Abstract
Background
Probiotic lactic acid bacteria (LAB) have been used in the anti-inflammation and anti-infection process of various diseases, including inflammatory bowel disease (IBD). Vitamin D receptor (VDR) plays an essential role in pathogenesis of IBD and infectious diseases. Previous studies have demonstrated that the human VDR gene is a key host factor to shape gut microbiome. Furthermore, intestinal epithelial VDR conditional knockout (VDRΔIEC) leads to dysbiosis. Low expressions of VDR is associated with impaired autophagy, accompanied by a reduction of ATG16L1 and LC3B. The purpose of this study is to investigate probiotic effects and mechanism in modulating the VDR-autophagy pathways.
Methods
Five LAB strains were isolated from Korean kimchi. Conditional medium (CM) from these strains was used to treat a human cell line HCT116 or intestinal organoids to measure the expression of VDR and autophagy. Mouse embryonic fibroblast (MEF) cells with or without VDR were used to investigate the dependence on the VDR signaling. To test the role of LAB in anti-inflammation, VDR+/+ organoids were treated with 121-CM before infection with Salmonella enterica serovar Enteritidis. In vivo, the role of LAB in regulating VDR-autophagy signaling was examined using LAB 121-CM orally administrated to VDRLoxp and VDRΔIEC mice.
Results
The LAB-CM-treated groups showed higher mRNA expression of VDR and its target genes cathelicidin compared with the control group. LAB treatment also enhanced expressions of Beclin-1 and ATG16L1 and changed the ratio of LC3B I and II, indicating the activation of autophagic responses. Furthermore, 121-CM treatment before Salmonella enterica serovar Enteritidis infection dramatically increased VDR and ATG16L1 and inhibited the inflammation. Administration of 121-CM to VDRLoxp and VDRΔIEC mice for 12 and 24 hours resulted in an increase of VDR and LC3B II:I ratio. Furthermore, we identified that probiotic proteins P40 and P75 in the LAB-CM contributed to the anti-inflammatory function by increasing VDR.
Conclusions
Probiotic LAB exert anti-inflammation activity and induces autophagy. These effects depend on the VDR expression. Our data highlight the beneficial effects of these 5 LAB strains isolated from food in anti-infection and anti-inflammation.
Keywords: autophagy, lactic acid bacteria, inflammation, organoids, probiotics, salmonella enteritidis, vitamin D, vitamin D receptor
Lactic acid bacteria from Korean kimchi increase vitamin D receptor and autophagy signaling. Proteins P40 and P75 in lactic acid bacteria contributed to the anti-inflammatory function by increasing VDR. This study indicates the beneficial effects of probiotics in anti-infection and anti-inflammation.
INTRODUCTION
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.1 It is known that probiotics and their fermented food products are beneficial for health.2 Researchers have reported success with in the probiotic treatment of inflammatory bowel disease (IBD).3, 4 However, the clinical outcomes were not consistent, and the mechanisms of probiotics are still not well understood.5–8 In IBD, for example, the intestinal microbiota composition showed a decreased Clostridium IXa and IV groups and Bifidobacterium and an increase in members of a detrimental microbiota like Escherichia coli and sulfate-reducing bacteria. The beneficial effects attributed to specific probiotic strains in this case are related to the stabilization of the intestinal microbiota, which is a very difficult and subjective parameter to evaluate—or measure.9
Vitamin D receptor (VDR) is a nuclear receptor that mediates most functions of vitamin D.10 Vitamin D deficiency has been implicated in patients with IBD.11–15 It also plays a protective role in infection and inflammation.16 Vitamin D receptor is identified as an IBD risky gene.17–21 Because VDR exerts cell differentiation, growth, anti-inflammatory actions, and microbiome in the intestine, there is extensive therapeutic exploitation of VDR ligands for the treatment of inflammatory conditions. Dysfunction of vitamin/VDR signaling is reported in patients with chronic inflammation.22, 23 Hence, restoration of the function of VDR to control inflammation and infection is desirable.
The purpose of this study is to investigate probiotic effects and mechanisms in modulating the VDR-autophagy pathways. Five lactic acid bacteria (LAB) strains (Lactobacillus paracasei DKL121, L. casei DK128,24L. plantarum DKL109 and DKL119,24 and L. acidophilus DKLLa5) were isolated from Korean kimchi.24 Our previous study reported that the heat-killed DK128 had protective effects against the influenza virus.25 However, it is not clear whether these LAB strains have the protective roles in intestinal inflammation and bacterial infection. In the current study, we hypothesized that conditional medium from LAB could enhance the VDR expression, thus enhancing the protective functions against inflammation and infection. We generated conditional medium (CM) from different LAB strains for the in vitro and in vivo studies because we hoped to identify the universal beneficial factor(s) in the LAB strains. We used the CM in the current study to avoid potential variation in bacterial cultures. Our data indicate that LAB induces autophagy and exerts anti-inflammation and anti-infection activities. These effects depend on the VDR expression.
MATERIALS AND METHODS
Animals
The VDR+/- and VDR−/− mice on a C57BL6 background were obtained by breeding heterozygous VDR+/− mice.26 The VDRLoxP mice were originally reported by Dr. Geert Carmeliet.26 The VDRΔIEC mice were obtained by crossing the VDRLoxP mice with villin-cre mice (Jackson Laboratory, 004586), as we previously reported.27 Experiments were performed on mice that were 2 to 3 months old, including male and female. Mice were provided with water ad libitum and maintained in a 12-hour dark/light cycle. The animal work was approved by the UIC Office of Animal Care. Euthanasia method was sodium pentobarbital (100 mg per kg body weight) intraperitoneal injection (I.P.) followed by cervical dislocation. All experiments were carried out in accordance with the approved guidelines.
Lactic Acid Bacteria Culture
Five LAB strains (Lactobacillus paracasei DKL121, L. casei DK128,24L. plantarum DKL109 and DKL11924, and L. acidophilus DKLLa5) were isolated from homemade or commercial Korean kimchi from different regions in Korea, as reported in a previous study.24 Bacteria were cultured in Lactobacillus MRS broth (Difco, MD, USA) at 37°C overnight. As the bacterial density reached 109 U/mL, all the conditional medium25 were collected and filtered using 0.22μm filters. The supernatants were stored at −80°C for indicated studeis.
Cell Culture
Human epithelial HCT116 cells were maintained in DMEM supplemented with 10% fetal bovine serum, penicillin-streptomycin (Penicillin, 100 IU/mL, Streptomycin, 100µg/mL), and L-glutamine (4.5 g/L), as previously described.28, 29 Mouse embryonic fibroblasts (MEFs) were cultured in high glucose Dulbecco’s Modified Eagle Medium (DMEM) with 5% FBS (vol/vol) and streptomycin-penicillin.27, 30
Salmonella Enteritidis Infection
Salmonella enterica serovar Enteritidis wild-type (SE-WT) was used in the process of infection, as we described in a previous study.31 Nonagitated microaerophilic bacteria were recovered in Luria-Bertani (LB) broth (1:100, vol/vol) after overnight incubation (about 16~18 h) at 37°C according to the previous study. The bacterial culture suspensions were centrifuged at 5000 g for 10 minutes. Hank’s balanced salt solution (HBSS) with 10 mM HEPES (pH 7.4) was used to resuspend the bacterial cultures. Then the resuspension of SE-WT (0.75 x 107 CFU) was added in the cell culture plate of HCT116, MEFs, or organoid. Half an hour later, the bacteria were removed and washed 3 times by HBSS. After that, cells were incubated with routine medium-containing gentamicin (500 μg/mL) for 1 hour to control the extracellular bacteria.
Protection of 121 Conditional Medium on Salmonella Invasion in Cultured Organoids
Organoids from small intestines of VDR+/+ and VDR-/- mice were prepared and maintained as previously described.16, 32, 33 Mini gut medium (advanced DMEM/F12 supplemented with HEPES, L-glutamine, N2, and B27) was added to the culture, along with R-Spondin, Noggin, and epidermal growth factor (EGF). Organoid cells (6 days after passage) were incubated with or without 10% 121 conditional medium (121-CM) in mini gut media for 2 hours and washed with HBSS. After 121-CM pretreatment, organoids were infected with SE-WT for 30 minutes, washed with HBSS, and incubated in mini gut media containing gentamicin (500 mg/mL) for 1 hour. After extensive HBSS washing, the samples were collected for Western blots.
Mouse Colonic Epithelial Cells
Mouse colonic epithelial cells were collected by scraping the tissue from the colon of the mouse, including the proximal and distal regions.28, 34 The cells were sonicated in lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA, 1 mM EGTA, pH 8.0, 0.2 mM sodium ortho-vanadate, and protease inhibitor cocktail). The total protein concentration in the samples was measured using the BioRad Protein Assay Dye Reagent (BioRad, Hercules, CA, USA).
Immunoblotting
Cultured cells were rinsed twice with ice-cold HBSS, lysed in protein-loading buffer (50 mM Tris, pH 6.8, 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue, 10% glycerol), and then sonicated. Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and immunoblotted with primary antibodies. The membrane was incubated with the following primary antibodies: anti-VDR (Santa Cruz Biotechnology Inc., CA, USA), anti-Beclin-1, anti-LC3B, anti-ATG16L1, anti-tumor necrosis facto (TNF)-α (Cell Signal, Beverly, MA, USA), anti-P40, and anti-P75 (kindly provided by Prof. F. Yan),35 anti-IκBα (Cell Signal, Beverly, MA, USA), and anti-β-actin (Sigma-Aldrich, Milwaukee, WI, USA) antibodies and visualized by ECL. Membranes that were probed with more than 1 antibody were stripped before reprobing.
Immunofluorescence
Cells were fixed with 100% ethanol for 30 minutes and permeabilized with 0.1% Triton X-100 for 10 minutes. Normal goat serum was performed to block the nonspecific antigen. The primary antibody against ATG16L1 was added to incubate the cells overnight at 4°C. After incubating with Alexa Fluor 549 anti-Rabbit secondary antibody for 1 hour, coverslips were mounted by fluoromount-G (SouthernBiotech, AL, USA). Images were photographed using Zeiss laser scanning microscope LSM 710 (Carl Zeiss Inc., Oberkochen, Germany).
Real Time Quantitative Polymerase Chain Reaction
Total cellular RNA was obtained by TRIzol reagent (Thermo Fisher, MA, USA) according to the manufacturer’s protocol. Vitamin D receptor and its target genes CYP24, cathelicidin, autophagic markers ATG16L1, and LC3B were measured by real-time polymerase chain reaction (PCR). APRIL is a protein of the TNF superfamily and measured by real-time PCR. The sequences of the primers were shown in Table 1. All the gene expression levels were normalized to GAPDH through the 2-ΔΔCt method.
Table 1.
Real-time PCR Primers
| Primers name | Sequence |
|---|---|
| β-actin F | 5′-TGTTACCAACTGGGACGACA-3′ |
| β-actin R | 5′-CTGGGTCATCTTTTCACGGT-3′ |
| VDR F | 5′-GAATGTGCCTCGGATCTGTGG-3′ |
| VDR R | 5′-ATGCGGCAATCTCCATTGAAG-3′ |
| Cathelicidin F | 5′-GGCTGTGGCGGTCACTATC-3′ |
| Cathelicidin R | 5′-GTCTAGGGACTGCTGGTTGAA-3′ |
| Cyp24 F | 5′-GCTGATGACCGACGGTGAG-3′ |
| Cyp24 R | 5′-GTGCGGTACAGAGCTTCCAG-3′ |
| LC3 F | 5′-GACCGCTGTAAGGAGGTGC-3′ |
| LC3 R | 5′-CTTGACCAACTCGCTCATGTTA-3′ |
| ATG16L1 F | 5′-CAGAGCAGCTACTAAGCGACT-3′ |
| ATG16L1 R | 5′-AAAAGGGGAGATTCGGACAGA-3′ |
| April F | 5′-CTTTCGGTTGCTCTTTGGTTG-3′ |
| April R | 5′-CGACAGCACAAGTCACAGC-3′ |
| IFNγ F | 5-GCCACGGCACAGTCATTGA-3 |
| IFNγ R | 5-TGCTGATGGCCTGATTGTCTT-3 |
| IL1β F | 5-GAAATGCCACCTTTTGACAGTG-3 |
| IL1β R | 5-TGGATGCTCTCATCAGGACAG-3 |
| IL8 F | 5-TTTTGCCAAGGAGTGCTAAAGA-3 |
| IL8 R | 5-AACCCTCTGCACCCAGTTTTC-3 |
Autophagy Activity Culture36
Autophagy activity was quantified using the commercial Cyto ID autophagy detection kit (ENZO Life Sciences, ENZ-51,031-K200) while following the manufacturer’s protocol. This kit contains a 488 nm excitable green fluorescent detection reagent that becomes brightly fluorescent when incorporated into the vesicles produced during autophagy. Then the specimens were examined with a Zeiss laser scanning microscope LSM 710 (Carl Zeiss Inc.).
P40 and P75 Proteins
Cloning of the encoding genes in vector pQE80e over expression in E. coli BL21 and purification of the complete mature proteins P40 and P75 were carried out in as described before.37 Briefly, specific primers were designed to amplify the target regions of L. casei BL23 chromosomal DNA and clone them in the BamHI and SmaI restriction sites of pQE80e that carried a RGS-His encoding region. They were then ligated and cloned in an intermediate E. coli DHB10 host, and after checking the sequences, they were subcloned in E. coli BL21(BE3)-[pLysS]. Nucleic acid manipulation and cloning procedures were carried out as previously described.16 For protein purification, E. coli BL21 clones carrying the selected plasmids were grown in batches of 500 mL of LB at 37ºC 100 μg/mL ampicillin and 20 μg/mL chloramphenicol until OD600 reached 0.4, when 1 mM IPTG was added to induce expression. Then bacteria were collected by centrifugation, lysed by sonication, and centrifuged, and supernatants were loaded onto HisTrapTM FF Crude Column (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) in an ÅktaPrimeTM Plus chromatography system (GE Healthcare Bio-Sciences AB). His-tagged P40 and P75 were separated, eluted, and collected according to the instructions of the manufacturer. For the correct preservation of the proteins, they were subject to lyophilization. For this purpose, 0.5 mL aliquots of both proteins were prepared at 0.5 mg/mL in freeze drying buffer (50 mM tris pH 8.0, 50 mM NaCl, 30 mM sucrose, and 0.01% tween 80) and dried in a Virtis Genesis freeze drier (SP Scientific, Stone Ridge, NY, USA).
To study the protective role of P40 and P75 proteins, the organoid from ileum tissue were pretreated with P40 or P75 (100 ng/mL) for 24 hours. Then the organoids were added with TNF-α 0.5ng/mL for 30 minutes. The expressions of VDR and IκBα were detected by Western blots.
Statistical Analysis
All data are expressed as the mean ± SD. All statistical tests were 2-sided. The P values <0.05 were considered statistically significant. Differences between 2 groups were analyzed using 2-sample Welch t test, 1-way ANOVA for more than 2 groups, and 2 time points, respectively. For multiple comparisons, either the Tukey method or the Dunnett test was used for adjusting the P values, depending on if the pairwise or treatment (follow-up time points) vs control (baseline) was compared. Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
RESULTS
121 Conditional Medium Upregulates mRNA Levels of VDR and Autophagy Regulators in HCT116
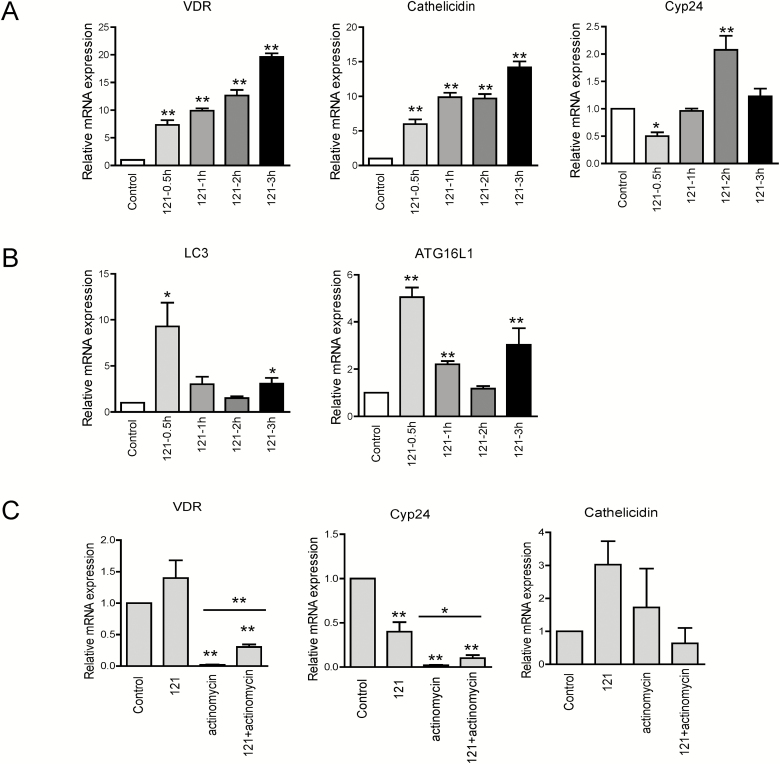
To determine the effect of the conditional medium from Lactobacillus paracasei DKL121 (121-CM) on VDR expression in HCT116 cells, we firstly assessed whether 121-CM alters the transcriptional levels of VDR and its target gene cyp24, Cathelicidin in human HCT116 cells. As compared with the control group, 121-CM treatment increased VDR mRNA levels at different times (0.5 h, 1 h, 2 h, 3 h), which presented a time-dependent trend. The 121-CM upregulated the level of cathelicidin over all time points, and the level of cyp24 was decreased at 0.5 hour but increased at 2 hours (Fig. 1A). Meanwhile, the autophagy response was measured, which presented that the mRNA levels of LC3 and ATG16L1 were elevated at 0.5 hour and 3 hours (Fig. 1B). These findings indicate that 121-CM might induce VDR upregulation in vitro. To further verify the action of 121-CM, the DNA inhibitor actinomycin was added to suppress mRNA levels of VDR. As shown in Figure 1C, 121-CM could reverse the effect of downregulating VDR and its target gene expression caused by actinomycin D treatment. These results confirmed that 121-CM promoted VDR at the RNA level and its target gene expression.
Figure 1.
mRNA expression of VDR and autophagy marker in 121-CM-treated HCT116 compared with nontreated cells. A, mRNA levels of VDR and its target genes in 121-CM-treated HCT116 for 0.5 hour, 1 hour, 2 hours, and 3 hours. Data are expressed as mean ± SD; n = 3, 1-way ANOVA test, *P < 0.05, **P < 0.01, ***P < 0.001 compared with control group. B, mRNA expression of LC3 and ATG16L1 in HCT116 after 121-CM treatment for 0.5 hour, 1 hour, 2 hours, and 3 hours. Data are expressed as mean ± SD; n = 3, 1-way ANOVA test, *P < 0.05, **P < 0.01, ***P < 0.001 compared with control group. C, Transcriptional level of VDR and its target genes in actinomycin (10 μg/mL)-treated HCT116 before coculturing with 121-CM for 3 hours. Data are expressed as mean ± SD; n = 3, 1-way ANOVA test, *P < 0.05, **P < 0.01, ***P < 0.001 compared with no treatment.
121 Conditional Medium Elevates Protein Levels of VDR and Autophagy Regulators in HCT116
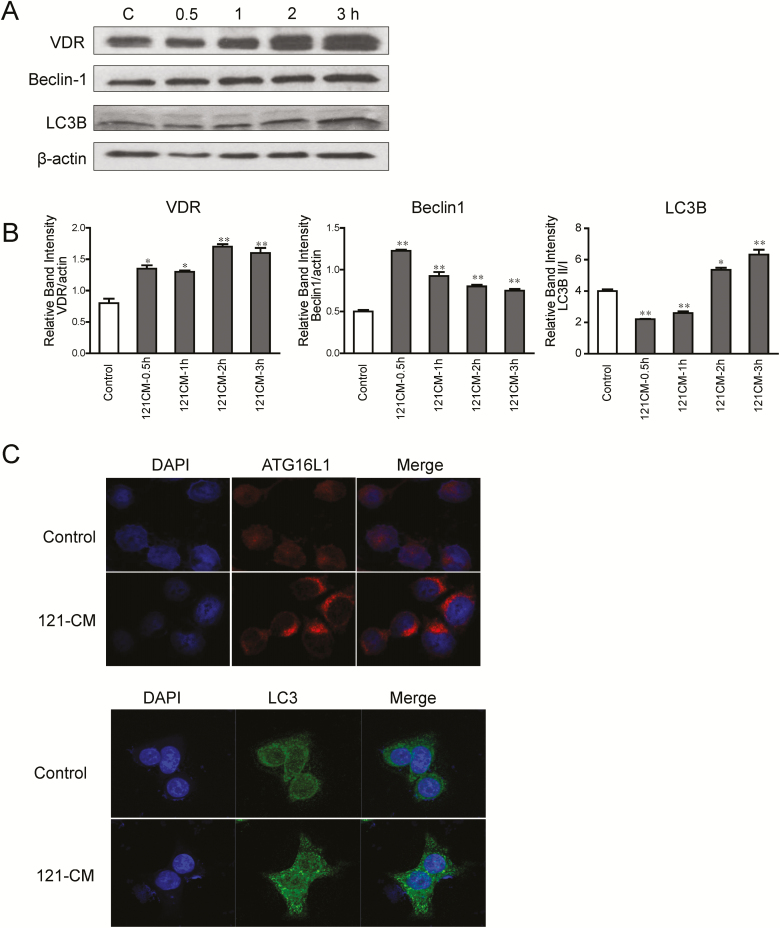
To determine whether 121-CM affects the VDR and autophagic markers at the protein levels, we examined the expression of VDR, Beclin-1, and LC3B after 121-CM treatment by Western blots. As presented in Figure 2A and Figure 2B, the protein levels of VDR and Beclin-1 were increased in 121-CM-treated HCT116 cells at 0.5, 1 , 2, and 3 hours. The 121-CM treatment could downregulate the protein expression of LC3B at 0.5 and 1 hour, whereas it increased the LC3B protein level at 2 and 3 hours. At 3 hours, 121-CM increased ATG16L1 and LC3 abundance in human HCT116 cells (Fig. 2C). These results demonstrated that autophagic response was activated during the process of 121-CM upregulating VDR expression.
Figure 2.
Protein expression of VDR and autophagy regulators in 121-CM-treated HCT116. A, Protein levels of VDR, LC3B and Beclin-1 in 121-CM-treated HCT116 for 0.5 hour, 1 hour, 2 hours, and 3 hours. B, The graph represents the quantification of VDR, LC3BII/I, and Beclin-1 protein levels. Data are expressed as mean ± SD; n = 3, 1-way ANOVA test, *P < 0.05, **P < 0.01 compared with no treatment. C, Expression of ATG16L1 and LC3 were increased after 121-CM treatment (3 hours) in the HCT116 cells.
121 Conditional Medium Administration Upregulates VDR and Autophagy in Ileum of VDRloxp/loxp Mice
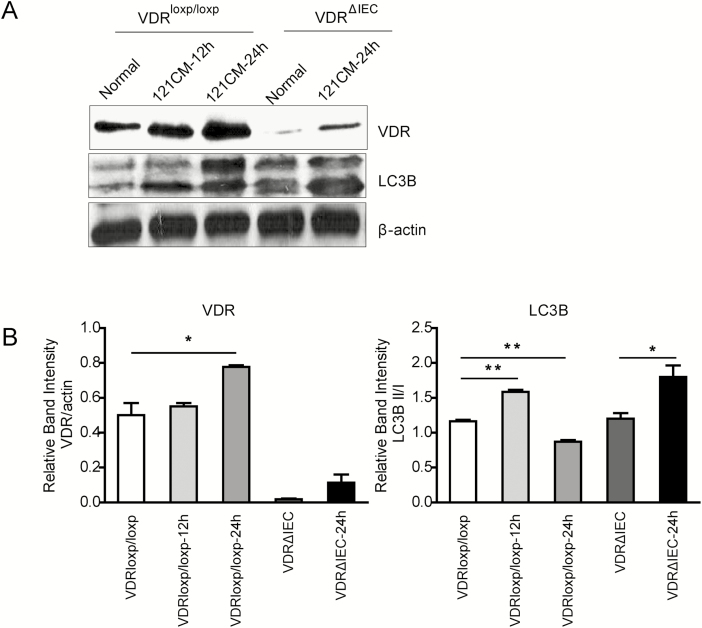
To determine the function of 121-CM-induced VDR expression in vivo, we measured the effect of 121-CM administration on VDR expression and the activation of autophagy in the ileum of VDRloxp/loxp and VDRΔIEC mice. Compared with vehicle control (nontreated group), 121-CM upregulated the level of VDR and LC3B after administrating the 121-CM for 24 hours in VDRΔIEC mice. In VDRloxp/loxp mice, VDR expression was increased at 24 hours. Meanwhile, it also upregulated the levels of LC3B at 12 hours, though decreased at 24 hours (Fig. 3). The data further indicated that 121-CM significantly promoted VDR and autophagy in vivo.
Figure 3.
Expression of VDR and autophagy-related LC3B in ileum of VDRloxp and VDRΔIEC mice orally administrated with 121-CM for 12 hours and 24 hours. A, Changes of VDR and LC3B expression after 121 administration for 12 hours and 24 hours. B, The graph represents the quantification of VDR and LC3B II:I ratio. Data are expressed as mean ± SD; n = 6, 1-way ANOVA test, *P < 0.05, **P < 0.01 compared with normal (No treatment).
Four Other LAB Strains Have Similar Effects on VDR Expression
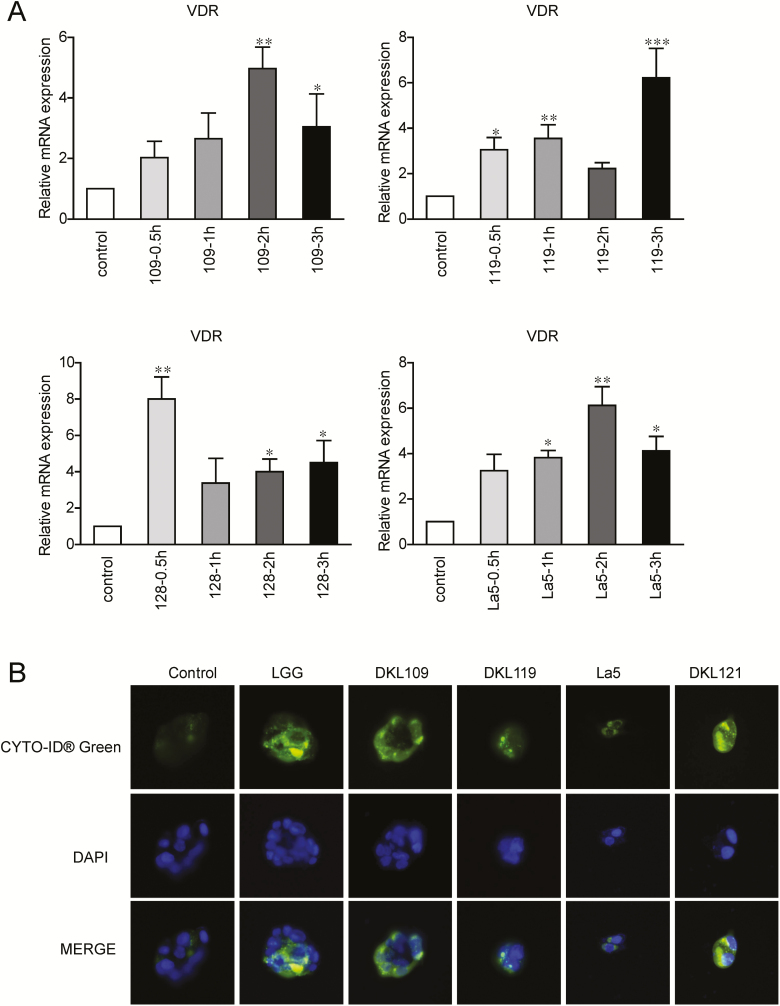
To detect the cellular function of 4 other LAB strains on VDR expression in HCT116 cells, we analyzed the mRNA expression of VDR in HCT116 at different times (0.5 hour, 1 hour, 2 hours and 3 hours) after incubation with the conditional medium from 4 other LAB strains (109-CM, 119-CM, 128-CM, and La5-CM). As shown in Figure 4A, VDR expression was significantly increased in LAB-CM-treated cells. Autophagy activity was also detected by CYTO-ID Autophagy detection kit for the autophagy marker LC3. We found that the autophagy activity was increased after treatment with the LAB strains (Fig. 4B).
Figure 4.
mRNA expression of VDR in HCT116 treated with 4 other LAB stains for different time. A, VDR expression in HCT116 treated with conditional medium of 4 other LAB stains: DKL109, DKL119, DKL128, and DKLLa-5. Data are expressed as mean ± SD; n = 6, 1-way ANOVA test, *P < 0.05, **P < 0.01 compared with normal (no treatment). B, Autophagy activity was increased after LAB strains treated. Autophagy activity was detected by CYTO-ID Autophagy detection kit.
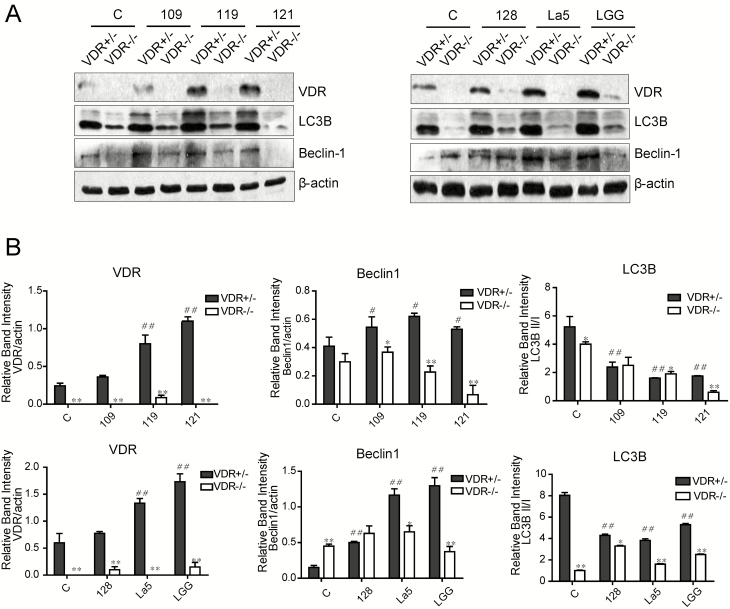
LAB-CM-enhanced VDR and Beclin-1/LC3B Is Abolished in the VDR-/- MEF Cells
To analyze whether LAB-CM enhanced Beclin-1 and LC3B depending VDR, we chose VDR+/- MEF and VDR-/- MEF cells to coculture with LAB-CM. The VDR expression was upregulated in 119-CM-treated, 121-CM-treated, and La5-CM-treated VDR+/- MEFs, which was similar with the effect of positive control lactobacillus rhamnosus GG (LGG)-CM-treated group. It also showed that autophagy was modulated by LAB-CM. The expression of Beclin-1 was elevated after LAB-CM (109-CM, 119-CM, 121-CM, 128-CM, and La5-CM) stimulation in VDR+/- MEFs. Elevation of LC3B II/I happened in 119-CM-treated, 121-CM-treated, and LGG-CM-treated VDR+/- MEFs (Fig. 5). Otherwise, VDR deletion in VDR-/- MEF cells could abolish the effect of LAB-CM on VDR expression and autophagy response. These data illustrated that VDR was required in the action of LAB-CM for the promotion of VDR and activation of autophagy.
Figure 5.
Effects of VDR deletion on 121-CM-induced increase of VDR and autophagy. A, VDR, LC3B, and Beclin-1 expression in 121-CM-treated VDR+/- and VDR-/- MEFs. B, The graph represents the quantification of VDR, Beclin-1, and LC3B II:I ratio. Data are expressed as mean ± SD; n = 3, 1-way ANOVA test, #P < 0.05, ##P < 0.01 compared with no treatment; Student t test, *P < 0.05, **P < 0.01.
We further tested the effects of LAB on the VDR expression and activation of autophagy in macrophages (Supplemental Figs. 1 and 2). However, we did not observe the robust responses that we observed in the epithelial cells. Thus, we focused on the intestinal epithelial studies using organoids and intestinal tissues.
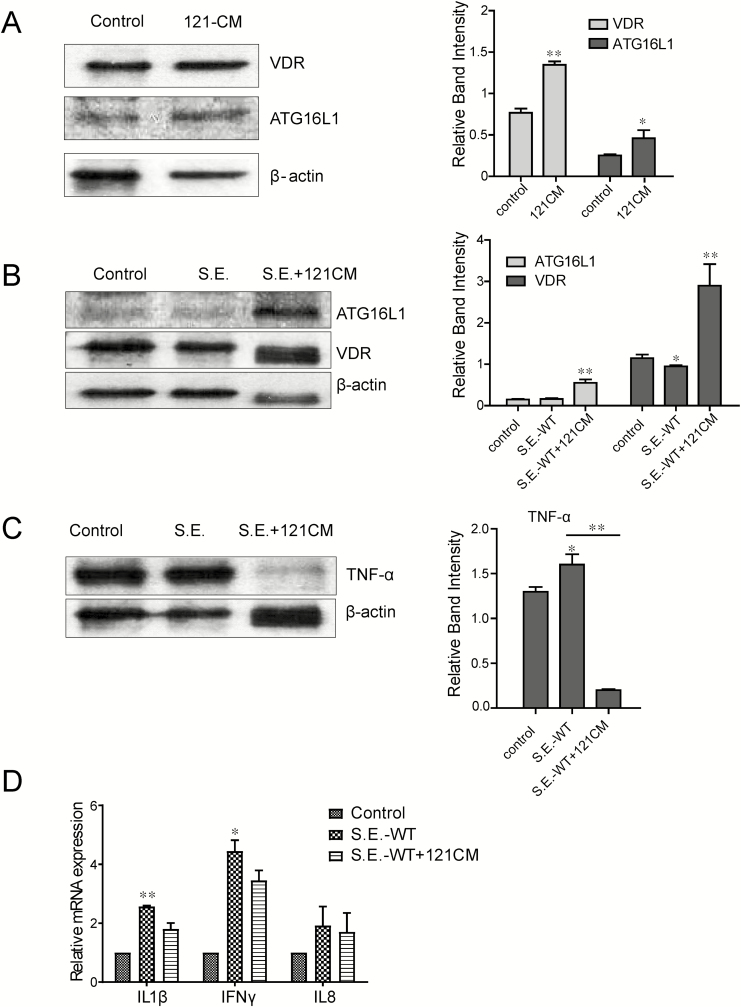
121 Conditional Medium Increased Expressions of VDR in VDR+/+ Organoids and Suppresses Salmonella-induced Inflammation
We have reported a Salmonella Typhimurium–infected organoid culture system suitable for studying host-bacterial interactions.16 Here, we further used the Salmonella enterica serovar Enteritidis wild-type (SE-WT) strain in the infected organoids (Fig. 6A). The 121-CM postincubation apparently induced the expression of VDR and ATG16L1 in VDR+/+ organoids after treating with 121-CM for 3 hours. This result was accordance with the previously described data, which further confirmed the function of 121-CM on promoting VDR-ATG16L1 expression.
Figure 6.
Protein level of VDR and ATG16L1 expression in the 121-CM-treated organoids. A, VDR and ATG16L1 expression is determined by Western blots after treatment with 121-CM for 3 hours. Data are expressed as mean ± SD; n = 3, Student t test, *P < 0.05, **P < 0.01 compared with control group. B, Expression of VDR, ATG16L1, and inflammation molecules in organoids infected with Samonella enterica serovar Enteritidis before 121-CM treatment. VDR and ATG16L1 expression in (0.5 hour) organoids infected with S. Enteritidis before 121-CM treatment for 3 hours. Data are expressed as mean ± SD; n = 3, 1-way ANOVA test, *P < 0.05, **P < 0.01 compared with control (no treatment). C, TNF-α protein levels in organoids after treatment with 121-CM plus S. Enteritidis Data are expressed as mean ± SD; n = 3, 1-way ANOVA test, *P < 0.05, **P < 0.01 compared with control (no treatment). D, mRNA levels of IL1β, IFN γ, and IL8 gene expression in the organoids after treatment with 121-CM plus S. Enteritidis Data are expressed as mean ± SD; n = 3, 1-way ANOVA test, *P < 0.05, **P < 0.01 compared with 121 CM treatment.
We then hypothesized that 121-CM protects the host from Salmonella infection. A Salmonella-infected model was used in an organoid isolated from the ileum of VDR+/+ mouse.
Before SE-WT infection, 121-CM was used to stimulate the organoids for 3 hours. We found that the levels of VDR and ATG16L1 in SE-WT infection plus 121-CM-treated organoids were higher than that in the group infected with SE-WT. The SE-WT infection could obviously promote the expression of TNF-α. Interestingly, 121-CM downregulated the infection-induced TNF-α (Figs. 6B and 6C). The 121-CM treatment also reduced the expression levels of interleukin (IL)-1β and IFNγ (Fig. 6D). Thus, our data indicate that 121-CM not only increased VDR and ATG16L1 but also inhibited the inflammatory response induced by Salmonella infection.
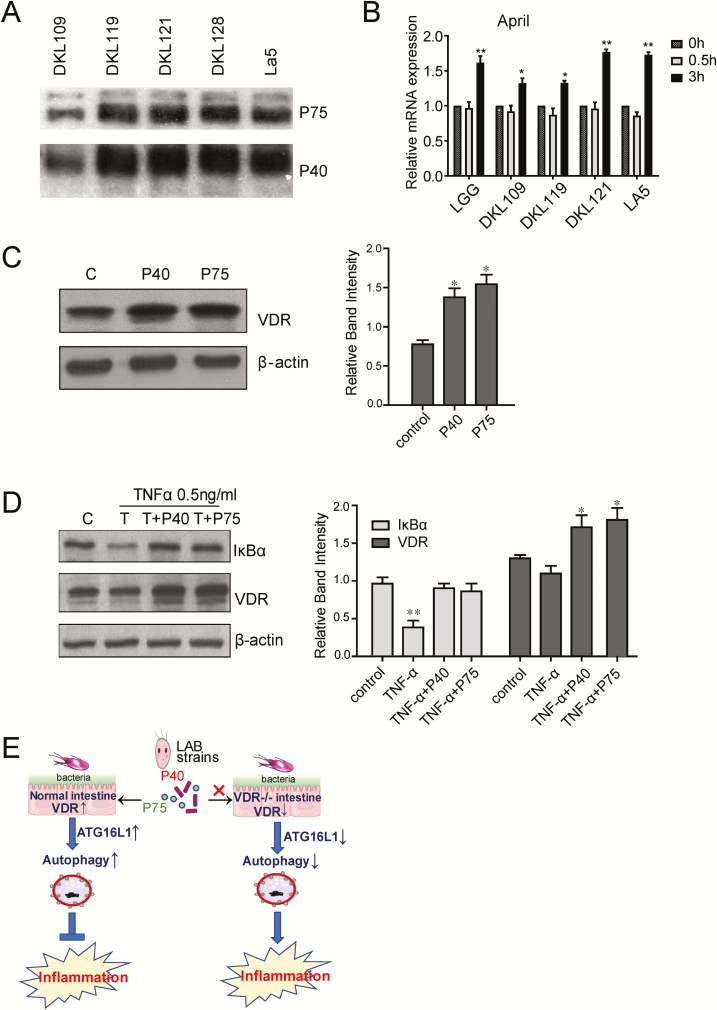
P40 and P75 of the LAB Strains Can Protect the Organoids from Inflammatory Response Induced by TNF-α
Probiotic LGG proteins P40 and P75 are known to be key functional proteins in inhibiting inflammation.35, 38 According to their polyacrilamide gel electrophoresis (PAGE) migration, these 2 proteins have apparent sizes of 40 kDa and 75 kDa, and they were first isolated from LGG culture supernatants. The P40 protein has more potent effects on intestinal epithelial cells as compared with P75. The P40 protein activates epidermal growth factor receptor (EGFR), leading to Akt. It is not clear whether P40 or P75 directly regulate VDR expression. We have tested the expression levels of P40 and P75 in the conditional medium of probiotic strains. We have also purified proteins to treat the organoids and determine the change of VDR. Proteins P40 and P75 were detected in the LAB strains (Fig. 7A). APRIL is a known cytokine secreted by intestinal epithelial cells to trigger IgA production. The P40 protein upregulated the mRNA level of APRIL (Fig. 7B). We also treated the organoids with P40 or P75 (100 ng/mL) for 24 hours. The expressions of VDR were increased by P40 or P75 treatment (Fig. 7C). Excitingly, P40 and P75 can protect the organoids from inflammatory response induced by TNF-α (Fig. 7D). The expression of IκBα was not reduced by TNF-α in organoids with P40 or P75 treatment.
Figure 7.
The P40 and P75 of the LAB strains increase VDR and protect the organoids from inflammatory response induced by TNF-α. A, P40 and P75 proteins can be detected in the LAB strains. B, LAB increased the expression of APRIL. Data are expressed as mean ± SD; n = 3, 1-way ANOVA test, *P < 0.05, **P < 0.01 compared with 0h group. C, The expression of VDR in organoids were upregulated after P40 or P75 treatment (100ng/mL for 24 hours). Data are expressed as mean ± SD; n = 3, 1-way ANOVA test, *P < 0.05 compared with control group. D, Organoid from ileum tissue WAS treated with P40 or P75 (100 ng/mL) for 24 hours, Then added with TNF-α 0.5 ng/mL for 30 minutes. Proteins P40 and P75 upregulated the expression of VDR. The expression of IκBα was decreased after TNF-α treatment but not in the groups with P40 or P75 treatment. Data are expressed as mean ± SD; n = 3, 1-way ANOVA test, *P < 0.05, **P < 0.01 compared with control group. E, A working model of probiotic upregulation of VDR through P40 and P75 to stimulate autophagic responses and inhibit inflammation.
DISCUSSION
In the current study, we demonstrate the protective role of LAB strains from Korean kimchi in inflammatory responses. We found a robust increase of VDR and autophagy signaling when cells treated with the conditional medium from these probiotic strains. The LAB-CM treatment induced higher VDR and its target genes cathelicidin compared with the control group. Lactic acid bacteria also enhanced autophagy responses in vitro and in vivo. The 121-CM treatment before Salmonella infection dramatically increased VDR and ATG16L1 and inhibited the inflammation. Furthermore, we identified that P40 and P75 in the LAB-CM contributed to the anti-inflammatory function by increasing VDR (Fig. 7E). Our study highlights the beneficial effects of probiotics from food in anti-infection and anti-inflammation, suggesting that they may have therapeutic and possibly preventive efficacy in IBD.
Our study has shown that probiotic LGG treatment protected hosts from Salmonella Typhimurium infection.39 Here, we found that probiotics also protect hosts from Salmonella Enteritidis–induced inflammation and infection. These protective effects depend on VDR. Vitamin D receptor regulates host response to invasive pathogens, commensal bacteria, and probiotics in innate and adaptive immunity.40–48 Absence of VDR in the intestine leads to activation of NF-κB and higher risk of chronic inflammation.49, 50 Vitamin D receptor deletion leads to more severe inflammation and bacterial invasion. Here, functionally, we find the LAB-CM treatment increased VDR and autophagy responses. Mechanistically, we identified that the soluble proteins P40 and P75 from LAB strains contribute to enhance VDR and inhibit inflammation. Thus, this study highlights an important mechanism for probiotics through the VDR/autophagy regulation.
Probiotics are not equal; they may use various strategies to interact with the host cells. They have specific features that can distinguish the mode of action of different species and even strains from the same species. In IBD patients, the responses to probiotic treatment and clinical outcomes are inconsistent.5–8 What remains unknown is how probiotics specifically work on VDR signaling and effectively play the anti-inflammatory role. Our data indicate that the probiotic function of 5 LAB strains involves VDR signaling. A VSL3#, a mixture of 8 probiotics, resulted in upregulation of antagonists of NF-κB inflammatory pathways, including the VDR signature.51 Specific probiotic strains exert specific effects in IBD therapy; however, the anti-inflammatory role of probiotics remains unclear.52 Elucidating how probiotics specifically regulate signaling pathways, including VDR, will advance our understanding of bacterial-host interaction in inflammation.
Ours and others’ studies support the critical role of intestinal VDR in maintaining intestinal homeostasis, with its dysregulation possibly contributing to human IBD.27, 53 Based on our study here, changes in the expression of the VDR can be a sensitive measurable parameter to evaluate clinical outcomes of probiotics health effects.
It is important to point out ingestion of probiotics has many forms, such as in foodstuff (eg, cheese, yogurt, fermented milk, fruit juice, or chewing gum) or as a constituent of tablets and capsules. Probiotic activity depends very much on a daily and continued ingestion. It should be part of an individual’s diet and, as such, either incorporated in fermented or supplemented food products. Moreover, some food ingredients might protect probiotic strains against the stressful digestive conditions, increase their activity, or act as gene promoters and should be tested to optimize probiotic effects. Our study found functional proteins P40 and P75 in both probiotic strains and culture media. The functional components of probiotics are present in both culture media of LAB and ingested kimchi. The beneficial role of the lactic acid bacteria isolated from Korean kimchi provides positive evidence of ingestion of probiotics from food. It was reported that probiotics isolated from kimchi can be used in yogurt manufacturing as a starter culture.24 These probiotic strains could be mixed into dairy products to benefit diets.
In conclusion, we tested 5 LAB strains from Korean kimchi in anti-infection and inflammatory responses. Using conditional VDRΔIEC mice, organoids, and cultured human intestinal epithelial cells, we performed a series of molecular and biochemical experiments in vivo and in vitro to investigate probiotic regulation of VDR and autophagy signaling in infection and inflammation. We investigated the mechanisms on how VDR and autophagy responses are related in probiotic-treated cells. We highlight the complex role of VDR in bacterial infection and chronic inflammation.54 Our findings reveal a novel role of LAB from nutriment factors in food in regulating VDR and autophagy responses in inflammation and infectious diseases. This knowledge can be expanded to define novel strategies to prevent and treat various human diseases, including IBD and infectious diseases.
Supplementary Material
ACKNOWLEDGEMENTS
Authors would like to acknowledge the NIDDK/National Institutes of Health grant R01 DK105118, R01DK114126, and DOD BC160450P1 to Jun Sun.
Author Contribution: RL, MS, YZ, SG, DB, and YJ contributed to the acquisition, analysis, and interpretation of data and drafting of the manuscript. YX contributed to the statistical analysis and drafting of the manuscript. JS contributed to the study concept and design, analysis, and interpretation of data, obtained funding, supervised the study, and wrote the manuscript. CP and GPM contributed to the critical materials and intelligent contribution to probiotic proteins. CK and SK helped with critical materials and gave intelligent contribution of probiotic strains. All authors read the manuscript.
REFERENCES
- 1. Hill C, Guarner F, Reid G, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. [DOI] [PubMed] [Google Scholar]
- 2. Parvez S, Malik KA, Ah Kang S, et al. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol. 2006;100:1171–1185. [DOI] [PubMed] [Google Scholar]
- 3. Lee ES, Song EJ, Nam YD, et al. Probiotics in human health and disease: from nutribiotics to pharmabiotics. J Microbiol. 2018;56:773–782. [DOI] [PubMed] [Google Scholar]
- 4. Sartor RB. Efficacy of probiotics for the management of inflammatory bowel disease. Gastroenterol Hepatol. 2011;7:606–608. [PMC free article] [PubMed] [Google Scholar]
- 5. Sood A, Midha V, Makharia GK, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–9, 1209.e1. [DOI] [PubMed] [Google Scholar]
- 6. Garcia Vilela E, De Lourdes De Abreu Ferrari M, Oswaldo Da Gama Torres H, et al. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand J Gastroenterol. 2008;43:842–848. [DOI] [PubMed] [Google Scholar]
- 7. Fujimori S, Tatsuguchi A, Gudis K, et al. High dose probiotic and prebiotic cotherapy for remission induction of active Crohn’s disease. J Gastroenterol Hepatol. 2007;22:1199–1204. [DOI] [PubMed] [Google Scholar]
- 8. Rahimi R, Nikfar S, Rahimi F, et al. A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn’s disease. Dig Dis Sci. 2008;53:2524–2531. [DOI] [PubMed] [Google Scholar]
- 9. LeBlanc JG, de Moreno de LeBlanc A. Probiotics in inflammatory bowel diseases and cancer prevention. In: Watson R, Preedy V, eds. Bioactive Foods in Promoting Health: Probiotics, Prebiotics, and Synbiotics. 2nd ed.Oxford: Elsevier; 2016:345–360. [Google Scholar]
- 10. Demay MB. Mechanism of vitamin D receptor action. Ann N Y Acad Sci. 2006;1068:204–213. [DOI] [PubMed] [Google Scholar]
- 11. Sentongo TA, Semaeo EJ, Stettler N, et al. Vitamin D status in children, adolescents, and young adults with Crohn disease. Am J Clin Nutr. 2002;76:1077–1081. [DOI] [PubMed] [Google Scholar]
- 12. Abreu MT, Kantorovich V, Vasiliauskas EA, et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004;53:1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim WC, Hanauer SB, Li YC. Mechanisms of disease: vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:308–315. [DOI] [PubMed] [Google Scholar]
- 14. Wang TT, Dabbas B, Laperriere D, et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285:2227–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rufo PA, Bousvaros A. Current therapy of inflammatory bowel disease in children. Paediatr Drugs. 2006;8:279–302. [DOI] [PubMed] [Google Scholar]
- 16. Zhang YG, Wu S, Xia Y, Sun J. Salmonella-infected crypt-derived intestinal organoid culture system for host-bacterial interactions. Physiological Reports. 2014;2:e12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simmons JD, Mullighan C, Welsh KI, et al. Vitamin D receptor gene polymorphism: association with Crohn’s disease susceptibility. Gut. 2000;47:211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pei FH, Wang YJ, Gao SL, et al. Vitamin D receptor gene polymorphism and ulcerative colitis susceptibility in Han Chinese. J Dig Dis. 2011;12:90–98. [DOI] [PubMed] [Google Scholar]
- 19. Naderi N, Farnood A, Habibi M, et al. Association of vitamin D receptor gene polymorphisms in Iranian patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2008;23:1816–1822. [DOI] [PubMed] [Google Scholar]
- 20. Kim JH, Yamaori S, Tanabe T, et al. Implication of intestinal VDR deficiency in inflammatory bowel disease. Biochim Biophys Acta. 2013;1830:2118–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hughes DJ, McManus R, Neary P, et al. Common variation in the vitamin D receptor gene and risk of inflammatory bowel disease in an Irish case-control study. Eur J Gastroenterol Hepatol. 2011;23:807–812. [DOI] [PubMed] [Google Scholar]
- 22. Lu R, Wu S, Xia Y, et al. The vitamin D receptor, inflammatory bowel diseases, and colon cancer. Curr Colorectal Cancer Rep. 2012;8:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mangin M, Sinha R, Fincher K. Inflammation and vitamin D: the infection connection. Inflamm Res. 2014;63:803–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cho YH, Hong SM, Kim CH. Isolation and characterization of lactic acid bacteria from Kimchi, Korean traditional fermented food to apply into fermented dairy products. Korean J Food Sci 2013;33:75–82. [Google Scholar]
- 25. Jung YJ, Lee YT, Ngo VL, et al. Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci Rep. 2017;7:17360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Cromphaut SJ, Dewerchin M, Hoenderop JG, et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci U S A. 2001;98:13324–13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu S, Zhang YG, Lu R, et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut. 2015;64:1082–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun J, Hobert ME, Duan Y, et al. Crosstalk between NF-kappaB and beta-catenin pathways in bacterial-colonized intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G129–G137. [DOI] [PubMed] [Google Scholar]
- 29. Lu R, Wu S, Liu X, et al. Chronic effects of a Salmonella type III secretion effector protein AvrA in vivo. Plos One. 2010;5:e10505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun J, Kong J, Duan Y, et al. Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab. 2006;291:E315–E322. [DOI] [PubMed] [Google Scholar]
- 31. Lin Z, Zhang YG, Xia Y, et al. Salmonella enteritidis effector AvrA stabilizes intestinal tight junctions via the JNK Pathway. J Biol Chem. 2016;291:26837–26849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu R, Voigt RM, Zhang Y, et al. Alcohol injury damages intestinal stem cells. Alcohol Clin Exp Res. 2017;41:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang YG, Zhu X, Lu R, et al. Intestinal epithelial HMGB1 inhibits bacterial infection via STAT3 regulation of autophagy. Autophagy. 2019;11:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duan Y, Liao AP, Kuppireddi S, et al. beta-Catenin activity negatively regulates bacteria-induced inflammation. Lab Invest. 2007;87:613–624. [DOI] [PubMed] [Google Scholar]
- 35. Yan F, Cao H, Cover TL, et al. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. Research Support, N.I.H., Extramural Review. 2008;4:151–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bäuerl C, Abitayeva G, Sosa-Carrillo S, et al. P40 and P75 are singular functional muramidases present in the Lactobacillus casei /paracasei/rhamnosus Taxon. Front Microbiol. 2019;10:1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shen X, Liu L, Peek RM, et al. Supplementation of p40, a Lactobacillus rhamnosus GG-derived protein, in early life promotes epidermal growth factor receptor-dependent intestinal development and long-term health outcomes. Mucosal Immunol. 2018;11:1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu S, Yoon S, Zhang YG, et al. Vitamin D receptor pathway is required for probiotic protection in colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G341–G349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ogura M, Nishida S, Ishizawa M, et al. Vitamin D3 modulates the expression of bile acid regulatory genes and represses inflammation in bile duct-ligated mice. J Pharmacol Exp Ther. 2009;328:564–570. [DOI] [PubMed] [Google Scholar]
- 41. Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl). 2010;88:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Waterhouse JC, Perez TH, Albert PJ. Reversing bacteria-induced vitamin D receptor dysfunction is key to autoimmune disease. Ann N Y Acad Sci. 2009;1173:757–765. [DOI] [PubMed] [Google Scholar]
- 43. Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends Mol Med. 2007;13:117–124. [DOI] [PubMed] [Google Scholar]
- 44. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. Faseb J. 2005;19:1067–1077. [DOI] [PubMed] [Google Scholar]
- 45. Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–G216. [DOI] [PubMed] [Google Scholar]
- 46. Lagishetty V, Misharin AV, Liu NQ, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151:2423–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu S, Bruce D, Froicu M, et al. Failure of T cell homing, reduced CD4/CD8alphaalpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc Natl Acad Sci U S A. 2008;105:20834–20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu S, Liao AP, Xia Y, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol. 2010;177:686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sun J, Mustafi R, Cerda S, et al. Lithocholic acid down-regulation of NF-kappaB activity through vitamin D receptor in colonic cancer cells. J Steroid Biochem Mol Biol. 2008;111:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reiff C, Delday M, Rucklidge G, et al. Balancing inflammatory, lipid, and xenobiotic signaling pathways by VSL#3, a biotherapeutic agent, in the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1721–1736. [DOI] [PubMed] [Google Scholar]
- 52. Walker A. Genome watch: probiotics stick it to the man. Nat Rev Microbiol. 2009;7:843. [DOI] [PubMed] [Google Scholar]
- 53. Liu W, Chen Y, Golan MA, et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest. 2013;123:3983–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang YG, Wu S, Sun J. Vitamin D, vitamin D receptor, and tissue barriers. Tissue Barriers. 2013;1:e23118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.