Abstract
Background and Aims
Poor sleep quality in Crohn’s disease (CD) is associated with histologic activity and clinical relapse. We sought to characterize sleep dysfunction and determine the effect of poor sleep quality on risk for hospitalization and surgery.
Methods
Clinical data were collected for CD subjects including the Pittsburgh Sleep Quality Index (PSQI) and Harvey-Bradshaw index (HBI). The PSQI score and a brief medical history were obtained for control subjects. The PSQI and HBI correlation was tested at an initial clinic visit and at follow-up. Crohn’s disease subjects with and without poor sleep were compared for risk of hospitalization or surgery by Kaplan–Meier and Cox proportional hazards.
Results
Ninety-two CD and 82 control subjects were included. Crohn’s disease and control subjects shared similar baseline characteristics and PSQI (8.3 vs 7.8, P = 0.31), and 77% of the CD population had PSQI >5. Crohn’s disease subjects with PSQI >5 more often had inflammatory phenotypes and reported increased benzodiazepine and psychiatric medication use. Crohn’s disease subjects with PSQI >5 also reported more night awakenings due to pain and bathroom use. The PSQI correlated with HBI (r = 0.256, P = 0.014), and ΔPSQI on follow-up correlated with ΔHBI (r = 0.47, P = 0.002). Cox proportional hazards model for hospitalization or surgery showed that PSQI >8 was predictive of surgery or hospitalization (hazards ratio 5.37; 95% confidence interval, 1.39–27.54).
Conclusion
There is a high burden of poor sleep quality in CD, which is associated with risk for adverse outcomes. Sleep quality may identify CD patients at risk for complications and have prognostic value in CD.
Keywords: inflammatory bowel disease, Crohn’s disease, sleep, outcomes
Outpatients with Crohn’s disease have a high rate of sleep dysfunction using the Pittsburgh Sleep Quality Index. Sleep quality is sensitive to changes in disease activity, and poor sleep quality (PSQI >8) is independently associated with hospitalization or surgery.
INTRODUCTION
Crohn’s disease (CD) is a chronic, progressive, inflammatory condition of the gastrointestinal tract that has numerous effects on the function and quality of life of more than 3 million Americans.1 Among the extraintestinal manifestations of CD, sleep disturbances are a common source of reduced quality of life.2, 3 Sleep physiology is intimately integrated into immune physiology, and there is a bidirectional relationship between sleep disturbances and immune dysregulation.
Sleep is a manifestation of the natural circadian rhythm of the brain, which cycles between periods of wakefulness and sleep. Sleep is defined as a state of reduced consciousness, decreased movement, and slowed metabolism.4 Divided into rapid eye movement (REM) and non-REM sleep, of which there are 4 stages, sleep is a dynamic neurologic and metabolic state. Evidence shows that the master circadian regulator, the supra-chiasmatic nucleus, coordinates circadian rhythm from the brain throughout the body.5 Previous studies show that tumor necrosis factor alpha (TNF-α) follows a circadian rhythm in the brain and peripheral circulation.6 Notably, even small disturbances in sleep habits can alter circulating monocytes to express more TNF-α.7 There is additional evidence that sleep habits change other manifestations of immune system activity, such as interleukin (IL)-6.8
An estimated 51%–80% of people with CD suffer from poor sleep.3 Prior studies show that this is a source of reduced quality of life but also reflects disease activity and prognosis.9 People with poor sleep, as measured by the Pittsburgh Sleep Quality Index (PSQI), are more likely to have histologic disease activity.10 Inflammatory bowel disease patients who self-report poor sleep quality in the setting of clinical remission are also at higher likelihood of suffering clinical disease relapse by 6 months.2 We sought to determine the factors associated with poor sleep quality in CD and assess the effect of poor sleep quality on disease outcomes.
METHODS
Patient and Control Subject Recruitment
Patients with CD were enrolled during a standard-of-care clinic appointment at a tertiary care inflammatory bowel disease center. Inclusion in the study required that the patient be 18 years or older and have a diagnosis of CD. Patients were excluded from the study if they were currently pregnant or within 1 month of an intestinal operation for Crohn’s disease.
Patients were then asked to refer a person to serve as a control subject. Control subjects were required to be 18 years or older and were excluded if they had a diagnosis of CD or ulcerative colitis, were currently pregnant, or underwent an intestinal operation within the past month.
Data Acquisition
After obtaining consent, subjects completed a questionnaire that included the Pittsburgh Sleep Quality Index (PSQI).11 Information about medication, tobacco, and alcohol use were included in the questionnaire. Clinical data were abstracted from electronic health records including CD Montreal classification, medical history, medications, age, body mass index (BMI), and gender. Medications were categorized according to class, indication, and mechanism of action. Benzodiazepines categorized separately from other medications with psychiatric indications. Nonbenzodiazepine psychiatric medications included selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and amphetamine derivative stimulant medications. A Charlson comorbidity score was assigned for each patient based on review of the electronic health records. Clinical disease activity was assessed at the clinic appointment and measured by Harvey-Bradshaw index (HBI).12 Recent steroid use was defined as any reported use of oral or rectal prednisone, budesonide, or methylprednisolone in the past month. Alcohol use was defined as patient report of at least 1 alcoholic drink per week. Clinical remission was defined as total HBI score <5.13
Control subjects recruited by enrolled patients completed an online questionnaire that included the PSQI and a brief description of their medical history, including prior medical diagnoses, surgical history, current medications, smoking status, alcohol use, recent steroid use, gender, and age. Control subjects reported their height and weight in order to calculate BMI. A Charlson comorbidity score was assigned to each control subject on the basis of their reported medical history.
Information gathered from the study subject questionnaire, control questionnaire, and review of the medical record was then compiled into a secure database using the Research Electronic Data Capture (REDCap) system.14 Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Chicago.
Study subjects were followed prospectively for up to 1 year after completion of the survey. The study subjects were then given a follow-up questionnaire at their next standard-of-care clinic appointment. The follow-up questionnaire included a repeat PSQI, and an HBI was recorded from the medical record. In addition to repeat PSQI, the patients were followed for the clinical outcome of either hospitalization for Crohn’s disease or surgical intervention for Crohn’s disease. The time interval between enrollment and the outcome was then recorded.
Statistical Methods
Study subjects and control subjects were compared according to age, sex, BMI, smoking status, alcohol use, Charlson comorbidity index, and PSQI using Student t test, Pearson χ 2, and Fisher exact test. They were also compared for the proportion of control and study subjects with PSQI <5 by Pearson χ 2.
Patients with a PSQI score >5 were determined to have poor sleep quality.11 Patients with poor sleep quality were then compared with patients without poor sleep quality by Student t test, Pearson χ 2, and Fisher exact test for baseline demographics, Charlson comorbidity index, disease activity, surgical history, and medication use. Pearson χ 2 and Fisher exact tests were performed to assess for differences in the given answers to individual questions about sleep disturbances in the PSQI. Sleep efficiency was compared using Student t test. A Spearman correlation coefficient was calculated to assess the relationship between PSQI and HBI, PSQI and C-reactive protein (CRP) at the time of clinic visit, and the change in PSQI and change in HBI on follow-up questionnaire. A receiver operating characteristic (ROC) was calculated for the performance of PSQI to indicate the presence of clinically active disease. Clinically active disease was defined as a total HBI ≥5. The Youden index was calculated from the ROC to determine the PSQI value that maximizes the performance of PSQI to detect clinically active disease. Univariate and multivariate logistic regression analysis was performed to determine the association between baseline clinical and demographic characteristics with poor sleep quality. A Kaplan-Meier survival analysis compared outcome of hospitalization or surgery between CD subjects with a PSQI score >5 with CD subjects with a PSQI score ≤5. A multivariate Cox proportional hazards model was performed for the outcome of hospitalization or surgery. The included independent variables were current steroid use, current smoking, history of prior surgery, clinical remission at the time of survey, BMI, age, any alcohol use, and female sex. The first model included the variable a PSQI score >5. The second model used a PSQI score >8, which is a value proposed to suggest more objective sleep pathology.15 Statistical calculations were performed with JMP Version 13 (SAS Institute Inc., Cary, NC, 1989–2007) and R version 3.3.2 (R Foundation, Vienna, Austria) using the “survival” and “Epi” packages.16–19
Ethical Considerations
The study was approved by the University of Chicago institutional review board under the study protocol 15-1786.
RESULTS
Baseline Characteristics
Overall, 92 patients with CD and 82 control subjects were included in the analysis (Table 1). The 2 populations were similar in age, sex, BMI, smoking status, and Charlson comorbidity index. The share of controls that reported any alcohol use was greater than the share of study subjects with CD (60% vs 42%, P = 0.03). The CD population mean PSQI score was 8.3, and mean HBI score was 2.2. There was no statistically significant difference between the CD and control subjects’ mean PSQI score (8.3 vs 7.8, P = 0.31) or the percentage with a PSQI score >5 (77% vs 81%, P = 0.73). Subjects with CD were more frequently using antiemetics, opioids, benzodiazepines, nonbenzodiazepine psychiatric medications, biologic medications, azathioprine, methotrexate, antibiotics, antidiarrheals, and prescription sleep aids (Table 2). The proportion of patients using stimulant amphetaminederived medications within the class of nonbenzodiazepine psychiatric medications were similar between control and CD subjects (6.1% vs 6.5%, P = 0.92).
Table 1.
Baseline Characteristics and Comparison Between Control and Study Subjects and Between Study Subjects With Poor Sleep Quality and Those Without
| Control Subjects | CD Overall | P | CD PSQI >5 | CD PSQI ≤5 | P | |
|---|---|---|---|---|---|---|
| (n = 82) | (n = 92) | (n = 71) | (n = 21) | |||
| Age (mean, range) | 45.5 (18–97) | 42.9 (18–82) | 0.30 | 42.9 | 43.0 | 0.97 |
| Sex (% female) | 68% | 62% | 0.47 | 66.2% | 47.6% | 0.20 |
| BMI (mean, range) | 27.2 (17.3–48.8) | 26.6 (17.8–51.2) | 0.49 | 27.4 | 23.7 | 0.03 |
| Current smoker (%) | 5% | 5% | 1.00 | 7.0% | 0.0% | 0.59 |
| Current alcohol (%) | 60% | 42% | 0.03 | 38.0% | 57.1% | 0.19 |
| Charlson | ||||||
| 0 | 80% | 75% | 0.11 | 85.7% | 71.8% | 0.53 |
| 1 | 15% | 11% | 4.8% | 12.7% | ||
| 2 | 5% | 14% | 9.5% | 15.5% | ||
| PSQI (mean, range) | 7.8 (3–19) | 8.3 (2–20) | 0.31 | 9.6 | 4.0 | <0.001 |
| Poor sleep (PSQI >5) (%) | 81% | 77% | 0.73 | — | — | — |
| HBI (mean, range) | — | 2.2 (0–14) | — | 2.3 | 2.0 | 0.66 |
| In clinical remission (HBI < 5) (%) | — | 85% | — | 84.5% | 85.7% | 1.00 |
| Number of prior CD surgeries (mean, range) | — | 1.2 (0–7) | — | 1.2 | 1.2 | 0.87 |
| Age at diagnosis (mean, range) | — | 26.3 (3–65) | — | 27.3 | 22.8 | 0.19 |
| Montreal A (%) | ||||||
| 1 | — | 24% | — | 21.1% | 33.3% | 0.55 |
| 2 | — | 63% | — | 64.8% | 57.1% | |
| 3 | — | 13% | — | 14.1% | 9.5% | |
| Montreal L (%) | ||||||
| 1 | — | 22% | — | 23.9% | 14.3% | 0.53 |
| 2 | — | 23% | — | 23.9% | 19.0% | |
| 3 | — | 55% | — | 52.1% | 66.7% | |
| L4 modifier (%) | — | 8% | — | 7.0% | 9.5% | 0.65 |
| Montreal B (%) | ||||||
| 1 | — | 52% | — | 59.2% | 28.6% | 0.03 |
| 2 | — | 17% | — | 17.0% | 19.0% | |
| 3 | — | 30% | — | 23.9% | 52.4% | |
| Perianal modifier (%) | — | 34% | — | 32.4% | 38.1% | 0.82 |
Table 2.
Medication Use Overall and Compared Between Control and Study Subjects and Between Study Subjects With Poor Sleep Quality and Those Without
| Control Subjects | CD—Overall | P | CD PSQI >5 | CD PSQI ≤5 | P | |
|---|---|---|---|---|---|---|
| (n = 82) | (n = 92) | (n = 71) | (n = 21) | |||
| Steroid | 13.6% | 22.8% | 0.17 | 23.9% | 19.0% | 0.77 |
| Stool softener or Laxative | 1.2% | 6.5% | 0.12 | 8.5% | 0.0% | 0.33 |
| Antiemetic | 1.2% | 21.7% | <0.001 | 25.4% | 10.5% | 0.15 |
| Oral Narcotic | 1.2% | 15.2% | 0.002 | 18.3% | 4.8% | 0.18 |
| Benzodiazepine | 3.8% | 15.2% | 0.019 | 20.0% | 0.0% | 0.03 |
| Psychiatric Medication | 17.1% | 32.6% | 0.029 | 39.4% | 9.5% | 0.02 |
| NSAID | 6.6% | 3.3% | 0.48 | 4.2% | 0.0% | 1.00 |
| Biologic Medication | 0.0% | 65.2% | <0.001 | 66.2% | 61.9% | 0.92 |
| Oral 5-ASA | 0.0% | 4.3% | 0.12 | 4.3% | 4.8% | 1.00 |
| Azathioprine | 0.0% | 33.7% | <0.001 | 36.6% | 23.8% | 0.41 |
| Methotrexate | 0.0% | 20.7% | <0.001 | 18.3% | 28.6% | 0.36 |
| Calcineurin inhibitor | 2.4% | 2.2% | 1.00 | 1.4% | 4.8% | 0.41 |
| Antibiotic | 1.2% | 18.5% | <0.001 | 19.7% | 14.3% | 0.75 |
| Budesonide | 1.2% | 7.6% | 0.07 | 7.6% | 9.5% | 0.66 |
| Anti-diarrheal | 0.0% | 12.0% | 0.004 | 8.5% | 23.8% | 0.12 |
| Prescription sleep aid | 0.0% | 8.8% | 0.007 | 8.6% | 9.5% | 1.00 |
There were 71 (77%) people in the CD population with PSQI score >5 and 21 (23%) people in the CD population with PSQI score ≤5 (Table 1). The 2 groups were similar in age, sex, smoking status, alcohol use, and Charlson comorbidity index. The mean BMI was significantly greater in the CD subjects with poor sleep (27.4 vs 23.7, P = 0.03). There was no difference between the mean HBI of the 2 groups (2.3 vs 2.0, P = 0.66) or the percentage in clinical remission (84.5% vs 85.7%, P = 1.00). Crohn’s disease Montreal classification differed according to phenotype. Those CD subjects with a PSQI score >5 were a majority B1 (59.2%), whereas those CD subjects with a PSQI score ≤5 were a majority B3 (52.4%, P = 0.03). Otherwise, there was no significant difference between the groups according to age at diagnosis, number of prior CD surgeries, Montreal A classification, Montreal L classification, Montreal L4 classification, or perianal disease modifier. The CD subjects with a PSQI score >5 were more likely than those with a PSQI score ≤5 to be using benzodiazepines and nonbenzodiazepine psychiatric medications (Table 2). There was no difference between the 2 groups for use of prescription sleep aids or stimulant medications (PSQI >5, 7.0% vs PSQI ≤5, 4.8%; P = 0.71).
Medication Use and Somatic Symptoms Contribute to Poor Sleep Quality in Crohn’s Disease
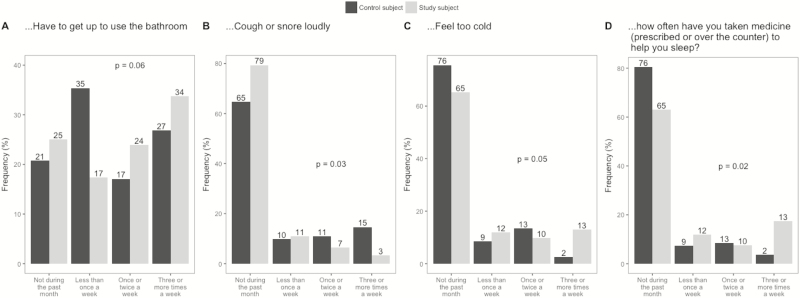
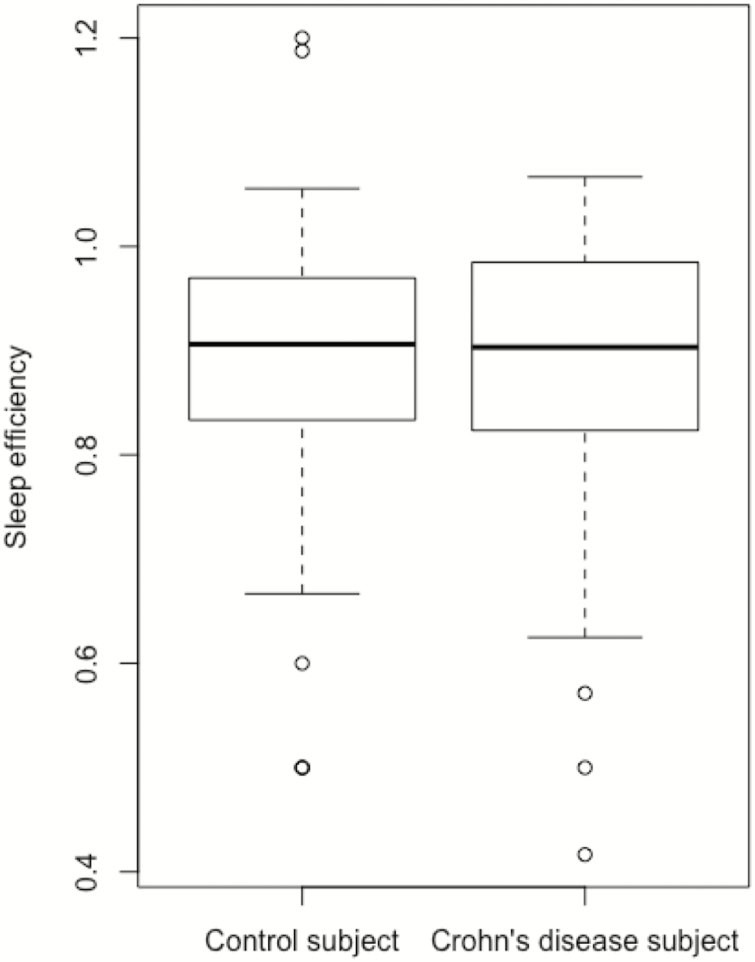
Crohn’s disease subjects (Fig. 1) were more likely than control subjects to report that their sleep was disturbed by feeling too cold (P = 0.05) and less likely than control subjects to report that they cough or snore loudly (P = 0.03). Crohn’s disease subjects were also more likely to report using medication to help sleep (P = 0.02). There was a numeric difference in the perception of needing to use the bathroom that did not reach statistical significance (P = 0.06). Mean sleep efficiency in control subjects was 0.892 compared with a mean of 0.883 in study subjects (Fig. 2, P = 0.66).
Figure 1.
Differences between control and CD responses to the specific questions of sleep quality.
Figure 2.
Comparing sleep efficiency measures.
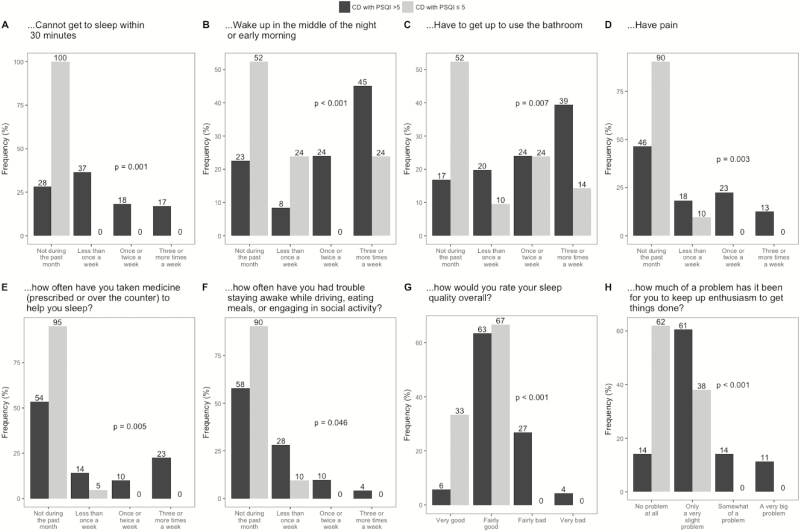
Crohn’s disease subjects with a PSQI score >5 were more likely to report difficulty falling asleep within 30 minutes, waking in the middle of night, waking to use the bathroom, having nighttime pain, using medications to help with sleep, and staying awake than CD subjects with a PSQI score ≤5 (Fig. 3). They were also more likely to report having poorer sleep quality overall and difficulty with anhedonia.
Figure 3.
Comparing PSQI answers between CD patients with and without poor sleep (PSQI >5).
Sleep Quality Is Associated With Clinical Disease Activity
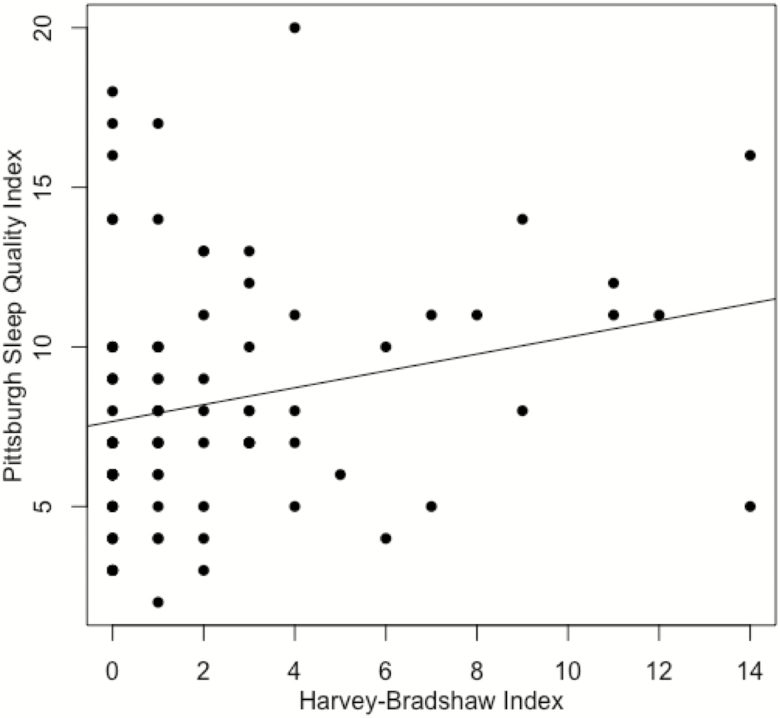
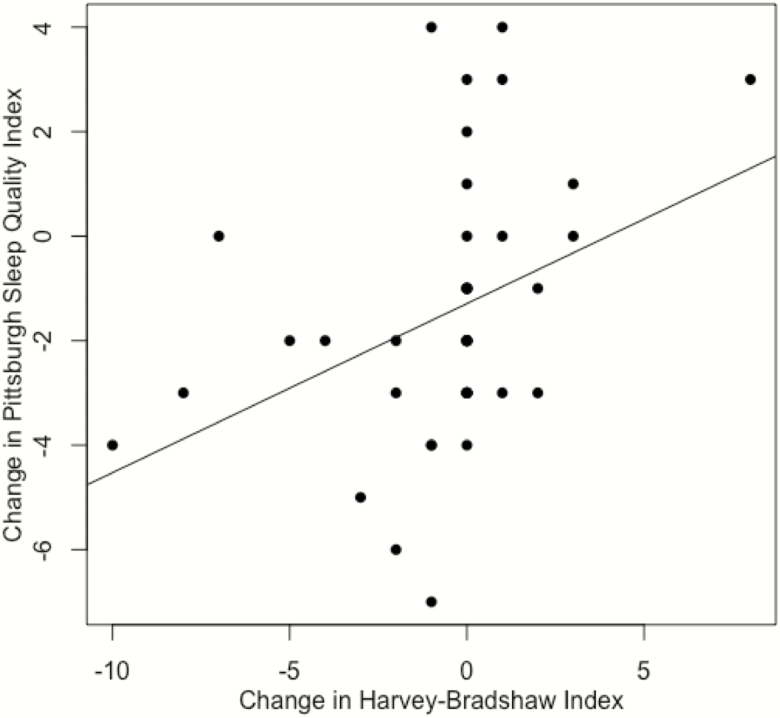
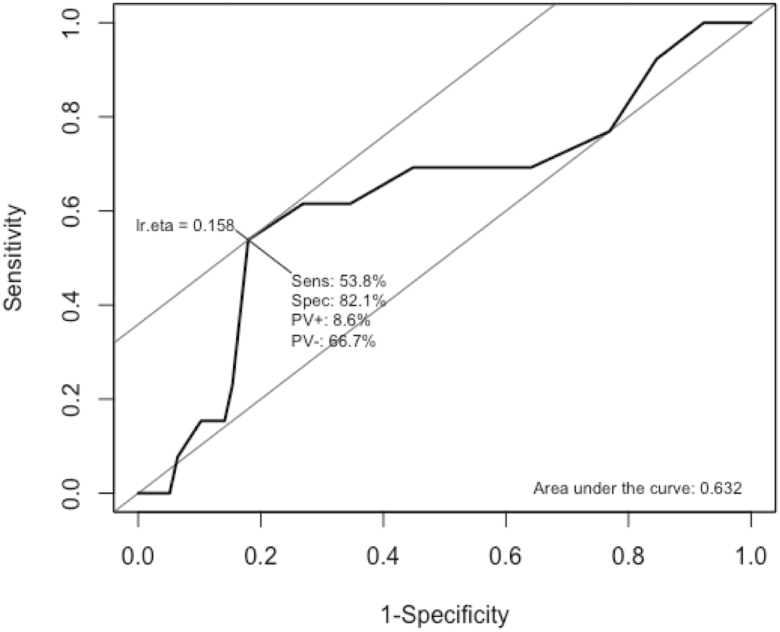
There was a significant positive correlation between the PSQI and HBI during the initial clinic visit (Fig. 4, rho = 0.256, P = 0.014). Among the 40 subjects with CD who were tested for CRP levels at the time of the clinic visit, there was no significant correlation between CRP values and PSQI scores (rho = 0.12, P = 0.47). There were 42 subjects with CD that received a second questionnaire at their next follow-up appointment. The change in PSQI score was significantly correlated with the change in HBI (Fig. 5, rho = 0.47, P = 0.002). The ROC for PSQI to predict clinically active disease had an area under the curve of 0.632 (Fig. 6). The PSQI score with a maximal Youden index was a PSQI score of 10. A PSQI score of 10 was determined to have a sensitivity of 53.8% and specificity of 82.1% for detecting clinically active disease.
Figure 4.
Correlation between PSQI and HBI among study subjects.
Figure 5.
Correlation between delta PSQI and delta HBI on follow-up cohort.
Figure 6.
ROC curve for PSQI predicting clinical disease activity. Optimal cut point for PSQI predicting clinical disease activity is 10.
Crohn’s Disease Phenotype and Psychiatric Medication Use Is Associated With Poor Sleep Quality
In a univariate logistic regression, an increasing BMI and the use of a nonbenzodiazepine psychiatric medication conferred greater odds of a PSQI score >5 (Table 3). A Montreal classification of B3 conferred lower odds of a PSQI score >5 compared with Montreal classification of B1. A multivariate logistic regression using these inputs showed that a Montreal classification of B3 and the use of a nonbenzodiazepine psychiatric medication were significantly associated with a PSQI score >5.
Table 3.
Logistic Regression Analysis for Predictors of Poor Sleep
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | Lower 95% | Upper 95% | P | OR | Lower 95% | Upper 95% | P | |
| Age | 0.99 | 0.97 | 1.03 | 0.96 | ||||
| Male sex | 0.46 | 0.17 | 1.24 | 0.13 | ||||
| BMI | 1.12 | 1.02 | 1.25 | 0.03 | 1.12 | 1.01 | 1.27 | 0.053 |
| Current smoker | * | * | * | * | ||||
| Current alcohol | 0.46 | 0.17 | 1.22 | 0.12 | ||||
| Charlson score | 1.60 | 0.58 | 6.24 | 0.42 | ||||
| HBI | 1.03 | 0.89 | 1.25 | 0.66 | ||||
| In clinical remission | 1.00 | 0.33 | 2.52 | 1.00 | ||||
| # prior CD surgery | 1.03 | 0.73 | 1.55 | 0.87 | ||||
| Age at diagnosis | 1.03 | 0.99 | 1.07 | 0.19 | ||||
| Montreal A | ||||||||
| 1 | Reference | |||||||
| 2 | 1.79 | 0.58 | 5.33 | 0.30 | ||||
| 3 | 2.33 | 0.45 | 17.87 | 0.35 | ||||
| Montreal L | ||||||||
| 1 | Reference | |||||||
| 2 | 0.75 | 0.13 | 3.90 | 0.73 | ||||
| 3 | 0.47 | 0.10 | 1.67 | 0.28 | ||||
| L4 | 0.79 | 0.25 | 3.25 | 0.71 | ||||
| Montreal B | ||||||||
| 1 | Reference | Reference | ||||||
| 2 | 0.43 | 0.10 | 1.90 | 0.24 | 0.65 | 0.14 | 3.27 | 0.593 |
| 3 | 0.22 | 0.07 | 0.67 | 0.009 | 0.29 | 0.08 | 0.97 | 0.048 |
| Perianal | 0.83 | 0.41 | 1.75 | 0.63 | ||||
| Steroid | 1.23 | 0.55 | 3.18 | 0.64 | ||||
| Stool softener or Laxative | * | * | * | * | ||||
| Antiemetic | 2.29 | 0.87 | 8.75 | 0.14 | ||||
| Oral Narcotic | 2.89 | 0.86 | 22.94 | 0.16 | ||||
| Benzodiazepine | * | * | * | * | ||||
| Psychiatric Medication | 3.63 | 1.41 | 13.75 | 0.02 | 3.09 | 1.14 | 12.07 | 0.049 |
| NSAID | * | * | * | * | ||||
| Biologic Medication | 1.14 | 0.54 | 2.31 | 0.72 | ||||
| Oral 5-ASA | 0.92 | 0.21 | 7.91 | 0.93 | ||||
| Azathioprine | 1.54 | 0.73 | 3.62 | 0.28 | ||||
| Methotrexate | 0.66 | 0.30 | 1.52 | 0.31 | ||||
| Calcineurin inhibitor | 0.41 | 0.04 | 4.13 | 0.38 | ||||
| Antibiotic | 1.31 | 0.54 | 3.92 | 0.58 | ||||
| Budesonide | 0.79 | 0.25 | 3.25 | 0.71 | ||||
| Anti-diarrheal | 0.42 | 0.17 | 1.10 | 0.07 | ||||
| Prescription sleep aid | 0.92 | 0.31 | 3.72 | 0.89 |
*Indicates variables for which univariate logistic regression was not possible due to all instances occurring in one category but not the other.
Pittsburgh Sleep Quality Index Greater Than 8 Is Associated With Worse Outcomes
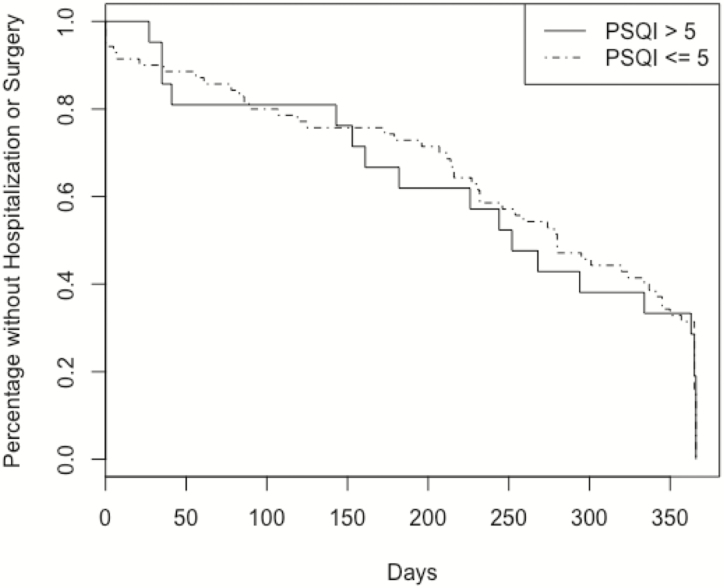
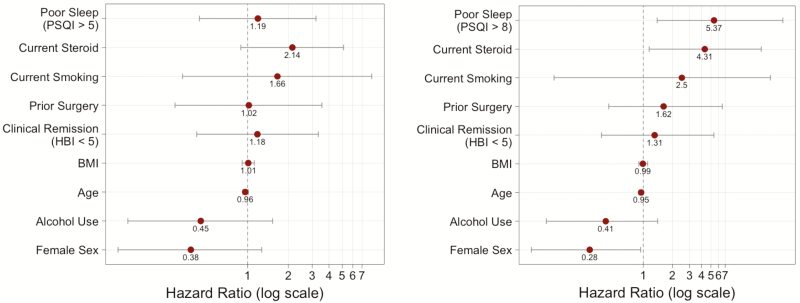
The patients were prospectively followed over a mean of 258 days. During follow-up, 13 patients were hospitalized, and 7 patients underwent surgery. Overall, the combined outcome of either hospitalization or surgery occurred in 14 patients within 1 year (Fig. 7). There was no significant difference between CD subjects with a PSQI score >5 compared with CD subjects with a PSQI score <5 (P = 0.906). In a multivariate Cox proportional hazards model, using a PSQI score >5 was not predictive of outcomes (hazards ratio [HR] 1.19; 95% confidence interval [CI], 0.44–3.20) (Fig. 8a). However, a PSQI score >8 in the same model was significantly predictive of the outcome of hospitalization or surgery (HR 5.37; 95% CI, 1.39–27.54) (Fig. 8b).
Figure 7.
KM curve for PSQI >5 or ≤5 (P = 0.906).
Figure 8.
Hazard ratios for multivariate Cox proportional hazards model for the outcome of hospitalization or surgery.
DISCUSSION
Sleep dysfunction is an important contributor to quality of life in CD. We observed a high frequency of sleep dysfunction in this population, affecting 77% of patients surveyed. Addressing sleep quality may be an opportunity to improve not only quality of life but also clinical outcomes.
Previous studies identified that poor sleep quality was a risk factor for clinical disease relapse.2, 9, 20 In our cohort of patients with CD, a PSQI score >8 was associated with a higher risk for hospitalization or surgery in a multivariate Cox proportional hazards model that included clinical disease activity. A previous study evaluating the PSQI cut-off values suggested that a PSQI score >8 may be a more appropriate indicator of sleep pathology.15 The relationship between sleep and intestinal inflammation is likely bidirectional. In one direction, clinical symptoms such as abdominal pain and diarrhea have the ability to disrupt sleep. An association between sleep quality and histologic inflammation underscores this objective connection with disease activity.10 In a prospective study comparing individuals starting vedolizumab or an anti-TNF-α medication for active IBD, there were parallel improvements in sleep function with both treatments.21 This follows clinical experience that medication-induced symptom management can improve sleep dysfunction.
However, disordered sleep may be characteristic of the psychologic and environmental exposures that can precipitate inflammatory activity through neurohormonal pathways. Studies identify numerous inflammatory mediators that can be affected by changes in sleep, including tumor necrosis factor α (TNF-α), interleukin-6, and interleukin-12.7, 22–25 Cortisol release patterns change according to environmental and psychological stresses. An artificially induced stress response can objectively alter intestinal permeability, which is a proposed contributor to Crohn’s disease.26 Tryptophan metabolism through the kynurenine pathway is altered in both ulcerative colitis and Crohn’s disease.27, 28 As an essential amino acid and precursor of serotonin and melatonin, tryptophan metabolism alterations have the potential of connecting mood disorders, sleep dysfunction, and intestinal inflammation at the cellular level, independent of clinical symptom effects on sleep. Future studies investigating sleep dysfunction in Crohn’s disease should consider targeted interventions for individuals in clinical remission who report sleep dysfunction for assessment of subclinical disease activity.
In our study, poor sleep quality was associated with more severe CD-related outcomes, but the rate of poor sleep quality was not different between patients with CD and health controls. The true effect of CD on sleep quality may be obscured by responses to the question about needing to use the bathroom at night. This component of the PSQI is not sensitive to the difference between bowel movements and urinary habits. Although the proportion of CD subjects experiencing poor sleep quality was similar to control subjects, there were differences in the associated symptoms that led to poor sleep, including feeling too cold, coughing, or snoring that is disturbing. The pathophysiology of these complaints is not known. The effect of bowel function is probably more accurately captured when comparing CD subjects with and without poor sleep. In that comparison, night awakenings to use the bathroom were more commonly reported in the CD population with poor sleep quality. This is further supported by the correlation between the HBI clinical disease activity score and PSQI, where liquid stool number is a major component of the HBI. As in other studies, we show that clinical disease activity is associated with poor sleep quality.3, 29 Furthermore, changes in PSQI and HBI show a significant correlation in follow-up. This lends further support to prioritizing disease or bowel symptom management before addressing sleep quality. In contrast, the subset of patients with a CRP tested at the clinic visit did not have a significant correlation between CRP and PSQI score. This may be due to the insensitivity of CRP for clinically active CD and heterogeneity in the study population, or it may suggest that an inflammatory component of CD does not contribute as much to the poor sleep quality as bowel frequency.
Several differences arise when comparing the CD subjects with poor sleep with those without poor sleep. Patients with an inflammatory phenotype (Montreal classification B1) were more often represented in the group with poor sleep, and those with penetrating phenotype (Montreal classification B3) were more often represented in the group without poor sleep. There were also medication use differences between the 2 groups, with greater use of benzodiazepines and psychiatric medications in the group with poor sleep. Benzodiazepines may be used as sleep aids but are not considered “first-line” due to concerns about habit formation, tolerance, and side effects.30 It is not known whether these patients were using benzodiazepines for anxiety or as a sleep aid, but the high frequency of patients reporting that they take medications to help sleep suggests some overlap. Psychiatric manifestations of major depression and anxiety often involve poor sleep. The association with psychiatric medication use suggests there may be a burden of mood disorders associated with poor sleep quality in this population with CD.
This prospective cohort study is strengthened by the comprehensive collection of demographic, phenotypic, and medical data for each of the CD subjects and subsequent follow-up sleep quality assessments in many participants. The control group was statistically similar to the CD subjects despite being remotely recruited and surveyed using an online instrument. The control subjects provided medical, surgical, and medication histories that were used in the analysis. That this information could not be verified by a review of professionally documented medical records introduces the possibility that control subjects did not fully report some diagnoses or medications. In addition, the bed partner or housing relationship between the study subject and referred control was not assessed. This introduces the possibility that shared sleep behaviors could affect both persons or cause one person to affect the other. The observational study design can only assess for associations and cannot elucidate causality. Another limitation is that a significant portion of the CD patients were in clinical remission. This may decrease the sensitivity of our analyses for finding sleep changes associated with disease activity at baseline. Nevertheless, the multivariate Cox proportional hazards model did detect an association between poor sleep quality and the risk for hospitalization or surgery despite the sample size. Sample size may have limited the ability of the model to fully assess risk because traditional risk factors including smoking status and clinical disease activity were not identified as significant.
In conclusion, there is a high prevalence of poor sleep quality in this cohort of patients with CD. Although sleep quality is overall similar to controls, CD patients face different circumstances leading to sleep dysfunction. Clinicians should be aware of the associations between sleep dysfunction, medication use, and disease phenotype. Sleep quality may be a previously underappreciated risk factor for negative outcomes. Future investigations should consider incorporating sleep assessments into clinical practice to identify opportunities for intervention.
Author contributions: MAS, AML, and DTR contributed to the study concept and design, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. NZ and EP contributed to the acquisition of data and technical support. RK contributed to the study concept and design, analysis and interpretation of data, and drafting of the manuscript.
Supported by: The REDCap database platform is supported by NIH CTSA UL1 TR000430.
Conflicts of Interest: MAS, AML, NZ, EP and RK have no relevant disclosures. DTR is a consultant and has received grant support from Abbvie, Merck & Co., Janssen, Takeda and Pfizer.
REFERENCES
- 1. Dahlhamer JM, Zammitti EP, Ward BW, et al. Prevalence of inflammatory bowel disease among adults aged ≥18 years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1166–1169. [DOI] [PubMed] [Google Scholar]
- 2. Ananthakrishnan AN, Long MD, Martin CF, et al. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2013;11:965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Graff LA, Vincent N, Walker JR, et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1882–1889. [DOI] [PubMed] [Google Scholar]
- 4. Zisapel N. Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci. 2007;64:1174–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwartz WJ. Suprachiasmatic nucleus. Curr Biol. 2002;12:R644. [DOI] [PubMed] [Google Scholar]
- 6. Bredow S, Guha-Thakurta N, Taishi P, et al. Diurnal variations of tumor necrosis factor alpha mRNA and alpha-tubulin mRNA in rat brain. Neuroimmunomodulation. 1997;4:84–90. [DOI] [PubMed] [Google Scholar]
- 7. Irwin MR, Wang M, Campomayor CO, et al. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. [DOI] [PubMed] [Google Scholar]
- 8. Späth-Schwalbe E, Hansen K, Schmidt F, et al. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998;83:1573–1579. [DOI] [PubMed] [Google Scholar]
- 9. Ali T, Madhoun MF, Orr WC, et al. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:2440–2443. [DOI] [PubMed] [Google Scholar]
- 10. Qazi T, Farraye FA. Sleep and inflammatory bowel disease: an important bi-directional relationship. Inflamm Bowel Dis. 2019;25:843–852. [DOI] [PubMed] [Google Scholar]
- 11. Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 12. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 13. Vermeire S, Schreiber S, Sandborn WJ, et al. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8:357–363. [DOI] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh sleep quality index. J Psychosom Res. 1998;45:5–13. [DOI] [PubMed] [Google Scholar]
- 16. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 17. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 18.Therneau TM. A Package for Survival Analysis in S. 2015. [Google Scholar]
- 19. Carstensen B, Plummer M, Laara E, Hills M.. Epi: A Package for Statistical Analysis in Epidemiology. 2018. [Google Scholar]
- 20. Ali T, Madhoun MF, Crosby A, et al. 43 Poor sleep quality predicts disease relapse in patients with inflammatory bowel disease. Gastroenterology. 2013;144:S12. [Google Scholar]
- 21. Stevens BW, Borren NZ, Velonias G, et al. Vedolizumab therapy is associated with an improvement in sleep quality and mood in inflammatory bowel diseases. Dig Dis Sci. 2017;62:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Born J, Lange T, Hansen K, et al. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–4464. [PubMed] [Google Scholar]
- 23. Vgontzas AN, Bixler EO, Lin HM, et al. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12:131–140. [DOI] [PubMed] [Google Scholar]
- 24. Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–2607. [DOI] [PubMed] [Google Scholar]
- 25. Lange T, Dimitrov S, Fehm HL, et al. Shift of monocyte function toward cellular immunity during sleep. Arch Intern Med. 2006;166:1695–1700. [DOI] [PubMed] [Google Scholar]
- 26. Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293–1299. [DOI] [PubMed] [Google Scholar]
- 27. Sofia MA, Ciorba MA, Meckel K, et al. Tryptophan metabolism through the kynurenine pathway is associated with endoscopic inflammation in ulcerative colitis. Inflamm Bowel Dis. 2018;24:1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta NK, Thaker AI, Kanuri N, et al. Serum analysis of tryptophan catabolism pathway: correlation with Crohn’s disease activity. Inflamm Bowel Dis. 2012;18:1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ranjbaran Z, Keefer L, Farhadi A, et al. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol. 2007;22:1748–1753. [DOI] [PubMed] [Google Scholar]
- 30. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an american academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307–349. [DOI] [PMC free article] [PubMed] [Google Scholar]