived-bound polyphenols in LPS-induced HT-29 cell via ROS/miR-149/Akt/NF-κB signaling pathway. Oncotarget. 2017;8:74582–74594. doi: 10.18632/oncotarget.20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren X., Yin R., Hou D. The glucose-lowering effect of foxtail millet in subjects with impaired glucose tolerance: a self-controlled clinical trial. Nutrients. 2018;10:1509. doi: 10.3390/nu10101509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Chen J., Xie H., Ju X., Liu R.H. Phytochemical profiles and antioxidant activity of adlay varieties. J Agric Food Chem. 2013;61:5103–5113. doi: 10.1021/jf400556s. [DOI] [PubMed] [Google Scholar]
- 14.Zhao M., Zhu D., Sun-Waterhouse D. In vitro and in vivo studies on adlay-derived seed extracts: phenolic profiles, antioxidant activities, serum uric acid suppression, and xanthine oxidase inhibitory effects. J Agric Food Chem. 2014;62:7771–7778. doi: 10.1021/jf501952e. [DOI] [PubMed] [Google Scholar]
- 15.Kuo C.C., Shih M.C., Kuo Y.H., Chiang W. Antagonism of free-radical-induced damage of adlay seed and its antiproliferative effect in human histolytic lymphoma U937 monocytic cells. J Agric Food Chem. 2001;49:1564–1570. doi: 10.1021/jf001215v. [DOI] [PubMed] [Google Scholar]
- 16.Lee M.Y., Tsai S.H., Kuo Y.H., Chiang W. Anti-tumor and anti-inflammatory activity of the methanol extracts from adlay bran. Food Sci Biotechnol. 2008;17:1265–1271. [Google Scholar]
- 17.Lu X., Liu W., Wu J. A polysaccharide fraction of adlay seed (Coixlachryma-jobi L.) induces apoptosis in human non-small cell lung cancer A549 cells. Biochem Biophys Res Commun. 2013;430:846–851. doi: 10.1016/j.bbrc.2012.11.058. [DOI] [PubMed] [Google Scholar]
- 18.Chung C.P., Hsu C.Y., Lin J.H., Kuo Y.H., Chiang W., Lin Y.L. Antiproliferative lactams and spiroenone from adlay bran in human breast cancer cell lines. J Agric Food Chem. 2011;59:1185–1194. doi: 10.1021/jf104088x. [DOI] [PubMed] [Google Scholar]
- 19.Chen H.J., Hsu H.Y., Chiang W. Allergic immune-regulatory effects of adlay bran on an OVA-immunized mice allergic model. Food Chem Toxicol. 2012;50:3808–3813. doi: 10.1016/j.fct.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi M., Konno C., Hikino H. Isolation and hypoglycemic activity of coixans A, B and C, glycans of Coix lachryma-jobi var. ma-yuen seeds. Planta Med. 1986;52:64–65. doi: 10.1055/s-2007-969074. [DOI] [PubMed] [Google Scholar]
- 21.Yeh P.H., Chiang W., Chiang M.T. Effects on dehulled adlay on plasma and lipid concentrations in streptozotocin-induced diabetic rats fed a diet enriched in cholesterol. Int J Vitam Nutr Res. 2006;76:299–305. doi: 10.1024/0300-9831.76.5.299. [DOI] [PubMed] [Google Scholar]
- 22.Chung C.P., Hsia S.M., Lee M.Y. Gastroprotective activities of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) on the growth of the stomach cancer AGS cell line and indomethacin-induced gastric ulcers. J Agric Food Chem. 2011;59:6025–6033. doi: 10.1021/jf2009556. [DOI] [PubMed] [Google Scholar]
- 23.Reeves P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127:838–841. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 24.Konturek P.C., Brzozowski T., Ptak A. Nitric oxide releasing aspirin protects the gastric mucosa against stress and promotes healing of stress-induced gastric mucosal damage: role of heat shock protein 70. Digestion. 2002;66:160–172. doi: 10.1159/000066762. [DOI] [PubMed] [Google Scholar]
- 25.Nie S.N., Qian X.M., Wu X.H. Role of TFF in healing of stress-induced gastric lesions. World J Gastroenterol. 2003;9:1772–1776. doi: 10.3748/wjg.v9.i8.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 27.Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 28.Dahlqvist A. Assay of intestinal disaccharidases. Scand J Clin Lab Investig. 1984;44:169–172. doi: 10.3109/00365518409161400. [DOI] [PubMed] [Google Scholar]
- 29.Lowry O.H., Rosenbrough N.Y., Farr A.L., Randall R.Y. Proteins measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Rauscher E., Bulow S., Hagele E.O., Shaich U.E. Ethylidene protected substrate for the assay of human amylase. Fresenius Z Anal Chem. 1986;324:304–305. [Google Scholar]
- 31.Ziegenhorn J., Neumann U., Knitsch K.W., Zwez W. Determination of serum lipase. Clin Chem. 1979;25:1067. [Google Scholar]
- 32.Lin S.H., Van Wart H.E. Effect of cryosolvents and subzero temperatures on the hydrolysis of L-leucine-p-nitroanilide by porcine kidney leucine aminopeptidase. Biochemistry. 1982;21:5528–5533. doi: 10.1021/bi00265a023. [DOI] [PubMed] [Google Scholar]
- 33.Adinortey M.B., Ansah C., Galyuon I., Nyarko A. In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers. 2013;2013:796405. [Google Scholar]
- 34.Yasukawa K., Kasazaki K., Hyodo F., Utsumi H. Non-invasive analysis of reactive oxygen species generated in rats with water immersion restraint-induced gastric lesions using in vivo electron spin resonance spectroscopy. Free Radic Res. 2004;38:147–155. doi: 10.1080/1071576036001641196. [DOI] [PubMed] [Google Scholar]
- 35.Tandon R., Khanna H.D., Dorababu M., Goel R.K. Oxidative stress and antioxidants status in peptic ulcer and gastric carcinoma. Indian J Physiol Pharmacol. 2004;48:115–118. [PubMed] [Google Scholar]
- 36.Ock C.Y., Hong K.S., Kim J.H., Hahm K.B. Free radicals and gastric mucosal injury. Front Gastrointest Res. 2011;29:97–110. [Google Scholar]
- 37.Podoprigorova V.G., Khibin L.S., Kozlov N.B., Barsel’ V.A. Effectiveness of synthetic antioxidants in treatment of patients with peptic ulcer an open randomized controlled trial. Klin Med (Mosc) 1999;77:32–35. [PubMed] [Google Scholar]
- 38.Liu R.H. Whole grain phytochemicals and health. J Cereal Sci. 2007;46:207–219. [Google Scholar]
- 39.Chandrasekara A., Shahidi F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J Agric Food Chem. 2010;58:6706–6714. doi: 10.1021/jf100868b. [DOI] [PubMed] [Google Scholar]
- 40.Li S.C., Chen C.M., Lin S.H., Chiang W., Shih C.K. Effects of adlay bran and its ethanolic extract and residue on preneoplastic lesions of the colon in rats. J Sci Food Agric. 2011;91:547–552. doi: 10.1002/jsfa.4219. [DOI] [PubMed] [Google Scholar]
- 41.Chung C.P., Hsu H.Y., Huang D.W. Ethyl acetate fraction of adlay bran ethanolic extract inhibits oncogene expression and suppresses DMH-induced preneoplastic lesions of the colon in F344 rats through an anti-inflammatory pathway. J Agric Food Chem. 2010;58:7616–7623. doi: 10.1021/jf101084e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.