Abstract
Despite significant recent therapeutic advances, complete mucosal healing remains a difficult treatment target for many patients with inflammatory bowel diseases (IBD) to achieve. Our review focuses on the translational concept of promoting resolution of inflammation and repair as a necessary adjunctive step to reach this goal. We explore the roles of inflammatory cell apoptosis and efferocytosis to promote resolution, the new knowledge of gut monocyte-macrophage populations and their secreted prorepair mediators, and the processes of gut epithelial repair and regeneration to bridge this gap. We discuss the need and rationale for this vision and the tangible steps toward integrating proresolution therapies in IBD.
Keywords: inflammatory bowel disease, Crohn, ulcerative colitis, mucosal healing, inflammation
This review focuses on the development of therapeutic approaches that are geared toward the resolution of inflammation and repair in IBD. We discuss key targets and pathways, new understandings in the complex inflammatory landscape in IBD, and the practical considerations required for tangible clinical translation toward complete mucosal healing.
BACKGROUND
Inflammation is a protective host response to “danger”.1-3 Once the threat or insult, usually infection or trauma, is neutralized, a coordinated and active process of resolution and repair begins to restore tissue integrity and function.4, 5 Ulcerative colitis (UC) and Crohn disease (CD) are characterized by immune-mediated nonresolving chronic inflammation of the gastrointestinal tract, which if untreated will progress toward the natural complications of uncontrolled inflammation, namely the development of fibrosis (strictures in CD; “hose-pipe” colon in UC) or organ damage and subsequent organ failure (abscess and fistula formation in CD; toxic megacolon in UC). Many factors (host genetics, the complex gut tissue environment, microbial dysbiosis, impaired gut barrier function, and dysregulated innate/adaptive immune system) drive the pathogenic immune response and underlie the emphatic failure to resolve gut inflammation in IBD.6-8
From general anti-inflammatory and immunosuppressive therapies using 5-aminosalicylates, corticosteroids, and thiopurines in the pre-2000s, we have moved toward biologic therapies that enable specific targeting of proinflammatory mediators such as tumor necrosis factor (TNF) and interleukin (IL)-12/23p40 and toward integrins to reduce inflammatory cell migration. More recently, a new family of small molecules to block Janus kinase signaling, which regulates multiple inflammatory pathways,9, 10 have also become available in clinics. These therapies have undoubtedly been successful, and many more new drugs are on the horizon.11
Despite these promising advances, there is a consistent “therapeutic ceiling” 12-17 to the ability of such approaches to bring about complete mucosal healing—the total resolution and healing of ulcerations and a full return to healthy gut mucosa. Complete mucosal healing is the most coveted treatment target with the best long-term implication in prognosis.18 It is particularly noteworthy that this is only achieved in about 50% of patients with moderate to severe IBD, despite intensive medical therapy.13, 19, 20 The CALM study in CD, for example, showed that even with early aggressive medical therapy using anti-TNF and azathioprine and guided by biomarkers (fecal calprotectin [S100a8/9] and C-reactive protein), endoscopic mucosal healing was seen in less than 50% of patients after 48 weeks of treatment.13 In the conventionally managed group from the same study, this rate was even lower at 30%. Three recent keynote clinical trials in UC from 2017 to 2019, the VARSITY,14 UNIFI,21 and OCTAVE22 studies, showed endoscopic mucosal improvements of 39.7% vs 27.7% (vedolizumab vs adalimumab), 51.1% vs 28.6% (ustekinumab vs placebo), and 54.7% vs 13.1% (tofacitinib vs placebo), respectively, after almost a year of continuous treatment. These studies highlight the present-day situation and the limitations of available medical treatments.
Because the key driving factors that initiate and perpetuate IBD mucosal inflammation are not fully known, the dominant drug development model so far has been based on the principle of long-term continuous inhibition of the abnormal immune response in IBD.23 Whether such an approach, by further intensification (eg, combining different biologics) or stratification (eg, using biomarkers to select treatment), can provide the game-changing improvement is questionable. In this context, our review focuses on the therapeutic concept of promoting inflammation resolution as the extra factor required to overcome the therapeutic ceiling phenomenon. In essence, we posit a simple dual-approach model where complete mucosal healing = anti-inflammatory + proresolution therapy. We discuss this with a particular focus on key immune cells, such as monocytes, macrophages, and neutrophils, and the importance of dismantling the inflammatory mucosal environment as the prerequisite to mucosal healing. Despite a developing mature field in the resolution of inflammation, there are no present treatments that are wholly focused on this resolution in IBD, or indeed in any other major chronic inflammatory conditions. Here we discuss the tangible routes toward translation in IBD.
INFLAMMATION: FROM INITIATION TO RESOLUTION
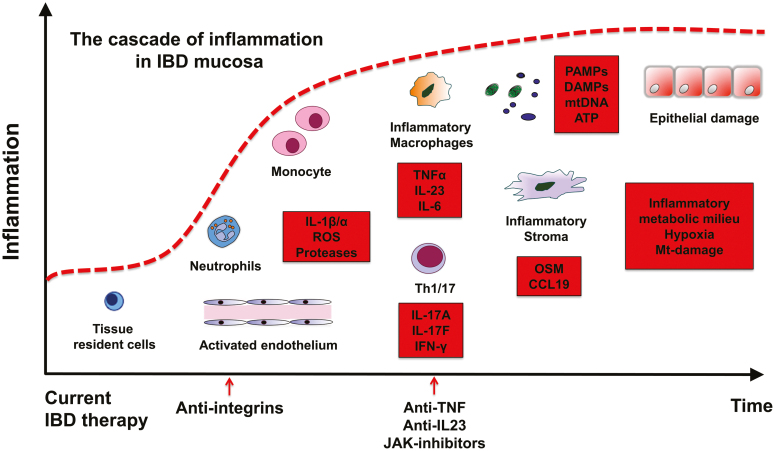
In general, the immediate inflammatory response is mediated by receptors of the innate immune system, such as toll-like receptors (TLRs) and nucleotide-binding oligomerization-domain protein–like receptors.24 In such settings, tissue-resident immune cells contribute by producing inflammatory mediators, including cytokines such as chemokines, eicosanoids, and products of proteolytic cascades. The main effect of these mediators is to elicit an inflammatory environment: plasma proteins and leukocytes (mainly neutrophils) that are normally restricted to the blood vessels gain access. The activated endothelium allows recruitment and selective extravasation of neutrophils into the tissues. Here they become activated and exert their effector functions by releasing the contents of their granules, potent proteases, and oxidants that are damaging in an indiscriminate manner25, 26 (Fig. 1).
FIGURE 1.
From initiation to the development of the chronic nonresolving inflammatory milieu in the IBD gut mucosa. Main biologic treatments such as anti-integrins, anti-TNF, IL-23p40, and Janus kinase inhibitors block single factors to reduce inflammation. Potentiating factors in the red box. ATP indicates adenosine triphosphate; mtDNA, mitochondrial DNA.
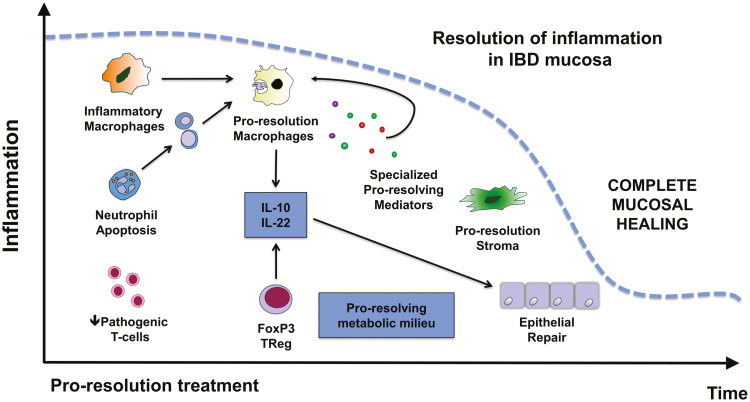
A successful acute inflammatory response results in the elimination of the harmful stimuli (eg, infectious organisms such as bacteria, fungi, parasites, and viruses) followed by a resolution and repair phase, which is thought to be mediated mainly by tissue-resident and recruited macrophages.27 Neutrophils undergo apoptosis, a process that promotes several proresolution pathways, particularly when paired with their uptake via phagocytosis by macrophages (also known as efferocytosis). This leads to neutrophil clearance and further release of anti-inflammatory and reparative cytokines and mediators.28 Macrophages play a further role to dampen inflammation and initiate wound repair by clearing debris and producing growth factors and mediators that provide trophic support to the tissue environment29 (Fig. 2).
FIGURE 2.
Promoting resolution of inflammation in IBD. Key events and pathways that can be targeted to promote and accelerate resolution and repair following IBD treatment.
MUCOSAL INFLAMMATION LANDSCAPE IN IBD
Although these steps from inflammation initiation to resolution are reasonably well defined in infection or tissue injury, they are poorly understood in the context of IBD—in particular, regarding the timeline of key immunological events that shape the complex IBD mucosal milieu.30, 31 A persistent innate inflammatory process acquires new pathogenic characteristics. Excessive production of IL-1, IL-6, IL12/23, and TNFα result in polarizing cytokine conditions that drive distinct abnormal T-cell responses7 and subsequent uncontrolled tissue remodeling, such as fibrosis.32 Recent advances in single-cell technology are now providing novel insights into the IBD inflammatory landscape.33-36 Three recent studies have presented comprehensive high-resolution cell-type mapping of the inflamed mucosal milieu in UC and CD using a single-cell RNA-sequencing approach (scRNAseq).33, 35, 36 Of interest, they showed a few findings with common themes for UC and CD relevant to this review.
First, this mucosal milieu is unsurprisingly complex. By harnessing the power of single-cell analysis and rather than focusing on discrete cells or mediators, researchers have identified “modules” of proinflammatory cells that are bound by transcriptional functional programs. For instance, Martin et al36 described a module comprising inflammatory macrophages, activated dendritic cells, T-cells, and stromal cells with highly correlated transcriptional profiles occupying the inflamed ileum affected by CD, which they termed the GIMAT module (IgG plasma cells, inflammatory mononuclear phagocytes, and activated T- and stromal cells). In UC, such a complex modular inflammatory network is also present. By using receptor-ligand pair analyses to construct cell-cell interaction network, Smillie and colleagues35 showed that the most dominant modules in UC are also focused around inflammatory macrophages and stromal cell populations. These studies extend previous findings using more classical approaches to show the accumulation of phenotypically distinct monocytes/macrophages in both CD and UC.37-39
Second, inflammatory macrophages and stromal cells that dominate the IBD mucosal milieu have high expressions of the proinflammatory cytokines oncostatin M (OSM), IL-23, IL-6, IL-1β, IL-1α, and TNF.36 In particular, stromal cells appear to form part of a positive feedback loop to maintain an inflammatory environment through the expression of monocyte [chemokine (C-C motif) ligand] CCL2/CCL7 and neutrophil [chemokine (C-X-C motif) ligand] CXCL2, CXCL3, and CXCL8 chemoattractants. Earlier genomic-expression analysis showed significant enrichment of IBD-associated genetic variants to be associated with these immune processes, and in particular the function of macrophages.40-42 These included GPR65, an IBD-risk gene shown to inhibit proinflammatory cytokine production in macrophages; 43GBP5, which promotes NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome activation; 44 and MAFB, a transcription factor controlling macrophage differentiation and self-renewal.45
Third, 2 scRNA studies consistently showed that enrichment of such cell types or their mediator profile (high GIMAT and OSM-OSM receptor expressions, respectively) are associated with resistance to anti-TNF treatment in UC and CD.33, 36 In essence, such a complex modular system underlies the treatment-resistant inflammatory gut landscape. The role for OSM was shown in an earlier study by West et al46 showing that high stromal OSM expression predicted anti-TNF failure in UC. In an independent cohort of 441 patients, the GIMAT module was also associated with anti-TNF nonresponse. Taken together, these studies demonstrate that dysregulated macrophage and stromal cell activity are prominent features of the IBD mucosal milieu and represent a roadblock to complete mucosal healing and restoration of homeostasis.
PROMOTING RESOLUTION OF INFLAMMATION AND REPAIR AS A THERAPEUTIC CONCEPT
The challenge is to develop effective strategies that will first disrupt the self-perpetuating environment that sustains immune cell activation and initiate and then accelerate the process of inflammation resolution to achieve complete mucosal healing (Box 1). The concept of proactive reparative immunology has been covered recently.47 Here we align some of these ideas with IBD-specific pathogenic factors and discuss the translational opportunities that can be developed in conjunction with this goal.
BOX 1. RESOLUTION OF INFLAMMATION AS A THERAPEUTIC CONCEPT.
-
Disrupt the mucosal environment that sustains the activation of innate immune cells:
Interrupt key signaling network in inflammatory modules in the IBD mucosal milieu.
Manipulate inflammatory and/or epigenetic cues that shape the differentiation of inflammatory monocytes/macrophages—for example, metabolism, hypoxia, reactive oxygen species, short-chain fatty acids.
Block the signaling and recruitment of key inflammatory cells to the inflamed gut, such as proinflammatory monocyte-derived macrophages.
-
Initiate and accelerate the process of inflammation resolution:
Promote neutrophil apoptosis such as cyclin-dependent kinase (CDK)-inhibitor drugs.
Promote macrophage efferocytosis and its proresolution macrophage phenotype.
Directly harness proresolution macrophage products such as resolvins, protectins, and maresins.
-
Promote a state that allows deep repair and complete mucosal healing:
Encourage epithelial repair and regeneration such as IL-22.
Tackle the inflammatory signals from the stroma that mediate resistance to current biologic therapy in IBD such as OSM and CCL19.
Neutrophils in the Resolution of Inflammation
Neutrophils constitute 60% to 70% of circulating leukocytes in human blood. These short-lived “first responder” cells are recruited in abundance in IBD mucosa (particularly in UC) and are equipped with an arsenal of proteases and oxidants to execute host defense duties during the onset of inflammation.48, 49 Although much prevailing data are focused on the role of neutrophils in acute inflammation, they have an increasingly recognized contribution to chronic inflammation.50 Neutrophils deposit a trail of granule proteins such as α-defensins and CXCL12 that recruit monocytes to inflammatory sites; 51 cathelicidins that are present in neutrophils (LL-37 in humans; cathelicidin-related antimicrobial peptide (CRAMP) in mice) and promote the adhesion of monocytes via formylated peptide receptor 2 (FPR2)52; and neutrophil alarmins such as S100a8/9, mitochondrial DNA, and high-mobility group box-1 that all enhance the inflammatory function of macrophages.53-55 The IBD mucosa is associated with prolonged neutrophil survival56 and possibly proinflammatory neutrophil cell death such as (neutrophil extracellular trap) NETosis in UC.57, 58 However, in CD, a defect in acute inflammation has been suggested.59 It is purported that neutrophils fail to migrate to the inflammatory site, resulting in impaired bacterial clearance, which then sustains a chronic inflammatory response.
The mechanism of neutrophil death is important to the resolution process.60 Neutrophil death via apoptosis, a process of programmed cell death, prevents the release of its toxic contents and is the first step to turning off inflammation. Apoptotic neutrophils are taken up by macrophages (efferocytosis), initiating a feed-forward proresolution program that is characterized by the release of tissue-repairing cytokines, such as transforming growth factor-β (TGFβ) and IL-10, that counteract proinflammatory pathways.27, 28 Uptake of apoptotic neutrophils by macrophages also suppresses the transcription of Il23, which encodes for the IL-23 protein, a key IBD proinflammatory cytokine.61 This central tenet provides the platform for therapeutic intervention. Neutrophil apoptosis can be induced pharmacologically (eg, using cyclin-dependent kinase inhibitors such as R-roscovitine, tanshinone IIA, and ectoine).62-65 Of interest, tanshinone IIA, a Chinese medicinal herb identified from a large compound screen, was shown to potently stimulate egress of neutrophils from sites of inflammation in a zebrafish injury model.64 Anti-oxidants, such as N-acetylcysteine, also promote apoptotic cell clearance by inhibiting Ras homolog family member A (RhoA) and reactive oxygen species production.66 These drugs have the therapeutic potential to accelerate resolution of inflammation.67
Whereas neutrophils drive inflammation at one end of the spectrum, they are also important in tissue repair, and specific aspects of this built-in biological response can be exploited.68 Of the approximately 300 proteins contained within neutrophils, some have properties that are important in repair.69 One such protein, annexin A1 (ANXA1), is released by dying neutrophils, where it interacts with FPR2 to attenuate chemokine-triggered activation of integrins, thereby reducing further inflammatory cell recruitment.70 The ANXA1/FPR2 interaction also promotes macrophage efferocytosis.71, 72 Lipoxin A4, a proresolving lipid mediator released by the neutrophils, is a major “stop signal” for neutrophil migration.73 Of interest, the production of lipoxin A4 is reduced in IBD,74 and its administration is beneficial in a hapten-induced mouse colitis model.75 Αlpha-defensins are also released from neutrophils, with a functional effect of increasing the phagocytic capacity of macrophages and dampening their release of inflammatory mediators.76 These proresolution angles coupled with approaches to inhibit neutrophil-mediated chronic inflammatory functions (eg, inhibiting the neutrophil alarmins and NETosis) offer a rich ream of targets for IBD treatment.
Inflammatory Monocyte Recruitment
In health, intestinal macrophages are relatively anti-inflammatory and hyporesponsive to microbial stimuli, an adaptation that allows them to exist in an antigen- and microbe-rich environment. They are vital for the maintenance of intestinal homeostasis through the removal of apoptotic and senescent cells and the production of regulatory cytokines that also limit collateral damage associated with excessive inflammation.77, 78 Like macrophages in other tissues, those in the gut wall are epigenetically shaped by local environmental cues.77, 79 In IBD there are marked changes to the macrophage compartment resulting from increased immigration of classical (CD14hi) monocytes, leading to the accumulation of proinflammatory CD11chi monocytes/macrophages in the inflamed colon.37, 38, 80, 81 In CD, paired blood and gut scRNAseq analysis showed that increased gut inflammatory macrophages were associated with a depletion of circulating monocytes in patients enriched with the GIMAT module.36
Targeting leukocyte—and specifically monocyte—recruitment has been widely considered in IBD.82 Research has reported that CCR2 is the essential chemokine receptor that mediates the entry of monocytes into the circulation and subsequent recruitment into the site of the inflamed gut.83, 84 Genetic ablation and antibody-mediated blockade of CCR2 is protective against mouse experimental colitis.84-86 Potential therapies could inhibit the recruitment of proinflammatory monocytes and their inflammatory products, but a few complexities are evident. For instance, it is not clear whether monocyte blockade would have a collateral effect on the maintenance of the resident macrophage pool, which also relies on CCR2-dependent monocytes, or if distinct monocyte subsets are recruited to healthy vs inflamed/repairing tissue. Moreover, the fate of monocytes in the IBD mucosa remains poorly understood, and it is plausible that although initially proinflammatory, monocytes may be conditioned by the local gut environment to become proresolving macrophages over time. In this scenario, monocyte blockade may prove counterproductive.
Anti-α4β7 integrin vedolizumab, a current IBD biologic treatment, is thought to exert its effect by inhibiting the adhesion of α4β7-expressing lymphocytes to gut mucosal vascular addressin cell adhesion molecule 1.87, 88 A recent study showed that vedolizumab also blocks gut homing for α4β7-expressing monocytes.89 Of particular interest, vedolizumab was shown to affect the recruitment of nonclassical (CD14+CD16++) monocytes, which preferentially develop into wound-healing macrophages. Vedolizumab resulted in poor gut wound healing in vivo,89 which has a potential clinical impact, such as healing following IBD surgery.90 Moreover, a recent study examining monocyte heterogeneity in mice described the presence of prorepair monocytes during the recovery phase of dextran sulfate sodium (DSS)–induced colitis that were marked by their high expression of Ym-1.91 In a further study, CCR2-deletion in a mouse model of surgery delayed recovery from inflammation and postoperative ileus,92 again supporting a proresolution role for these cells. Hence, targeting monocytes may be more complicated than first thought. Cellular lineage tracing in experimental models may provide further information regarding monocyte heterogeneity and temporal recruitment mechanism(s). Timing of blocking monocyte recruitment is potentially critical in developing this approach in the clinic.
Macrophage Efferocytosis of Apoptotic Cells
Macrophages are incredibly plastic and adapt in response to signals received from their immediate microenvironment. This biological feature provides an angle for potential intervention.93 Although historically considered as either proinflammatory or anti-inflammatory, macrophages also exhibit prowound healing and antifibrotic, proresolving, and tissue-regenerating characteristics.29 A key event in the programming of macrophages begins following the uptake of apoptotic cells, which reduces the expression of proinflammatory cytokines and chemokines from macrophages94 while promoting production of the immunoregulatory cytokines TGF-β and IL-10.27, 28 Macrophage efferocytosis also results in activation of peroxisome proliferator-activated receptor-γ and liver X-receptor, which enhance the expression of the phagocytic receptors CD36 and Mer proto-oncogene tyrosine kinase,95, 96 further enhancing the potential to clear dying cells. Moreover, major transcriptomic changes occur in intestinal macrophages following the uptake of apoptotic intestinal epithelial cells, including the downregulation of inflammatory genes (several of these are IBD susceptibility genes: Lsp1, Mrpl20, S100a10/11, and Sept1), pattern recognition receptors (Clec4a, Clec4b1, Cd209a, and Tlr2), and inflammatory leukotriene biosynthesis (Alox5ap).97 Such data provide the framework to understand how macrophages are programmed toward a specific beneficial phenotype.
Trained Macrophage Immunity
Immunological memory is thought to be a defining feature of the adaptive immune system. However, recent work has shown that myeloid cells of the innate immune system may be able to “remember” the stimuli they encounter by undergoing functional, metabolic, and epigenetic reprogramming, which facilitates an altered response upon restimulation—a phenomenon that has become known as trained immunity.98 This response further opens an additional translational avenue that involves suppressing or enhancing the trained proinflammatory and prorepair macrophage functions, respectively.99 Exploring the factors that shape monocyte-macrophage function in the gut, such as the epigenetic modifications that monocytes undergo as they are recruited into the IBD gut, offers tractable targets.100-102 These targets include manipulating the environmental cues, tissue factors, or epigenetic signals that confer their inflammatory properties. The roles of immune metabolism,103 tissue hypoxia,104 presence of extravasated pathogen-associated molecular patterns (PAMPs), and damage-associated molecular patterns (DAMPs; bacterial lipopolysaccharide, mitochondrial DNA, and reactive oxygen species [ROS])105, 106 are also tractable targets for interventions to disrupt the proinflammatory features of monocytes and their progeny. Exposure to butyrate, a short-chain fatty acid produced by gut bacteria, during monocyte-macrophage differentiation enhances monocyte antimicrobial activity via the histone-deacetylase-3 epigenetic regulation of S100a8/9.107 As discussed herein, such potential translation will require further understanding of the macrophage populations that persist in the inflamed gut during the evolution of IBD.108
Macrophage-Specific Therapeutics
In defining specific molecular targets for monocytes or macrophages, the next challenge is to find ways to deliver therapeutics to enable this objective. Parallel developments in oncology, where there is a need to target tumor-associated macrophages (TAMs), in a similarly complex tumor microenvironment, provide some insights into how this might work in IBD.109 For example, macrophage-targeting nanoparticles (liposomes/PEGylated nanoparticles/folate receptor targeting agents) could be synthesized to deliver specific pathway inhibitors or agonists to promote the differentiation of inflammatory myeloid cells into macrophages with a proresolving phenotype.110-113 Nanoparticles are emerging as key translational moieties in targeting TAMs. For examples in cancer, immunonanomedicines target TAMs primarily by blocking specific TAM survival or affecting their signaling cascades, restricting their recruitment to tumors, and re-educating tumor-promoting TAMs to the tumoricidal phenotype.114 In another development, nanobiologics exploit high-density lipoprotein properties to deliver specific therapeutics.115 These areas remain in early stages of translation but are likely to become one of the next important steps to precision medicine in IBD.
Products of the Proresolving Macrophage as IBD Treatment
Downstream from this secreted products of the proresolving macrophage are closer to translation. Proresolving macrophages secrete resolvins, protectins, and maresins, long-chain fatty acid mediators (secreted proresolving mediators [SPMs]) shown to promote resolution of tissue injury in a wide variety of pathologies.116 The SPMs are a large class of signaling molecules produced by macrophages through the metabolism of ω-3 polyunsaturated fatty acids. The specific SPMs protectin; resolvin D1, D2, E1; and maresins have been shown to attenuate mouse colitis.117-120 They act by blocking neutrophil recruitment and mediating the phagocytosis and clearance of apoptotic neutrophils by macrophages. It is noteworthy that these resolving bioactive lipids are synthesized from ω-6 and ω-3 polyunsaturated fatty acids (PUFAs). High dietary ω-6 PUFA with proinflammatory potential is associated with increased risk of UC,121 but ω-3 PUFA has proresolution properties.122 However, ω-3 PUFA dietary treatment has not been shown to be effective in IBD,123–125 most likely reflecting the complex and context-specific nature of integrating metabolites and immunity.
Macrophages also produce a variety of growth factors, such as insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF)-α, TGF-β, and Wnt proteins that regulate epithelial and endothelial cell proliferation, myofibroblast activation, stem and tissue progenitor cell differentiation, and angiogenesis.29 A cocktail of macrophage products (termed SuperMAPO) obtained through a culture of macrophages with apoptotic thymic cells has been shown to be effective in ameliorating mouse models of arthritis and IBD.126 In a further study, Yoon et al127 showed that instillation of apoptotic leukocytes can also induce the production of proresolving cytokines in an acute pulmonary inflammation model, although whether this cytokine production relies on local tissue macrophage activity remains unclear.
Inflammatory Stroma and Activated Endothelium
The advent of single-cell technologies has led to a much deeper understanding of the role of the intestinal stroma in intestinal inflammation. Indeed, UC is characterized by major changes in the composition and inflammatory status of the stromal compartment,33 which is becoming recognized as an amplifier and driver of disease chronicity in IBD. Many potential specific targets in the IBD stroma recently, for example, endothelial atypical chemokine receptor 1 (ACKR1), stromal OSM,36, 46 and CCL19/CCL21.33 These appear to be key factors that maintain the IBD milieu and resistance to anti-TNF treatment. West et al46 showed that in a mouse model of anti-TNF-resistant IBD, mice lacking OSM developed significantly less disease than their wild-type counterparts, with reduced colonic chemokine and cytokine production accompanied by attenuated signs of inflammation. This was shown especially in the late disease course and could be recapitulated by OSM neutralization. In previous research, OSM has been targeted for rheumatoid arthritis in phase 1 and 2 clinical trials using a humanized anti-OSM monoclonal antibody, but little clinical efficacy was seen for this chronic inflammatory condition.128 Regardless, OSM remains a good potential therapeutic target and is a proven biomarker for anti-TNFα treatment failure.
Epithelial Repair
A healthy intestinal mucosa is maintained by (1) a secreted barrier, a generous mucus layer laced with antimicrobial peptides, and (2) a physical one, comprising epithelial cells with tight-junctions and innate immune receptors such as TLR2 and TLR5 that can initiate a response when the barrier is breached.129 Intestinal epithelial cell regeneration and differentiation occur at the intestinal stem cells located at the crypt base driven by 4 important signaling molecules: Wnt, Notch, bone morphogenic proteins, and hedgehog.130 Given that barrier dysfunction precedes the development of clinical IBD131, 132 and that incomplete mucosal healing is associated with a high risk of relapse, treatments that are focused on epithelial repair are important components of a proresolution treatment approach.133
The mechanisms of epithelial repair can be divided into 3 phases. The first phase is epithelial restitution, where epithelial cells lose their columnar polarity and migrate to the site of the injury to rapidly seal the defective barrier.134 Note that TGF-α/β, trefoil factors, and galectin-2 and -4 are vital for this process.135, 136 Second, epithelial cells receive signals, such as cytokines, growth factors, and bacterial products that lead to the induction of transcription factors, such as signal transducer and activator of transcription (STAT)-3 and -5 and nuclear factor-kappa B (NF-κB) that promote epithelial homeostasis.133, 137 Finally, the new epithelial cells follow a well-defined differentiation process into mature intestinal epithelial cells (IECs) of either the absorptive or secretory lineage.138
The concept of epithelial repair in IBD is not new and has been countenanced upon for more than 20 years.139 Such therapies have included trefoil factors,140 neutrophil-borne defensins and cathelicidins,141 ANXA-1 and its mimetic peptide Ac2-26,142 lipoxins,143 EGF,144 fish oil,145 and probiotics.146 Despite positive EGF clinical trial data in UC,144 further clinical development has been stymied by the fear of overstimulation of epithelial proliferation and subsequent colorectal cancer development.147 Recent work in an scRNAseq study focusing on the colonic IECs in UC has uncovered novel leads, such as WAP four-disulfide core domain 2 (WFDC2),34 an anti-protease molecule that inhibits bacterial growth and is involved in the repositioning of goblet cells in UC. Further work from Parikh et al34 showed that WAP four-disulfide core domain 2 is an important goblet cell–secreted antibacterial factor that is required to prevent colonization and invasion during epithelial barrier breakdown.
The identification of IL-22 (of the IL-10 family) as a prorepair cytokine has generated significant interest. In 2008, Sugimoto et al148 showed that IL-22 could ameliorate intestinal inflammation. Further studies have shown that IL-22 activates the epithelial STAT-3 pathway to promote intestinal stem cell, antimicrobial peptide, and mucin production and the subsequent expansion and survival of the epithelial cells.149, 150 In addition, IL-22 has a major protective role in intestinal graft-vs-host disease.151 Some of the beneficial effects of anti-TNFα have been ascribed to increased IL-22 production.152 However, there is a fine balance to maintain because IL-22 is potentially a key factor in colon cancer153 and has been shown to promote colitis in some settings.154 Upregulating IL-22 function can be achieved by using the endogenous inhibitor IL-22BP, directly via IL-22-Fc fusion protein UTR1147A or by using a ligand-based approach to activate the Aryl hydrocarbon receptor (AhR)-IL-22 pathway. A clinical trial using the traditional herbal remedy indigo naturalis to activate the AhR-IL-22 pathway has been shown to induce a clinical response in active UC, but this is limited by potential adverse effects, such as liver and lung toxicity.155 Other cytokines including TGFβ and IL-28 also have roles in mediating epithelial repair. For example, IL-28 induces IEC proliferation and promotes wound healing via STAT1 signaling.156 Inhibition of Smad7, a blocker of the TGFβ receptor, via antisense oligonucleotides showed initially positive results in CD, but its clinical development was terminated because of lack of efficacy.157, 158
The rationale for epithelial repair/regeneration treatment is strong, but its place within the IBD treatment armamentarium requires further thought. As this area continues to grow, the practical place for such an approach may require stratification involving patients with severe IBD with extensive gut tissue damage, ideally with a more specific drug delivery mechanism that avoids systemic exposure and in a time-limited fashion.
CONCLUDING REMARKS
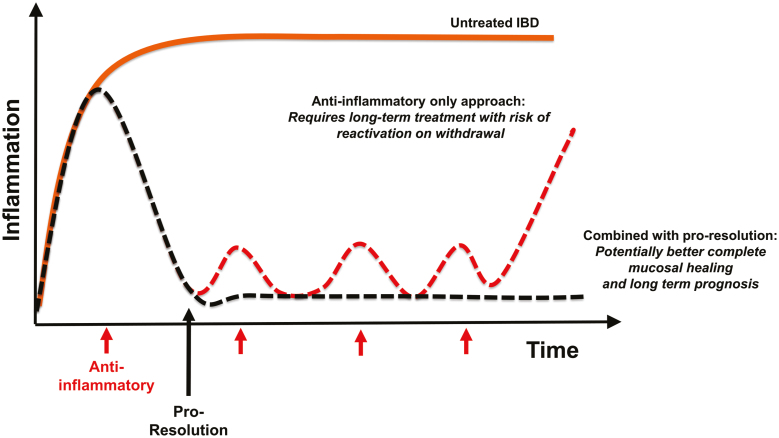
The therapeutic ceiling of current IBD treatments indicates strongly that a combined approach targeting anti-inflammatory, proresolution, and repair processes is necessary (Fig. 3). This mature field of inflammation resolution offers many potential therapeutic targets (Table 1), yet it is pertinent to note that there are surprisingly no current bona fide proresolution or repair treatments available to patients with IBD. The tangible route to clinical translation poses several challenges (Box 2).
FIGURE 3.
Therapeutic positioning of proresolution/repair approaches in IBD. Combined anti-inflammatory and proresolution therapy potential to significantly improve beyond current rates of complete mucosal healing in IBD.
TABLE 1.
Proresolution and Repair Targets in IBD
| IBD Inflammation Resolution and Repair Targets* | ||||
|---|---|---|---|---|
| Target Category/Method | Specific Target | Condition/Model | Species Studied (in vivo unless stated) | Reference |
| Targeting immune cell recruitment | ||||
| Block or ↓production of monocyte and neutrophil chemo-attractants | Theoretical (CCL2/CCL7) (CXCL2, CXCL3, and CXCL8) | Targets identified but not evaluated in IBD | n/a | 36 |
| ↓Proinflammatory monocyte-derived macrophages | CCR2-blocking | DSS colitis | Mouse | 84-86 |
| Resolvins, protectins, and maresins | See use of proresolving macrophage products below. | |||
| Lipoxin A4 | Hapten-induced colitis | Mouse | 75 | |
| Annexin A1 | Annexin A1 | Not evaluated in IBD | n/a | 72 |
| ↓Inflammatory signals from the stroma and endothelium | Endothelial AKTB1, stromal OSM CCL19/CCL21 | Targets identified in scRNA in people with IBD | Human | 33, 36, 46 |
| Stromal cytokine OSM genetic deletion or blockade | IBD mouse model; Helicobacter hepaticus infection and systemic IL-10 receptor blockade | Mouse, wild-type, and OSM-/- | 46 | |
| OSM targeting with anti-OSM monoclonal antibody | Patients with rheumatoid arthritis; not evaluated in IBD | Human | 128 | |
| Clearance of inflammatory cells | ||||
| ↑Granulocyte/neutrophil death | CDKI | Several inflammatory mouse models (not IBD) | Mouse | 62, 63 |
| N-acetylcysteine, inhibiting RHOA, and reactive oxygen species production | Xenobiotic-induced lung inflammation; not evaluated in IBD | Human in vitro; rat in vivo | 65 | |
| ↑Granulocyte/neutrophil egress | CDKI | Tail fin resection | Zebrafish | 64 |
| ↑Efferocytosis and other effete inflammatory cells | Apoptotic neutrophil cell therapy | Acute pulmonary inflammation model; not evaluated in IBD | Mouse | 127 |
| Apoptotic neutrophil products, annexin A1 | Not evaluated in IBD | Human in vitro | 71 | |
| Apoptotic neutrophil products, α-defensins | Thioglychollate model of peritonitis; not evaluated in IBD | Human in vitro; mouse | 76 | |
| Promoting proresolving immune cells | ||||
| ↑Monocytes; wound healing macrophages | Increasing adhesion of α4β7-expressing monocytes; nonclassical (CD14+CD16++) monocytes | Findings from vedolizumab-treated IBD patients; blocking in mice with intestinal surgery | Human; mouse | 89, 90 |
| Increasing prorepair monocytes | Theoretical; prorepair cells identified in DSS-induced colitis | Mouse | 91 | |
| Increasing CCR2-dependent monocyte-derived macrophages† | Blocking delayed recovery from postoperative ileus in mouse | Mouse | 92 | |
| Modulating macrophage function to proresolving | ||||
| ↑Efferocytosis | CDKI | Pneumonia model; not evaluated in IBD | Mouse | 62 |
| Change immune metabolism | Block succinate dehydrogenase | Not evaluated in IBD | n/a Mouse in vitro; | 103 |
| Butyrate | Not evaluated in IBD | In vitro monocyte- macrophage differentiation | 107 | |
| Macrophage-targeting nanoparticles | Liposomes/PEGylated nanoparticles/folate receptor targeting agents | Evidence from targeting TAMs; not evaluated in IBD | n/a | 110 |
| ↓Macrophage inflammatory signature | Macrophage uptake of apoptotic intestinal epithelial cells | DSS colitis | Mouse | 97 |
| Exploiting pro-resolving macrophage products | ||||
| Delivery of resolvins, protectins, and maresins (SPMs) | Resolvin E1 | DSS colitis | Mouse | 117 |
| Resolvin D1 and Resolvin D2 | DSS colitis | Mouse | 118 | |
| Maresin 1 | DSS- and 2,4,6-trinitrobenzene sulfonic acid–induced colitis | Mouse | 119, 159 | |
| Lipoxin A4 | Lipoxin A4 | Human IBD expression data; hapten-induced colitis | Human Mouse | 74, 75 |
| Macrophage (SuperMAPO) products | Products from macrophages after culture with apoptotic cells | Arthritis model; DSS colitis | Mouse | 126 |
| Accelerating epithelial cell growth/regeneration | ||||
| IL-22 | IL-22 and STAT3 | Human IBD expression data; DSS colitis | Human and mouse in vitro; mouse in vivo | 149-151 |
| Activate AhR-IL-22 pathway | Multicenter, double-blind trial, human UC | Human | 155 | |
| TNFα | Anti-TNFα Increased IL-22 | CD patients receiving anti-TNF monoclonal antibody | Human | 152 |
| IL-28 | IL-28 via STAT1 signaling | Patients with IBD; DSS colitis | Human; mouse | 156 |
*Although presented in isolation based on published findings, the targets listed below should be considered as potentially interconnected.
†CCR2 deletion has both potential anti-inflammatory effects and causes delayed wound healing; the monocyte-derived macrophage department is very complex.
n/a, not available.
BOX 2. CHALLENGES IN TRANSLATING PRORESOLUTION AND REPAIR IN IBD.
-
Where to position in clinical trials in IBD?
These treatments are likely to play an adjunctive role to current treatments that inhibit the proinflammatory mucosal response (eg, anti-TNFα). Treatment duration should be short-term and focused on patients with active IBD at an early stage. Study endpoints like mucosal healing rather than clinical response are likely to be more informative.
-
Is there a dominant proresolution or repair mechanism to target?
It is unclear if there is a hierarchy of importance and whether there are likely to be UC- or CD-specific therapies. Some such as neutrophil-targeted approaches may be better for UC whereas stratification according to biological response, such IL-22 levels, may guide therapeutic decision-making.
-
Do we have tools to accurately to monitor whether complete mucosal healing has been achieved?
More comprehensive ways to assess mucosal healing (combined radiological, histologic, and endoscopic methods and biomarkers such as C-reactive protein and S100a8/9) are necessary. More specific mechanistic biomarkers may be needed depending on the type of treatment, such as cytokine-based therapies.
-
How to deliver proresolution and repair treatments in the clinical setting?
Gut mucosal drug delivery systems (such as 5-aminosalicylic acid/mesalazine) are necessary to ensure that adequate levels of proresolution/repair drugs reach the inflamed mucosa. Their effects may be different in inflamed vs normal tissue states. More cell-specific delivery methods (eg, macrophage nanobiologics) as discussed are areas for further studies.
-
What are the potential unintended consequences of proresolution and repair treatments?
Neoplasia and fibrotic complications may be factors to consider. A potential mitigation step is to ensure that these therapies are time-limited to the active and early phase of IBD (eg, at time of diagnosis) and that drugs are delivered to inflamed rather than normal tissue.
One key step for therapeutic progression is to generate the willingness to develop high-quality clinical trials to test the efficacy of potential proresolution/repair treatments. Such studies should be adjunctive in nature: for example, can they improve complete mucosal healing in conjunction with current biologic therapy? Some prior considerations are necessary. First, current trial endpoints of response that are heavily based on clinical activity (eg, the CD Activity Index and the Mayo Score for CD and UC, respectively) may not reflect the true efficacy of proresolution/repair treatments. Here, objective read-outs of mucosal and histological inflammation and healing may be more instructive. Second, useful molecular tools or systems to track mucosal healing in a dynamic manner in IBD are lacking. Measurements of S100a8/9 are increasingly adopted in IBD clinical trials but are less useful in CD. Third, novel delivery systems with specificity for the inflamed gut only—or better, to the implicated cell type—are fundamental. This specificity would reduce concern over potential proresolution/repair treatment effects on normal tissue, particularly the risk of neoplasia.
Although the direction of translation is clear, many of the concepts of resolution and repair are derived from acute inflammatory or injury models and conditions. These findings may not be generalizable to an immune-mediated condition like IBD where the gut mucosa is in close apposition with the complex luminal environment. New studies, such as the important scRNAseq studies, continue to reveal further complexities in both UC and CD, raising the prospect of disease-specific proresolution treatment. It is clear that there is some ground to cover with regard to understanding IBD-specific inflammation resolution; nevertheless, there is enormous potential to develop new simple treatments that harness the resolution and repair process. In conjunction with current available treatments, there is a real possibility to break the current therapeutic ceiling, to finally facilitate universal complete mucosal healing for all patients with IBD.
Supported by: GTH is supported by the Leona M. and Harry B. Helmsley Charitable Trust, the Jon Moulton Foundation, Crohn’s Colitis UK, Guts Charity UK, the Chief Scientist Office (CSO) Scotland, and the Medical Research Council (MRC). JAC is supported by the Wellcome Trust (Grant No. 108906/Z/15/Z). EJT is supported by a European Research Council Consolidator grant (771443). CCB holds a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant No. 206234/Z/17/Z). AGR is supported by a MRC Programme grant (MR/K013386/1) and the MRC SHIELD consortium (MRNO2995X/1).
Conflicts of interest: There are no conflicts of interests to declare from all authors.
REFERENCES
- 1. Netea MG, Balkwill F, Chonchol M, et al. . A guiding map for inflammation. Nat Immunol. 2017;18:826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. [DOI] [PubMed] [Google Scholar]
- 3. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. [DOI] [PubMed] [Google Scholar]
- 4. Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15:551–567. [DOI] [PubMed] [Google Scholar]
- 5. Buckley CD, Gilroy DW, Serhan CN, et al. . The resolution of inflammation. Nat Rev Immunol. 2013;13:59–66. [DOI] [PubMed] [Google Scholar]
- 6. Schirmer M, Garner A, Vlamakis H, et al. . Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 2019;17:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019;50:992–1006. [DOI] [PubMed] [Google Scholar]
- 8. Boyapati R, Satsangi J, Ho GT. Pathogenesis of Crohn’s disease. F1000prime Rep. 2015;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paramsothy S, Rosenstein AK, Mehandru S, et al. . The current state of the art for biological therapies and new small molecules in inflammatory bowel disease. Mucosal Immunol. 2018;11:1558–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Shea JJ, Schwartz DM, Villarino AV, et al. . The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sabino J, Verstockt B, Vermeire S, et al. . New biologics and small molecules in inflammatory bowel disease: an update. Therap Adv Gastroenterol. 2019;12:1756284819853208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Danese S, Sandborn WJ, Colombel JF, et al. . Endoscopic, radiologic, and histologic healing with vedolizumab in patients with active Crohn’s disease. Gastroenterology. 2019;157:1007–1018.e7. [DOI] [PubMed] [Google Scholar]
- 13. Colombel JF, Panaccione R, Bossuyt P, et al. . Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–2789. [DOI] [PubMed] [Google Scholar]
- 14. Sands BE, Peyrin-Biroulet L, Loftus EV Jr, et al. ; VARSITY Study Group Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019;381:1215–1226. [DOI] [PubMed] [Google Scholar]
- 15. Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 16. Panaccione R, Ghosh S, Middleton S, et al. . Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400.e3. [DOI] [PubMed] [Google Scholar]
- 17. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 18. Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6–19. [DOI] [PubMed] [Google Scholar]
- 19. Frøslie KF, Jahnsen J, Moum BA, et al. ; IBSEN Group Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. [DOI] [PubMed] [Google Scholar]
- 20. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. . Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut. 2014;63:88–95. [DOI] [PubMed] [Google Scholar]
- 21. Sands BE, Sandborn WJ, Panaccione R, et al. ; UNIFI Study Group Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 22. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 23. Abraham C, Dulai PS, Vermeire S, et al. . Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:374–388.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barton GM. A calculated response: control of inflammation by the innate immune system. J Clin Invest. 2008;118:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. [DOI] [PubMed] [Google Scholar]
- 26. Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17:1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watanabe S, Alexander M, Misharin AV, et al. . The role of macrophages in the resolution of inflammation. J Clin Invest. 2019;129:2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. [DOI] [PubMed] [Google Scholar]
- 29. Vannella KM, Wynn TA. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol. 2017;79:593–617. [DOI] [PubMed] [Google Scholar]
- 30. Plichta DR, Graham DB, Subramanian S, et al. . Therapeutic opportunities in inflammatory bowel disease: mechanistic dissection of host-microbiome relationships. Cell. 2019;178:1041–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. ; IBDMDB Investigators Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kinchen J, Chen HH, Parikh K, et al. . Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018;175:372– 386.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parikh K, Antanaviciute A, Fawkner-Corbett D, et al. . Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature. 2019;567:49–55. [DOI] [PubMed] [Google Scholar]
- 35. Smillie CS, Biton M, Ordovas-Montanes J, et al. . Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178:714–730.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin JC, Chang C, Boschetti G, et al. . Single-cell analysis of Crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell. 2019;178:1493–1508.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamada N, Hisamatsu T, Okamoto S, et al. . Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bain CC, Scott CL, Uronen-Hansson H, et al. . Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thiesen S, Janciauskiene S, Uronen-Hansson H, et al. . CD14(hi)HLA-DR(dim) macrophages, with a resemblance to classical blood monocytes, dominate inflamed mucosa in Crohn’s disease. J Leukoc Biol. 2014;95:531–541. [DOI] [PubMed] [Google Scholar]
- 40. Peters LA, Perrigoue J, Mortha A, et al. . A functional genomics predictive network model identifies regulators of inflammatory bowel disease. Nat Genet. 2017;49:1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jostins L, Ripke S, Weersma RK, et al. ; International IBD Genetics Consortium Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baillie JK, Arner E, Daub C, et al. ; FANTOM Consortium Analysis of the human monocyte-derived macrophage transcriptome and response to lipopolysaccharide provides new insights into genetic aetiology of inflammatory bowel disease. Plos Genet. 2017;13:e1006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Onozawa Y, Fujita Y, Kuwabara H, et al. . Activation of T cell death-associated gene 8 regulates the cytokine production of T cells and macrophages in vitro. Eur J Pharmacol. 2012;683:325–331. [DOI] [PubMed] [Google Scholar]
- 44. Shenoy AR, Wellington DA, Kumar P, et al. . GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. 2012;336:481–485. [DOI] [PubMed] [Google Scholar]
- 45. Soucie EL, Weng Z, Geirsdóttir L, et al. . Lineage-specific enhancers activate self-renewal genes in macrophages and embryonic stem cells. Science. 2016;351:aad5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. West NR, Hegazy AN, Owens BMJ, et al. ; Oxford IBD Cohort Investigators Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017;23:579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schett G, Neurath MF. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat Commun. 2018;9:3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Park S, Abdi T, Gentry M, et al. . Histological disease activity as a predictor of clinical relapse among patients with ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2016;111:1692–1701. [DOI] [PubMed] [Google Scholar]
- 49. Bressenot A, Geboes K, Vignaud JM, et al. . Microscopic features for initial diagnosis and disease activity evaluation in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1745–1752. [DOI] [PubMed] [Google Scholar]
- 50. Soehnlein O, Steffens S, Hidalgo A, et al. . Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol. 2017;17:248–261. [DOI] [PubMed] [Google Scholar]
- 51. Soehnlein O, Zernecke A, Eriksson EE, et al. . Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wantha S, Alard JE, Megens RT, et al. . Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ Res. 2013;112:792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Soehnlein O, Kai-Larsen Y, Frithiof R, et al. . Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages. J Clin Invest. 2008;118:3491–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lood C, Blanco LP, Purmalek MM, et al. . Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Venereau E, Casalgrandi M, Schiraldi M, et al. . Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. [DOI] [PubMed] [Google Scholar]
- 57. Dinallo V, Marafini I, Di Fusco D, et al. . Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohns Colitis. 2019;13:772–784. [DOI] [PubMed] [Google Scholar]
- 58. Angelidou I, Chrysanthopoulou A, Mitsios A, et al. . REDD1/autophagy pathway is associated with neutrophil-driven IL-1beta inflammatory response in active ulcerative colitis. J Immunol. 2018;200:3950–3961. [DOI] [PubMed] [Google Scholar]
- 59. Marks DJ, Harbord MW, MacAllister R, et al. . Defective acute inflammation in Crohn’s disease: a clinical investigation. Lancet. 2006;367:668–678. [DOI] [PubMed] [Google Scholar]
- 60. Wang J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018;371:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol. 2008;181:5183–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cartwright JA, Lucas CD, Rossi AG. Inflammation resolution and the induction of granulocyte apoptosis by cyclin-dependent kinase inhibitor drugs. Front Pharmacol. 2019;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rossi AG, Sawatzky DA, Walker A, et al. . Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–1064. [DOI] [PubMed] [Google Scholar]
- 64. Robertson AL, Holmes GR, Bojarczuk AN, et al. . A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Sci Transl Med. 2014;6:225ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sydlik U, Peuschel H, Paunel-Görgülü A, et al. . Recovery of neutrophil apoptosis by ectoine: a new strategy against lung inflammation. Eur Respir J. 2013;41:433–442. [DOI] [PubMed] [Google Scholar]
- 66. Moon C, Lee YJ, Park HJ, et al. . N-acetylcysteine inhibits RhoA and promotes apoptotic cell clearance during intense lung inflammation. Am J Respir Crit Care Med. 2010;181:374–387. [DOI] [PubMed] [Google Scholar]
- 67. Jones HR, Robb CT, Perretti M, et al. . The role of neutrophils in inflammation resolution. Semin Immunol. 2016;28:137–145. [DOI] [PubMed] [Google Scholar]
- 68. Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest. 2019;129:2629–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dalli J, Montero-Melendez T, Norling LV, et al. . Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol Cell Proteomics. 2013;12:2205–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Drechsler M, de Jong R, Rossaint J, et al. . Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ Res. 2015;116:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Scannell M, Flanagan MB, deStefani A, et al. . Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol. 2007;178:4595–4605. [DOI] [PubMed] [Google Scholar]
- 72. Perretti M, Flower RJ. Annexin 1 and the biology of the neutrophil. J Leukoc Biol. 2004;76:25–29. [DOI] [PubMed] [Google Scholar]
- 73. Takano T, Clish CB, Gronert K, et al. . Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mangino MJ, Brounts L, Harms B, et al. . Lipoxin biosynthesis in inflammatory bowel disease. Prostaglandins Other Lipid Mediat. 2006;79:84–92. [DOI] [PubMed] [Google Scholar]
- 75. Fiorucci S, Wallace JL, Mencarelli A, et al. . A beta-oxidation-resistant lipoxin A4 analog treats hapten-induced colitis by attenuating inflammation and immune dysfunction. Proc Natl Acad Sci U S A. 2004;101:15736–15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Miles K, Clarke DJ, Lu W, et al. . Dying and necrotic neutrophils are anti-inflammatory secondary to the release of alpha-defensins. J Immunol. 2009;183:2122–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Smythies LE, Sellers M, Clements RH, et al. . Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Uderhardt S, Martins AJ, Tsang JS, et al. . Resident macrophages cloak tissue microlesions to prevent neutrophil-driven inflammatory damage. Cell. 2019;177:541–555.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mowat AM, Scott CL, Bain CC. Barrier-tissue macrophages: functional adaptation to environmental challenges. Nat Med. 2017;23:1258–1270. [DOI] [PubMed] [Google Scholar]
- 80. Ogino T, Nishimura J, Barman S, et al. . Increased Th17-inducing activity of CD14+ CD163 low myeloid cells in intestinal lamina propria of patients with Crohn’s disease. Gastroenterology. 2013;145:1380–91.e1. [DOI] [PubMed] [Google Scholar]
- 81. Bernardo D, Marin AC, Fernández-Tomé S, et al. . Human intestinal pro-inflammatory CD11c(high)CCR2(+)CX3CR1(+) macrophages, but not their tolerogenic CD11c(-)CCR2(-)CX3CR1(-) counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol. 2018;11:1114–1126. [DOI] [PubMed] [Google Scholar]
- 82. Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. 2019;20:970–979. [DOI] [PubMed] [Google Scholar]
- 83. Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. [DOI] [PubMed] [Google Scholar]
- 84. Zigmond E, Varol C, Farache J, et al. . Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. [DOI] [PubMed] [Google Scholar]
- 85. Waddell A, Ahrens R, Steinbrecher K, et al. . Colonic eosinophilic inflammation in experimental colitis is mediated by Ly6C(high) CCR2(+) inflammatory monocyte/macrophage-derived CCL11. J Immunol. 2011;186:5993–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Platt AM, Bain CC, Bordon Y, et al. . An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J Immunol. 2010;184:6843–6854. [DOI] [PubMed] [Google Scholar]
- 87. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. [DOI] [PubMed] [Google Scholar]
- 88. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 89. Schleier L, Wiendl M, Heidbreder K, et al. . Non-classical monocyte homing to the gut via α4β7 integrin mediates macrophage-dependent intestinal wound healing. Gut. 2020;69:252–263. [DOI] [PubMed] [Google Scholar]
- 90. Lightner AL, Mathis KL, Tse CS, et al. . Postoperative outcomes in vedolizumab-treated patients undergoing major abdominal operations for inflammatory bowel disease: retrospective multicenter cohort study. Inflamm Bowel Dis. 2018;24:871–876. [DOI] [PubMed] [Google Scholar]
- 91. Ikeda N, Asano K, Kikuchi K, et al. . Emergence of immunoregulatory Ym1(+)Ly6C(hi) monocytes during recovery phase of tissue injury. Sci Immunol. 2018;3. doi: 10.1126/sciimmunol.aat0207. [DOI] [PubMed] [Google Scholar]
- 92. Farro G, Stakenborg M, Gomez-Pinilla PJ, et al. . CCR2-dependent monocyte-derived macrophages resolve inflammation and restore gut motility in postoperative ileus. Gut. 2017;66:2098–2109. [DOI] [PubMed] [Google Scholar]
- 93. Poon IK, Lucas CD, Rossi AG, et al. . Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. A-Gonzalez N, Quintana JA, García-Silva S, et al. . Phagocytosis imprints heterogeneity in tissue-resident macrophages. J Exp Med. 2017;214:1281–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. A-Gonzalez N, Bensinger SJ, Hong C, et al. . Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mukundan L, Odegaard JI, Morel CR, et al. . PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009;15:1266–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cummings RJ, Barbet G, Bongers G, et al. . Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature. 2016;539:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Netea MG, Joosten LA, Latz E, et al. . Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mulder WJM, Ochando J, Joosten LAB, et al. . Therapeutic targeting of trained immunity. Nat Rev Drug Discov. 2019;18:553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lavin Y, Mortha A, Rahman A, et al. . Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15:731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol. 2016;17:18–25. [DOI] [PubMed] [Google Scholar]
- 102. Wu F, Zikusoka M, Trindade A, et al. . MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635.e24. [DOI] [PubMed] [Google Scholar]
- 103. Mills EL, Kelly B, Logan A, et al. . Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–470.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17:774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Boyapati RK, Dorward DA, Tamborska A, et al. . Mitochondrial DNA is a pro-inflammatory damage-associated molecular pattern released during active IBD. Inflamm Bowel Dis. 2018;24:2113–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Boyapati RK, Rossi AG, Satsangi J, et al. . Gut mucosal DAMPs in IBD: from mechanisms to therapeutic implications. Mucosal Immunol. 2016;9:567–582. [DOI] [PubMed] [Google Scholar]
- 107. Schulthess J, Pandey S, Capitani M, et al. . The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50:432–445.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Placek K, Schultze JL, Aschenbrenner AC. Epigenetic reprogramming of immune cells in injury, repair, and resolution. J Clin Invest. 2019;129:2994–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mantovani A, Marchesi F, Malesci A, et al. . Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Singh Y, Pawar VK, Meher JG, et al. . Targeting tumor associated macrophages (TAMs) via nanocarriers. J Control Release. 2017;254:92–106. [DOI] [PubMed] [Google Scholar]
- 111. Bu L, Gao M, Qu S, et al. . Intraperitoneal injection of clodronate liposomes eliminates visceral adipose macrophages and blocks high-fat diet-induced weight gain and development of insulin resistance. Aaps J. 2013;15:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Piaggio F, Kondylis V, Pastorino F, et al. . A novel liposomal clodronate depletes tumor-associated macrophages in primary and metastatic melanoma: anti-angiogenic and anti-tumor effects. J Control Release. 2016;223:165–177. [DOI] [PubMed] [Google Scholar]
- 113. Marra M, Salzano G, Leonetti C, et al. . New self-assembly nanoparticles and stealth liposomes for the delivery of zoledronic acid: a comparative study. Biotechnol Adv. 2012;30:302–309. [DOI] [PubMed] [Google Scholar]
- 114. Ovais M, Guo M, Chen C. Tailoring nanomaterials for targeting tumor-associated macrophages. Adv Mater. 2019;31:e1808303. [DOI] [PubMed] [Google Scholar]
- 115. Mulder WJM, van Leent MMT, Lameijer M, et al. . High-density lipoprotein nanobiologics for precision medicine. Acc Chem Res. 2018;51:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128:2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ishida T, Yoshida M, Arita M, et al. . Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflamm Bowel Dis. 2010;16:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bento AF, Claudino RF, Dutra RC, et al. . Omega-3 fatty acid-derived mediators 17-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol. 2011;187:1957–1969. [DOI] [PubMed] [Google Scholar]
- 119. Marcon R, Bento AF, Dutra RC, et al. . Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J Immunol. 2013;191:4288–4298. [DOI] [PubMed] [Google Scholar]
- 120. Gobbetti T, Dalli J, Colas RA, et al. . Protectin D1n-3 DPA and resolvin D5n-3 DPA are effectors of intestinal protection. Proc Natl Acad Sci U S A. 2017;114:3963–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. de Silva PS, Olsen A, Christensen J, et al. . An association between dietary arachidonic acid, measured in adipose tissue, and ulcerative colitis. Gastroenterology. 2010;139:1912–1917. [DOI] [PubMed] [Google Scholar]
- 122. Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. [DOI] [PubMed] [Google Scholar]
- 123. Lev-Tzion R, Griffiths AM, Leder O, et al. . Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2014:CD006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Turner D, Shah PS, Steinhart AH, et al. . Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): a systematic review and meta-analyses. Inflamm Bowel Dis. 2011;17:336–345. [DOI] [PubMed] [Google Scholar]
- 125. Feagan BG, Sandborn WJ, Mittmann U, et al. . Omega-3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC randomized controlled trials. Jama. 2008;299:1690–1697. [DOI] [PubMed] [Google Scholar]
- 126. Bonnefoy F, Gauthier T, Vallion R, et al. . Factors produced by macrophages eliminating apoptotic cells demonstrate pro-resolutive properties and terminate ongoing inflammation. Front Immunol. 2018;9:2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yoon YS, Kim SY, Kim MJ, et al. . PPARγ activation following apoptotic cell instillation promotes resolution of lung inflammation and fibrosis via regulation of efferocytosis and proresolving cytokines. Mucosal Immunol. 2015;8:1031–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Choy EH, Bendit M, McAleer D, et al. . Safety, tolerability, pharmacokinetics and pharmacodynamics of an anti-oncostatin M monoclonal antibody in rheumatoid arthritis: results from phase II randomized, placebo-controlled trials. Arthritis Res Ther. 2013;15:R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. [DOI] [PubMed] [Google Scholar]
- 130. Vanuytsel T, Senger S, Fasano A, et al. . Major signaling pathways in intestinal stem cells. Biochim Biophys Acta. 2013;1830:2410–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hollander D, Vadheim CM, Brettholz E, et al. . Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. [DOI] [PubMed] [Google Scholar]
- 132. Irvine EJ, Marshall JK. Increased intestinal permeability precedes the onset of Crohn’s disease in a subject with familial risk. Gastroenterology. 2000;119:1740–1744. [DOI] [PubMed] [Google Scholar]
- 133. Martini E, Krug SM, Siegmund B, et al. . Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2017;4:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14:348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Beck PL, Rosenberg IM, Xavier RJ, et al. . Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hoffmann P, Zeeh JM, Lakshmanan J, et al. . Increased expression of transforming growth factor alpha precursors in acute experimental colitis in rats. Gut. 1997;41:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Waldner MJ, Neurath MF. Mechanisms of immune signaling in colitis-associated cancer. Cell Mol Gastroenterol Hepatol. 2015;1:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Taniguchi K, Wu LW, Grivennikov SI, et al. . A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Podolsky DK. Healing the epithelium: solving the problem from two sides. J Gastroenterol. 1997;32:122–126. [DOI] [PubMed] [Google Scholar]
- 140. Aamann L, Vestergaard EM, Grønbæk H. Trefoil factors in inflammatory bowel disease. World J Gastroenterol. 2014;20:3223–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Otte JM, Zdebik AE, Brand S, et al. . Effects of the cathelicidin LL-37 on intestinal epithelial barrier integrity. Regul Pept. 2009;156:104–117. [DOI] [PubMed] [Google Scholar]
- 142. Leoni G, Alam A, Neumann PA, et al. . Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Anbazhagan AN, Priyamvada S, Gujral T, et al. . A novel anti-inflammatory role of GPR120 in intestinal epithelial cells. Am J Physiol Cell Physiol. 2016;310:C612–C621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sinha A, Nightingale J, West KP, et al. . Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med. 2003;349:350–357. [DOI] [PubMed] [Google Scholar]
- 145. Belluzzi A, Brignola C, Campieri M, et al. . Effect of an enteric-coated fish-oil preparation on relapses in Crohn’s disease. N Engl J Med. 1996;334:1557–1560. [DOI] [PubMed] [Google Scholar]
- 146. Cinque B, La Torre C, Lombardi F, et al. . VSL#3 probiotic differently influences IEC-6 intestinal epithelial cell status and function. J Cell Physiol. 2017;232:3530–3539. [DOI] [PubMed] [Google Scholar]
- 147. Farrell RJ. Epidermal growth factor for ulcerative colitis. N Engl J Med. 2003;349:395–397. [DOI] [PubMed] [Google Scholar]
- 148. Sugimoto K, Ogawa A, Mizoguchi E, et al. . IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lindemans CA, Calafiore M, Mertelsmann AM, et al. . Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Neufert C, Pickert G, Zheng Y, et al. . Activation of epithelial STAT3 regulates intestinal homeostasis. Cell Cycle. 2010;9:652–655. [DOI] [PubMed] [Google Scholar]
- 151. Hanash AM, Dudakov JA, Hua G, et al. . Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Fang L, Pang Z, Shu W, et al. . Anti-TNF therapy induces CD4+ T-cell production of il-22 and promotes epithelial repairs in patients with Crohn’s disease. Inflamm Bowel Dis. 2018;24:1733–1744. [DOI] [PubMed] [Google Scholar]
- 153. Kirchberger S, Royston DJ, Boulard O, et al. . Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med. 2013;210:917–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kamanaka M, Huber S, Zenewicz LA, et al. . Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J Exp Med. 2011;208:1027–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Naganuma M, Sugimoto S, Mitsuyama K, et al. ; INDIGO Study Group Efficacy of Indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology. 2018;154:935–947. [DOI] [PubMed] [Google Scholar]
- 156. Chiriac MT, Buchen B, Wandersee A, et al. . Activation of epithelial signal transducer and activator of transcription 1 by interleukin 28 controls mucosal healing in mice with colitis and is increased in mucosa of patients with inflammatory bowel disease. Gastroenterology. 2017;153:123–138 e8. [DOI] [PubMed] [Google Scholar]
- 157. Monteleone G, Neurath MF, Ardizzone S, et al. . Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N Engl J Med. 2015;372:1104–1113. [DOI] [PubMed] [Google Scholar]
- 158. Feagan BG, Sands BE, Rossiter G, et al. . Effects of mongersen (GED-0301) on endoscopic and clinical outcomes in patients with active Crohn’s disease. Gastroenterology. 2018;154:61–64.e6. [DOI] [PubMed] [Google Scholar]
- 159. Schwanke RC, Marcon R, Bento AF, et al. . EPA- and DHA-derived resolvins’ actions in inflammatory bowel disease. Eur J Pharmacol. 2016;785:156–164. [DOI] [PubMed] [Google Scholar]