Abstract
Background
Anti–tumor necrosis factor–α (anti-TNF-α) treatments are increasingly used to treat pediatric Crohn’s disease, even without a prior trial of immunomodulators, but the cost-effectiveness of such treatment algorithms has not been formally examined. Drug plan decision-makers require evidence of cost-effectiveness to inform funding decisions. The objective was to assess the incremental cost-effectiveness of early intervention with anti-TNF-α treatment vs a conventional step-up strategy per steroid-free remission-week gained from public health care and societal payer perspectives over 3 years.
Methods
A probabilistic microsimulation model was constructed for children with newly diagnosed moderate to severe Crohn’s disease receiving anti-TNF-α treatment and concomitant treatments within the first 3 months of diagnosis compared with children receiving standard care consisting of steroids and/or immunomodulators with the possibility of anti-TNF-α treatment after 3 months of diagnosis. A North American multicenter observational study with 360 patients provided input into clinical outcomes and health care resource use.
Results
Early intervention with anti-TNF-α treatment was more costly, with an incremental cost of CAD$31,112 (95% confidence interval [CI], $2939–$91,715), and more effective, with 11.3 more weeks in steroid-free remission (95% CI, 10.6–11.6) compared with standard care, resulting in an incremental cost per steroid-free remission-week gained of CAD$2756 from an Ontario public health care perspective and CAD$2968 from a societal perspective. The incremental cost-effectiveness ratio was sensitive to the price of infliximab.
Conclusions
The results suggest that although early anti-TNF-α was not cost-effective, it was clinically beneficial. These findings, along with other randomized controlled trial evidence, may inform formulary decision-making.
Keywords: economic evaluation, pediatric Crohn’s disease, anti-TNF-α, infliximab, cost-effectiveness analysis
Intervention with anti-TNF-α within 3 months of diagnosis in pediatric CD results in 11.3 additional weeks of steroid-free remission over 3 years compared with step-up care, but is costly, with an incremental cost per steroid-free remission-week gained of CAD$2756.
INTRODUCTION
The incidence of inflammatory bowel disease (IBD) among children is increasing globally, but there is a lack of high-quality studies comparing treatments.1 There is a keen interest in optimizing treatment early, aiming to both control symptoms and achieve intestinal healing because of the lifelong burden of disease and its sustained impact on quality of life for children and their families.2, 3
The traditional treatment of pediatric Crohn’s disease (CD) involves a stepwise approach, or “step-up” strategy, including several classes of drugs such as corticosteroids, immunomodulators (IM)s, and tumor necrosis factor alpha antagonist (anti-TNF-α) biologics. Children who do not respond adequately to corticosteroids and IMs or who cannot tolerate them are subsequently prescribed anti-TNF-α drugs such as infliximab or adalimumab, as was endorsed in the recent Canadian clinical practice guidelines for medical management of pediatric CD.4 These guidelines also suggested use of anti-TNF-α as the firstline therapy in patients at high risk for disabling disease.4 An observational cohort study by Walters et al. showed 85% of children with CD achieved steroid-free clinical remission at 12 months if they received anti-TNF-α drugs within the first 3 months of diagnosis (early treatment), compared with 60% of children receiving early IM therapy.5 Another observational study in pediatric CD showed sustained effectiveness of infliximab in children and adolescents with luminal CD with enhanced linear growth, particularly when therapy was initiated within the first 18 months of diagnosed disease.6 Although these studies suggest that anti-TNF-α drugs are better at achieving remission than traditional therapies, the medications are very expensive for drug plans and for uninsured families.
To date, a randomized controlled trial (RCT) of an anti-TNF-α treatment administered within 3 months after initial corticosteroid remission (early introduction of anti-TNF-α) compared with a step-up strategy (previously standard care) with the later introduction of anti-TNF-α treatment after immunomodulator treatment has not been completed in newly diagnosed children with CD. An RCT in adult CD patients examining early intervention with 3 induction doses of anti-TNF-α followed by thiopurine monotherapy (“top-down” approach) showed a greater proportion of patients (60.0%) in remission at week 26 compared with the standard care (“step-up”) group (35.9%) and a greater likelihood of sustained mucosal healing.7, 8 Another trial in adult CD patients showed similar remission rates (>60% of patients in remission) between the early intervention and step-up comparator groups at 12 months, but a lower adverse event rate at 24 months in the early anti-TNF-α intervention group.9 A review of predominantly adult studies concluded that there is insufficient evidence to warrant a change in treatment practices from the traditional “step-up” standard to a “top-down” approach with an early intervention of anti-TNF-α treatments.10 A more recent review concluded that early combination therapy of biologics and immunomodulators was found to be effective at improving patient outcomes, but early biologics monotherapy did not have a clear benefit over step-up therapy and evidence supporting a top-down approach with biologics in pediatric CD populations was inconclusive.11 That review also concluded that there was insufficient evidence to draw any conclusions on the cost-benefits of a top-down treatment strategy.11 Hence the debate between “top-down” and “step-up” approaches with anti-TNF-α treatments persists.
A systematic review of economic evaluations comparing the treatment of biological therapies with standard care in moderate to severe CD in adults (and 1 pediatric study) found that biological treatments were cost-effective and reduced health care resource use compared with standard therapy in certain situations, such as luminal CD, when used as induction treatment.12 It was suggested that intervention with anti-TNF-α treatment within the first 2 years of diagnosis could result in cost-savings over the lifetime of the IBD patient.13 To date, there have been no economic evaluations comparing the early use of anti-TNF-α treatments with the traditional “step-up” strategy (standard care) in pediatric CD. Furthermore, drug re-imbursement policies are not aligned with an early or firstline introduction of anti-TNF-α treatments for moderate to severe pediatric CD. This study aimed to perform an economic evaluation of early anti-TNF-α treatment in pediatric CD by taking provincial and societal perspectives to generate evidence to inform clinical and policy decision-makers regarding the optimal placement of anti-TNFα drugs in the management of pediatric CD. The objective was to determine the incremental costs of early intervention with anti-TNF-α treatment (top-down approach) compared with the conventional step-up strategy per steroid-free remission-week gained in newly diagnosed moderate to severe pediatric CD over a 3-year time horizon from the public health care payer and societal perspectives.
METHODS
Study Design
A cost-effectiveness analysis was conducted by creating a 2-dimensional probabilistic microsimulation Markov model for children with newly diagnosed moderate to severe CD. The study compared early treatment with anti-TNF-α treatment and other concomitant medications with standard care over a 3-year time horizon. As suggested by international guidelines,14–16 a public health care payer perspective (Ontario, Canada) and a societal payer perspective were taken. For economic evaluations of pediatric interventions, a societal perspective is also recommended, as parental caregiving and time losses are often substantial.15, 17 Weeks in steroid-free remission was the primary outcome. The target population consisted of newly diagnosed children with moderate to severe CD aged 4–17 years.
Comparator Interventions
The experimental group consisted of early intervention with anti-TNF-α, defined as an induction phase of anti-TNF-α with or without concomitant immunomodulators, and/or corticosteroids, and/or enteral nutrition, administered within 3 months of diagnosis but without prior failure of corticosteroids/enteral nutrition and immunomodulators, followed by a maintenance phase of anti-TNF-α treatment with or without concomitant immunomodulators.
The control group consisted of step-up strategy (traditional standard care), defined as an induction phase of enteral nutrition or corticosteroids with or without concomitant immunomodulators administered within 3 months of diagnosis, followed by a maintenance phase with immunomodulators, followed by anti-TNF-α treatment (with or without concomitant immunomodulators) ≥3 months after diagnosis if deemed necessary.
Data Sources
Clinical and outcomes data were principally derived from the RISK-PROKIIDS study,18 a prospective, multicenter North American observational cohort study of children with inflammatory bowel disease involving 25 US and 3 Canadian clinical centers that started in 2008 and followed enrolled children for a minimum of 3 years. As a pragmatic, observational study, children in the RISK-PROKIIDS study were treated according to recommended guidelines at the discretion of the treating physicians, providing high external validity. The broad RISK-PROKIIDS study data were used to extract patient-level data (analyzed retrospectively) from a subset of CD patients to inform clinical health information, such as whether they were in active or remission states of disease and treatment patterns. Included subjects had a confirmed CD diagnosis, at least 3 years of follow-up data from the date of diagnosis, and determinable health state information at approximately 6-month intervals for at least 3 years from the date of diagnosis. A total of 573 subjects were initially included from the RISK-PROKIIDS study. The health state for each patient visit was based primarily on the determined weighted Pediatric Crohn’s Disease Activity Index (wPCDAI) score, or on Physician Global Assessment (PGA) values if wPCDAI was indeterminable. These data were supplemented by other published literature.
Propensity score matching was conducted to create comparator groups for the economic evaluation (Supplementary Data, “Propensity Score Analysis Methods”). The matched data set contained 360 total subjects with 237 standard care subjects and 123 early anti-TNF-α intervention subjects. Baseline characteristics of the patient population are listed in Table 1.
TABLE 1.
Patient Characteristics in Propensity-Matched RISK-PROKIIDS Comparator Groups
| Characteristic | Standard Care | Early Intervention With Biologics | P |
|---|---|---|---|
| n = 237, No. (%) | n = 123, No. (%) | ||
| Sex, female | 75 (31.6) | 41 (33.3) | 0.837 |
| Age at diagnosis, mean (SD), y | 12.27 (2.65) | 12.28 (2.70) | 0.964 |
| Albumin, mean (SD), g/dL | 3.46 (0.59) | 3.43 (0.62) | 0.717 |
| Height Z score, mean (SD) | –0.38 (1.08) | –0.45 (1.21) | 0.577 |
| Family history of IBD | 0.164 | ||
| No 1st-degree relative | 185 (78.1) | 107 (87.0) | |
| One 1st-degree relative | 41 (17.3) | 14 (11.4) | |
| Two 1st-degree relatives | 3 (1.3) | 0 (0.0) | |
| Unknown | 8 (3.4) | 2 (1.6) | |
| Ethnicity | 0.335 | ||
| Caucasian | 176 (74.3) | 88 (71.5) | |
| African | 21 (8.9) | 12 (9.8) | |
| Mixed | 18 (7.6) | 7 (5.7) | |
| Other | 10 (4.2) | 9 (7.3) | |
| Unknown | 12 (5.1) | 7 (5.7) | |
| Presence of perianal disease | 0.048* | ||
| No | 169 (71.3) | 72 (58.5) | |
| Yes | 66 (27.8) | 50 (40.7) | |
| Unknown | 2 (0.8) | 1 (0.8) | |
| Disease activity at diagnosis (Physician Global Assessment) | 0.235 | ||
| None | 3 (1.3) | 0 (0.0) | |
| Mild | 59 (24.9) | 23 (18.7) | |
| Moderate | 121 (51.1) | 74 (60.2) | |
| Severe | 54 (22.8) | 26 (21.1) | |
| Disease location | 0.962 | ||
| No L1–L3 disease | 0 (0.4) | 1 (0.8) | |
| L1 | 33 (13.9) | 17 (13.8) | |
| L2 | 48 (20.3) | 23 (18.7) | |
| L3 | 136 (57.4) | 70 (56.9) | |
| Unknown | 19 (8.0) | 12 (9.8) |
Chi-square test, t test, and Kruskal-Wallis rank-sum tests were used for comparisons between groups depending on continuous or noncontinuous variables.
*P < 0.05.
Effectiveness Outcome
Weeks in steroid-free remission was the primary clinical outcome. The number of subjects in steroid-free remission from each intervention arm was assessed at 6, 12, 18, 24, and 36 months based on RISK-PROKIIDS data.
Model Structure
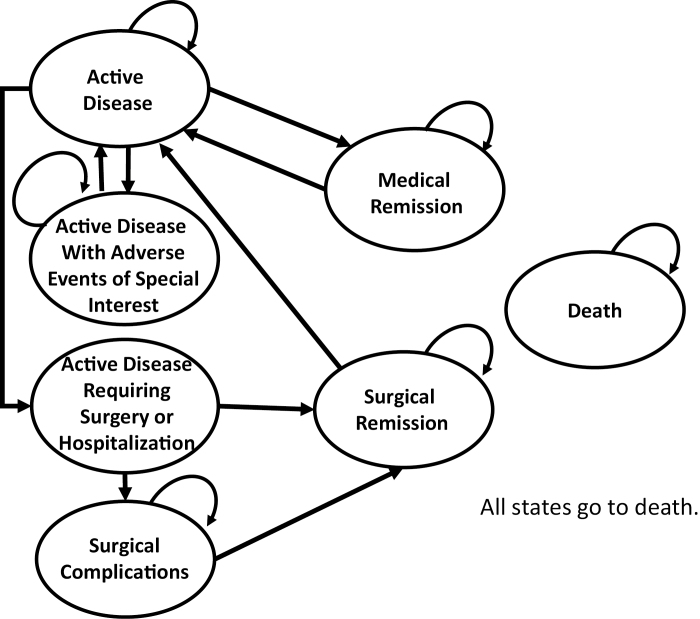
A health state transition (Markov) microsimulation model was created to model 7 health states experienced by CD patients: (1) active disease, (2) active disease experiencing adverse events of special interest, (3) active disease requiring surgery or hospitalization, (4) medical remission, (5) surgical remission, (6) surgical complications, and (7) death (Fig. 1). A 1-week cycle length was modeled to reflect the approximate minimum time within a health state. The health state of active disease in moderate to severe CD was defined as having active disease flare-up with a weighted Pediatric Crohn’s Disease Activity Index (wPCDAI) score ≥12.5 or a Physician Global Assessment (PGA) score >1. It was assumed that people started in active disease and entered the induction phase of treatment. Costs associated with the induction phase and maintenance phase of treatment were assigned to the active disease phase if a subject exhibited an active disease state symptomatically. Medical remission was defined as a state with a wPCDAI of ≤12.5 and a PGA of 1. Maintenance phase costs of treatment were assigned to the medical remission state. The state of adverse events of special interest described a state where treatment could result in possible treatment-related events of concern such as opportunistic infection, lymphoma, or an antibody reaction. Costs associated with active disease requiring surgery/hospitalization health state included hospitalization and surgery costs. After surgery, a person entered the state of surgical remission or surgical complications. Surgical remission was defined as remission achieved through surgery. The state of surgical remission had remission phase drug treatment costs assigned to it; however, time in surgical remission was not included as time in remission for the purposes of calculating the primary outcome. Sepsis was considered the major surgical complication after surgery.19 Hospitalization and treatment costs associated with sepsis were assigned to the surgical complications health state. Patients could remain in the surgical complications health state a maximum of 4 weeks to reflect average treatment time for this health state. All states eventually went to death as the absorbing state, which assumed no costs and assumed that transition probabilities were reflective of age-specific all-cause mortality.20
FIGURE 1.
The Crohn’s disease health state transition model. Ovals represent health states. Arrows show the direction of moving from one health state to another. The curved arrows indicate remaining in the same health state.
An individual-level microsimulation model21 to account for the heterogeneity in patient age, sex, and disease progression was constructed using TreeAge Pro software v.2018. Ten thousand individual-level, first-order Monte Carlo microsimulations were conducted. A probabilistic analysis was undertaken, whereby all distributions were sampled using a 2-dimensional microsimulation that sampled 50 samples of 10,000 individuals.22, 23 Transition probabilities are listed in Table 2.
TABLE 2.
Health State Transition Probabilities
| Event (Strategy) | Probability | SD | Time | Source | Distribution |
|---|---|---|---|---|---|
| Active disease to medical remission (early anti-TNF-α) | 0.553 | 0.003 | 0 to 6 mo | Kugathasan et al., 200818 | Beta |
| 0.396 | 0.004 | 6 to 12 mo | |||
| 0.500 | 0.005 | 12 to 18 mo | |||
| 0.400 | 0.006 | 18 to 24 mo | |||
| 0.355 | 0.007 | 24 to 30 mo | |||
| 0.529 | 0.007 | 30 to 36 mo | |||
| Active disease to medical remission (standard care) | 0.515 | 0.001 | 0 to 6 mo | Kugathasan et al., 200818 | Beta |
| 0.452 | 0.002 | 6 to 12 mo | |||
| 0.495 | 0.002 | 12 to 18 mo | |||
| 0.526 | 0.003 | 18 to 24 mo | |||
| 0.467 | 0.003 | 24 to 30 mo | |||
| 0.507 | 0.004 | 30 to 36 mo | |||
| Continued medical remission (early anti-TNF-α) | 0.794 | 0.002 | 6 to 12 mo | Kugathasan et al., 200818 | Beta |
| 0.747 | 0.002 | 12 to 18 mo | |||
| 0.899 | 0.001 | 18 to 24 mo | |||
| 0.828 | 0.002 | 24 to 30 mo | |||
| 0.843 | 0.002 | 30 to 36 mo | |||
| Continued medical remission (standard care) | 0.680 | 0.002 | 6 to 12 mo | Kugathasan et al., 200818 | Beta |
| 0.644 | 0.002 | 12 to 18 mo | |||
| 0.781 | 0.001 | 18 to 24 mo | |||
| 0.835 | 0.001 | 24 to 30 mo | |||
| 0.850 | 0.001 | 30 to 36 mo | |||
| Active disease to active disease requiring surgery or hospitalization (early anti-TNF-α) | 0.046 | 0.000 | 3 y | Kugathasan et al., 200818 | Beta |
| Active disease to active disease requiring surgery or hospitalization (standard care) | 0.030 | 0.000 | 3 y | Kugathasan et al., 200818 | Beta |
| Surgical remission to active disease (early anti-TNF-α) | 0.001 | 0.001 | 3 y | Blackburn, et al., 201419 | Beta |
| Surgical remission to active disease (standard care) | 0.000 | 0.000 | 3 y | Kugathasan et al., 200818 | Beta |
| Serious infection on CS | 0.070 | 0.014 | 1 y | Dulai et al., 201424 | Beta |
| Serious infection on IM | 0.033 | 0.007 | 1 y | ||
| Serious infection on anti-TNF-α | 0.032 | 0.006 | 1 y | ||
| Lymphoma on anti-TNF-α | 0.00021 | 0.00004 | 1 y | ||
| Lymphoma on IM | 0.00045 | 0.00009 | 1 y | ||
| Antibody reaction on infliximab | 0.00036 | 0.001 | 1 wk | Hyams et al., 201125 | Beta |
| Surgical complications | 0.058 | 0.002 | 1 wk | Leonor et al., 200726 | Beta |
| Death from lymphoma (female) | 0.000053 | 0.00001 | 1 y | Canadian Cancer Society, 201727 |
Beta |
| Death from lymphoma (male) | 0.000084 | 0.00002 | 1 y |
Costs
Direct health care costs included all intervention costs, physician services, and hospitalizations. The treatment patterns of the RISK-PROKIIDS population informed the treatment resource use over time for each comparator group and the probability of changing treatments. Treatment classes included biologic treatments (anti-TNF-α treatments), corticosteroids (CS), immunomodulators (IM), 5-aminosalicylates (5-ASAs), antibiotics, and enteral nutrition (EN). Treatments were dynamic and often included concomitant medications, which changed over time for each subject. The proportions of subjects in each drug class per week over a 3-year period were used to calculate the weighted average utilization per drug class per week for each comparator group. Standard growth charts were used to estimate mean sex-specific and age-related weights and body surface area to determine treatment doses, which were based on standard clinical practice.28
All direct drug and medical procedure costs are shown in Supplementary Table 1. Dosing was based on limited available data from the RISK-PROKIIDS study and clinical practice guidelines. To reflect standard initial treatment, infliximab was assumed to be given as a 5-mg/kg infusion at 0, 2, and 6 weeks, then as 5 mg/kg every 8 weeks thereafter. Adalimumab dosing was a subcutaneous injection of 160 mg at week 0, 80 mg at week 2, and 40 mg every 2 weeks thereafter. Enteral nutrition estimates were based on estimated energy requirements for sedentary activity level29 and local Ontario prices (see Supplementary Table 1 for EN costs). Mean costs per week of EN per age and sex were calculated. All drug treatment costs were assigned a gamma distribution for the probabilistic analysis.21
To create a general model for each health state in CD, the difference in visit frequency and testing during remission and during active disease (more frequent visits) in this particular cohort was estimated based on observable patient-level data, as seen in the RISK cohort and based on expert opinion. With regard to medical services, it was assumed that all subjects underwent 1 tuberculosis test, 1 chest x-ray, 1 colonoscopy, and 1 gastroscopy upon entry into the model, with an initial gastroenterologist consultation and a subsequent assessment within a month. To reflect a typical visit schedule, if patients continued with active disease, they would see the gastroenterologist at 3 months, 6 months, and 12 months. Subjects in remission would see the gastroenterologist every 6 months. Physician fees were considered fixed for the probabilistic analysis based on the Ontario Schedule of Benefits for Physician Services (see Supplementary Table 1 for physician costs). Costs of medical procedures were assigned a gamma distribution, and average costs for all age ranges were assumed, as pediatric-specific cost data were unavailable. Hospital inpatient procedures including surgeries were estimated using the Ontario Case Costing Initiative based on ICD-10 codes for Crohn’s disease (see Supplementary Table 1 for inpatient costs). The treatment for an antibody reaction to infliximab was assumed to be a change in treatment to adalimumab.
All treatment prices, service fees, and procedural costs were based on 2017 prices and costs or were adjusted to 2017 Canadian dollars using the Canadian Consumer Price Index for health and personal care (CAD$1 = US$0.77 average 2017 rate). A 1.5% discount rate was used.14
Indirect Costs
Costs from a societal perspective included all health care payer costs described above and costs associated with caregiver (such as a parent or guardian) time losses (indirect costs). Each medical procedure and physician visit was assumed to consume 4 hours of caregiver time for pediatric CD patients. A day in the hospital was assumed to consume 8 hours of caregiver time. The mean length of stay for ICD10 codes for Crohn’s disease in the Ontario Case Costing Initiative database was used to calculate loss of hours for caregivers during hospitalization and medical procedures. The Human Capital Approach was used in the economic evaluation, which incorporated direct costs and indirect productivity losses.21 The caregiver labor wage was based on the average Canadian hourly wage rate for people aged 24–54 in January 2018, which was listed as CAD$29.04.30
Model Assumptions
The treatment pathways outlined in this model represent common clinical practice for pediatric CD patients recommended by clinical practice guidelines and as observed by the RISK-PROKIIDS study.18, 31, 32 It was assumed that the RISK-PROKIIDS study data were representative of the most common drugs prescribed to pediatric CD patients. It was assumed that all patients complied with treatment regimens. It was assumed that CD and taking the prescribed medications did not increase the risk of death in the pediatric patients. It was assumed that all forms of intestinal resection (including ileal resection) were the most common form of surgery and that the probability of surgery did not increase within the 3-year time horizon regardless of having a previous surgery, as observed in the RISK-PROKIIDS data. Private health insurance out-of-pocket costs for co-payments or deductibles were not included due to the variation in individual plans. Administration costs for health insurance claims were not considered. It was assumed that only treatment strategy, and no other correlating factors within comparator groups, affected clinical outcomes.
Model Validation and Calibration
The model was internally calibrated using observed outcomes from the RISK-PROKIIDS study data set due to a lack of external studies available for calibration. The number of subjects in medical remission at 1 year served as an external parameter for model validation and was compared with published observational studies of early intervention with infliximab in pediatric CD and an earlier study of a portion of the RISK-PROKIIDS subjects.5, 33, 34 The percentage of pediatric CD surgeries among CD patients in Ontario aged >3 years (14.7%) was obtained from an epidemiological study that served as an external validation target for the model and was comparable to the 15.3% of CD patients across both comparator groups who had surgery within 3 years in our model.35
Incremental Cost-effectiveness Analysis
The incremental cost-effectiveness ratio (ICER) was the ratio of the difference in cost between early intervention with anti-TNF-α and standard care to the difference in outcome between the 2 strategies, as measured by weeks in steroid-free medical remission. In the reference case (primary) analysis, the cost and outcomes for each strategy were averaged for each of 50 samples of 10,000 individual microsimulations. Multiple sampling from each specified distribution was performed for a probabilistic 2-dimensional microsimulation for the reference case.
Uncertainty Analysis
Parameter uncertainty was ascertained by conducting a probabilistic analysis via a 2-dimensional Monte Carlo microsimulation where all variable distributions were varied simultaneously in the model. Cost-effectiveness acceptability curves (CEACs) of the 2-D microsimulation illustrated uncertainty in the estimated costs, outcomes, and incremental cost-effectiveness ratio for the early anti-TNF-α intervention and standard care (step-up) treatment strategies from health care public payer and societal perspectives. Uncertainty in the discount rate was assessed in a 1-way sensitivity analysis using discount rates of 0% and 3%.14
Scenario Analysis
Scenario analyses were conducted to examine the sensitivity of the ICER to key parameters, including the price of infliximab, the price of immunomodulators, and the rate of switching to anti-TNF-α (in the step-up group), as these may be primary drivers of uncertainty in the model. Infliximab was the chosen target, as it was the predominant anti-TNF-α treatment used in the RISK-PROKIIDS study. A 1-way sensitivity analysis was conducted by varying the price of infliximab by 150%, 50%, 37.5%, and 25% of the reference case (CAD$987.56). These ranges were used to examine at what range biologic treatment could potentially be cost-effective. A 1-way sensitivity analysis was conducted by varying the price of infliximab by a multiplier. The 1-way sensitivity was restricted to a 1-dimensional Monte Carlo analysis with 10,000 trials using Tree Age 2018 software and was conducted from the health care public payer perspective. Similarly, the cost of immunomodulators was based on the average cost of the drug class per week, and this average cost was multiplied by a factor of 2 or a factor of 0.5 in the sensitivity analysis. It was hypothesized that the rate of escalation to anti-TNF-α treatment in the standard care group introduced uncertainty in the model, as it was based on the RISK-PROKIIDS study and may not have reflected more current rates of adoption of anti-TNF-α, which are unknown. The probability of being on anti-TNF-α treatment in the step-up group was multiplied by a factor of 0.5× and 4× the assessed weekly probability of not switching to anti-TNF-α.
ETHICAL CONSIDERATIONS
The study was approved by the Research Ethics Board of the Hospital for Sick Children, Toronto, Canada, file #1000047757, and the University of Toronto Research Ethics Board. Data for children whose parents consented to the RISK-PROKIIDS study and RISK ancillary studies were transferred to Sick Kids for analysis. A waiver for consent was approved by the Sick Kids Research Ethics Board for conducting the economic evaluation using de-identified patient information.
RESULTS
Characteristics of the Matched Patient Population
Patient and disease characteristics at diagnosis are listed in Table 1. Health status at 6, 12, 18, 24, and 36 months postdiagnosis was determined for each comparator group in the propensity-matched RISK-PROKIIDS population and is shown in Table 3. At 6 months, in the matched early anti-TNF-α group, 52.8% were in steroid-free remission, and in the standard care group, 39.2% were in steroid-free remission (P = 0.019). Patients in the early anti-TNF-α group had a mean number of 3.98 steroid-free remission semesters and 3.61 consecutive steroid-free remission semesters over 36 months compared with the patients in the standard care group, who had a mean number of 3.59 steroid-free remission semesters (P = 0.036) and a mean number of 3.02 consecutive steroid-free remission semesters (P = 0.003).
TABLE 3.
The Health Status at 6, 12, 18, 24, 30, and 36 Months Postdiagnosis in the Matched RISK-PROKIIDS Comparator Groups
| Health Status | Standard Care | Early Intervention With Biologics | P |
|---|---|---|---|
| n = 237 | n = 123 | ||
| No. subjects in steroid-free remission at 6 mo (%) | 93 (39.2) | 65 (52.8) | 0.019* |
| No. subjects in steroid-free remission at 12 mo (%) | 125 (52.7) | 75 (61.0) | 0.168 |
| No. subjects in steroid-free remission at 18 mo (%) | 132 (55.7) | 82 (66.7) | 0.058 |
| No. subjects in steroid-free remission at 24 mo (%) | 154 (65.0) | 92 (74.8) | 0.075 |
| No. subjects in steroid-free remission at 30 mo (%) | 167 (70.5) | 87 (70.7) | 1 |
| No. subjects in steroid-free remission at 36 mo (%) | 177 (74.7) | 88 (71.5) | 0.607 |
| No. steroid-free remission semesters in 36 mo per group, mean (SD) | 3.59 (1.61) | 3.98 (1.86) | 0.036* |
| Maximum consecutive steroid-free remission semesters in 36 mo per group, mean (SD) | 3.02 (1.66) | 3.61 (1.97) | 0.003* |
| Total days in hospital at 36 mo per group, mean (SD) | 3.76 (10.51) | 3.97 (13.37) | 0.872 |
| No. subjects hospitalized (%) | 84 (35.4) | 45 (36.6) | 0.922 |
Health state at diagnosis was based on the weighted Pediatric Crohn’s Disease Activity Index and the steroid state of the patients.
*P < 0.05.
Treatment Patterns in the Matched Patient Population
The classes of medications that each RISK-PROKIIDS patient was taking over 3 years informed the total cost of drugs for each comparator group at a given time. At 3 years postdiagnosis, 46% of early anti-TNF-α patients were on anti-TNF-α monotherapy and 39% were on anti-TNF-α with immunomodulators, and in the standard care group, 23% of patients were on anti-TNF-α monotherapy, 19% were on anti-TNF-α with immunomodulators, and 17% were on immunomodulator monotherapy. Over 90% of patients in the early anti-TNF-α intervention group remained on biologic treatment with or without other medications over 3 years postdiagnosis.
Three months postdiagnosis, 11% of patients in the standard care group began taking an anti-TNF-α, which increased to 27% of patients by the first year, 46% of patients at the second year, and 54% of patients by the end of the third year. Approximately 56% of patients in the early anti-TNF-α intervention group were on corticosteroids at the beginning of the study, but use tapered to <10% of patients after 6 months and <5% for the rest of the 3 years. Similarly, 65% of patients in the standard care group were initially on corticosteroids, which decreased to 5% by the third year postdiagnosis. Nine percent of patients in the early anti-TNF-α intervention group were taking immunomodulators at the beginning of the study, and use increased steadily to 39% by 3 years. In the standard care group, 59% of patients were on immunomodulators at the end of the first year, decreasing only slightly to 54% of patients taking immunomodulators by the end of the third year after diagnosis. In the early anti-TNF-α group, ≤2% of patients ever used EN. In the standard care group, 7% of patients started using EN in the first month, decreasing to 2% by the end of the third year. Forty-one percent of patients in the early anti-TNF-α group were taking antibiotics initially, which decreased to 5% by 3 years. In the standard care group, 27% of patients were on antibiotics in the first month, decreasing to 14% by the end of the third year. The use of 5-ASAs in the early TNF-α group started with 15% of patients and decreased to 10% at 3 years. In the standard care group, 34% of patients were on 5-ASA initially, which decreased to 27% 3 years after diagnosis.
Although early treatment was defined for the purpose of this study as receipt of anti-TNF-α within the first 3 months, the effects of early intervention defined by other intervals would be of interest. In the RISK cohort, most patients who received early anti-TNF-α did so within the first 3 months, and extending the interval to 6 months added <7% of total patients, with no effect on outcomes (results not shown).
Cost-effectiveness Analysis
The results of the cost-effectiveness analysis are summarized in Table 4. In the reference case, from a public health care perspective, early anti-TNF-α intervention was on average more costly than the standard care intervention over 3 years, by CAD$31,112. Early anti-TNF-α intervention was also on average more effective, with 11.3 more weeks in steroid-free medical remission. The incremental cost per steroid-free remission week gained was CAD$2756 for the early anti-TNF-α intervention for the reference case from a health care payer perspective. The wide confidence interval for the incremental cost in Table 4 reflects the large range of costs incurred by individuals in each comparator arm. Higher costs were accrued by individuals who experienced surgeries or multiple adverse events in either comparator group.
TABLE 4.
Cost-effectiveness Analysis Results Summary
| Perspective | Strategy | Mean Cost, CAD$ | Incremental Cost (95% CI), CAD$ | Mean Effectiveness, Wk in Steroid-Free Remission | Incremental Effectiveness (95% CI) | Incremental Cost-effectiveness Ratio (ICER) |
|---|---|---|---|---|---|---|
| Public health care payer | Standard care (step-up) | 96,516 | 31,112 (2939–91,715) | 83.07 | 11.29 (10.60–11.59) | |
| Early anti-TNF-α intervention | 127,628 | 94.36 | 2756 | |||
| Societal | Standard care (step-up) | 100,956 | 33,508 (5436–94,308) | 83.07 | 11.29 (10.60–11.59) | |
| Early anti-TNF-α intervention | 134,464 | 94.36 | 2968 |
Probabilistic analysis of a 2-dimensional microsimulation model with 50 samples of 10,000 microsimulations. Costs are presented in 2017 Canadian dollars.
The cost-effectiveness analysis from the societal perspective was identical to the health care public payer perspective, except for the addition of costs associated with caregiver productivity time losses. Therefore, the ICER from the societal perspective was slightly higher than that of the health care public payer perspective at CAD$2968 per additional week gained in steroid-free remission for the early anti-TNF-α intervention group. From a societal perspective, early anti-TNF-α intervention was on average more costly than the standard care intervention over 3 years by CAD$33,508 and on average more effective, with 11.3 more weeks in steroid-free medical remission.
Uncertainty Analysis
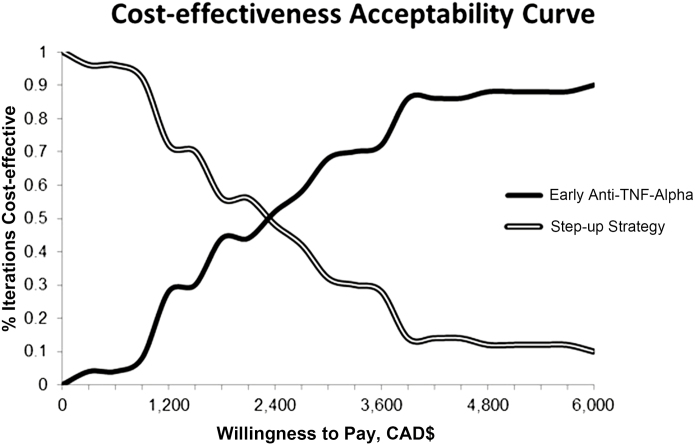
The CEAC from the 2-D microsimulation for the reference case from a health care payer perspective (Fig. 2) is reflective of uncertainty in the ICER and represents the proportion of microsimulations wherein each strategy is cost-effective (the dollar value of effectiveness exceeds the costs) over a range of willingness-to-pay thresholds. Although an actual willingness to pay for a week in steroid-free remission is unknown, at a theoretical willingness-to-pay threshold of approximately CAD$2500 per week in steroid-free remission, neither strategy is dominant over the other, as both strategies are cost-effective 50% of the time. Above a willingness-to-pay threshold of CAD$3500, the early anti-TNF-α intervention becomes increasingly cost-effective compared with standard care. Above a willingness-to-pay of CAD$6000 per week in steroid-free remission, the early anti-TNF-α intervention becomes the dominant strategy. From a societal perspective, at a willingness-to-pay threshold of approximately CAD$5000 per week in steroid-free remission, neither strategy was dominant over the other, as both strategies were cost-effective 50% of the time.
FIGURE 2.
Cost-effectiveness acceptability curve of the probabilistic (2-D) cost-effectiveness analysis of early anti-TNF-α intervention vs standard care from a public health care payer perspective. The curves, reflective of uncertainty in the ICER, represent the proportion of microsimulations wherein each strategy is cost-effective over a range of willingness-to-pay (in Canadian dollars) thresholds. Although a willingness-to-pay threshold for a week in steroid-free remission is unknown in Canada, at a theoretical willingness-to-pay threshold of CAD$2500 per week in steroid-free remission, neither strategy is dominant.
The incremental costs of early anti-TNF-α treatment per additional steroid-free remission-week gained compared with the standard care intervention using discount rates of 0% and 3% from a health care public perspective were CAD$2740 and CAD$2771, respectively, and from a societal perspective they were CAD$2954 and CAD$2982, respectively. Varying the discount rates did not have a major effect on the ICER, as the time horizon was only 3 years.
Scenario Analysis
Scenario analyses were conducted to examine how the cost of infliximab, the anti-TNF-α adoption rate in the step-up group, and the cost of immunomodulators affect the incremental cost-effectiveness ratio. It was hypothesized that the cost of anti-TNF-α treatment, particularly the cost of infliximab, could be a major source of the uncertainty in the ICER. Based on our model, an increase in the price of infliximab to 150% of its current price increased the ICER to CAD$4782. Reducing the price of infliximab to 50% and 37.5% of its current price reduced the ICER to CAD$659 and CAD$143, respectively. Reducing the price of infliximab to 25% of its current price resulted in a savings of CAD$372 per additional steroid-free remission-week gained. A similar 1-way sensitivity analysis demonstrated that doubling or halving the costs of immunomodulators had a negligible effect on the ICER. Reducing the rate of switching to anti-TNF-α in the standard care group by 0.5× increased the ICER for the early anti-TNF-α strategy to CAD$3175, and increasing the rate of switching to anti-TNF-α by 4× increased the ICER only slightly to CAD$2750. As expected, not switching to anti-TNF-α in the step-up group increased the ICER to CAD$6291. The ICER was most sensitive to the price of infliximab compared with the other factors.
DISCUSSION
This study represents the first cost-effectiveness analysis examining early intervention with anti-TNF-α treatment in treatment-naïve pediatric CD compared with standard care, defined as traditional step-up therapy with biologics from both societal and health care system payer perspectives. It was found that from a public health care perspective, early anti-TNF-α intervention was on average more costly than the standard care intervention over 3 years by CAD$31,112, but more effective, with 11.3 more weeks in steroid-free medical remission, resulting in an incremental cost per steroid-free remission-week gained of CAD$2756. From a societal perspective, the ICER was CAD$2968 per additional week gained in steroid-free remission for the early anti-TNF-α intervention group.
This economic evaluation is novel in that it represents the traditional standard care of step-up therapy in which pediatric patients can be placed on anti-TNF-α treatment later in their course of treatment after trying other nonbiologic treatments. Another unique feature was the use of steroid-free remission-weeks rather than the number of patients in remission at a certain time as the primary outcome measure. Steroid-free remission-weeks are particularly relevant for a pediatric population given the adverse effects of chronic steroid use on linear growth.36 Our results suggested a steroid-sparing benefit, which is particularly relevant for a pediatric population with early anti-TNF-α treatment, as there were 11.3 more weeks in steroid-free remission compared with standard step-up care. Growth in pediatric CD was reviewed in Gasparetto and Guariso.37 Whether an additional 11 weeks of steroid-free remission over the course of 3 years is clinically meaningful is unknown, as studies correlating weeks in steroid-free remission to a particular clinical outcome such as the degree of linear growth over a short period of time have not been conducted in CD. Heuschkel et al. suggested that growth failure in IBD can be described in terms of height-for-age standard deviation score (z score) or by variations in growth velocity over a period of 3–4 months, implying that growth velocity is not usually measured over periods of <3 months.38 Walters et al. found that early intervention with anti-TNF monotherapy improved height z score by a mean of +0.14 points in the first year.5 Hence, 11 weeks of steroid-free remission may or may not correspond to a variation in growth velocity. However, growth velocity was not calculated over 3 years in the present study due to limitations in data availability. Although mucosal healing was not assessed in this study, time in steroid-free remission may result in improved intestinal mucosal healing.39 Froslie et al. showed that medical treatment without steroids was a predictor for mucosal healing in adults with CD.40
In a review of economic evaluations in adult and pediatric inflammatory bowel disease, Lachaine et al. observed that no studies comparing biologic treatments with standard care in CD resulted in an ICER below a CAD$100,000 per quality-adjusted life-year (QALY) willingness-to-pay threshold in the Canadian setting, which makes it difficult to deem biologic treatments cost-effective at this threshold despite clinical effectiveness.41 Recent reviews of cost-effectiveness studies, predominantly in adults, of biological agents including anti-TNF-α treatments for the treatment of inflammatory bowel disease have shown a wide range of costs per QALY, owing to diverse outcomes, treatment paradigms, and patient populations.41–43 A review examining the efficacy of early anti-TNF-α treatment vs a step-up strategy for the treatment of Crohn’s disease concluded that for pediatric patients, early treatment (top-down) demonstrated positive clinical outcomes in larger studies but was inconclusive in smaller studies; however, only 6 studies were reviewed.11 The only economic evaluation of biological treatments in pediatric CD, from a public payer perspective, found that scheduled maintenance therapy with 5 mg/kg of infliximab was cost-effective, assuming a £30,000/QALY willingness-to-pay threshold, compared with standard care in refractory pediatric CD patients.44 In an adult study, from a Canadian perspective, comparing infliximab or adalimumab with non-anti-TNF-α usual care in refractory adult CD patients over a 5-year time horizon found that the cost per QALY gained for infliximab therapy compared with usual care was $222,955 with a 0.166 QALY gain.45 Overall, research and evidence supporting top-down therapy remain limited in adult and pediatric CD, and further studies are needed to determine the most appropriate CD patients to receive a top-down treatment approach.11
The present study used a large multicenter North American observational study with a comparatively large number of pediatric patients to inform model inputs to increase generalizability. The data were pragmatic and reflected actual clinical practice as opposed to an RCT. The use of patient-level data in an individual microsimulation model allowed the sampling of a wide range of patient ages and health experiences over time, as opposed to a cohort model. The model reflected the average costs over a 3-year period, stating an incremental cost per week in steroid-free remission as the outcome. Due to the frequent movement between health states, the cost for percentage in remission was not determined, as this varied from semester to semester. The average costs accrued in a particular health state and for each treatment group were similar over time.
A major research gap identified by our study is the lack of health utilities for the calculation of QALYs in children with CD, resulting in the use of surrogate measures, such as adult CD utilities being used in cost-utility analyses in children.44 This points to the critical need to ascertain utilities for pediatric CD, particularly as the disease can manifest itself slightly differently in adults and children as children are developing.46 Due to the potential effect on growth, a child’s preference-based quality of life may be affected differently than an adult’s; thus adult utilities for CD health states are an inappropriate substitute for a child’s health state preferences. Eliciting generic pediatric health utilities can be a challenge in children, as completing a standard gamble or time trade-off task is limited by their developing cognitive abilities.47 Nevertheless, the determination of QALYs specific to children in CD will allow the comparison of CD treatments with treatments for other diseases, so that treatments in pediatric CD can be evaluated more effectively in economic evaluations by policy-makers making decisions about treatment coverage.17
A limitation of the study was that the study population may not reflect rural communities, as recruiting sites were in major urban centers and in academic teaching hospitals. Although this study took a Canadian health care payer perspective, practice patterns may have been skewed toward US practice, as most of the sites were in the United States. Upon close examination, there was no major difference between the health states and treatment patterns of Canadian and American RISK-PROKIIDS study patients. The entry of new biologics and biosimilars into the market was not included in the study and may affect the external validity of the study. However, the impact of changes in the prices of these agents was tested in the scenario analysis. Similarly, newer practices such as therapeutic drug monitoring were not included in the study, but these are not yet considered standard practice. Another limitation was that, due to a lack of data, the increasingly important treatment targets of endoscopic improvement and mucosal healing were not assessed. Also due to limitations in the data, it was not possible to adequately further subclassify patients on the basis of disease severity and provide generalizable results. The RISK-PROKIIDS patients moved frequently and unpredictably between active and remission phases of disease. Hence, the number of people in steroid-free remission at any one time could vary and may not be an adequate end point on its own to measure efficacy of treatment. Multiple clinical end points should be considered and reported to avoid reporting bias. The absence of QALYs as the primary effectiveness measure limit the comparability of the treatments to other treatments. A limitation of this study may be that for a chronic lifelong illness, a 3-year time horizon is too short and a lifetime horizon would be more suitable. However, data beyond 3 years in newly diagnosed children with CD prescribed firstline anti-TNF-α therapy are very limited to reasonably inform a longer-term model. Although the microsimulation model reflected a 3-year time horizon, it is likely that patients will be on anti-TNF therapy beyond 3 years and this will increase total costs over the long term. It can be assumed that additional patients in the step-up group would switch to anti-TNF beyond 3 years, and thus average treatment costs per patient between the 2 groups would equalize. However, long-term data would be required to estimate cost-effectiveness over longer time horizons.
From a clinical management perspective, our results are supportive of the use of anti-TNF-α treatments within the first 3 months of diagnosis to improve clinical outcomes. However, due to public payer drug formularies, such as in Canada and the United Kingdom, restricting anti-TNF-α use to second-line therapy, and according to their licensed indications, it may be difficult to implement such a strategy, as it would still be considered off-label use. Nevertheless, policies should ensure that once treatment with anti-TNF-α is warranted, that it is accessed quickly and that administrative delays in processing and approving applications for special access and reimbursement are avoided, as delays have been observed in Ontario.48
CONCLUSIONS
The purpose of this study was to provide additional rigorous evidence to support policy decision-making regarding the use and timing of anti-TNF-α treatments for the treatment of moderate to severe pediatric CD. Cost-effectiveness analyses from both the public health care payer and societal perspectives provide a more comprehensive analysis of the costs and consequences involved in the management of pediatric CD with anti-TNF-α therapy. Uncertainty surrounding the ICER made it difficult to state unequivocally that the early anti-TNF-α strategy was cost-effective compared with standard care, particularly as the willingness to pay for a week in steroid-free remission is unknown. The results are nevertheless of value to drug plan decision-makers, as they show that early anti-TNF-α intervention can be clinically beneficial to children with CD and that the cost of anti-TNF-α is a major driver in its cost-effectiveness. Based on our model, infliximab would likely be cost-effective at 25% of its current Ontario price. Biosimilar infliximab is 53% of the cost of originator infliximab in the Ontario Drug Formulary. Negotiating much lower costs for these treatments could ultimately benefit patients and payers.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge with thanks data provided for this study from the RISK-PROKIIDS study. The RISK Study and Dataset were solely and fully funded by the Crohn’s and Colitis Foundation of America under the umbrella of the PRO-KIIDS pediatric research network. We would like to thank Dr. Mary-Ellen Hogan, Mr. Austin Nam, Dr. Petros Pechlivanoglou, Dr. Lusine Abrahamyan, and Dr. Eleanor Pullenayegum for their help and suggestions.
Supported by: This work was supported by the Canadian Institutes of Health Research (CIHR) operating grant (#137137) to W. Ungar and the Ontario Ministry of Health and Long-term Care Drug Innovation Fund Grant (#2010-001). Additional financial support for this work was provided to N. Bashir in the form of a Hospital for Sick Children Restracomp Doctoral Scholarship, a University of Toronto Institute of Health Policy, Management and Evaluation (IHPME) Graduate Fellowship, the Eugene Vayda Award, the Dr. Robert Duff Barron Graduate Scholarship in Public Health Policy Award, a Doctoral Completion Award, an IHPME Travel Award, the Canadian Federation of University Women Dr. Alice B. Wilson Award, and a CIHR Travel Award. Dr. Wendy Ungar is supported by a Canada Research Chair in Economic Evaluation and Technology Assessment in Child Health.
Conflicts of interest: Naazish Bashir has no conflicts of interest. Thomas Walters has served on advisory boards for AbbVie and Janssen, received research support from AbbVie, and participated in speakers’ bureaus for AbbVie, Janssen, and Nestle Health Sciences. Anne Griffiths has been a consultant for Abbvie, Ferring, Gilead, Janssen, Lilly, Merck, and Roche, has received speaker fees from Abbvie, Janssen, and Shire, and has received research support from Abbvie. Shinya Ito has no conflicts of interest. Wendy Ungar has no conflicts of interest.
Author contributions: Naazish Bashir was involved in the study concept and design, acquisition of data, statistical analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, approval of the final manuscript, and obtaining funding support. Thomas Walters was involved in study supervision, study concept and design, acquisition of data, interpretation of data, critical revision of the manuscript for important intellectual content, and approval of the final manuscript. Anne Griffiths was involved in study supervision, study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content, and approval of the final manuscript. Shinya Ito was involved in study supervision, critical revision of the manuscript for important intellectual content, approval of the final manuscript, and obtaining funding support. Wendy Ungar was involved in study supervision, study concept and design, acquisition of data, interpretation of data, critical revision of the manuscript for important intellectual content, approval of the final manuscript, and obtaining funding support.
REFERENCES
- 1. Benchimol EI, Fortinsky KJ, Gozdyra P, et al. . Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423–439. [DOI] [PubMed] [Google Scholar]
- 2. Yang LS, Alex G, Catto-Smith AG. The use of biologic agents in pediatric inflammatory bowel disease. Curr Opin Pediatr. 2012;24:609–614. [DOI] [PubMed] [Google Scholar]
- 3. Colombel J-F, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology. 2017;152:351–361.e5. [DOI] [PubMed] [Google Scholar]
- 4. Mack DR, Benchimol EI, Critch J, et al. . Guidelines for the medical management of pediatric luminal Crohn’s disease from the Canadian children inflammatory bowel disease network and the Canadian Association of Gastroenterology Toronto Consensus. Gastroenterology. 2019;157:320–348. [DOI] [PubMed] [Google Scholar]
- 5. Walters TD, Kim MO, Denson LA, et al. ; PRO-KIIDS Research Group Increased effectiveness of early therapy with anti-tumor necrosis factor-α vs an immunomodulator in children with Crohn’s disease. Gastroenterology. 2014;146:383–391. [DOI] [PubMed] [Google Scholar]
- 6. Church PC, Guan J, Walters TD, et al. . Infliximab maintains durable response and facilitates catch-up growth in luminal pediatric Crohn’s disease. Inflamm Bowel Dis. 2014;20:1177–1186. [DOI] [PubMed] [Google Scholar]
- 7. D’Haens G, Baert F, van Assche G, et al. . Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–667. [DOI] [PubMed] [Google Scholar]
- 8. Baert F, Moortgat L, Van Assche G, et al. . Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463–468; quiz e10. [DOI] [PubMed] [Google Scholar]
- 9. Khanna R, Bressler B, Levesque BG, et al. . REACT Study Investigators Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet. 2015;386:1825–1834. [DOI] [PubMed] [Google Scholar]
- 10. Spurio F. Early treatment in Crohn’s disease: do we have enough evidence to reverse the therapeutic pyramid? J Gastrointestin Liver Dis. 2012;21:67–73. [PubMed] [Google Scholar]
- 11. Tsui JJ, Huynh HQ. Is top-down therapy a more effective alternative to conventional step-up therapy for Crohn’s disease? Ann Gastroenterol. 2018;31:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang DH, Harrington AR, Lee JK, et al. . A systematic review of economic studies on biological agents used to treat Crohn’s disease. Inflamm Bowel Dis. 2013;19:2673–2694. [DOI] [PubMed] [Google Scholar]
- 13. Beilman CL, Kirwin E, Ma C, et al. . A103 early initiation of anti-TNF therapy is cost-saving compared to late initiation for patients with Crohn’s disease. J Can Assoc Gastroenterol. 2018;1:177–178. [Google Scholar]
- 14. Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the Economic Evaluation of Health Technologies: Canada. 4th ed Ottawa: Canadian Agency for Drugs and Technologies in Health; 2017. https://www.cadth.ca/about-cadth/how-we-do-it/methods-and-guidelines/guidelines-for-the-economic-evaluation-of-health-technologies-canada. Accessed September 7, 2018. [Google Scholar]
- 15. Neumann PJ, Sanders GD, Basu A, et al. . Recommendations on perspectives for the reference case. In: Neumann PJ, Ganiats TG, Russell LB, Sanders GD, Siegel JE, eds. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 2016:68–74. [Google Scholar]
- 16. National Institute of Health and Care Excellence (NICE). Guide to the Methods of Technology Appraisal 2013. London, UK: National Institute of Health and Care Excellence; 2013. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed July 25, 2019. [PubMed] [Google Scholar]
- 17. Ungar WJ, Gerber A. The uniqueness of child health and challenges to measuring costs and consequences. In: Ungar WJ, ed. Economic Evaluation in Child Health. New York: Oxford University Press; 2010:3–32. [Google Scholar]
- 18. Kugathasan S, Walters T, Dubinsky M, et al. . RISK Cohort Study in Crohn’s Disease 2008 https://prokiids.com/RISK_Study_Description.html. Accessed 25 July 2019.
- 19. Blackburn SC, Wiskin AE, Barnes C, et al. . Surgery for children with Crohn’s disease: indications, complications and outcome. Arch Dis Child. 2014;99:420–426. [DOI] [PubMed] [Google Scholar]
- 20. Department of Demography Université de Montréal (Canada) Canadian Human Mortality Database. www.demo.umontreal.ca/chmd/. Accessed November 29, 2014.
- 21. Drummond MF, Sculpher MJ, Torrance GW, et al. . Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005. [Google Scholar]
- 22. Halpern EF, Weinstein MC, Hunink MG, et al. . Representing both first- and second-order uncertainties by Monte Carlo simulation for groups of patients. Med Decis Making. 2000;20:314–322. [DOI] [PubMed] [Google Scholar]
- 23. O’Hagan A, Stevenson M, Madan J. Monte Carlo probabilistic sensitivity analysis for patient level simulation models: efficient estimation of mean and variance using ANOVA. Health Econ. 2007;16:1009–1023. [DOI] [PubMed] [Google Scholar]
- 24. Dulai PS, Thompson KD, Blunt HB, et al. . Risks of serious infection or lymphoma with anti-tumor necrosis factor therapy for pediatric inflammatory bowel disease: a systematic review. Clin Gastroenterol Hepatol. 2014;12:1443–1451; quiz e88. [DOI] [PubMed] [Google Scholar]
- 25. Hyams J, Walters TD, Crandall W, et al. . Safety and efficacy of maintenance infliximab therapy for moderate-to-severe Crohn’s disease in children: REACH open-label extension. Curr Med Res Opin. 2011;27:651–662. [DOI] [PubMed] [Google Scholar]
- 26. Leonor R, Jacobson K, Pinsk V, et al. . Surgical intervention in children with Crohn’s disease. Int J Colorectal Dis. 2007;22:1037–1041. [DOI] [PubMed] [Google Scholar]
- 27. Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2017. Toronto: Canadian Cancer Society; 2017. http://www.cancer.ca/~/media/cancer.ca/CW/publications/Canadian%20Cancer%20Statistics/Canadian-Cancer-Statistics-2017-EN.pdf. Accessed December 14, 2017. [Google Scholar]
- 28. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. . CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 29. Aquilina A, Bisson R, Brennan J, et al. . Guidelines for the Administration of Enteral and Parenteral Nutrition in Paediatrics. 3rd ed Toronto: Hospital for Sick Children; 2007. [Google Scholar]
- 30. Statistics Canada. Table 14-10-0320-02 Average Usual Hours and Wages by Selected Characteristics, Monthly, Unadjusted for Seasonality (x 1000). Ottawa, Canada: Statistics Canada; 2014. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410032002 Accessed February 18, 2015. [Google Scholar]
- 31. Ruemmele FM, Veres G, Kolho KL, et al. . European Crohn’s and Colitis Organisation; European Society of Pediatric Gastroenterology, Hepatology and Nutrition Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8:1179–1207. [DOI] [PubMed] [Google Scholar]
- 32. Rufo PA, Denson LA, Sylvester FA, et al. . Health supervision in the management of children and adolescents with IBD: NASPGHAN recommendations. J Pediatr Gastroenterol Nutr. 2012;55:93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim MJ, Lee JS, Lee JH, et al. . Infliximab therapy in children with Crohn’s disease: a one-year evaluation of efficacy comparing ‘top-down’ and ‘step-up’ strategies. Acta Paediatr. 2011;100:451–455. [DOI] [PubMed] [Google Scholar]
- 34. Lee YM, Kang B, Lee Y, et al. . Infliximab “top-down” strategy is superior to “step-up” in maintaining long-term remission in the treatment of pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2015;60:737–743. [DOI] [PubMed] [Google Scholar]
- 35. Benchimol EI, Mack DR, Nguyen GC, et al. . Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology. 2014;147:803–813.e7; quiz e14. [DOI] [PubMed] [Google Scholar]
- 36. Ruemmele FM, Hyams JS, Otley A, et al. . Outcome measures for clinical trials in paediatric IBD: an evidence-based, expert-driven practical statement paper of the paediatric ECCO committee. Gut. 2015;64:438–446. [DOI] [PubMed] [Google Scholar]
- 37. Gasparetto M, Guariso G. Crohn’s disease and growth deficiency in children and adolescents. World J Gastroenterol. 2014;20:13219–13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heuschkel R, Salvestrini C, Beattie RM, et al. . Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:839–849. [DOI] [PubMed] [Google Scholar]
- 39. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619–1635. [DOI] [PubMed] [Google Scholar]
- 40. Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. [DOI] [PubMed] [Google Scholar]
- 41. Lachaine J, Miron A, Beauchemin C, et al. . Economic evaluations of treatments for inflammatory bowel diseases: a literature review. Can J Gastroenterol Hepatol. 2018;2018:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huoponen S, Blom M. A Systematic review of the cost-effectiveness of biologics for the treatment of inflammatory bowel diseases. PLoS One. 2015;10:e0145087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pillai N, Dusheiko M, Burnand B, et al. . A systematic review of cost-effectiveness studies comparing conventional, biological and surgical interventions for inflammatory bowel disease. PLoS One. 2017;12:e0185500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Punekar YS, Sunderland T, Hawkins N, et al. . Cost-effectiveness of scheduled maintenance treatment with infliximab for pediatric Crohn’s disease. Value Health. 2010;13:188–195. [DOI] [PubMed] [Google Scholar]
- 45. Blackhouse G, Assasi N, Xie F, et al. . Canadian cost-utility analysis of initiation and maintenance treatment with anti-TNF-α drugs for refractory Crohn’s disease. J Crohns Colitis. 2012;6:77–85. [DOI] [PubMed] [Google Scholar]
- 46. Kelsen J, Baldassano RN. Inflammatory bowel disease: the difference between children and adults. Inflamm Bowel Dis. 2008;14(Suppl 2):S9–11. [DOI] [PubMed] [Google Scholar]
- 47. Ungar WJ. Challenges in health state valuation in paediatric economic evaluation: are QALYs contraindicated? Pharmacoeconomics. 2011;29:641–652. [DOI] [PubMed] [Google Scholar]
- 48. Office of the Auditor General of Ontario. Ontario Public Drug Programs. Toronto: Ontario Ministry of Health and Long-Term Care; 2017. http://www.auditor.on.ca/en/content/annualreports/arreports/en17/v1_309en17.pdf. Accessed July 31, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.