Abstract
Background
Peripheral and mucosal eosinophilia may be associated with more aggressive disease in inflammatory bowel disease (IBD) patients. Vedolizumab blocks T lymphocytes, eosinophil adhesion, and extravasation in the gastrointestinal tract. It is not known if mucosal eosinophilia is a predictor for the therapeutic efficacy of vedolizumab.
Methods
This was a retrospective cohort study of IBD patients with ileal or colonic biopsies who were off steroids before starting vedolizumab. Biopsies were rereviewed by pathologists, and mean eosinophil density was quantified. Patient characteristics and steroid-free clinical response 6 months after beginning vedolizumab were determined. Features were compared between nonresponders and responders, and multivariable logistic regression was performed to identify predictors of clinical response.
Results
Of 251 IBD patients starting vedolizumab therapy, 65 patients (48% Crohn’s disease, 52% ulcerative colitis) met inclusion criteria. All IBD patients not responding to vedolizumab were more likely to have a higher baseline mean eosinophil count (340 ± 156 vs 236 ± 124; P = 0.004), be previously exposed to an anti-TNF (96% vs 56%; P = 0.001), and be male (58% vs 28%; P = 0.02). Mean eosinophil counts were significantly increased in colonic biopsies in UC nonresponders (438 ± 149 vs 299 ± 145; P = 0.01). A similar trend was seen in CD nonresponders. On multivariable analysis, colonic eosinophil density and prior anti-TNF exposure—and the combination of both—were independent predictors of response.
Conclusion
In ulcerative colitis, colonic eosinophilia and prior anti-TNF exposure were independent predictors of 6-month clinical nonresponse to vedolizumab. Mucosal eosinophil density as a novel biomarker should be explored in larger patient cohorts.
Aside from the previous anti-TNF exposure, eosinophil density in the colon of patients with UC is a negative predictor for a steroid-free long-term response to vedolizumab. The degree colonic eosinophilia may be a novel biomarker that should be further explored.
Keywords: inflammatory bowel diseases, Crohn’s disease, ulcerative colitis, eosinophil, vedolizumab, biomarker
Aside from the previous anti-TNF exposure, eosinophil density in the colon of patients with UC is a negative predictor for a steroid-free long-term response to vedolizumab. The degree colonic eosinophilia may be a novel biomarker that should be further explored.
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) are clinical separate disease entities and belong to the group of inflammatory bowel diseases (IBDs).1 The exact cause of IBD is unknown, but the evolution of chronic intestinal inflammation is most likely due to an aberrant interaction between genetic susceptibility, environmental factors, gut flora, and an abnormal immune response.2 Athough CD and UC have distinct clinical, endoscopic, radiological, and histological findings, in both diseases the migration of leukocytes, including T lymphocytes and eosinophils, to the intestinal site of inflammation is one of the pathophysiological key events. For both UC and CD, histologically the presence of neutrophils and eosinophils in the intestinal mucosa is a hallmark of active disease.3
In the absence of a curative IBD therapy, current medical approaches aim to either block broadly inflammatory pathways such as steroids or immunomodulators or more selectively modulate intestinal inflammation by blocking critical inflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-12, and IL-23.4 An alternative therapeutic approach is the blockade of adhesion molecules preventing adhesion and extravasation of lymphocytes and other cells into inflamed tissue. Vedolizumab is a monoclonal antibody that binds to the α4β7 integrin, which mediates T lymphocyte and eosinophil-selective migration and adhesion to the gastrointestinal (GI) tract.5 Binding of this integrin allows vedolizumab to block lymphocytic and eosinophil adhesion and extravasation into the bowel. Eosinophils are usually present in low numbers throughout the GI tract (except for the esophagus), but their number increases in disease states.6 Gut eosinophilia is common in IBD, and eosinophils are thought to have a role in IBD pathogenesis.7–9 Additionally, peripheral blood eosinophilia in patients with IBD has shown to be associated with more complicated disease course, which indicates that there can be a relationship between disease severity and the degree of eosinophilia.10 Due to the specific mechanism of vedolizumab blocking the adhesion and extravasation of eosinophils, we hypothesized that eosinophil density in the GI tract could be a biomarker for vedolizumab efficacy, with higher mucosal eosinophil density being predictive of response to vedolizumab. This study aimed to analyze the association of small bowel and colonic mucosal eosinophil density in IBD patients before the start of vedolizumab and associate the density with the clinical response to vedolizumab therapy during the initial 6 months of treatment.
METHODS
Patient Population
We performed a retrospective cohort study examining adult patients with IBD initiated on vedolizumab therapy within the University of North Carolina (UNC) Hospitals system between 2014 and 2017. Patients were identified for inclusion in the study using the International Classification of Diseases 9th and 10th Clinical Modification (ICD-9-CM and ICD-10-CM) coding (556.xx and K51.xx) and the Informatics for Integrating Biology and the Bedside (i2b2) platform through the Carolina Data Warehouse for Health. The i2b2 platform was developed by the i2b2 Center, a National Institutes of Health (NIH)–funded National Center for Biomedical Computing based at Partners HealthCare System in Boston, Massachusetts.11
Patients were eligible for inclusion based on the following criteria: 1) 18 years of age or older, 2) an existing diagnosis of UC or CD, 3) initiation of treatment with vedolizumab between July 1, 2014, and December 31, 2017, and available follow-up documentation of therapy and treatment outcome in the electronic medical records at our center, and 4) available colonic and/or ileal biopsies that were taken during an index colonoscopy in a time frame of up to 12 months before initiation of vedolizumab therapy. Patients with intestinal biopsies but steroid therapy within 4 weeks before the index colonoscopy were excluded from these analyses.
Outcome of Interest
The primary aim of this study was to evaluate the clinical response to vedolizumab at 6 months in relation to the mean eosinophil density in intestinal biopsies at the start of vedolizumab therapy. Clinical response was defined as any improvement in stool frequency, blood in bowel movements, abdominal pain, and general wellbeing after initiation of vedolizumab as documented by the treating physician in the electronic medical record and the persistence of vedolizumab therapy at 6 months without the need for steroids.
Covariates
Multiple variables were analyzed to evaluate their potential relationship with a clinical response with vedolizumab. These variables included sex, time since diagnosis, disease location according to the Montreal classification,12 body mass index (BMI) at the time of initiation of vedolizumab, and prior therapies.
Evaluation of Eosinophil Number and Density
Colonoscopy and pathology reports were reviewed. All IBD patients who had an index colonoscopy in a time frame of up to 12 months before initiation of vedolizumab therapy and were on no systemic or topical steroids within 4 weeks before this procedure were included in the study. The biopsies had been collected in the setting of clinical care in a nonstandardized fashion, and only the information about location of the biopsies (small or large bowel) and the presence of inflammation but no detailed descriptions of the biopsy site were available. Archived hematoxylin and eosin slides generated from biopsies taken from inflamed colonic or small bowel in IBD patients were pulled and digitized, and the digital slides were rereviewed by pathologists to determine the eosinophil count as per our previously published protocol for use in the GI tract.13, 14 In brief, the biopsy was first examined at low power to determine areas of eosinophil infiltration. Then, using a high-power setting on the imaging software, the number of eosinophils were quantified in 5 microscopy high-power fields (HPFs), each with a measured area, such that the eosinophil density could be calculated (eosinophils/mm2). The mean eosinophil count from these 5 HP’s was our primary study measure. In addition to the eosinophil count, peak eosinophil density (highest count of all of the 5 HPFs examined), eosinophil degranulation, and eosinophil microabscesses (defined as clusters of 4 or more eosinophils) were quantified.
Laboratory Data
If available, peripheral eosinophil counts were retrieved from the laboratory data if available at index colonoscopy. All other lab values were collected on the first vedolizumab infusion, at which at our institution a complete blood count, C-reactive protein, and liver function tests are drawn in the setting of the standard of care.
Statistical Analysis
Continuous variables are presented using means and standard deviations and compared using Student t tests or Wilcoxon-rank-sum testing, as appropriate. Proportions are used to express categorical variables, which were analyzed using χ2 testing or Fisher exact test, as appropriate. Potential predictors of inadequate response to vedolizumab were evaluated using univariable and multivariable logistic regression to adjust for confounders. In multivariable logistic regression models, all variables included were selected a priori given their suspected relevance to potential response to vedolizumab. A backward elimination approach was used to reduce the model to identify independent predictors. In addition, receiver operator characteristic (ROC) curves were constructed and the area under the curve (AUC) calculated. A 2-tailed P value of 0.05 was chosen as the threshold for statistical significance for all tests. Unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) are presented. All analyses were performed using Stata (version 9) statistical software (StataCorp, College Station, TX, USA).
Ethical Considerations
The study protocol was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill (IRB No 17-3321).
RESULTS
Between 2014 and 2017, 251 patients were treated with vedolizumab at our institution. Of these, 186 patients were excluded from the analyses due to no available biopsies in the predefined timeframe before the start of vedolizumab, concomitant steroid therapy at the index colonoscopy before the initiation of vedolizumab, or the presence of a pouch. The remaining 65 IBD patients (CD, 31of 65 [48%] and UC, 34 of 65 [52%]) were included in the study (Table 1). The majority of CD patients (94%) had ileocolonic or colonic involvement (L2 or L3), and 58% were classified as inflammatory phenotype (B1).12 More than 60% of UC patients had pancolitis, and only 1 patient (3%) suffered from proctitis only. The majority of patients had been treated with immunomodulators (64%), and at least 1 anti-TNF therapy (72%) before starting vedolizumab.
TABLE 1.
Demographics of All Patients (n = 65)
| Characteristic | |
|---|---|
| Age at vedolizumab start (mean yrs ± SD) | 38.1 ± 16.8 |
| Female (n, %) | 39 (60) |
| BMI (mean kg/m2 ± SD) | 28.8 ± 8.1 |
| IBD type (n, %)a | |
| Crohn’s disease | 31 (48) |
| A1 | 10 (32) |
| A2 | 14 (45) |
| A3 | 7 (23) |
| L1 | 2 (6) |
| L2 | 11 (36) |
| L3 | 18 (58) |
| L4 | 0 (0) |
| B1 | 18 (58) |
| B2 | 8 (26) |
| B3 | 5 (16) |
| perianal disease | 2 (6) |
| Ulcerative colitis | 34 (52) |
| E1 | 1 (3) |
| E2 | 12 (35) |
| E3 | 21 (62) |
| Laboratory values | |
| Hemoglobin (mean ± SD) (n = 64) | 12.4 ± 1.9 |
| CRP (mean ± SD) (n = 50) | 8.0 ± 12.1 |
| Peripheral eosinophil count (mean ± SD) (n = 40) | 0.25 ± 0.19 |
| Prior medications (n, %) | |
| Any prior anti-TNF | 47 (72) |
| Any prior immunomodulator | 42 (64) |
aAccording to the Montreal classification12
Clinical Response and Persistence of Vedolizumab Therapy at 6 Months
Overall, 39 of 65 (60%) of IBD patients achieved a clinical response and were still on vedolizumab 6 months after the initiation of this therapy (Table 2). Patients with CD showed slightly higher response rates (20 of 31, 65%) compared with UC patients (19 of 34, 55%). Vedolizumab was administered with concomitant immunomodulators in comparable frequency in both groups (15 of 39 [38%] and 10 of 26 [38%] for nonresponders and responders, respectively). In 6 patients (4 CD and 2 UC), the vedolizumab dose was increased to 4 weekly applications, which restored the clinical response in 50% (3 of 6, 2 CD and 1 UC).
TABLE 2.
Demographics and Mucosal Eosinophil Density of Nonresponder and Responders to Vedolizumab Therapy
| No response (n = 26) | Response (n = 39) | P | |
|---|---|---|---|
| Age at vedolizumab start (mean yrs ± SD) | 37.6 ± 18.9 | 38.4 ± 15.6 | 0.86 |
| Female (n, %) | 11 (42) | 28 (72) | 0.02 |
| BMI (mean kg/m2 ± SD) | 26.4 ± 6.2 | 30.4 ± 8.9 | 0.05 |
| Nonsmoker (n, %) | 20 (77) | 30 (77) | 0.77 |
| IBD type (n, %) | |||
| Crohn’s | 11 (42) | 20 (51) | 0.66 |
| UC | 15 (58) | 19 (49) | |
| Disease duration (mean yrs ± SD) | 9.9 ± 8.3 | 9.8 ± 8.6 | 0.97 |
| Hemoglobin (n = 64;mean mg/dl ± SD) | 12.4 ± 2.2 | 12.4 ± 1.7 | 0.98 |
| CRP (n = 50; mean mg/dl ± SD) | 10.0 ± 16.7 | 6.6 ± 7.6 | 0.52 |
| Concomitant immunomodulators with vedolizumab | |||
| Azathioprine, 6-mercaptopurine (n, %) | 4 (15) | 7 (18) | 0.82 |
| Methotrexate (n, %) | 6 (23) | 8 (21) | 0.78 |
| Prior Biologics (n, %) | |||
| Any prior anti-TNF (n, %) | 25 (96) | 22 (56) | 0.001 |
| More than one prior anti-TNF (n, %) | 16 (62) | 12 (31) | 0.01 |
| More than one prior class of biologic (n, %) | 1 (4) | 0 (0) | 0.22 |
| Any prior immunomodulator (n, %) | 19 (73) | 23 (29) | 0.24 |
| Any prior anti-TNF and immunomodulator (n, %) | 19 (73) | 16 (41) | 0.01 |
| Eosinophil counts | |||
| Peripheral eosinophil count (n = 40;mean eos x 109/L ± SD) | 260 ± 180 | 250 ± 210 | 0.80 |
| Mean mucosal eosinophil density (mean eos/mm2 ± SD) | 339.5 ± 155.7 | 235.2 ± 123.6 | 0.004 |
| Other histologic findings (n, %) | |||
| Eosinophil degranulation | 25 (96) | 35 (90) | 0.34 |
| Eosinophil microabscesses | 20 (77) | 27 (68) | 0.50 |
| Diffuse eosinophils on biopsy | 18 (69) | 27 (69) | 1.0 |
| Extensive epithelial damage | 13 (50) | 17 (45) | 0.63 |
| Severe architectural changes | 9 (36) | 16 (41) | 0.80 |
| Diffuse polymorphonuclear neutrophils on biopsy | 7 (27) | 5 (13) | 0.24 |
| Erosions/ulcers | 18 (72) | 25 (64) | 0.51 |
| Granulomas (CD) | 6 (23) | 8 (21) | 0.85 |
| ≥50% crypts involved (UC) | 6 (40) | 9 (45) | 0.71 |
Eosinophil Density in Colonic and Small Bowel Biopsies
The overall mean mucosal eosinophil density in colonic and small bowel biopsy samples was 268 ± 146 eosinophils/mm2. The mean density was highest in colonic biopsies from UC patients (312 ± 152), whereas a slightly lower eosinophil density was observed in biopsies taken from the colon and small bowel of CD patients (272 ± 164 and 243 ± 145, respectively). The mean mucosal eosinophil density in all nonresponders was significantly increased compared with responders (Table 2). There was no difference between nonresponders and responders regarding eosinophil degranulation or microabscesses and other histologic measures of inflammation and the peripheral eosinophil count.
When analyzing UC and CD separately, the mean eosinophil density was significantly increased in UC nonresponders vs responders (438 ± 149 vs 299 ± 145), and there was a trend to an increased eosinophil density in colonic biopsies from CD nonresponders (353 ± 173 vs 232 ± 149), whereas no such difference could be detected in biopsies from nonresponding and responding CD patients (Table 3).
TABLE 3.
Peak and Mean Eosinophil Density in Nonresponder and Responders to Vedolizumab Therapy
| Eosinophil counts (mean eos/mm2 ± SD) | No response | Response | P |
|---|---|---|---|
| Colonic (UC + CD) | n = 23 | n = 35 | |
| Mean eosinophil density | 408.7 ± 159.6 | 268.4 ± 148.6 | 0.001 |
| UC colon | n = 15 | n = 19 | |
| Mean eosinophil density | 438.4 ± 149.4 | 298.9 ± 145.3 | 0.01 |
| CD colon | n = 8 | n = 16 | |
| Mean eosinophil density | 352.9 ± 172.9 | 232.0 ± 148.7 | 0.09 |
| CD small bowel | n = 10 | n = 9 | |
| Mean eosinophil density | 235.0 ± 156.4 | 220.2 ± 122.2 | 0.83 |
Disease Characteristics as Predictors of Nonresponse to Vedolizumab
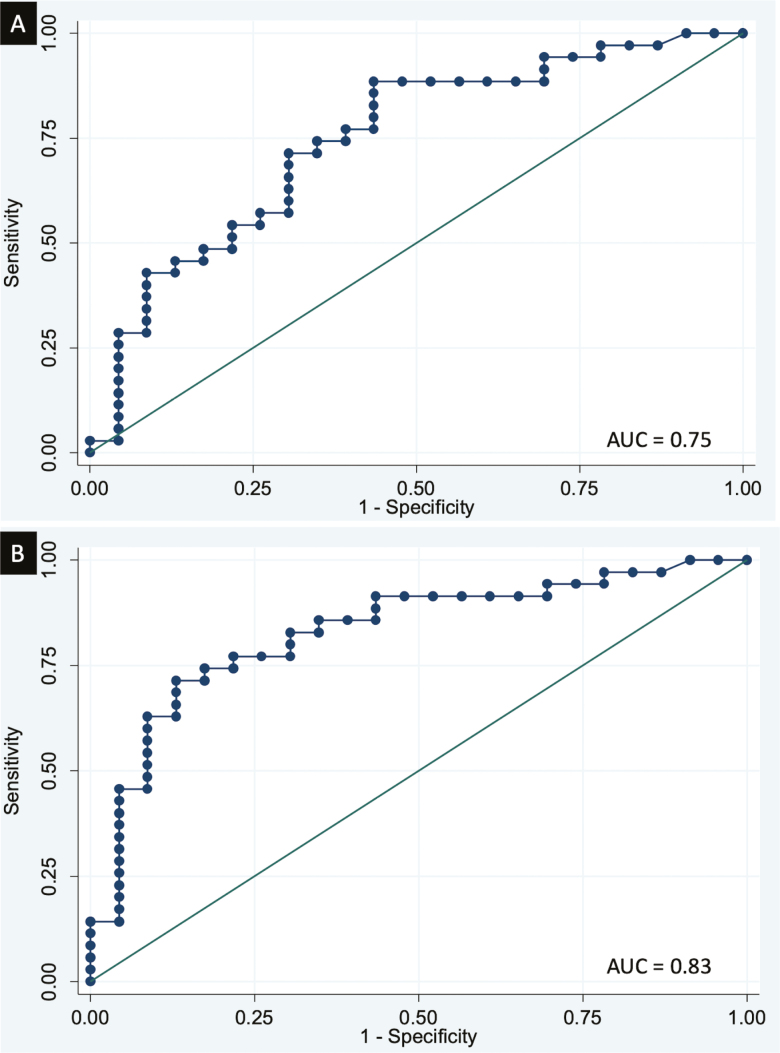
Predictors of nonresponse to vedolizumab after 6 months of therapy were male sex, a lower BMI, prior anti-TNF therapy, and higher mucosal eosinophil density (Table 2). Stratifying patients by previous TNF exposure revealed a significantly higher mean eosinophil density compared with patients with response, but no such difference could be observed in the anti-TNF-naïve population (Table 4). In an adjusted multivariate analysis, prior anti-TNF exposure, an increased eosinophil count, and male sex remained negative predictors for a clinical response to vedolizumab (Table 5). The odds ratio for colonic eosinophil density was calculated as a change per 25 eosinophils/mm2, implying that for every increase in 25 eosinophils/mm2, the odds of response decrease by 14% in the unadjusted and by 13% in the adjusted analysis. Receiver operating characteristic curves showed an area under the curve for prediction of nonresponse vs response to vedolizumab for previous anti-TNF exposure of 0.69, for the mean eosinophil count of 0.75, and both anti-TNF failure and eosinophil count of 0.83. (Fig. 1)
TABLE 4.
Peak and Mean Eosinophil Density in Nonresponder and Responders to Vedolizumab Therapy Stratified According to Previous anti-TNF Therapy Exposure
| Entire population eosinophil counts (mean eos/mm2 ± SD) | No response | Response | P |
|---|---|---|---|
| Anti-TNF naive | n = 1 | n = 15 | |
| Mean eosinophil density | 267.1 ± 0 | 278.0 ± 125.5 | |
| Anti-TNF exposed | n = 22 | n = 22 | |
| Mean eosinophil density | 415.1 ± 160.2 | 261.1 ± 166.7 | 0.004 |
TABLE 5.
Unadjusted and Adjusted Odds Ratios for the Relationship Between Colonic Eosinophil Count and Response to Vedolizumab in IBD Patients
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | |
|---|---|---|
| Female sex (vs male) | 3.47 (1.22–9.87) | 3.45 (0.87–13.7) |
| Any prior anti-TNF | 0.05 (0.01–0.42) | 0.06 (0.01–0.58) |
| UC (vs Crohns) | 0.70 (0.26–1.89) | — |
| Mean eosinophil density | 0.86 (0.78–0.95) | 0.994 (0.990–0.999) |
asex, any prior anti-TNF, and mean eososinophil density included in the model analysis
FIGURE 1.
ROC curves of (A) mean colonic eosinophil count and (B) mean colonic eosinophil count and prior anti-TNF exposure
DISCUSSION
With the rapidly increasing targeted therapeutic options in IBD, one of the ultimate goals of IBD therapy is the prediction of treatment response to specific therapies such as vedolizumab. Our study suggests that aside from previous anti-TNF therapy, mucosal eosinophil density predicts the 6 months therapeutic response of vedolizumab therapy, especially in patients with UC. A higher mucosal eosinophil density was independently associated with treatment failure, and a combination of colonic mucosal eosinophil density and previous anti-TNF exposure predicted nonresponse with high accuracy.
The vedolizumab steroid-free response rate in our cohort was similar to other reported real-world, steroid-free response and remission rates, which range between 40%–60%.15–17 Our findings of an inferior response to vedolizumab after the previous failure or exposure of anti-TNF therapies are confirmed by similar reports in both CD and UC patients. The studies of vedolizumab in patients with moderate to severe ulcerative colitis and moderate to severe Crohn’s disease—GEMINI 1, 2 and 3—demonstrated that previous treatment with TNF antagonists affect the efficacy of vedolizumab in patients with CD (GEMINI 2 and 3) but not with UC (GEMINI 1).18–20 However, subsequent analyses of the UC GEMINI 1 study revealed an inferior early response to vedolizumab compared with placebo in patients previously exposed to anti-TNF agents.21 Additionally, the recently reported results of VARSITY (An Efficacy and Safety Study of Vedolizumab Intravenous [IV] Compared with Adalimumab Subcutaneous [SC] in Participants With Ulcerative Colitis) and real-world cohorts also reported a lower efficacy of vedolizumab in patients with a history of previous anti-TNF failure compared with biologic-naïve patients.15, 22 The reasons for the lower response to vedolizumab after the failure of anti-TNF is not yet known, but the duration of disease, especially in CD, may play a role.23 Aside from the negative impact of previous anti-TNF exposure on the overall clinical efficacy of vedolizumab in both UC and CD, additional predictors for CD patients have been described utilizing large cohorts.23–25 An inferior response to vedolizumab has been demonstrated in CD patients with a history of previous surgery, low baseline C-reactive protein (CRP), lower albumin levels, fistulizing disease, and a disease duration of more than 2 years. Due to the small sample size with low numbers of the individual CD risk factors, none of these predictors reached statistical significance.
The finding of an increased eosinophil density in the gastrointestinal tract is a common feature of immunoglobulin (Ig)E-mediated and non-IgE-mediated digestive disorders, including eosinophilic gastroenteritis, eosinophilic esophagitis, gastroesophageal reflux disease, and inflammatory bowel diseases.26 Eosinophils are activated through the engagement of receptors for cytokines, immunoglobulins, and complement, which leads to a release of an array of pro- or anti-inflammatory cytokines (IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, IL-16, IL-18, TGF-a, and TGF-b), chemokines (RANTES and eotaxin-1), lipid mediators (platelet-activating factor and leukotriene C4), histamine, proteolytic enzymes, and antimicrobial factors.27 In the normal healthy gastrointestinal tract, eosinophils are thought to be protective by attenuating inflammatory responses, but in excess numbers, eosinophils contribute to inflammatory damage and tissue remodeling.28 Tissue remodeling may lead to stricture formation, as shown in a recent study linking ileal eosinophilia to a fibrostenosing phenotype in CD.29 Eosinophils are not pathognomonic of IBD but represent a major component of the inflammatory infiltrate. Increased mucosal eosinophil numbers in patients in both active and inactive UC or CD have been described.9, 30–32 Increased mucosal eosinophils have been associated with treatment response in ulcerative proctosigmoiditis.33 In CD, increased mucosal eosinophil density or increased peripheral eosinophilia have been found to be predictive of clinical relapse and a more refractory disease course, respectively.10, 34
The association of an increased eosinophil density with a lower response rate to vedolizumab therapy has not been previously reported. The higher mucosal eosinophil density in the nonresponders to vedolizumab therapy did not correlate with peripheral eosinophilia. Similarly, it has been shown that in IBD patients, mucosal eosinophilia does not correspond with the degree of peripheral eosinophil count or products of eosinophil activation.35 In the multivariate analysis, mucosal eosinophilia was independently associated with nonresponse to vedolizumab therapy. The mechanism underlying this association is speculative, but we can offer a few hypotheses. Early clinical investigations attempted to determine whether IBD was an allergic disorder, but such an association could never be confirmed.36 However, a higher incidence of eosinophilic esophagitis in patients with IBD has been reported and was associated with worse clinical outcome, which implicates environmental triggers in both diseases.37, 38 Akin to eosinophilic esophagitis, food antigens may trigger an increased eosinophil influx in the small and large bowel and thus hamper the medical control of the intestinal inflammation.39 Recently, an association of depression and colonic eosinophilia has been described for patients with irritable bowel syndrome.40 Depression has also been associated with worse outcome in IBD, including increased risk of relapse, hospitalization, and surgery.41 Due to the retrospective nature of our cohort, we were not able to evaluate if an increased eosinophil density was correlated with a higher frequency of depression.
Our study has several strengths. We used a standardized quantitative evaluation for tissue eosinophil density, which has been previously validated with excellent interobserver agreements.13, 14 Second, we used stringent inclusion criteria in our cohort, excluding all patients with steroid exposure for at least 4 weeks before the collection of biopsies. Eosinophils are highly susceptible to steroids, and as such, the density cannot reliably be analyzed if steroids were used shortly before the biopsies were taken.42 Third, because we included only patients who were treated continuously at our institution, the persistence of vedolizumab therapy and the prescription of steroids could be reliably verified in our electronic medical record system (EPIC).
This study also has several limitations, including the sample size. The main reason for the small number of patients included in this cohort was the stringent inclusion criteria, which necessitated the exclusion of many patients either due to concomitant steroid therapy at the index colonoscopy, or the availability of biopsies before the start of vedolizumab therapy. Second, due to the retrospective nature of the clinical data collection, no standardized prospectively collected indices, such as the Mayo score or Crohn’s disease activity index score, were available. Clinical response was judged by the physician’s impression rather than an objective scale. The subjective definition for response has the potential for a bias in the assessment of individual providers when evaluating the response of patients. However, the definition of response used in this study was strengthened by the hard outcome of no use of steroids at 6 months after initiation of treatment with vedolizumab and the persistence of vedolizumab infusion therapy. Third, biopsies were collected in a nonstandardized fashion, and only the information about location of the biopsies (small or large bowel) and the presence of inflammation but no detailed descriptions of the biopsy site were available.
In conclusion, colonic eosinophil density was significantly negatively associated with clinical response to vedolizumab therapy at 6 months. The negative impact of an increased mucosal eosinophil density on clinical vedolizumab efficacy was independent of the previously described lower response to vedolizumab in patients who were previously exposed to anti-TNF therapy. Thus, eosinophil density may present a novel mucosal biomarker predicting the treatment success of vedolizumab, which should be explored in larger patient cohorts.
ACKNOWLEDGMENTS
The i2b2 software was used in conducting this study. i2b2 is the flagship tool developed by the i2b2 (Informatics for Integrating Biology and the Bedside) Center, a National Institutes of Health (NIH)–funded National Center for Biomedical Computing based at Partners HealthCare System. The i2b2 instance at the University of North Carolina is supported by the National Center for Advancing Translational Sciences (NCATS), NIH, through Grant Award Number UL1TR002489.
Supported by: NIH T35 DK007386 (EMK).
Conflicts of Interest: HHH received consulting fees from Alivio, AMAG, Finch, Gilead, Lycera, Merck, Otsuka, Pfizer, PureTech, and Seres and research support from Pfizer and Artizan Biosciences. ESD received consulting fees from Adare, Aimmune, Alivio, Allakos, AstraZeneca, Banner, Biorasi, Calypso, Celgene/Receptos, Enumeral, EsoCap, Gossamer Bio, GSK, Regeneron, Robarts, Salix, and Shire; ESD received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; ESD received educational grants from Allakos, Banner, and Holoclara. EMK, CR, RB, SK AL, MF report no disclosures or conflicts of interest relevant to this study.
REFERENCES
- 1. Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. [DOI] [PubMed] [Google Scholar]
- 2. Sartor RB, Wu GD. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology. 2017;152:327–339.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019;50:992–1006. [DOI] [PubMed] [Google Scholar]
- 4. Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14:269–278. [DOI] [PubMed] [Google Scholar]
- 5. Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10:1437–1444. [DOI] [PubMed] [Google Scholar]
- 6. Mehta P, Furuta GT. Eosinophils in gastrointestinal disorders: eosinophilic gastrointestinal diseases, celiac disease, inflammatory bowel diseases, and parasitic infections. Immunol Allergy Clin North Am. 2015;35:413–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker MM, Potter MD, Talley NJ. Eosinophilic colitis and colonic eosinophilia. Curr Opin Gastroenterol. 2019;35:42–50. [DOI] [PubMed] [Google Scholar]
- 8. Filippone RT, Sahakian L, Apostolopoulos V, et al. . Eosinophils in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:1140–1151. [DOI] [PubMed] [Google Scholar]
- 9. Bischoff SC, Wedemeyer J, Herrmann A, et al. . Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology. 1996;28:1–13. [DOI] [PubMed] [Google Scholar]
- 10. Click B, Anderson AM, Koutroubakis IE, et al. . Peripheral eosinophilia in patients with inflammatory bowel disease defines an aggressive disease phenotype. Am J Gastroenterol. 2017;112:1849–1858. [DOI] [PubMed] [Google Scholar]
- 11. Murphy S, Wilcox A. Mission and sustainability of informatics for integrating biology and the bedside (i2b2). EGEMS (Wash DC). 2014;2:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Satsangi J, Silverberg MS, Vermeire S, et al. . The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dellon ES, Fritchie KJ, Rubinas TC, et al. . Inter- and intraobserver reliability and validation of a new method for determination of eosinophil counts in patients with esophageal eosinophilia. Dig Dis Sci. 2010;55:1940–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rusin S, Covey S, Perjar I, et al. . Determination of esophageal eosinophil counts and other histologic features of eosinophilic esophagitis by pathology trainees is highly accurate. Hum Pathol. 2017;62:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plevris N, Chuah CS, Allen RM, et al. . Real-world effectiveness and safety of vedolizumab for the treatment of inflammatory bowel disease: the Scottish vedolizumab cohort. J Crohns Colitis. 2019;13:1111–1120. [DOI] [PubMed] [Google Scholar]
- 16. Schreiber S, Dignass A, Peyrin-Biroulet L, et al. . Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol. 2018;53:1048–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dulai PS, Singh S, Jiang X, et al. . The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY consortium. Am J Gastroenterol. 2016;111:1147–1155. [DOI] [PubMed] [Google Scholar]
- 18. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 19. Sands BE, Sandborn WJ, Van Assche G, et al. . Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis. 2017;23:97–106. [DOI] [PubMed] [Google Scholar]
- 20. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. [DOI] [PubMed] [Google Scholar]
- 21. Feagan BG, Lasch K, Lissoos T, et al. . Rapid response to vedolizumab therapy in biologic-naive patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:130–138.e7. [DOI] [PubMed] [Google Scholar]
- 22. Sands BE, Peyrin-Biroulet L, Loftus EV, et al. . Vedolizumab shows superior efficacy versus adalimumab: results of varsity-the first head-to-head study of biologic therapy for moderate-to-severe ulcerative colitis. Gastroenterology. 2019;156:S81. [Google Scholar]
- 23. Faleck DM, Winters A, Chablaney S, et al. . Shorter disease duration is associated with higher rates of response to vedolizumab in patients with Crohn’s disease but not ulcerative colitis. Clin Gastroenterol Hepatol. 2019. doi:10.1016/j.cgh.2018.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dulai PS, Boland BS, Singh S, et al. . Development and validation of a scoring system to predict outcomes of vedolizumab treatment in patients with Crohn’s disease. Gastroenterology. 2018;155:687–695.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shmidt E, Kochhar G, Hartke J, et al. . Predictors and management of loss of response to vedolizumab in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:2461–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hogan SP. Functional role of eosinophils in gastrointestinal inflammation. Immunol Allergy Clin North Am. 2009;29:129–140, xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hogan SP, Waddell A, Fulkerson PC. Eosinophils in infection and intestinal immunity. Curr Opin Gastroenterol. 2013;29:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Masterson JC, McNamee EN, Fillon SA, et al. . Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut. 2015;64:1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Masterson JC, Capocelli KE, Hosford L, et al. . Eosinophils and IL-33 perpetuate chronic inflammation and fibrosis in a pediatric population with stricturing Crohn’s ileitis. Inflamm Bowel Dis. 2015;21:2429–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lampinen M, Backman M, Winqvist O, et al. . Different regulation of eosinophil activity in Crohn’s disease compared with ulcerative colitis. J Leukoc Biol. 2008;84:1392–1399. [DOI] [PubMed] [Google Scholar]
- 31. Lampinen M, Rönnblom A, Amin K, et al. . Eosinophil granulocytes are activated during the remission phase of ulcerative colitis. Gut. 2005;54:1714–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stasikowska-Kanicka O, Danilewicz M, Głowacka A, et al. . Mast cells and eosinophils are involved in activation of ulcerative colitis. Adv Med Sci. 2012;57:230–236. [DOI] [PubMed] [Google Scholar]
- 33. Heatley RV, James PD. Eosinophils in the rectal mucosa. A simple method of predicting the outcome of ulcerative proctocolitis? Gut. 1979;20:787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brennan GT, Melton SD, Spechler SJ, et al. . Clinical implications of histologic abnormalities in ileocolonic biopsies of patients with Crohn’s disease in remission. J Clin Gastroenterol. 2017;51:43–48. [DOI] [PubMed] [Google Scholar]
- 35. Dainese R, Galliani EA, De Lazzari F, et al. . Role of serological markers of activated eosinophils in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2012;24:393–397. [DOI] [PubMed] [Google Scholar]
- 36. Bischoff SC. Mucosal allergy: role of mast cells and eosinophil granulocytes in the gut. Baillieres Clin Gastroenterol. 1996;10:443–459. [DOI] [PubMed] [Google Scholar]
- 37. Fan YC, Steele D, Kochar B, et al. . Increased prevalence of esophageal eosinophilia in patients with inflammatory bowel disease. Inflamm Intest Dis. 2019;3:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Limketkai BN, Shah SC, Hirano I, et al. . Epidemiology and implications of concurrent diagnosis of eosinophilic oesophagitis and IBD based on a prospective population-based analysis. Gut. 2019: gutjnl-2018-318074. [DOI] [PubMed] [Google Scholar]
- 39. Cotton CC, Durban R, Dellon ES. Illuminating elimination diets: controversies regarding dietary treatment of eosinophilic esophagitis. Dig Dis Sci. 2019;64:1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andreasson A, Walker MM, Agreus L, et al. . Colonic eosinophilia is associated with current but not incident depression independent of IBS status. Gastroenterology. 2019;156:S52. [Google Scholar]
- 41. Kochar B, Barnes EL, Long MD, et al. . Depression is associated with more aggressive inflammatory bowel disease. Am J Gastroenterol. 2018;113:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. [DOI] [PubMed] [Google Scholar]