Abstract
Background
Evidence supports establishing a continuum of care from stroke rehabilitation (SR) to cardiac rehabilitation programs (CRPs). It is not known to what extent people poststroke are being integrated. This study aimed to determine the proportion of CRPs that accept referrals poststroke, barriers/facilitators, and eligibility criteria.
Methods
A web-based questionnaire was sent to CRPs across Canada.
Results
Of 160 questionnaires sent, 114 representatives (71%) of 130 CRPs responded. Of respondents, 65% (n = 74) reported accepting people with a diagnosis of stroke and doing so for a median of 11 years, 11 offering stroke-specific classes and an additional 6 planning inclusion. However, 62.5% of CRPs reported that < 11 patients participated in the last calendar year despite 88.5% reporting no limit to the number they could enroll. Among CRPs, 25% accepted only patients with concurrent cardiac diagnoses, living in the community (47.8%), and without severe mobility (70.1%), communication (80.6%), or cognitive (85.1%) deficits. The 2 most influential barriers and facilitators among all CRPs were funding and staffing. The fourth greatest barrier was lack of poststroke referrals, and third to sixth facilitators were SR/CRP collaboration to ensure appropriate referrals (third) and to increase referrals (sixth), toolkits for prescribing resistance (fourth), and aerobic training (fifth). CRP characteristics associated with accepting stroke were a hybrid program model, a medium program size, and having a falls prevention component.
Conclusions
Most CRPs accept patients poststroke, but few participate. Therefore, establishing SR/CRP partnerships to increase appropriate referrals, using a toolkit to help operationalize exercise components, and allocating funding/resources to CRPs may significantly increase access to secondary prevention strategies.
Résumé
Contexte
Les données recueillies appuient la continuité des soins entre les programmes de réadaptation après un accident vasculaire cérébral (PR-AVC) et les programmes de réadaptation cardiaque (PRC). On ne sait toutefois pas dans quelle mesure les patients qui ont subi un accident vasculaire cérébral (AVC) sont intégrés à un PRC. L’étude visait donc à déterminer la proportion de PRC admettant les patients ayant subi un AVC, les obstacles à l’intégration de ces derniers et les éléments qui la facilitent, ainsi que les critères d’admissibilité.
Méthodologie
Des responsables de PRC de partout au Canada ont été invités à répondre à un questionnaire en ligne.
Résultats
Au total, 160 invitations ont été envoyées et 114 (71 %) responsables rattachés à 130 PRC y ont répondu. Parmi ces répondants, 65 % (n = 74) ont dit que leur programme admettait depuis un nombre médian de 11 ans les patients ayant reçu un diagnostic d’AVC; 11 programmes offraient des cours spécialement destinés aux patients ayant subi un AVC et 6 autres prévoyaient de le faire. Toutefois, 62,5 % des répondants ont souligné que moins de 11 patients avaient participé à leur programme au cours de l’année qui précédait, malgré le fait que dans 88,5 % des cas, il n’y avait pas de limite au nombre de participants admis. Parmi les PRC, 25 % n’admettaient que des patients ayant aussi reçu un diagnostic d’atteinte cardiaque, vivant dans la collectivité (47,8 %) et n’ayant pas de déficit sévère sur les plans de la mobilité (70,1 %), de la communication (80,6 %) ou de la fonction cognitive (85,1 %). Dans tous les cas, les deux facteurs influant le plus (positivement ou négativement, selon le cas) sur l’intégration des patients ayant subi un AVC étaient les ressources financières et les ressources humaines. Le quatrième obstacle en importance était le faible nombre de patients ayant subi un AVC orientés vers les programmes; les autres éléments facilitateurs également recensés étaient la collaboration entre les PR-AVC et les PRC afin d’assurer l’orientation des patients concernés (3e place), l’augmentation du nombre de ces orientations (6e place), ainsi que les outils permettant de prescrire un programme d’entraînement musculaire (4e place) et un programme d’entraînement aérobique (5e place). Les PRC admettant des patients ayant subi un AVC avaient en commun les caractéristiques suivantes : ils reposaient sur un modèle hybride, ils étaient de taille moyenne et ils comprenaient un volet sur la prévention des chutes.
Conclusions
Si la plupart des PRC admettent les patients qui ont subi un AVC, ces derniers sont peu nombreux à y participer. L’établissement de partenariats entre les PR-AVC et les PRC afin d’augmenter le nombre de patients orientés, la mise en place d’outils facilitant l’exécution de programmes d’exercice physique et l’affectation de fonds et de ressources aux PRC pourraient donc augmenter considérablement l’accès aux stratégies de prévention secondaire.
Approximately 405,000 people in Canada are living with the effects of stroke,1 with 75% having persisting neurological impairments that affect functional abilities.2 A sedentary lifestyle increases the risk of stroke, with sedentary individuals being 25% to 30% more susceptible to having a stroke or dying than their physically active counterparts.3 Unfortunately, this inactivity persists after a stroke, leading to further cardiorespiratory deconditioning,4, 5, 6, 7 as well as muscle atrophy and weakness that lead to deterioration in metabolic, cardiorespiratory, and functional health.8, 9, 10, 11, 12, 13 Thus, individuals are at increased risk of cardiovascular disease and recurrent stroke.14,15 Indeed, cardiac disease and recurrent stroke are the leading causes of mortality after stroke.16, 17, 18 Regular physical activity after stroke is associated with improvements in cardiovascular disease risk factors and reduced 3-year risk for recurrent stroke, myocardial infarction, and vascular death, and independently associated with lower all-cause mortality.6,19, 20, 21, 22 Resistance training (RT) when combined with aerobic training (AT) yields a greater increase in lean mass, anaerobic threshold, and muscular strength than AT alone after stroke.21 Therefore, AT in combination with RT has been recommended in the American Heart Association/American Stroke Association Guidelines for Adult Stroke Rehabilitation and Recovery as a Class IIA, level A recommendation.23
Unfortunately, traditional stroke rehabilitation (SR) programs face several challenges for incorporating exercise programming into practice. Recent survey data of Canadian SR programs revealed that 28% of programs exclude individuals with cardiac conditions and that patients may not receive appropriate electrocardiogram (ECG), blood pressure, or blood glucose monitoring.24 In a separate study, the most frequently cited barrier to prescribing AT identified by Canadian neurological rehabilitation therapists was concern for patient safety because of cardiac conditions.25 Further, the 2 most influential barriers to prescribing AT cited by Canadian and US physical therapists/managers were insufficient time within therapy sessions and insufficient length of stay.24,26
Comprehensive cardiac rehabilitation programs (CRPs) can overcome the barriers faced by SR programs. CRPs include AT and RT, nutrition and psychosocial counseling and education, plasma glucose and lipid monitoring, risk factor modification education and counseling, and cardiac exercise assessments and screening.27,28 The benefits of attending CRPs are not restricted to the cardiac population. People poststroke share many of the same health hazards as patients with cardiac disease and several groups have reported significant improvements in these, including cardiorespiratory fitness, anaerobic threshold, blood lipids, lean mass, and body mass index with minimal or absent adverse outcomes in people with stroke attending CRPs.20,29, 30, 31, 32 In addition, when given the opportunity, the proportion of eligible patients who have had a stroke and who enroll in CRPs (71%) is superior to that reported in patients with cardiac disease (40%) with superior completion rates (82%) than reported in the population with cardiac disease (60%-70%).28,32, 33, 34
Although current evidence supports establishing a continuum of care from SR to CRPs, it is not clear if Canada’s healthcare system is adequately integrating this population into CRP facilities. To our knowledge, no study has identified the number of people poststroke who participate, the eligibility criteria, the program characteristics associated with inclusion (exploratory), or the barriers and facilitators to including people poststroke in Canadian CRPs. Therefore, we conducted a pan-Canadian cross-sectional questionnaire-based study to examine these factors.
Materials and Methods
Study design and program eligibility criteria
This was a cross-sectional study using a web-based questionnaire administered to CRPs across Canada. CRPs were eligible to participate if they included a structured exercise component and at least 1 other strategy to control cardiovascular risk factors. For the purposes of the study, structured exercise was defined as at least 1 on-site supervised AT session. Approval from the Ethics Review Board of the University of Toronto was obtained.
Recruitment
Recruitment was initiated in January and completed in April 2019. A directory from the Cardiac Health Foundation of Canada, past studies, program websites, and investigator contacts were used to ascertain CRP identification across Canada. Participants were screened for eligibility and invited through telephone calls. After telephone contact, an electronic invitation was sent via email with a hyperlink that directed participants to the web-based questionnaire. A modified Dillman approach was used to optimize the response rate.35 Two e-mail reminders were sent to those who had not completed the survey at 1-week intervals. For the Quebec participants, the telephone call was made by a French-speaking investigator, and all invitations and reminders were translated to French.
Questionnaire design
Questionnaire design was based on review of the literature, expert knowledge, and a previous questionnaire administered24 in consultation with a Certified Analytics and Insights Professional. The questionnaire consisted primarily of fixed categories response options with some free-text. The first of 3 subcategories of questions were related to stroke inclusion criteria, program delivery, and other CRP characteristics for those that included stroke. The second subcategory included perceived or actual barriers and facilitators to including patients with stroke, CRP, and sociodemographic characteristics for all programs to complete. A 5-point Likert scale was used for CRP representatives to assess the significance, if at all, of 17 listed barriers and 11 facilitators to inclusion across 3 mobility categories: no, moderate, and severe stroke-related mobility deficits. To our knowledge, there are no universally agreed upon definitions for mobility, cognition, and stroke-related communication deficits or for program models. Therefore, respondents were provided with definitions (Supplemental Appendix S1). A hybrid program was defined as a combination of home-based exercise (independent of rehabilitation staff) and regular supervised center-based exercise. A supervised-only program model was defined as all exercise sessions supervised and no home-based sessions prescribed. A home-based program model was defined as exercise conducted independently of rehabilitation staff and combined with periodic 1-on-1 supervised sessions with rehabilitation staff. The number of total patients enrolled in a year as a measure of program size was categorized on the basis of previous literature.36 Three kinesiologists from Quebec (1) and Ontario (2) working in CRPs participated in pilot testing to determine face and content validity, and appropriate modifications were made.
Statistical analysis
Differences between subgroups (CRPs that include vs not include stroke) were assessed using chi-square tests or Fisher exact tests as appropriate for categorical variables. The ranking of barriers and facilitators for each of the subgroups of including and not including stroke and mobility level was calculated by the proportion of programs that indicated the parameter as greater than somewhat of a barrier/facilitator to a very significant barrier/facilitator. All analyses were performed in SPSS (version 24.0, IBM Corp., Armonk, NY).
Results
Response rate and descriptive characteristics
CRPs are reported to be available in 10 of Canada’s 13 provinces and territories with no programs in the North.28 A total of 160 CRP representatives in Canada were contacted, and 114 (71.3%) representing 130 CRPs initiated the survey. Surveys were completed by CRP representatives from all 10 provinces (100%). It is reported that there are 182 CRPs in Canada;37 thus, our study sample represents 71.4% of all programs. There was a response rate of 96% (24/25) from Quebec, 83.3% (15/18) from Atlantic Provinces, 67.9% (19/28) from Prairie Provinces, 67.7% (44/65) from Ontario, and 50% (12/24) from British Columbia. There were 28 questionnaires that were incomplete (i.e., ≥25% of questions had not been responded to). There was no significant difference between complete and incomplete questionnaires by province (P = 0.6) or by CRPs that include or exclude stroke (P = 0.7).
Most of the responding programs were located in Ontario (38.6%), were in urban areas (44%), were hospital based/affiliated (59.5%), received funding from the hospital or clinical center (56.3%), enrolled a median of 144 patients annually, and were mainly at capacity (67.1%) with a wait-list between < 1 month and 2 months vs no wait-list (73.2% and 13.4%, respectively) (Table 1).
Table 1.
Descriptive characteristics of CRPs that did and did not include people poststroke across Canada
| Characteristic | CRPs n = 114 n (%) |
Include stroke n = 74 n (%) |
No stroke n = 40 n (%) |
P value |
|---|---|---|---|---|
| Province | n = 114 | n = 74 | n = 40 | 0.511 |
| Ontario | 44 (38.6) | 27 (36.5) | 17 (42.5) | |
| Quebec | 24 (21.1) | 18 (24.3) | 6 (15) | |
| Prairie∗ | 19 (16.7) | 14 (18.9) | 5 (12.5) | |
| Atlantic∗ | 15 (13.2) | 9 (12.2) | 6 (15) | |
| British Columbia | 12 (10.5) | 6 (8.1) | 6 (15) | |
| Location | n = 84 | n = 56 | n = 28 | 0.340 |
| Urban | 37 (44) | 28 (50.0) | 9 (32.1) | |
| Rural | 27 (32.1) | 15 (26.8) | 12 (42.9) | |
| Suburban | 18 (21.4) | 12 (21.4) | 6 (21.4) | |
| All of the above† | 2 (2.4) | 1 (1.8) | 1 (3.6) | |
| CRP facility setting | n = 84 | n = 56 | n = 28 | 0.415 |
| Hospital-based/affiliated | 50 (59.5) | 36 (64.3) | 14 (50.0) | |
| Community recreation/fitness center | 16 (19) | 10 (17.9) | 6 (21.4) | |
| University | 7 (8.3) | 5 (8.9) | 2 (7.1) | |
| Community health centre | 6 (7.1) | 2 (3.6) | 4 (14.3) | |
| Independent facility | 5 (6.0) | 3 (5.4) | 2 (7.1) | |
| Funding source‡ | n = 80 | n = 53 | n = 24 | |
| Hospital/clinical center funding, any | 45 (56.3) | 31 (57.4) | 14 (53.8) | 0.813 |
| Government/provincial health insurance, any | 35 (43.8) | 23 (42.6) | 12 (46.2) | 0.764 |
| User fee, any | 20 (25) | 12 (22.2) | 8 (30.8) | 0.408 |
| User fee only | 6 (7.5) | 4 (7.4) | 2 (7.7) | 0.964 |
| Private health insurance/industry, any | 2 (2.5) | 1 (1.9) | 1 (3.8) | 1.00 |
| Fundraising/foundation, any | 4 (5.0) | 3 (5.6) | 1 (3.8) | 1.00 |
| Research, any | 3 (3.8) | 1 (1.9) | 2 (7.7) | 0.198 |
| > 1 source of funding | 20 (25) | 12 (22.2) | 8 (30.8) | 0.408 |
| Distance to closest outpatient stroke program | n = 83 | n = 56 | n = 27 | |
| Within 25 km | 47 (56.6) | 33 (58.9) | 14 (51.9) | 0.660 |
| 26-50 km | 10 (12.0) | 5 (8.9) | 5 (18.5) | |
| > 50 km | 17 (20.5) | 12 (21.4) | 5 (18.5) | |
| I do not know | 9 (10.9) | 6 (10.7) | 3 (11.1) | |
| No. of patients enrolled in 1 y | n = 78 | n = 54 | n = 24 | 0.043 |
| 1-100 | 25 (32.1) | 14 (25.9) | 11 (45.8) | |
| 101-500 | 36 (46.2) | 30 (55.6) | 6 (25) | |
| > 500 | 17 (21.8) | 10 (18.5) | 7 (29.2) | |
| Capacity | n = 82 | n = 54 | n = 28 | 0.283 |
| Mainly under capacity | 11 (13.4) | 7 (13) | 4 (14.3) | |
| Mainly at capacity | 55 (67.1) | 39 (72.2) | 16 (57.1) | |
| Mainly over capacity | 16 (19.5) | 8 (14.8) | 8 (28.6) | |
| Wait-list | n = 82 | n = 55 | n = 27 | 0.297 |
| No wait-list | 11 (13.4) | 8 (14.5) | 3 (11.1) | |
| A wait-list but < 1 mo | 30 (36.6) | 23 (41.8) | 7 (25.9) | |
| 1-2 mo | 30 (36.6) | 19 (34.5) | 11 (40.7) | |
| 3+ mo | 11 (13.4) | 5 (9.1) | 6 (22.2) | |
| Healthcare professionals | n = 84 | n = 56 | n = 28 | |
| Dietitian | 67 (79.8) | 43 (76.8) | 24 (85.7) | 0.337 |
| Nurse or nurse practitioner | 64 (76.2) | 43 (76.8) | 21 (75) | 0.856 |
| Exercise professionals§ | 63 (75.0) | 44 (78.6) | 19 (67.9) | 0.285 |
| Physician, any | 62 (73.8) | 40 (71.4) | 22 (78.6) | 0.483 |
| Administrative | 48 (57.1) | 31 (55.4) | 17 (60.7) | 0.640 |
| Psych/social worker | 43 (51.2) | 26 (46.4) | 17 (60.7) | 0.217 |
| Physiotherapist | 40 (47.6) | 24 (42.9) | 16 (57.1) | 0.217 |
| Pharmacist | 32 (38.1) | 19 (33.9) | 13 (46.4) | 0.266 |
| Volunteers | 33 (39.3) | 23 (41.1) | 10 (35.7) | 0.636 |
| Program model | n = 84 | n = 56 | n = 29 | 0.049 |
| Hybrid program: Combination of home-based exercise independent of rehabilitation staff and regular supervised facility-based exercise | 58 (69) | 42 (75) | 16 (57.1) | |
| Web-based option (not included in analysis) | 3 (3.6) | 3 (5.4) | 0 | |
| Supervised, on-site sessions only | 23 (27.4) | 11 (19.6) | 12 (42.9) | |
| Web-based option (not included in analysis) | 2 (2.3) | 2 (3.6) | 0 | |
| Home-based alone (indirect supervision for most exercise with periodic 1-on-1 sessions with rehabilitation staff) | 3 (3.6) | 3 (5.4) | 0 | |
| Home-based (option offered by CRPs with a hybrid or on-site program but not offered as the only option) (not included in analysis) | 12 (14.3) | 8 (14.3) | 4 (13.8) | |
| Staff-to-patient ratio | n = 85 | n = 56 | n = 29 | 0.734 |
| Home-exercise only | 3 (3.5) | 3 (5.4) | 0 (0.0) | |
| 1:2-5 | 31 (36.5) | 19 (33.9) | 12 (41.4) | |
| 1:6-10 | 36 (42.4) | 25 (44.6) | 11 (37.9) | |
| 1:>10 | 13 (15.3) | 8 (14.3) | 5 (17.2) | |
| Other (varies, use ranking system based on risk and needs of clients) | 2 (2.4) | 1 (1.8) | 1 (3.4) | |
| Duration of CRP | n = 84 | n = 55 | n = 29 | 0.19 |
| 2-8 wk | 10 (11.9) | 4 (7.3) | 6 (20.7) | |
| 9-12 wk | 40 (47.6) | 25 (45.5) | 15 (51.7) | |
| 13-24 wk | 28 (33.3) | 21 (38.2) | 7 (24.1) | |
| Unlimited duration | 6 (7.1) | 5 (9.1) | 1 (13.8) | |
| No. of on-site sessions | n = 84 | n = 56 | n = 28 | 0.24 |
| 1 session per week | 12 (14.3) | 10 (17.9) | 2 (7.1) | |
| 2 sessions per week | 52 (61.9) | 30 (53.6) | 22 (78.6) | |
| 3 sessions per week | 14 (16.7) | 11 (19.6) | 3 (10.7) | |
| No regular on-site sessions (home exercise) | 3 (3.6) | 3 (5.4) | 0 (0.0) | |
| Unlimited | 3 (3.6) | 2 (3.6) | 1 (3.6) | |
| Maximum duration of sessions (including education) | n = 82 | n = 54 | n = 28 | 0.403 |
| ≤ 60 min | 33 (40.2) | 21 (38.9) | 15 (53.6) | |
| 61-90 min | 33 (40.2) | 23 (42.6) | 10 (35.7) | |
| > 90 min | 13 (15.9) | 10 (18.5) | 3 (10.7) | |
| Exercise prescription | ||||
| AT assessment | n = 83 | n = 55 | n = 28 | |
| Graded exercise stress test with ECG and BP | 47 (56.6) | 30 (54.5) | 17 (60.7) | 0.89 |
| 6MWT alone | 15 (18.1) | 10 (18.2) | 5 (17.9) | |
| ECG telemetry alone | 6 (7.2) | 4 (7.3) | 2 (7.1) | |
| No assessment | 15 (18.1) | 11 (20) | 4 (14.3) | |
| RT | n = 82 | n = 54 | n = 28 | |
| RT prescribed | 76 (92.7) | 50 (92.6) | 26 (92.9) | 1.0 |
| Strength assessment (1RM) n = 72/49/23 | 26 (36.1) | 16 (32.7) | 10 (43.5) | 0.37 |
| Assessments pre/post/during exercise | n = 84 | n = 56 | n = 28 | |
| Blood pressure (at least for those at risk for hypo/hypertension) | 80 (95.2) | 54 (96.4) | 26 (92.9) | 0.60 |
| Heart rate | 80 (95.2) | 54 (96.4) | 26 (92.9) | 0.60 |
| Blood glucose (at least for those at risk for hypoglycaemia) | 71 (84.5) | 47 (83.9) | 24 (85.7) | 1.00 |
| Oxygen saturation when appropriate | 73 (86.9) | 50 (89.3) | 23 (82.1) | 0.49 |
| ECG telemetry when needed | 24 (28.6) | 17 (30.4) | 7 (25.0) | 0.80 |
| Other services provided | n = 84-79 | n = 56-49 | n = 28-24 | |
| Educational sessions (eg, risk factor management) | 80 (95.2) | 53 (94.6) | 27 (96.4) | 1.0 |
| Nutrition counselling | 69 (83.1) | 45 (80.4) | 24 (88.9) | 0.53 |
| Medication counselling | 61 (75.3) | 38 (70.4) | 23 (85.2) | 0.18 |
| Follow-up assessment after completion of program | 58 (71.6) | 35 (66.0) | 23 (82.1) | 0.13 |
| Depression/psychological counselling | 53 (67.1) | 35 (67.3) | 18 (66.7) | 0.95 |
| Social services (eg, support groups, SW) | 36 (47.4) | 27 (51.9) | 9 (37.5) | 0.32 |
| Falls risk assessment/education | 34 (46.6) | 28 (57.1) | 6 (25.0) | 0.013 |
Proportions account for missing values.
AT, aerobic training; BP, blood pressure; CRP, cardiac rehabilitation program; ECG, electrocardiogram; 1RM, 1 repetition maximum; RT, resistance training; 6MWT, 6-minute walk test; SW, social worker.
Atlantic Provinces (Newfoundland and Labrador, Nova Scotia, Prince Edward Island, New Brunswick); Prairie (Alberta, Manitoba, and Saskatchewan).
Two respondents representing 16 programs.
Examined independently because there was considerable overlap in primary funding source.
Includes kinesiologists and exercise physiologists.
Inclusion of stroke
Of 114 respondents, 64.9% (n = 74) reported accepting referrals for people with a diagnosis of stroke. Of these, 14.9% (n = 11) reported offering a stroke-specific class with 10 of these also integrating patients into regular CRP classes. The provinces with the greatest proportion of CRPs that included stroke were Quebec (75%), Prairie Provinces (73.7%), and Ontario (61.4%). There were 6 more programs that reported considering including stroke > 1 year from completion of the questionnaire, with 2 of these planning to offer a stroke-specific class.
Of respondents, 62.5% (n = 35/56) reported that up to 10 people poststroke participated in the CRP and 88% (n = 44/50) indicated this to be < 11% of all patients participating in cardiac rehabilitation (CR) (Table 2). These data do not include the 11 and 6 programs that had no knowledge of the number or proportion admitted, respectively. Further, 88.5% (n = 54/61) of respondents reported no limit to the number of patients poststroke that they could admit to the CRP, and 2 programs (3.3%) had a limit of 200 patients. The median time accepting referrals for people poststroke was 11 years (interquartile range, 9 years, minimum 1 year and maximum 50 years) of n = 47 respondents.
Table 2.
Descriptive characteristics for CRPs that include people poststroke in regular CR classes
| Characteristic | CRPs that include stroke n = 74 n (%) |
|---|---|
| CRP stroke funding source n = 59 | |
| Hospital/clinical center funding | 31 (52.5) |
| +Health insurance (1) foundation (1) research (1) | |
| Government funding/health insurance | 21 (35.6) |
| +User fee (7) hospital (2) | |
| User fee as sole method of funding | 6 (10.2) |
| Fundraising/foundation only | 1 (1.7) |
| Eligibility criteria∗ (n = 67) | |
| Diagnosis of stroke alone is sufficient | 49 (73.1) |
| Only those with coronary artery disease | 17 (24.6) |
| Must be living in the community (not long-term care) | 32 (47.8) |
| Not currently participating in active SR | 15 (22.4) |
| Have own transportation to facility and able to get to treatment area from front door (e.g., 100 m, 10-min walk) | 5 (7.5) |
| Able to function in group setting | 5 (7.5) |
| Able to get on/off equipment independently | 5 (7.5) |
| Not a significant falls risk | 2 (3) |
| Other, independent toileting (1) no significant pain (1), complete a 6MWT (1), exercise for 0.5 to 1 h with breaks (1) | 4 (6) |
| Minimum days since stroke to be eligible (n = 68) | |
| Start any time after stroke | 26 (38.2) |
| Minimum 14 d | 1 (1.5) |
| Minimum 28-42 d | 12 (17.6) |
| Minimum 60-70 d | 2 (2.9) |
| When medically stable/referral from physician | 13 (19) |
| After completion of SR | 10 (14.7) |
| Other (ie, depends on the cardiac condition, discussed at team rounds, long wait-list) | 4 (5.9) |
| Upper limit of time since stroke, beyond which the patient is not eligible (n = 67) | |
| No upper limit | 57 (85.1) |
| 12 mo poststroke | 4 (6.0) |
| Other (unsure, based on physician referral, patient assessment [2], not specified [2]) | 6 (9.0) |
| Exercise program delivery model for people poststroke (n = 66) | |
| Integrated into CR class/sessions only | 55 (83.3) |
| Offers both stroke class/sessions separate from cardiac and integrated into CR | 10 (15.2) |
| Only offers stroke class separate from cardiac classes | 1 (1.5) |
| Model of supervision (n = 59) | |
| 1:1 beginning then group-based | 38 (64.4) |
| Entirely group-based | 20 (33.9) |
| 1:1 periodically with home-based exercise alone | 1 (1.7) |
| Program model (n = 42) | |
| Hybrid program: combination of home-based exercise independent of rehabilitation staff and regular supervised facility-based exercise | 29 (69) |
| Option of home-based with periodic 1:1 sessions | 8 (19.1) |
| Supervised on-site sessions only | 1 (2.4) |
| Telemedicine option | 3 (7.1) |
| Supervised on-site sessions only | 11 (26.2) |
| Home-based with periodic 1:1 session alone | 2 (4.8) |
| Combined with web-based | 1 (2.4) |
| Education specific to stroke, n = 61 | |
| Yes | 24 (39.3) |
| No | 36 (59) |
| Sometimes | 1 (1.6) |
| Proportion of people poststroke enrolled in CR program† (n = 61) | |
| < 1% | 10 (16.4) |
| 1%-2% | 7 (11.5) |
| 3%-4% | 17 (27.9) |
| 5%-10% | 10 (16.4) |
| > 10% | 6 (9.8) |
| I do not know | 11 (18.0) |
| No. of people poststroke accepted in last calendar year† (n = 62) | |
| 1-10 | 35 (56.5) |
| 11-20 | 9 (14.5) |
| 21-50 | 4 (6.4) |
| 50-100 | 4 (6.4) |
| > 100-200 | 4 (6.4) |
| I do not know | 6 (9.8) |
| Limit to No. of patients with stroke admitted (n = 61) | |
| No limit | 54 (88.5) |
| 200 patients | 2 (3.3) |
| 20 patients | 1 (1.6) |
| Other (availability space, personnel, 1/3 total volume, do not know) | 4 (6.6) |
| No. of years accepting patients with stroke into program† (n = 61) | |
| < 5 y | 10 (16.4) |
| 5-10 y | 11 (18.0) |
| > 10 y | 26 (42.6) |
| I do not know | 14 (23.0) |
| Wait-list of people poststroke† (n = 61) | |
| No wait-list | 31 (50.8) |
| 0-4 wk | 10 (16.4) |
| 5-8 wk | 14 (23.0) |
| > 8 wk | 4 (6.6) |
| I do not know | 2 (3.3) |
| No. of weekly classes available poststroke (n = 58) | |
| 1 class/wk | 10 (17.2) |
| 2-4 classes/wk | 35 (60.3) |
| 5-9 classes/wk | 8 (13.8) |
| 10-15 classes/wk | 2 (3.4) |
| > 15 classes/wk | 3 (5.2) |
| RT prescribed poststroke (n = 61) | |
| Yes | 50 (82) |
| No | 9 (14.8) |
| Other, classroom instruction but no supervised training (1) not specified (1) | 2 (3.3) |
| GXT and functional capacity tests (n = 59) | |
| GXT with ECG and BP for patients with and without mobility deficits | 13 (22.0) |
| GXT with ECG and BP only for patients with no mobility deficits | 16 (27.1) |
| GXT with ECG and BP only for patients with stroke in combination with cardiac conditions with or without mobility deficits | 5 (8.5) |
| No exercise stress tests conducted for CAD or stroke | 17 (28.8) |
| 6WMT for stroke with mobility deficits | 4 (6.8) |
| 6MWT alone or with DASI for any stroke | 3 (5.1) |
| ECG telemetry during 6MWT or during an exercise session for stroke with or without mobility deficits | 2 (3.3) |
| DASI alone | 1 (1.7) |
| Method of determining resistance intensity poststroke (n = 60) | |
| Not prescribed | 5 (8.3) |
| 1 repetition maximum (1RM) at least | 9 (15) |
| RPE | 33 (55) |
| Comfortable weight load alone | 11 (18.3) |
| Other (based on medical history and staff member determines resistance) | 2 (3.3) |
| Method of determining aerobic exercise intensity poststroke (n = 62) | |
| RPE (in combination with most methods below) | 56 (90.3) |
| Based on results of GXT data | 34 (54.8) |
| Based on functional test (i.e., 6MWT) | 27 (43.5) |
| 6MWT+GXT/6MWT+talk test | 12 (19.4)/1 (1.6) |
| Calculated age-adjusted target heart rate | 13 (21) |
| Estimated intensity based on clinical expertise | 27 (43.5) |
| Based on clinical expertise alone or with talk test or RPE | 7 (11.3) |
| Patient self-selected intensity | 18 (29) |
| Self-selected intensity alone | 1 (1.6) |
BP, blood pressure; CAD, coronary artery disease; CR, cardiac rehabilitation; CRP, cardiac rehabilitation program; DASI, Duke Activity Status Index (brief self-administered questionnaire to estimate functional capacity); ECG, electrocardiogram; GXT, graded exercise test; 1RM, 1 repetition maximum; RPE, rating of perceived exertion; RT, resistance training; 6MWT, 6-minute walk test; SR, stroke rehabilitation.
Program managers were instructed to choose all that apply.
Number based on responses for CRPs that include people poststroke in regular cardiac rehabilitation sessions only.
Descriptive characteristics of CRPs that did and did not include people poststroke are shown in Table 1. In exploratory analyses, the CRP model had a significant effect on inclusion of stroke into CRPs (P = 0.049), medium program size based on annual patients admitted (P = 0.043), and including a falls prevention assessment or education component (P = 0.013).
CRP eligibility criteria for including people poststroke
Of CRPs that included stroke, 24.6% accepted referrals only for those who also had a cardiac diagnosis (28% in Ontario;7/25), 47.8% would only take patients who were living in the community (not long-term care), and 22.4% would only take patients if they were not currently participating in active SR. Further, 39% reported no restriction on minimum number of days elapsed poststroke to start CR and 85% cited no upper limit (Table 2).
Figure 1 and Supplemental Table S1 illustrate eligibility criteria cited by program managers by type and severity of stroke-related deficits and requirement of caregiver support. Supplemental Table S1 shows the specific type of support required. Approximately half of CRPs accepting patients with moderately severe to severe mobility deficits required a caregiver to be on-site or to assist the patient with home exercise, 80% to 84% of CRPs required support for those with moderately severe to severe cognitive impairments, and 55.8% to 69.2% of CRPs required only on-site support for those with moderate to severe communication deficits.
Figure 1.
Eligibility of people poststroke for enrollment into CRPs by stroke-related impairments and caregiver support. Bottom portion of each bar (white) represents the proportion of CRPs accepting patients with impairment that require some level of caregiver support for the patient to be enrolled (support during the on-site program sessions, home exercise sessions, or both). Supplemental Table S1 shows more information. CRP, cardiac rehabilitation program.
Program model
Regarding CRP delivery, 83.3% integrated people poststroke into regular CR sessions, most had a staff to patient ratio of 1:1 at the beginning and then group-based exercise, and 39.3% offered stroke specific education, with the majority of CRPs (82.8%) offering more than 1 weekly class option for the patient to choose/be assigned to.
Preparticipation exercise assessments and intensity prescription
Of the 59 responding CRPs that included stroke, 58 (98.3%) reported conducting a preparticipation aerobic physical assessment (Table 2). Most of the assessments reported were graded exercise tests (GXTs) with ECG and blood pressure monitoring or ECG monitoring during exertion (n = 36/59; 61%) but with some restrictions. Specifically, 33.9% of all programs reported that people with mobility deficits were included in assessments with ECG monitoring. The remaining programs (8.5%; 5/59) conducted only 6-minute walk tests in those with or without mobility deficits. Overall, 42.4% of CRPs conducted GXT, ECG, or 6-minute walk tests for people with mobility deficits.
The AT intensity parameter was reported to be prescribed predominantly on the basis of the results of the GXT including the rating of perceived exertion (54.8%). Regarding RT, the weight/resistance load was determined using the rating of perceived exertion scale technique (55%), followed by a comfortable weight load (18.3%) and 1 repetition maximum testing (15%).
Barriers and facilitators
Supplemental Tables S2 and S3 present the perceived barriers and facilitators for the inclusion of people in CRPs by mobility deficits and by CRPs that include and do not include people poststroke. Figures 2 and 3 present the data in aggregate by the mean proportion of CRPs citing barriers and facilitators across all mobility levels.
Figure 2.
Barriers to including people poststroke into CRPs. Aggregate of the mean proportion of CRPs citing barriers across all mobility levels. Rank is based on the proportion of programs that indicated the parameter as somewhat of a barrier to a very significant barrier. AT, aerobic training; Ax, assessment; RT, resistance training; Rx, treatment.
Figure 3.
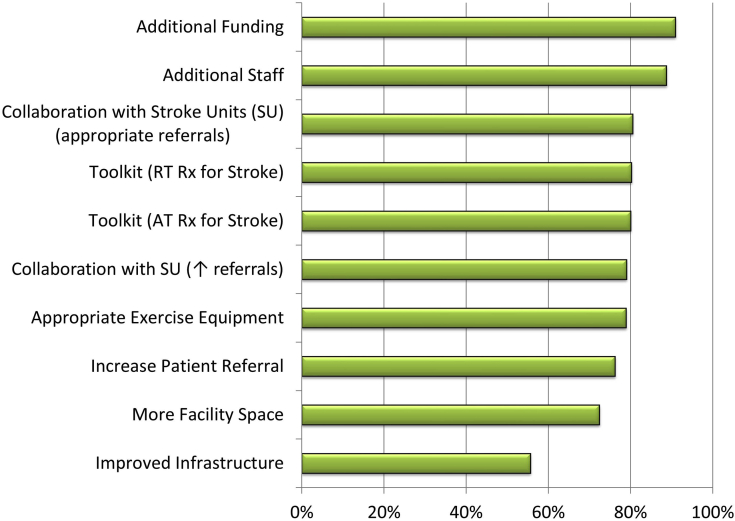
Facilitators to including people poststroke into CRPs. Aggregate of the mean proportion of CRPs citing facilitators across all mobility levels. Rank is based on the proportion of programs that indicated the parameter as somewhat of a facilitator to a very significant facilitator. AT, aerobic training; RT, resistance training; Rx, prescription.
Discussion
To our knowledge, this is the largest survey of CRPs in Canada and the first to measure the proportion of CRPs that accept people with a diagnosis of stroke, exploring the number of participants, eligibility criteria, and barriers and facilitators to inclusion. We reveal that although most CRPs accept referrals for people after stroke and have been doing so for a median of 11 years, few participate. Although this may be related in part to CRP inclusion/exclusion criteria, lack of referrals received is likely the most influential factor. This represents a lost opportunity for referral given that most CRP representatives reported no limit to the number of people poststroke that can be enrolled. However, a promising future direction revealed is to develop partnerships between CRP and SR professionals to increase the number of suitable referrals based on CRP inclusion/exclusion criteria. Providing a toolkit to help CRPs operationalize the RT and AT components is another highly rated strategy identified by CRP respondents.
Most CRPs in Canada accepted stroke referrals but had extensive eligibility criteria
Our result that 65% of 114 CRP representatives reported accepting referrals for people with a diagnosis of stroke was similar to a study published in 2018 reporting 61.5% of 64 CRPs accepting stroke referrals in Canada.37 The proportion of programs accepting referrals in Ontario, where the largest number of CRPs are located, has not changed much in a decade with 61.4% (n = 27/44) accepting stroke referrals in the current study and 60% (n = 24/40) in a 2009 study.36
Although these results provide some optimism for increasing access to secondary prevention services, we reveal restrictions in referral criteria and participation. Of programs that accepted stroke referrals, 25% only accepted people with a coexisting cardiac diagnosis (28% in Ontario), and approximately half of CRPs only accepted people living in the community and not in long-term care. This is less restrictive than reported in a 2009 survey of Ontario CRPs in which 33% only accepted people with a coexisting cardiac diagnosis. In addition, 70.1%, 80.6%, and 85.1% of CRPs in the current study reported not accepting people with severe mobility, communication, and cognitive deficits, respectively. This is similar to results from recent survey data from our group that 75% of SR programs in Canada do not prescribe AT to people with severe stroke.24
Few people participated in CRPs after stroke
Restrictions in eligibility criteria do not explain the finding that 63% of CRPs that admit stroke reported that only up to 10 patients poststroke participated in the previous calendar year representing approximately 10% or less of total patients enrolled. Recent survey data from Australia (2019) reveal that patients are even less likely to participate. Of 149 CRPs, only 6% had received a referral for people with stroke or transient ischemic attack (TIA) in the previous calendar year, with stroke/TIA representing < 2% of the patient population in > 90% of CRPs.38 Yet, in the current study, 89% of CRPs reported that there was no limit to the number of people poststroke that could be admitted. This raises the question of why people poststroke are not attending CRPs in higher numbers and what can be done to facilitate entry.
Barriers and facilitators to including people poststroke in CRPs
Lack of resources was a primary barrier to including stroke
Lack of funding was a more influential barrier to CRPs that do not include stroke compared with CRPs that do (ranked first to second vs sixth to ninth, respectively, across all mobility levels). Yet in bivariate analysis, none of the CRP characteristics related to resources were associated with including or not including stroke, specifically, CRP funding sources, facility setting, presence of a wait-list, length of a wait-list, duration of the program, and staff-to-patient ratio. However, we did not request programs to report an annual budget. Although the funding structure of CRPs is complex with one-quarter having multiple sources of funding, out-of-pocket expenses were reported to be required to attend CRPs by 25% of respondents, which might also pose an unmeasured barrier to access.
Facilitators to overcome lack of resources
Given that 3 of the most influential barriers were related to resources, it is not surprising that the 2 most cited facilitators to including stroke were additional funding and additional staff. However, with an overburdened healthcare system, other solutions to these issues should be explored. For example, we demonstrated a significant effect of program model on inclusion of stroke. Specifically, approximately three-quarters of programs with a hybrid model (combination of home-based and supervised center-based exercise) accepted stroke referrals, whereas only half of supervised-only on-site exercise program models accepted stroke. The possibility of more programs adopting a hybrid model provides an opportunity to decrease wait-times and resources. Research supports the adoption of hybrid versus supervised-only CRPs, finding that they are equally effective in improving clinical and health-related quality of life outcomes in patients with cardiac disease, although more research is required.39, 40, 41, 42 Indeed, participation in hybrid model CRPs for people with up to moderate motor impairments poststroke has resulted in improvements in cardiorespiratory fitness, muscle mass and strength, balance, mobility, cognition, sit-to-stand performance, upper and lower limb range-of-motion, plasma blood lipids, and quality of life.20,21,30,31,43
Lack of stroke referrals was the fourth most influential barrier to including stroke
Lack of referrals posed a significant barrier to Canadian CRPs for patients across all mobility levels. Australia, where only 6% of CRPs had received a referral for people with stroke or TIA, also reported lack of referrals as the fourth most influential barrier to including people poststroke.38
Facilitators to address lack of stroke referrals
The third and sixth most influential facilitators to inclusion of patients across all levels of mobility deficit was collaboration between healthcare professionals from CRPs and SR units to ensure appropriate referrals and to improve referral rate, respectively. Indeed, more than half of CRP respondents across Canada reported that there was an SR program within a 25-km radius of the CRP. This provides an opportunity to develop a seamless, automatic referral process from SR to CRP and significantly increase exposure to secondary prevention strategies for people poststroke. Soliciting referrals from stroke prevention clinics is an additional strategy to enhance referral. Another barrier encountered may be the complex and varying CRP eligibility criteria. This barrier may be mitigated by each CRP providing a detailed stroke referral form listing eligibility criteria to facilitate referral. A brochure describing the program, location, transportation resources, caregiver participation, and time commitment for the participant would also be of benefit. CRP websites should also list stroke as a referral population. Although these components have not been examined in a randomized controlled trial, a recent prospective study by our group demonstrated that collaboration between a CRP with a single SR program using the described recommendations resulted in 71% of all eligible patients poststroke being enrolled.33
Toolkits for prescribing resistance and AT were perceived as influential facilitators
Toolkits for RT and AT prescription were the fourth and fifth most important facilitators, respectively, for the inclusion of patients with stroke across all mobility levels. However, the toolkit should include instruction for prescribing RT and AT to patients with severe deficits because these were the most influential of all facilitators for patients with severe mobility deficits for 97.1% of programs that do not include stroke. A toolkit may also benefit programs across the continuum of care because in an earlier study, inpatient and outpatient SR program managers in Canada ranked lack of knowledge and skills of AT prescription for high-risk populations as the third most cited barrier to prescription.24
CRP model elements that overcome barriers reported by SR programs
Referral from SR to CRPs would help to fill a gap in care that is faced by Canadian SR programs to carrying out best practice recommendations for blood glucose and blood pressure monitoring, performing preparticipation cardiac assessments, and including patients with coexisting cardiac disease.24 The current survey demonstrated that blood glucose was measured by 83.9% and blood pressure was monitored by 95.2% of CRPs during exercise. Although 61% of CRPs that included stroke provided at least ECG monitoring during exertion, only 33.9% included these assessments for people with mobility deficits. Although this is still superior to that received in SR programs (9%),24 it is an area that can be improved on by using adaptive equipment, such as specialized pedals to secure the foot or leg during cycling. Indeed, lack of equipment to assess aerobic capacity was ranked as the third most important barrier to including stroke by CRP respondents. Furthermore, referring from SR to CRPs also offsets the 2 most frequently cited barriers to prescribing exercise reported by SR programs in both Canada and the United States. These included insufficient time within the therapy session and insufficient length of stay in rehabilitation.24, 25, 26 Indeed, patients after stroke referred to CRPs would have received extensive exposure to secondary prevention strategies with 93% of CRPs being ≥ 9 to 12 weeks in duration, with most (76.8%) including at least 2 on-site exercise sessions per week.
Limitations
This study had a number of limitations. We were unable to assess responder bias. Data collection relied on self-report, and there may have been ascertainment bias. Two of the respondents represented 16 programs, but we reported these as 2 responses. Examination of association between CRP characteristics and inclusion of stroke was exploratory and did not adjust for multiple comparisons. To our knowledge, there are no validated descriptions of severity of cognition, communication, and mobility stroke deficits; thus, our definitions may not match true clinical presentation.
Conclusion
Greater than half of eligible CRPs in Canada include people poststroke. However, few patients participate. Barriers were mostly related to lack of funding and resources, but also included lack of referrals. An important facilitator cited by programs was collaboration with SR units, which is a low-resource and feasible strategy for increasing referrals. Including the stroke population in CRPs is important, because these programs provide health resources, education, monitoring, and AT+RT programs that progress in intensity and duration/repetitions, and act to promote a nonsedentary lifestyle. Indeed, we demonstrated that most CRPs are medically supervised programs (71.4%) that offered education sessions on risk factor modification (94.6%), nutrition (80%), medication (70.4%), psychosocial counseling (67.3%), and falls risk assessment and education (57.1%). CRPs offer a safe environment to refer patients after stroke, and national strategies to subsidize CRPs could significantly help integrate these patients and have a significant effect on the health and quality of life trajectory of these patients.
Given the estimate that there are 182 CRPs in Canada,37 approximately 50,000 strokes occurring in Canada annually (Heart and Stroke Foundation of Canada, 2003), and approximately 72% of patients would be eligible to participate in CR,33 each CRP in Canada would need to accept approximately 198 stroke referrals annually. However, in the current study only 6.4% of programs that accepted stroke received > 100 to 200 patients annually after stroke. It should be noted that these are estimates, and the true incidence and prevalence of stroke in Canada or the proportion eligible for CR are not known. Nevertheless, these estimates provide a benchmark for policy makers when addressing secondary prevention strategies that would require collaboration between health organizations and CRPs, long-term care facilities, stroke prevention clinics, community exercise programs, and other institutions.
Funding Sources
There are no funding sources to declare.
Disclosures
The authors declare that there is no conflict of interest and have nothing to disclose.
Acknowledgements
The authors acknowledge Drs Paul Oh, Dylan Chipperfield, and Jane Brownrigg for help in locating programs in their provinces; Danielle Lawrence and Merrisa Martinuzzi for piloting the survey; Angela Marzolini for help with survey design; and the respondents for taking the time to complete the survey.
Footnotes
Ethics Statement: Approval from the Ethics Review Board of the University of Toronto was obtained.
See page 205 for disclosure information.
See editorial by Stone et al., pages189–191of this issue.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.01.007.
Supplementary Material
References
- 1.Krueger H., Koot J., Hall R.E. Prevalence of individuals experiencing the effects of stroke in Canada: trends and projections. Stroke. 2015;46:2226–2231. doi: 10.1161/STROKEAHA.115.009616. [DOI] [PubMed] [Google Scholar]
- 2.Hebert D., Lindsay M.P., McIntyre A. Canadian stroke best practice recommendations: stroke rehabilitation practice guidelines, update 2015. Int J Stroke. 2016;11:459–484. doi: 10.1177/1747493016643553. [DOI] [PubMed] [Google Scholar]
- 3.Physical Activity Guidelines Advisory Committee Report. US Department of Health and Human Services. Physical Activity Guidelines Advisory Committee; Washington, DC: 2008. [DOI] [PubMed] [Google Scholar]
- 4.Michael K.M., Macko R.F. Ambulatory activity intensity profiles, fitness, and fatigue in chronic stroke. Top Stroke Rehabil. 2007;14:5–12. doi: 10.1310/tsr1402-5. [DOI] [PubMed] [Google Scholar]
- 5.Rand D., Eng J.J., Tang P.F., Jeng J.S., Hung C. How active are people with stroke?: use of accelerometers to assess physical activity. Stroke. 2009;40:163–168. doi: 10.1161/STROKEAHA.108.523621. [DOI] [PubMed] [Google Scholar]
- 6.Towfighi A., Markovic D., Ovbiagele B. Impact of a healthy lifestyle on all-cause and cardiovascular mortality after stroke in the USA. J Neurol Neurosurg Psychiatry. 2012;83 doi: 10.1136/jnnp-2011-300743. 146-5. [DOI] [PubMed] [Google Scholar]
- 7.Brown C., Fraser J.E., Inness E.L. Does participation in standardized aerobic fitness training during inpatient stroke rehabilitation promote engagement in aerobic exercise after discharge? A cohort study. Top Stroke Rehabil. 2014;21:s42–s51. doi: 10.1310/tsr21S1-S42. [DOI] [PubMed] [Google Scholar]
- 8.Ryan A.S., Buscemi A., Forrester L., Hafer-Macko C., Ivey F.M. Atrophy and intramuscular fat in specific muscles of the thigh: associated weakness and hyperinsulinemia in stroke survivors. Neurorehabil Neural Repair. 2011;25:865–872. doi: 10.1177/1545968311408920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flansbjer U., Downham D., Lexell J. Knee muscle strength, gait performance, and perceived participation after stroke. Arch Phys Med Rehabil. 2006;87:974–980. doi: 10.1016/j.apmr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Lomaglio M., Eng J. Muscle strength and weight-bearing symmetry relate to sit-to-stand performance in individuals with stroke. Gait Posture. 2004;22:126–131. doi: 10.1016/j.gaitpost.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohannon R.W., Walsh S. Association of paretic lower extremity muscle strength and standing balance with stair-climbing ability in patients with stroke. J Stroke Cerebrovasc Dis. 1991;1:129–133. doi: 10.1016/S1052-3057(10)80004-7. [DOI] [PubMed] [Google Scholar]
- 12.Harris M., Polkey M., Bath P., Moxham J. Quadriceps muscle weakness following acute hemiplegic stroke. Clin Rehabil. 2001;15:274–281. doi: 10.1191/026921501669958740. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen L., Jacobsen B.K. Changes in muscle mass, fat mass, and bone mineral content in the legs after stroke: a 1 year prospective study. Bone. 2001;28:655–659. doi: 10.1016/s8756-3282(01)00434-3. [DOI] [PubMed] [Google Scholar]
- 14.Ivey F., Macko R., Ryan A., Hafer-Macko C. Cardiovascular health and fitness after stroke. Top Stroke Rehabil. 2005;12:1–16. doi: 10.1310/GEEU-YRUY-VJ72-LEAR. [DOI] [PubMed] [Google Scholar]
- 15.Hackam D.G., Spence J.D. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881–1885. doi: 10.1161/STROKEAHA.106.475525. [DOI] [PubMed] [Google Scholar]
- 16.Dhamoon M.S., Sciacca R.R., Rundek T., Sacco R.L., Elkind M.S. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology. 2006;66:641–646. doi: 10.1212/01.wnl.0000201253.93811.f6. [DOI] [PubMed] [Google Scholar]
- 17.Sacco R., Wolf P.A., Kannel W., McNamara P. Survival and recurrence following stroke. The Framingham study. Stroke. 1982;13:290–295. doi: 10.1161/01.str.13.3.290. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann A., Rundek T., Mast H. Mortality and causes of death after first ischemic stroke. The Northern Manhattan Stroke Study. Neurology. 2001;57:2000–2005. doi: 10.1212/wnl.57.11.2000. [DOI] [PubMed] [Google Scholar]
- 19.Naci H., Ioannidis J.P. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577. doi: 10.1136/bmj.f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prior P.L., Hachinski V., Unsworth K. Comprehensive cardiac rehabilitation for secondary prevention after transient ischemic attack or mild stroke I: feasibility and risk factors. Stroke. 2011;42:3207–3213. doi: 10.1161/STROKEAHA.111.620187. [DOI] [PubMed] [Google Scholar]
- 21.Marzolini S., Brooks D., Oh P. Aerobic with resistance training or aerobic training alone poststroke: a secondary analysis from a randomized clinical trial. Neurorehabil Neural Repair. 2018;32:209–222. doi: 10.1177/1545968318765692. [DOI] [PubMed] [Google Scholar]
- 22.Turan T.N., Nizam A., Lynn M.J. Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology. 2017;88:379–385. doi: 10.1212/WNL.0000000000003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winstein C.J., Stein J., Arena R. on behalf of the American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:e98–e169. doi: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 24.Nathoo C., Buren S., El-Haddad R. Aerobic training in Canadian stroke rehabilitation programs. J Neurol Phys Ther. 2018;42:248–255. doi: 10.1097/NPT.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 25.Doyle L., Mackay-Lyons M. Utilization of aerobic exercise in adult neurological rehabilitation by physical therapists in Canada. J Neurol Phys Ther. 2013;1:20–26. doi: 10.1097/NPT.0b013e318282975c. [DOI] [PubMed] [Google Scholar]
- 26.Boyne P., Billinger S., MacKay-Lyons M. Aerobic exercise prescription in stroke rehabilitation: a web-based survey of US physical therapists. J Neurol Phys Ther. 2017;41:119–128. doi: 10.1097/NPT.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mooren F.C. SpringerLink (Online service); NY: 2012. Encyclopedia of Exercise Medicine in Health and Disease. [Google Scholar]
- 28.Grace S.L., Bennett S., Ardern C.I., Clark A.M. Cardiac rehabilitation series: Canada. Prog Cardiovasc Dis. 2014;56:530–535. doi: 10.1016/j.pcad.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lennon O., Carey A., Gaffney N., Stephenson J., Blake C. A pilot randomized controlled trial to evaluate the benefit of the cardiac rehabilitation paradigm for the non-acute ischaemic stroke population. Clin Rehabil. 2008;22:125–133. doi: 10.1177/0269215507081580. [DOI] [PubMed] [Google Scholar]
- 30.Tang A., Marzolini S., Oh P.I., McIlroy W.E., Brooks D. Feasibility and effects of adapted cardiac rehabilitation after stroke: a prospective trial. BMC Neurol. 2010;10:40. doi: 10.1186/1471-2377-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marzolini S., Tang A., McIlroy W., Oh P.I., Brooks D. Outcomes in people after stroke attending an adapted cardiac rehabilitation exercise program: does time from stroke make a difference? Stroke Cerebrovasc. 2014;23:1648–1656. doi: 10.1016/j.jstrokecerebrovasdis.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Marzolini S. Integrating individuals with stroke into cardiac rehabilitation following traditional stroke rehabilitation: promoting a continuum of care. Can J Cardiol. 2018;34:S240–S246. doi: 10.1016/j.cjca.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Marzolini S., Fong K., Jagroop D. Eligibility, enrollment, and completion of exercise-based cardiac rehabilitation following stroke rehabilitation: what are the barriers? Phys Ther. 2020;100:44–56. doi: 10.1093/ptj/pzz149. [DOI] [PubMed] [Google Scholar]
- 34.Marzolini S., Oh P.I., McIlroy W., Brooks D. The feasibility of cardiopulmonary exercise testing for prescribing exercise to people after stroke. Stroke. 2012;43:1075–1081. doi: 10.1161/STROKEAHA.111.635128. [DOI] [PubMed] [Google Scholar]
- 35.Dillman D.A. John Wiley & Sons; Hoboken, NJ: 2011. Mail and Internet Surveys: The Tailored Design Method--2007 Update with New Internet, Visual, and Mixed-Mode Guide. [Google Scholar]
- 36.Tang A., Closson V., Marzolini S. Cardiac rehabilitation after stroke-need and opportunity. J Cardiopulm Rehabil. 2009;29:97–104. doi: 10.1097/HCR.0b013e31819a00d4. [DOI] [PubMed] [Google Scholar]
- 37.Tran M., Pesah E., Turk-Adawi K. Cardiac rehabilitation availability and delivery in Canada: how does it compare with other high-income countries? Can J Cardiol. 2018;34:S252–S262. doi: 10.1016/j.cjca.2018.07.413. [DOI] [PubMed] [Google Scholar]
- 38.Howes T., Mahenderan N., Freene N. Cardiac rehabilitation: are people with stroke or transient ischaemic attack being included? A cross-sectional survey. Heart Lung Circ. 2020;29:483–490. doi: 10.1016/j.hlc.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Kavanagh T., Cardiac Rehabilitation: Canada . Springer; 2007. Cardiovascular Prevention and Rehabilitation; pp. 37–40. [Google Scholar]
- 40.Hamm L., Kavanagh T., Campbell R.B. Timeline for peak improvements during 52 weeks of outpatient cardiac rehabilitation. J Cardiopulm Rehabil. 2004;24:374–380. doi: 10.1097/00008483-200411000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Farias-Godoy A., Chan S., Claydon V.E. The impact of reduced cardiac rehabilitation on maximal treadmill exercise time. J Cardiopulm Rehabil Prev. 2018;38:24–30. doi: 10.1097/HCR.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 42.Saeidi M., Soroush A., Komasi S., Singh P. A hybrid cardiac rehabilitation is as effective as a hospital-based program in reducing chest pain intensity and discomfort. Korean J Pain. 2017;30:265. doi: 10.3344/kjp.2017.30.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marzolini S., Oh P.I., McIlroy W., Brooks D. The effects of an aerobic and resistance exercise training program on cognition following stroke. Neurorehab Neural Repair. 2013;27:392–402. doi: 10.1177/1545968312465192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.