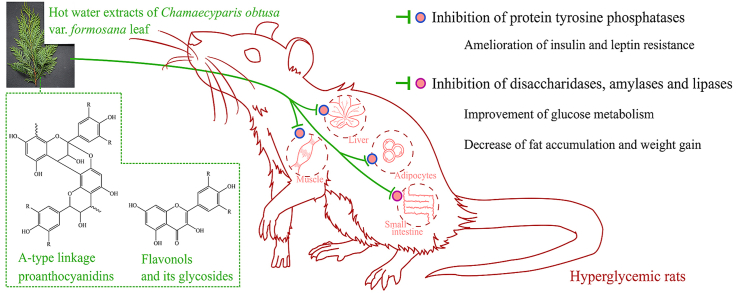
Abstract
Chamaecyparis obtusa var. formosana is a species indigenous to Taiwan and has been used as a medicinal plant. It has been claimed that the hot water extracts of C. obtusa var. formosana leaves (CoLE) with flavonoids and proanthocyanidins have anti-oxidant and anti-hyperglycemic activities in vitro. This study further examines the anti-hyperglycemic activity of CoLE and its possible mechanisms in hyperglycemic rats. Hyperglycemia of rats was induced by streptozotocin (STZ) and high-fat diets (HFD). Hyperglycemic rats treated orally with 30 and 150 mg/kg CoLE were classified into LCO and HCO groups, respectively. After three-month treatment, both LCO and HCO groups showed improved glucose metabolism in oral glucose tolerance and postprandial blood glucose tests. Decrease in HOMA-IR, leptin and adiponectin levels of the HCO group revealed amelioration of insulin and leptin resistance. Obesity and accumulation of visceral fats induced by STZ and HFD could be alleviated in both HCO and LCO groups. These anti-diabetic effects might be contributed by inhibition of intestinal digested enzymes and protein tyrosine phosphatases (PTPases). Although other studies are necessary, these findings suggest that CoLE could be potentially used as a health complement for treating diabetes without significant toxicity.
Keywords: Anti-diabetic activity, Anti-obesity activity, Chamaecyparis obtusa var. formosana, Disaccharidases, Proanthocyanidins, Protein tyrosine phosphatases
Graphical abstract
Highlights
-
•
Aqueous extract of C. obtusa var. formosana leaf (CoLE) is eco-friendly substances.
-
•
150 mg/kg treatment of CoLE is safe in rat model for 3 months.
-
•
CoLE ameliorates insulin resistance by inhibiting protein tyrosine phosphatases.
-
•
CoLE decreases blood glucose and weight gain by inhibiting intestinal enzymes.
List of abbreviations
- 2-h PG
2-h Plasma glucose
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- AUC
Area under the curve
- Cdx2
Caudal type homeobox 2
- CoLE
Hot water extracts of C. obtusa var. formosana leaves
- DC
Diabetic control
- FBG
Fasted blood sugar
- HCO
High-dosage C. obtusa var. formosana leaf extracts
- HDL-C
High density lipoprotein-cholesterol
- HFD
High-fat diets
- LCO
Low-dosage C. obtusa var. formosana leaf extracts
- LDL-C
Low density lipoprotein-cholesterol
- MAPK
Mitogen-activated protein kinases
- NC
Normal control
- OGTT
Oral glucose tolerant test
- PC
30 mg/kg of pioglitazone
- PTKs
Protein tyrosine kinases
- PTPases
Protein tyrosine phosphatases
- STZ
Streptozotocin
- TC
Total cholesterol
- TG
Triglyceride
1. Introduction
The World Health Organization estimated that diabetes was the seventh leading cause of death and killed 1.6 million people in 2016. A systematic review estimated that in 2017 the global prevalence of diabetes among adults over 18 years of age is 451 million, and was expected to increase to 693 million by 2045. The huge medical and societal expenditure of diabetes mellitus was estimated to be USD 850 billion in 2017.1 Type 2 diabetes accounts for 90–95% of all diabetes, and the cause of type 2 diabetes is peripheral insulin resistance. The risk of developing type 2 diabetes is associated with age, obesity, physical activity and ethnicity. Most but not all patients with type 2 diabetes are obese. In sedentary lifestyle, excess energy will deposit in adipocytes. Hypertrophy of adipocytes result in chronic inflammation and further insulin resistance in many organs.2,3
Several medicinal and edible plants possess anti-diabetic activities and their phytochemicals show synergistic and multi-target effects, leading to lower the adverse events in clinical trial.4 One of the ingredients suggested by the American Diabetes Association for reducing diabetes risk is tea (Camellia sinensis).5 In a randomized, double-blinded, and placebo-controlled trial, tea extract improved insulin resistance and increased glucagon-like peptide 1.6 Tea extract contains abundant phenolics, such as catechins, flavonol glycoside, flavone glycoside, theaflavins and proanthocyanidins.7 Not only green tea but herbal tea has potential to reduce risk of diabetes mellitus. Varied phytochemicals in herbal extracts have synergistic effects to enhance therapeutic outcomes, even mixed extracts of two or more plant species show synergism. In other words, synergism reduces side effects by lowering the dosage to reach threshold.8
From the substainable-untilization point of view, potential applications of renewable plant leaves become an imperative for the healthcare and pharmaceutical industries and warrant to be explored. Cupressaceae is a conifer family distributed throughout the world. Cupressaceae used to be construction timber, nevertheless, the anti-oxidant activities of its leaf extracts and essential oils have been widely investigated. Leaf extracts of Juniperus navicularis, J. oxycedrus, J. phoenicea and J. turbinata exhibited anti-oxidant and neuroprotective potential. All four species contained catechins, flavonol glycoside, flavone glycoside, biflavonoid and proanthocyanidins.9 Platycladus orientalis leaves have been used as traditional medicine in China. P. orientalis leaf extract showed the potent anti-oxidant, anti-inflammatory, anti-microbial and hair growth-promoting activity without toxicity.10 Leaf extracts of J. foetidissima and J. sabina exhibited better α-glucosidase inhibitory activity than fruit extracts, decreasing the blood glucose level in diabetic rats.11 α-Glucosidase in the small intestine is involved in breaking down carbohydrates to absorbable glucose. Acarbose, an anti-diabetic drug, slows down the rate of carbohydrate digestion and lowers the postprandial blood glucose by inhibiting α-glucosidase activity.12 Chamaecyparis obtusa var. formosana, called Taiwan hinoki, is an indigenous species and has been used as a medicinal plant in Taiwan. Flavonol glycoside derived from hot water extracts of C. obtusa var. formosana leaves (CoLE) showed great anti-oxidant activity in Caenorhabditis elegans model.13 Proanthocyanidins with A-type linkages derived from CoLE exhibited potent α-glucosidase inhibitory activity.14 However, in vivo anti-diabetic activity of Cupressaceae leaf extract has not been investigated.
Protein tyrosine phosphatases (PTPases) generally hinder the actions of protein tyrosine kinases (PTKs) and is associated with risk of diabetes. PTPN1 is one of the important contributors for metabolic regulation of type 2 diabetes. PTPN1 expression opposes the phosphorylation of Janus kinase 2 and insulin receptor to inhibit the signaling pathways of leptin and insulin receptors. In addition, plasma glucose levels of PTPN1-knockout mice are lower than wild-type mice after STZ-induced diabetes due to their higher rate of β-cell proliferation. Therefore, PTP1B inhibitors have potential application prospects in the therapy of type 2 diabetes.15,16
The aim of this study is to investigate the anti-diabetic activity of CoLE in a hyperglycemic rat model. Furthermore, effects of CoLE on digestive enzymes of the small intestine and PTPases of different tissues in hyperglycemic rats were also investigated.
2. Materials and methods
2.1. Sampling and preparation of plant extracts
Sampling and preparation of CoLE followed the method of Cheng et al. (2014).13 Briefly, fresh mature and healthy leaves of Chamaecyparis obtusa var. formosana were sampled from Mt. Chilan in Ilan County, Taiwan. The species was identified by Mr. Yen-Ray Hsui (Taiwan Forestry Research Institute). Fresh leaves were soaked in boiling deionized water for 6 h with vapor-cycle cooling system. The extract was decanted, filtered under vacuum, concentrated in a rotary evaporator and then freeze-dried. CoLE was reported that contained catechin (5.9 mg/g of crude), quercetin (4.2 mg/g) quercetin-3-O-α-rhamnopyranosid (17.7 mg/g), myricetin-3-O-α-rhamnopyranoside (6.8 mg/g), vanillic acid (2.3 mg/g) and 4-hydroxybenzoic acid (1.2 mg/g) [12]. The proanthocyanidins content of CoLE was 119.9 mg/g of crude (Supplementary Fig. S1).
2.2. Animals and diets
Eight-week old male Wistar rats (270–300 g) were purchased from BioLASCO (Taipei, Taiwan). All animals had free access to water and food and were housed in a laboratory room maintained under controlled temperature (22 ± 2 °C), humidity (65 ± 5%) and 12-h light–dark cycles. The AIN-76 diet was taken as the normal diet while the high-fat diet (HFD) consisted of 84.5% maintenance diet (Altromin #1320, Germany) and 15.5% lard. The composition of ingredients was detailed in Supplementary Table S1 and food intake of rats is shown in Supplementary Table S2. All animals were humanely treated in accordance with the animal protocol, reviewed and approved by the Institutional Animal Care and Use Committee, National Taiwan University.
2.3. Experimental design
After seven-day acclimatization, rats (n = 28) were fasted for 16 h and induced by a single intraperitoneal injection of 30 mg/kg STZ dissolved in citrate buffer (pH 4.0). The normal control (NC) group (n = 7) was fed with normal diet and given 0.1% (g/g of body weight) diluted water. On the 13th day, hyperglycemic rats were divided into groups according to the results of oral glucose tolerant test (OGTT) as follows: diabetic control (DC) group (n = 7), given 0.1% (g/g of body weight) diluted water; positive control (PC) group (n = 7), given 30 mg/kg pioglitazone; high-dosage CoLE (HCO) group (n = 7), given 150 mg/kg CoLE; low-dosage CoLE (LCO) group (n = 7), given 30 mg/kg CoLE, and these four groups fed with HFD. All five groups were treated by oral gavage. The duration of these experimental treatments was 12 weeks, and OGTT of experimental rats was tested for estimation of glucose tolerance every 4 weeks.
2.4. Tissue analysis
Biochemical parameters, insulin, leptin and adiponectin were analyzed at the termination of study. The 16-h-fasted rats were etherized by ethyl ether and sacrificed by cardiac exsanguination. Blood samples were placed in centrifuge tube for 30 min and then centrifuged at 3500g for 30 min at 4 °C to obtain serum. After laparotomy, internal organs (liver, kidney and pancreas), epididymal fat and perinephric fat were isolated and weighed. All isolated tissues and serum samples were stored at −80 °C before analysis. Biochemical parameters, such as fasted blood sugar (FBG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, bilirubin, triglyceride (TG), total cholesterol (TC), high density lipoprotein-cholesterol (HDL-C) and low density lipoprotein-cholesterol (LDL-C) in serum, were measured using a Roche Modular system and all the kits were purchased from Cobas (Roche, Mannheim, Germany). The rat insulin ELISA kit (Mercodia AB), rat leptin ELISA kit (BioVendor) and rat adiponectin ELISA kit (Assaypro) were employed to measure the insulin, leptin and adiponectin, respectively.
2.5. Oral glucose tolerance test
OGTT was performed on 16-h-fasted rats as described by Veerapur et al. (2012).17 Briefly, the rats were fed orally with 1.5 g glucose per kg of body weight. The blood glucose level was measured using the blood sample from the tail vein taken every 30 min in 2 h (at intervals of 0, 30, 60, 90 and 120 min). The results were represented by sum of area under the curve (AUC), which was calculated using formula of trapezoid rule as follows: sum of AUC of OGTT = [G0 + 2 * (G30 + G60 + G90) + G120] * 15. The G0-G120 represented the glucose level at different time points and the shape of OGTT curve was assessed for glucose metabolism.
2.6. Preparation of intestinal mucosa homogenate
Intestinal enzymes were collected in accordance with the method of Jurgoński et al. with slight modifications.18 Briefly, the small intestine was rinsed to remove lumen by cold physiological saline. The small intestine was divided into four equal segments, and with the second segment cut open from the stomach side. Mucosal samples were collected by scraping intestinal segment with glass slides on an iced plate. Then 10 mg of the mucosa sample was dissolved in 500 μL phosphate buffer (0.1 M, pH 7.0) and homogenized for detection of intestinal enzyme activity.
2.7. Assay of intestinal enzyme activity
Intestinal disaccharidase activities, such as maltase, sucrase, isomaltase and lactase, were analyzed following the method of Dahlqvist with some modifications.19 Briefly, 100 μL of 56 mM substrates, such as maltose, sucrose, isomaltulose and lactose, were added into 100 μL of mucosa homogenates and incubated for 1 h at 37 °C. The reaction was terminated after 3-min incubation at 100 °C. Then 10 μL of the mixture was added to 490 μL of the reagent from glucose kits (Randox GL2623, Amtrim, UK). After incubating for 10 min at room temperature, the absorbance was measured at 500 nm using ELISA. The amylase kit (Randox AY1580) and lipase kit (Randox LI188) were employed to determine the intestinal amylase and lipase activity in mucosa homogenate. Protein content of the homogenate was measured using protein assay kit (Bio-Rad) with bovine serum albumin used as a standard. The absorbance was measured at 595 nm using ELISA.
2.8. PTPases activity assasy
A modified assay of Wang et al. was adopted to examine the activity of PTPases.20 Briefly, 100 mg of the frozen tissue sample was homogenized in 1 mL ice-cold buffer solution containing 50 mM-Tris and 150 mM NaCl, and the homogenates were centrifuged at 14000g for 15 min at 4 °C. The supernatant homogenate was collected individually and the protein content was determined using Bio-Rad protein assay kit with bovine serum albumin as a standard. Then 100 μL of supernatant homogenate containing 20 μg protein was added 100 μL of 2 mM p-nitrophenyl phosphate in buffer solution. After incubation for 30 min at 37 °C, the reaction of PTPases was terminated by adding of 100 μL of 1 N NaOH. The absorbance was measured at 405 nm using ELISA.
2.9. Statistical analysis
The experimental data were expressed as means ± standard error (SE). Significant differences of plasma glucose level, AUC in OGTT, biochemical parameters, activities of intestinal enzyme and PTPases from the data of five groups were calculated using one-way ANOVA with post-hoc Tukey HSD test. Significance of result was defined as p < 0.05.
3. Results and discussion
3.1. Effect of treatments on hyperglycemic rats in oral glucose tolerance test
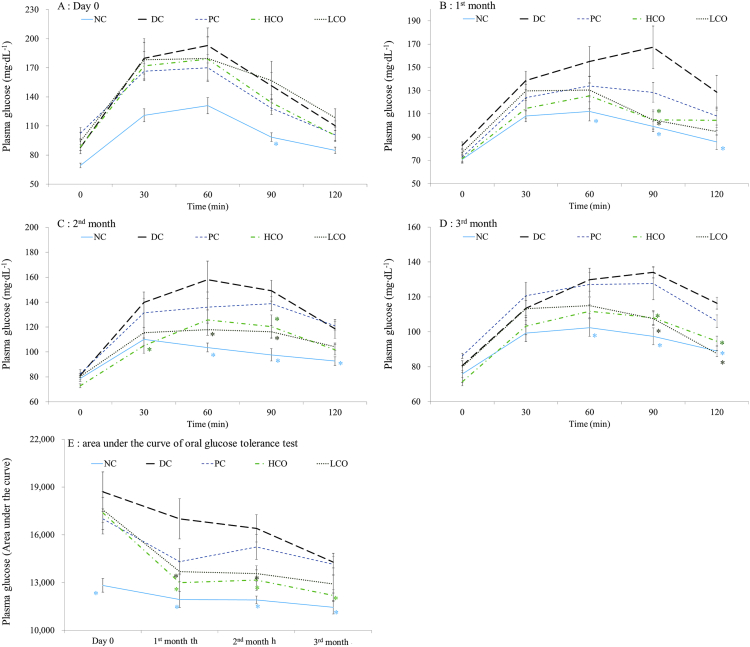
Fasting plasma glucose (FPG), 2-h plasma glucose (2-h PG), oral glucose tolerance test (OGTT) or A1C criteria could be diagnostic tests for diabetes.2 Recent reports showed OGTT to be more accurate than FPG and HbA1c tests for diagnosing diabetes.21,22 As shown in Fig. 1A, the plasma glucose of the DC group was significantly higher than that of the NC group at 0, 30, 60, 90 and 120 min on Day 0. The PC, HCO and LCO groups showed no difference from the DC group, indicating rats in STZ-induced groups suffered hyperglycemia.
Fig. 1.
Plasma glucose level in OGTT of different treatments during (A) day 0, (B) 1st month, (C) 2nd month, (D) 3rd month, and (E) sum of area under the curve in OGTT. *: p < 0.05 when compared with DC. NC: normal control, DC: diabetic control, PC: 30 mg/kg of pioglitazone, HCO: high-dosage C. obtusa var. formosana leaf extracts, LCO: low-dosage C. obtusa var. formosana leaf extracts.
In the first and second month of treatments (Fig. 1B and C), both HCO and LCO groups had significantly lower plasma glucose than the DC group at 90 min. In the third month of treatment (Fig. 1D), both HCO and LCO groups had significantly different plasma glucose level from the DC group at 90 and 120 min. The changes in AUC of OGTT during the three months of treatment are shown in Fig. 1E. Both LCO and HCO groups showed great decreases in AUC of OGTT, significantly different from those of DC group at first and second months of treatment. Furthermore, the HCO group showed significant difference from the DC group at the third month of treatment, with continuing improvement in hyperglycemia. Different time points of OGTT represented various functions of glucose metabolism. Early glycemia (30–60 min) is affected by glucose sensitivity while later glycemia (60–120 min) is influenced by insulin sensitivity.23 The shape of OGTT glucose curve of the DC group on day for the three months of treatment were all monophasic. The plasma glucose of HCO group at 90 min in the first and second months both decreased. After three months of treatment, both low-dosage and high-dosage of CoLE could decrease plasma glucose at 90 and 120 min, revealing that high-dosage CoLE could ameliorate insulin sensitivity sooner than low-dosage of CoLE.
3.2. Effect of treatments on biochemical parameters in hyperglycemic rats
As shown in Table 1, the DC group had the higher weight gain (157.4%), epididymal fat (2.6%), perinephric fat (3.8%), FBG (12.8 mmol/L) and HOMA-IR (10.9) than the NC group (144.7%, 1.4%, 1.6%, 6.9 mmol/L, 4.4, respectively). Insulin level in all five groups were similar.
Table 1.
Effects of different treatments on biochemical parameters in rats.
| Parameters | Treatments |
||||
|---|---|---|---|---|---|
| NC | DC | PC | HCO | LCO | |
| Weight gain (%) | 144.7 ± 1.5C | 157.4 ± 2.0B | 177.2 ± 2.7A | 142.9 ± 4.5C | 144.8 ± 2.5C |
| Epididymal fat (%) | 1.4 ± 0.1C | 2.6 ± 0.3A | 2.4 ± 0.1AB | 1.8 ± 0.2BC | 1.7 ± 0.2C |
| Perinephric fat (%) | 1.6 ± 0.1B | 3.8 ± 0.3A | 3.7 ± 0.2A | 2.4 ± 0.2B | 2.2 ± 0.2B |
| FBG (mmol/L) | 6.9 ± 0.7B | 12.8 ± 0.4A | 8.6 ± 0.4B | 9.5 ± 0.8B | 9.3 ± 1.0B |
| Insulin (mIU/L) | 14.1 ± 0.6A | 19.4 ± 1.9A | 14.8 ± 0.6A | 18.2 ± 2.4A | 16.7 ± 1.2A |
| HOMA-IR | 4.4 ± 0.6C | 10.9 ± 0.7A | 5.6 ± 0.3BC | 7.4 ± 0.5B | 6.7 ± 0.7BC |
| Leptin (ng/mL) | 1.7 ± 0.3B | 7.1 ± 0.3A | 4.4 ± 1.3AB | 3.2 ± 0.8B | 4.9 ± 0.9AB |
| Adponectin (μg/mL) | 9.6 ± 2.7C | 20.1 ± 2.1B | 30.4 ± 2.8A | 9.5 ± 0.6C | 12.4 ± 1.0BC |
| AST (UL) | 73.7 ± 6.1A | 61.7 ± 2.4A | 76.2 ± 2.9A | 66.2 ± 3.3A | 63.3 ± 4.2A |
| ALT (UL) | 42.2 ± 1.3A | 49.0 ± 3.4A | 49.2 ± 3.4A | 43.2 ± 3.0A | 44.8 ± 6.3A |
| Creatinine (mgdL) | 0.40 ± 0.02A | 0.39 ± 0.02A | 0.37 ± 0.02A | 0.36 ± 0.02A | 0.41 ± 0.03A |
| Bilirubin (mgdL) | 0.09 ± 0.01B | 0.06 ± 0.01A | 0.04 ± 0.00A | 0.09 ± 0.01B | 0.07 ± 0.01AB |
| TG (mg/dL) | 58.3 ± 4.0A | 54.7 ± 4.0A | 52.0 ± 4.9A | 47.5 ± 5.0A | 42.0 ± 1.8A |
| TC (mg/dL) | 75.8 ± 2.5A | 75.8 ± 2.8A | 77.7 ± 3.3A | 80.3 ± 4.7A | 78.7 ± 5.2A |
| HDL-C (mgdL) | 55.3 ± 2.2A | 57.7 ± 2.2A | 52.2 ± 2.3A | 59.7 ± 4.4A | 57.0 ± 2.9A |
| LDL-C (mgdL) | 15.8 ± 1.1AB | 14.0 ± 1.1B | 22.7 ± 2.0A | 16.0 ± 2.1AB | 16.7 ± 1.9AB |
Results are mean ± SE (n = 6–7). Different letters (A-C) are significantly different (p < 0.05, one-way ANOVA) according to the Tukey’s test. Weight gain = final weight/initial weight * 100, Epididymal and perinephric fat = fat weight/final weight * 100, AST: aspartate aminotransferase, ALT: alanine aminotransferase. TG: triglyceride, TC: total cholesterol, HDL-C: high-density lipoprotein-cholesterol, LDL-C: low-density lipoprotein-cholesterol, NC: normal control, DC: diabetic control, PC: 30 mg/kg of pioglitazone, HCO: high-dosage C. obtusa var. formosana leaf extracts, LCO: low-dosage C. obtusa var. formosana leaf extracts.
The FBG of PC (8.6 mmol/L), HCO (9.5 mmol/L) and LCO (9.3 mmol/L) group showed no significant difference from that of the NC group (6.9 mmol/L). HOMA-IR is a homeostasis model for evaluating insulin resistance. The HOMA-IR of PC (5.6), HCO (7.4) and LCO (6.7) groups were significantly different from that of the DC group (10.9). It was revealed that 30 mg/kg pioglitazone, 30 and 150 mg/kg CoLE could decrease blood sugar level, leading to improvement in insulin resistance. However, the PC group had the higher weight gain (177.2%) compared with the DC group (157.4%). By contrast, the weight gain of both HCO and LCO groups (142.9%, 144.8%) showed no significant difference from that of the NC group (144.7%). Taken together, these results indicated that CoLE had treatment potential for obese diabetes mellitus patients.
Leptin and adiponectin are adipokines secreted by adipocytes. The major function of leptin is increment of lipolysis while that of adiponectin is enhancement of insulin sensitivity.24 The DC group had higher leptin (7.1 mg/mL) and adiponectin (20.1 μg/mL) levels than the NC group (1.7 mg/mL, 9.6 μg/mL, respectively). Leptin and adiponectin levels of the HCO group (3.2 mg/mL, 9.5 μg/mL) were not significantly different from those of the NC group, revealing that hyperglycemic rats suffered insulin and leptin resistance, resulting in greater secretion of leptin and adiponectin. However, high-dosage of CoLE could ameliorate insulin and leptin resistance. AST, ALT and creatinine were common biomarkers for evaluation of hepatic and kidney functions. AST, ALT and creatinine in all five groups showed no significant difference. In addition, liver, kidney and pancreas of rats in all five groups showed no pathological incidence after three-month treatments (Supplementary Fig. S2). These results showed that high-dosage of CoLE treatment was safe on hyperglycemic rats. TG, TC, HDL-C and LDL-C in all five groups showed no significant difference, indicating that hyperglycemic rats have no symptom of dyslipidemia.
3.3. Effect of treatments on activities of intestinal enzymes in hyperglycemic rats
The activities of digestive enzymes in the small intestine are shown in Table 2. Maltase, amylase and lipase of the DC group was significantly higher than those of the NC group, indicating that more glucose and lipids released from HFD were absorbed in hyperglycemic rats. The HCO group showed that the significantly less activities of maltase, sucrase, lactase, amylase and lipase compared to the DC group. STZ-induced rats were suffered from insulin resistance. The mitogen-activated protein kinases (MAPK) signaling pathway are downregulated and RNA expression of caudal type homeobox 2 (Cdx2) are upregulated, bringing about increase in disaccharidase activities.25 Deng et al. reported that 0.75 g/kg (213 mg/kg of final weight) mixed extracts of Rhizoma coptidis and Radix rehmanniae attenuated intestinal maltase, sucrase and lactase in STZ-induced hyperglycemic rats.26 The above-mentioned studies revealed that hyperglycemia induced by STZ and HFD on rats, leading to increment in activity of disaccharidases and lipases. The treatment group of 150 mg/kg CoLE showed inhibitory effects on digestive enzymes compared with that using 0.75 g/kg mixed extracts of R. coptidis and R. rehmanniae in hyperglycemic rodent model. CoLE contained catechin, quercetin, quercetin-3-O-α-rhamnopyranoside, myricetin-3-O-α-rhamnopyranoside and proanthocyanidins suggesting the in vitro α-glucosidase, α-amylase and lipase inhibitory activity.13,27 This result demonstrates that CoLE have in vivo inhibitory effects on digestive enzymes and can decrease glucose, fructose, galactose and fat uptake.
Table 2.
Effects of different treatments on intestinal disaccharidases, amylase and lipase activities in rats.
| Parameters | Treatments |
||||
|---|---|---|---|---|---|
| NC | DC | PC | HCO | LCO | |
| Maltase (μmole glucose/min/g protein) | 175.7 ± 14.9B | 333.3 ± 41.7A | 340.3 ± 36.4A | 166.1 ± 19.1B | 259.6 ± 36.6AB |
| Sucrase (μmole glucose/min/g protein) | 62.5 ± 3.7AB | 77.1 ± 9.4A | 70.1 ± 10.7A | 31.7 ± 5.4B | 57.0 ± 8.2AB |
| Isomaltase (μmole glucose/min/g protein) | 31.3 ± 1.8A | 35.8 ± 6.7A | 31.0 ± 4.3A | 19.6 ± 2.4A | 23.4 ± 3.0A |
| Lactase (μmole glucose/min/g protein) | 10.7 ± 0.9AB | 14.1 ± 2.0A | 11.5 ± 1.9AB | 7.4 ± 0.9B | 6.7 ± 0.7B |
| Amylase (U/mg protein) | 180.0 ± 42.2B | 834.3 ± 174.7A | 860.1 ± 191.6A | 275.7 ± 68.0B | 286.2 ± 70.1B |
| Lipase (U/mg protein) | 207.8 ± 30.6C | 580.3 ± 52.0AB | 659.3 ± 115.1A | 284.7 ± 38.2C | 308.2 ± 73.3BC |
Results are mean ± SE (n = 6–7). Different letters (A-C) are significantly different (p < 0.05, one-way ANOVA) according to the Tukey’s test. NC: normal control, DC: diabetic control, PC: 30 mg/kg of pioglitazone, HCO: high-dosage C. obtusa var. formosana leaf extracts, LCO: low-dosage C. obtusa var. formosana leaf extracts.
3.4. Effect of treatments on PTPases in different tissues of hyperglycemic rats
The activities of PTPases in different tissues are shown in Table 3. There were significant differences in liver, muscle and epididymal fat between DC and NC groups, indicating that hyperglycemia increases the expression of PTPases in liver, muscle and epididymal fat primary. The HCO group showed significant differences in liver, muscle and epididymal fat compared with the DC group. Cremonini et al. also reported that consumption of HFD induced the expression of PTPN1 in liver and epididymal fat, leading to insulin resistance.28 Quercetin, kaempferol and myricetin showed the potent PTPN1 inhibitory activity as sodium orthovanadate, a phosphatase inhibitor.29 PTPN1 inhibitory activity was increased by the degree of polymerization of proanthocyanidins.30 CoLE contained flavonoids, flavonoid glycosides and proanthocyanidins.13,14 It was assumed that the PTPase inhibitory activity of CoLE treatment in rats was contributed by flavonoids and proanthocyanidins. CoLE treatment has potential to improve both insulin and leptin resistance. To the best of our knowledge, this is the first report on in vivo effects of Cupressaceae leaf extract on PTPases of rats.
Table 3.
Effects of different treatments on PTPase activities on different tissues in rats.
| Parameters | Treatments |
||||
|---|---|---|---|---|---|
| NC | DC | PC | HCO | LCO | |
| Liver (%) | 100.0 ± 3.0B | 129.3 ± 8.9A | 129.2 ± 3.4A | 100.9 ± 8.7B | 104.4 ± 3.4AB |
| Muscle (%) | 100.0 ± 3.3B | 117.8 ± 7.8A | 104.0 ± 1.7AB | 97.6 ± 3.4B | 108.5 ± 1.2AB |
| Perirenal fat (%) | 100.0 ± 6.7A | 94.9 ± 4.0A | 102.6 ± 5.8A | 84.8 ± 2.3A | 87.8 ± 3.7A |
| Epididymal fat (%) | 100.0 ± 8.8B | 160.4 ± 25.7A | 123.2 ± 5.9AB | 96.2 ± 3.0B | 89.0 ± 4.5B |
Results are mean ± SE (n = 6–7). Different letters (A-B) are significantly different (p < 0.05, one-way ANOVA) according to the Tukey’s test. PTPase: Protein tyrosine phosphatase, NC: normal control, DC: diabetic control, PC: 30 mg/kg of pioglitazone, HCO: high-dosage C. obtusa var. formosana leaf extracts, LCO: low-dosage C. obtusa var. formosana leaf extracts.
Results of OGTT glucose curve, HOMA-IR and leptin levels indicate that hyperglycemic rats have insulin and leptin resistance. Oral treatment of extract from C. obtusa var. formosana leaves improves the insulin and leptin insensitivity of hyperglycemic rats. CoLE contained catechin, quercetin, quercetin-3-O-α-rhamnopyranoside and myricetin-3-O-α-rhamnopyranoside.13 These compounds were reported to have PTPN1 and α-glucosidase inhibitory activities.29 The above-mentioned studies revealed that CoLE could alleviate the symptoms of diabetes by inhibiting activity of PTPases on liver, muscle and epididymal fat. The high AUC of OGTT and FBG of hyperglycemic rats are ameliorated by CoLE treatments. CoLE also contained A-type proanthocyanidins with structural units including (epi)afezelechin, (epi)catechin, (epi)gallocatechin and methyl (epi)catechin.14 Proanthocyanidins, also known as condensed tannins, could bind with protein to form aggregates. α-Glucosidase and α-amylase inhibitory activities of proanthocyanidins are increased by the degree of polymerizations.30,31 These findings indicated that CoLE could inhibit the intestinal disaccharidases and amylase activities to alleviate postprandial blood glucose level. Obesity of hyperglycemic rats was induced by intraperitoneal injection of STZ and uptake of HFD. CoLE could prevent the redundant weight gain and accumulation of visceral fats. Zhou et al. reported that leaf extract of Chinese bayberry containing abundant proanthocyanidins could prevent SD rats from obesity induced by HFD.32 Similar to the finding in the above-mentioned study, CoLE showed anti-obesity activity due to intestinal lipase inhibitory activity. Comparing with pioglitazone, an anti-diabetic drug, same dosage of CoLE exhibited better anti-hyperglycemic activities without side effects of weight gain.
Upon uptake, proanthocyanidins would go through the stomach intact to the small intestine. Only low-molecular-weight proanthocyanidins could be directly absorbed by enterocytes. Proanthocyanidins with degrees of polymerization exceeding 4 would go further to the colon. These proanthocyanidin polymers would be fermentable substrates for colonic microbiota and degraded into small-molecular-weight metabolites, which could be absorbed by colonocytes.33 It was indicated that proanthocyanidns of CoLE remained intact in the small intestine and interacted with intestinal enzymes. The chemical traits of proanthocyanidins lead to strong correlation between in vitro and in vivo tests of enzyme inhibitory activity. The low-molecular-weight compounds of CoLE, such as catechin, quercetin, quercetin-3-O-α-rhamnopyranoside and myricetin-3-O-α-rhamnopyranoside, could be absorbed into systemic circulation. Over expression of PTPases in the liver, muscle and epididymal fat of hyperglycemia may be alleviated by these flavonoids.
4. Conclusions
In summary, hot water extract of Chamaecyparis obtusa var. formosana leaves exhibited excellent anti-diabetic activity. The active compounds of C. obtusa var. formosana leaves could be extracted as herbal tea, which shows great potential as a health complement for diabetes. The metabolites of active compounds and the interaction with intestinal microbiota merit further investigation.
Declaration of competing interest
None.
Acknowledgements
This work was supported by Prof. James Swi-Bea Wu and Prof. Hen-Wei Wei (National Taiwan University) and are grateful to their constructive suggestions of rodent model test. Great thanks to Prof. Far-Ching Lin and Prof. Lih-Jih Wang (National Taiwan University) for their technical assistance on production of rat diet.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2019.11.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cho N.H., Shaw J.E., Karuranga S. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association 2 Classification and diagnosis of diabetes standards of medical care in diabetes—2018. Diabetes Care. 2018;41:S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 3.Reilly S.M., Saltiel A.R. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 4.Governa P., Baini G., Borgonetti V. Phytotherapy in the management of diabetes: a review. Molecules. 2018;23:105. doi: 10.3390/molecules23010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association 5 Prevention or delay of type 2 diabetes standards of medical care in diabetes—2018. Diabetes Care. 2018;41:S51–S54. doi: 10.2337/dc18-S005. [DOI] [PubMed] [Google Scholar]
- 6.Liu C.Y., Huang C.J., Huang L.H., Chen I.J., Chiu J.P., Hsu C.H. Effects of green tea extract on insulin resistance and glucagon-like peptide 1 in patients with type 2 diabetes and lipid abnormalities: a randomized, double-blinded, and placebo-controlled trial. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stodt U., Engelhardt U.H. Progress in the analysis of selected tea constituents over the past 20 years. Food Res Int. 2013;53:636–648. [Google Scholar]
- 8.Malongane F., McGaw L.J., Mudau F.N. The synergistic potential of various teas, herbs and therapeutic drugs in health improvement: a review. J Sci Food Agric. 2017;97:4679–4689. doi: 10.1002/jsfa.8472. [DOI] [PubMed] [Google Scholar]
- 9.Tavaresa L., McDougall G.J., Fortalezas S., Stewart R.D., Ferreira B., Santos C.N. The neuroprotective potential of phenolic-enriched fractions from four Juniperus species found in Portugal. Food Chem. 2012;135:562–570. doi: 10.1016/j.foodchem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Shan M.Q., Shang J., Ding A.W. Platycladus orientalis leaves: a systemic review on botany, phytochemistry and pharmacology. Am J Chin Med. 2014;42:523–542. doi: 10.1142/S0192415X14500347. [DOI] [PubMed] [Google Scholar]
- 11.Orhan N., Orhan D.D., Gökbulut A., Aslan M., Ergun F. Comparative analysis of chemical profile, antioxidant, in-vitro and in-vivo antidiabetic activities of Juniperus foetidissima Willd. and Juniperus sabina L. Iran J Pharm Res (IJPR) 2014;16:64–74. [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi S.R., Standl E., Tong N., Shah P., Kalra S., Rathod R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin Pharmacother. 2015;16:1959–1981. doi: 10.1517/14656566.2015.1070827. [DOI] [PubMed] [Google Scholar]
- 13.Cheng S.C., Li W.H., Shi Y.C. Antioxidant activity and delayed aging effects of hot water extract from Chamaecyparis obtusa var. formosana leaves. J Agric Food Chem. 2015;62:4159–4165. doi: 10.1021/jf500842v. [DOI] [PubMed] [Google Scholar]
- 14.Hsu C.Y., Lin G.M., Lin H.Y., Chang S.T. Characteristics of proanthocyanidins in leaves of Chamaecyparis obtusa var. formosana as strong α-glucosidase inhibitors. J Sci Food Agric. 2018;98:3806–3814. doi: 10.1002/jsfa.8894. [DOI] [PubMed] [Google Scholar]
- 15.Gurzov E.N., Stanley W.J., Brodnicki T.C., Thomas H.E. Protein tyrosine phosphatases: molecular switches in metabolism and diabetes. Trends Endocrinol Metab. 2015;26:30–39. doi: 10.1016/j.tem.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Ruiz R., Vieira E., Garcia-Roves P.M., Gomis R. Protein tyrosine phosphatase-1B modulates pancreatic beta-cell mass. PLoS One. 2015;9 doi: 10.1371/journal.pone.0090344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veerapur V.P., Prabhakar K.R., Thippeswamy B.S., Bansal P., Srinivasan K.K., Unnikrishnan M.K. Antidiabetic effect of Ficus racemosa Linn. stem bark in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rats: a mechanistic study. Food Chem. 2012;132:186–193. doi: 10.1016/j.foodchem.2011.10.052. [DOI] [PubMed] [Google Scholar]
- 18.Jurgoński A., Juśkiewicz J., Zduńczyk Z. Ingestion of black chokeberry fruit extract leads to intestinal and systemic changes in a rat model of prediabetes and hyperlipidemia. Plant Foods Hum Nutr (Dordr) 2008;63:176–182. doi: 10.1007/s11130-008-0087-7. [DOI] [PubMed] [Google Scholar]
- 19.Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968;22:99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- 20.Wang CD, Teng BS, He YM. Effect of a novel proteoglycan PTP1B inhibitor from Ganoderma lucidum on the amelioration of hyperglycaemia and dyslipidaemia in db/db mice. Br J Nutr. 2012;108:2014–2025. doi: 10.1017/S0007114512000153. [DOI] [PubMed] [Google Scholar]
- 21.Buse J.B., Kaufman F.R., Linder B., Hirst K., Ghormli L.E., Willi D. Diabetes screening with hemoglobin A1c versus fasting plasma glucose in a multiethnic middle-school cohort. Diabetes Care. 2013;36:429–435. doi: 10.2337/dc12-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D.L., Kim S.D., Kim S.K., Park S., Song K.H. Is an oral glucose tolerance test still valid for diagnosing diabetes mellitus? Diabetes Metab J. 2016;40:118–128. doi: 10.4093/dmj.2016.40.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrannini E., Mari A. β-Cell function in type 2 diabetes. Metabolism. 2014;63:1217–1227. doi: 10.1016/j.metabol.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Stern J.H., Rutkowski J.M., Scherer P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metabol. 2016;23:770–784. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L., Yu Y.L., Liu C., Wang X.T., Liu X.D., Xie L. Insulin deficiency induces abnormal increase in intestinal disaccharidase activities and expression under diabetic states, evidences from in vivo and in vitro study. Biochem Pharmacol. 2011;82:1963–1970. doi: 10.1016/j.bcp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Deng Y.X., Zhang X.J., Shi Q.Z., Chen Y.S., Qiu X.M., Chen B. Anti-hyperglycemic effects and mechanism of traditional Chinese medicine Huanglian Wan in streptozocin-induced diabetic rats. J Ethnopharmacol. 2012;144:425–432. doi: 10.1016/j.jep.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 27.Buchholz T., Melzig M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015;81:771–783. doi: 10.1055/s-0035-1546173. [DOI] [PubMed] [Google Scholar]
- 28.Cremonini E., Bettaieb A., Haj F.G., Fraga C.G., Oteiza P.I. (-)-Epicatechin improves insulin sensitivity in high fat diet-fed mice. Arch Biochem Biophys. 2015;599:13–21. doi: 10.1016/j.abb.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Wang X, Yue J, Sun Y, Zhang X, Zhao Y. Polyphenols from acorn leaves (Quercus liaotungensis) protect pancreatic beta cells and their inhibitory activity against α-glucosidase and protein tyrosine phosphatase 1B. Molecules. 2018;23:2167–2178. doi: 10.3390/molecules23092167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin G.M., Lin H.Y., Hsu C.Y., Chang S.T. Structural characterization and bioactivity of proanthocyanidins from indigenous cinnamon (Cinnamomum osmophloeum) J Sci Food Agric. 2016;96:4749–4759. doi: 10.1002/jsfa.7802. [DOI] [PubMed] [Google Scholar]
- 31.Frazier R.A., Deaville E.R., Green R.J. Interactions of tea tannins and condensed tannins with proteins. J Pharm Biomed Anal. 2010;51:490–495. doi: 10.1016/j.jpba.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X., Chen S., Ye X. The anti-obesity properties of the proanthocyanidin extract from the leaves of Chinese bayberry (Myrica rubra Sieb.et Zucc.) Food Funct. 2017;8:3259–3270. doi: 10.1039/c7fo00816c. [DOI] [PubMed] [Google Scholar]
- 33.Yang K., Chan C.B. Proposed mechanisms of the effects of proanthocyanidins on glucose homeostasis. Nutr Rev. 2017;75:642–657. doi: 10.1093/nutrit/nux028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.