Abstract
Background
Patients with obstructive sleep apnea (OSA) have increased sympathetic activity and frequently also have resistant hypertension (HTN). Treatment of OSA with continuous positive airway pressure (CPAP) decreases awake and sleep blood pressure (BP) and sympathetic activity. This study was designed to assess the effect of treatment of OSA with CPAP on sympathetic activity and BP in patients with diabetes mellitus (DM), chronic kidney disease (CKD), and resistant HTN.
Methods
This was a randomized, double-blind, sham-controlled trial. Patients with DM, CKD, and resistant HTN were randomized to treatment with a therapeutic or subtherapeutic CPAP for 6 weeks. They underwent 24-hour ambulatory BP monitoring and assessment of muscle sympathetic nerve activity before and after 6 weeks on treatment.
Results
Treatment with therapeutic CPAP caused significant decreases in awake systolic and diastolic BP from 144 to 136 mm Hg (P = 0.004) and from 79 to 74 mm Hg (P = 0.004) and in sleep BP from 135 to 119 mm Hg (P = 0.045) and from 75 to 65 mm Hg (P = 0.015) compared with treatment with subtherapeutic CPAP. In contrast, treatment with therapeutic CPAP did not decrease sympathetic activity as assessed from muscle sympathetic nerve activity.
Conclusions
Decrease in BP by treatment with CPAP in patients with DM, CKD, and OSA indicates the contribution of OSA to severity of HTN in this clinical scenario. Decrease in BP in the absence of changes in sympathetic activity is suggestive that other mechanisms induced by OSA play a larger role in the maintenance of HTN in these patients.
Résumé
Contexte
Les patients atteints d’apnée obstructive du sommeil (AOS) présentent une activité sympathique accrue qui est souvent accompagnée d’hypertension artérielle (HTA) réfractaire. Le traitement de l’AOS par ventilation en pression positive continue (CPAP, pour Continuous Positive Airway Pressure) diminue la pression artérielle (PA) à l’état de veille et durant le sommeil ainsi que l’activité sympathique. Cette étude était conçue pour évaluer l’effet du traitement de l’AOS par CPAP sur l’activité sympathique et la PA chez des patients atteints de diabète sucré (DS), d’insuffisance rénale chronique (IRC) et d’HTA réfractaire.
Méthodologie
Il s’agissait d’une étude à double insu et à répartition aléatoire contrôlée par simulation. Les patients atteints de DS, d’IRC et d’HTA réfractaire ont été répartis de façon aléatoire pour recevoir un traitement par CPAP thérapeutique ou subthérapeutique pendant 6 semaines. Une surveillance ambulatoire de la PA et une évaluation de l’activité nerveuse sympathique musculaire sur 24 h ont été effectuées chez les patients avant et après 6 semaines de traitement.
Résultats
Comparativement à la CPAP subthérapeutique, la CPAP thérapeutique a entraîné une diminution significative de la PA systolique et de la PA diastolique à l’état de veille, qui sont passées respectivement de 144 à 136 mmHg (p = 0,004) et de 79 à 74 mmHg (p = 0,004). La même observation a été faite à l’égard de la PA systolique et de la PA diastolique durant le sommeil, qui sont passées respectivement de 135 à 119 mmHg (p = 0,045) et de 75 à 65 mmHg (p = 0,015). En revanche, l’évaluation de l’activité nerveuse sympathique musculaire n’a révélé aucune diminution de l’activité sympathique associée au traitement par CPAP thérapeutique.
Conclusions
La diminution de la PA associée au traitement par CPAP chez les patients atteints de DS, d’IRC et d’AOS indique que l’AOS contribue à l’intensité de l’HTA dans ce scénario clinique. La diminution de la PA en l’absence de modification de l’activité sympathique laisse supposer que d’autres mécanismes induits par l’AOS jouent un rôle plus important dans le maintien de l’HTA chez ces patients.
Obstructive sleep apnea (OSA) has a high prevalence among patients with obesity, diabetes mellitus (DM), chronic kidney disease (CKD), and resistant hypertension (HTN).1, 2, 3, 4, 5, 6, 7 It is characterized by repeated upper airway obstructions during sleep, leading to hypopneic and apneic episodes associated with hypoxemia, triggering arousal and restoration of upper airway tone and airflow.8 These episodes evoke marked increases in sympathetic activity (SA) as assessed from muscle sympathetic nerve activity (MSNA) by peroneal nerve microneurography.8 They are also associated with nocturnal increases in blood pressure (BP) and heart rate.8,9 Prospective observational studies using treatment with continuous positive airway pressure (CPAP) have reported normalization of sleep architecture, SA, and decrease in BP during sleep and awake periods.10, 11, 12, 13, 14 However, findings from prospective randomized control trials (RCTs) reported more heterogeneity with regard to effect of CPAP on BP (as assessed from 24-hour ambulatory blood pressure monitoring [ABPM]).15, 16, 17, 18, 19 In a meta-analysis of 5 RCTs, the pooled difference in systolic BP on 24-hour ABPM was 4.78 mm Hg (95% confidence interval, −7.95 to −1.61), and study-level mean difference in systolic BP lowering ranged from +2.89 to −9.20 mm Hg.15 Two RCTs reported no decreases in BP in response to CPAP.19,20 The largest RCT, the Hipertension Arterial Resistente Control con CPAP (HIPARCO) trial, reported a decrease in diastolic BP, but no change in systolic BP.16 The other 2 trials reported decreases in systolic BP by 10.0 and 6.5 mm Hg, respectively.17,18 These trials did not evaluate the effect of treatment with CPAP on SA. Usui et al.21 reported a decrease in systolic BP from 134.9 ± 5.2 mm Hg to 119.5 ± 5.5 mm Hg (P = 0.03) and a significant decrease in MSNA from 58 ± 4 bursts/min to 48 ± 5 bursts/min (P < 0.001) in patients with chronic heart failure randomized to 4-week treatment with CPAP.21 In this study, less than half the patients randomized to treatment with CPAP also had HTN.
CKD is associated with a high prevalence of HTN and increased SA.22,23 Renal disease per se rather than the number of functioning nephrons or the severity of the renal disease appears to be the leading cause of an increased SA in patients with CKD via central sympathoexcitatory effects of renal afferents. This concept is based on studies showing high SA in patients at different stages of CKD, including patients with adult polycystic kidney disease with preserved renal function and patients receiving renal replacement therapy by hemodialysis.22, 23, 24, 25 In patients with kidney failure on hemodialysis, successful transplantation restores kidney function but does not decrease SA.23 In contrast, bilateral nephrectomy in these patients does normalize SA.23 Likewise, SA also increases in parallel with the severity of HTN and obesity.26
In animal models of OSA, renal sympathetic denervation attenuates BP increases and the incidence of secondary arrhythmias induced by apneic episodes.27 Likewise, in patients with severe OSA and HTN, renal sympathetic denervation decreases BP and severity of OSA syndrome (apneic-hypopneic index).28,29 However, the effect of renal denervation or treatment with CPAP on the interplay between renal and hypoxemic central sympatho-excitatory effects in relation to BP control has not been studied. Our study was designed to assess whether in the setting of high SA caused by OSA-related hypoxemia and renal disease–related central sympathoexcitatory effects, treatment of OSA with CPAP still decreases BP because it would eliminate/mitigate OSA-related central sympathoexcitatory stimuli but not central sympathoexcitatory effects caused by renal disease.
Materials and Methods
Study design
This study was a prospective, randomized, sham-controlled, double-blind trial. All the participants, the primary investigator, the research coordinators, and other personnel involved in the study conduct, data collection, and data analysis were blinded to allocation of therapeutic vs subtherapeutic CPAP. Only the sleep study physician was aware of the actual CPAP allocation and was also responsible for titration of CPAP (to ensure therapeutic vs nontherapeutic pressures, as detailed next).
Study population
Individuals with DM and CKD (creatinine clearance calculated according to Cockroft–Gault formula < 60 mL/min or albuminuria) and with resistant HTN (BP > 135/85 mm Hg on 24-hour ABPM while taking 3 or more BP-lowering drugs including a diuretic) were eligible and referred to have polysomnography. Patients found to have moderate or severe OSA (defined as apnea-hypopnea index [AHI] > 15/h) on a standard nocturnal polysomnogram test were invited to participate in the trial. Written informed consent was obtained from all participants enrolled in the trial.
Treatment protocol
Eligible and willing participants were randomized to treatment with therapeutic or subtherapeutic (sham) CPAP for 6 weeks. Randomization was done using computer-generated random numbers, and allocation was concealed. They underwent 24-hour ABPM, assessment of MSNA by peroneal nerve microneurography, and plasma sampling for catecholamines, renin, and aldosterone at baseline and 6 weeks after initiation of treatment with therapeutic or subtherapeutic CPAP. Fixed therapeutic CPAP pressure was determined by subsequent therapeutic polysomnogram. Sham therapy was determined as fixed therapeutic CPAP minus 10 cm of water (with lowest CPAP at 5 cm of water). Adherence was checked with download data on scheduled visits with the study coordinator during weeks 2 and 5. In addition, all patients were contacted by phone every week by the study coordinator to confirm adherence to the CPAP device. The minimum number of hours of use of the CPAP device was set at 5 per night. The specific studies were performed in the clinical research room at the University of Ottawa Heart Institute. On the day of the study, fasting patients presented in the morning to the clinical research room. After patients’ vital signs were determined, they underwent an assessment of MSNA by peroneal nerve microneurography, as described in detailed previously.30 MSNA was calculated from a 5-minute recording after 30 minutes of rest in a supine position in a quiet room with dim lights. At the end of this 5-minute period, blood samples for the assessment of plasma catecholamines, renin, and aldosterone were collected, processed, and evaluated as described previously.30 Patients then completed 24-hour ABPM with the SpaceLabs 90217 (Snoqualmie, WA). This was done at baseline and 6 weeks after CPAP initiation (therapeutic or subtherapeutic, as assigned). BP reading allocation as being asleep vs being awake was derived from individual patient logbooks.
The primary outcome measure was the change in nocturnal and daytime mean systolic BP as assessed from 24-hour ABPM in response to treatment with therapeutic vs subtherapeutic CPAP.
The secondary outcome measures included assessment of SA from MSNA and assessment of plasma epinephrine, norepinephrine, renin, and aldosterone in response to treatment with therapeutic vs subtherapeutic CPAP.
Statistical analysis
For assessment of BP effect, the minimal number of patients per group was determined as 21 to achieve 81% power to detect a difference of −10.3 mm Hg with an estimated standard deviation of 11.4 and a significance level of 0.05 using a 2-sided t test as derived from the report on BP changes in response to nasal CPAP by Becker et al.10 With regard to MSNA, in normotensive, otherwise healthy patients with OSA, prior literature reported 6-month treatment with nasal CPAP to result in a decrease in MSNA from 51 ± 2 bursts/min to 41 ± 2 bursts/min, whereas MSNA remained unchanged in untreated patients (45 ± 3 and 45 ± 5 bursts/min, respectively).31 Assuming similar changes (or lack thereof) in MSNA in the present study, we proposed a minimal number of patients 6 per group. Altogether, our initial plan was to recruit 54 patients for this study to allow for up to 25% dropout.
Given the small numbers and non-normally distributed data, nonparametric tests (Wilcoxon rank-sum) were used for assessment of therapeutic vs subtherapeutic CPAP on primary and secondary outcome measures. Data are presented as numbers and percentages for categorical variables and median (interquartile range [IQR]) for continuous variables. A P value < 0.05 was considered statistically significant. All analysis was done with JMP (version 8.0.2, SAS Institute Inc., Cary, NC).
Ethics approval was obtained from the Ottawa Health Sciences Research Ethics Board. The trial protocol is registered at clinicaltrials.gov (NCT number NCT01875341).
Results
Recruitment
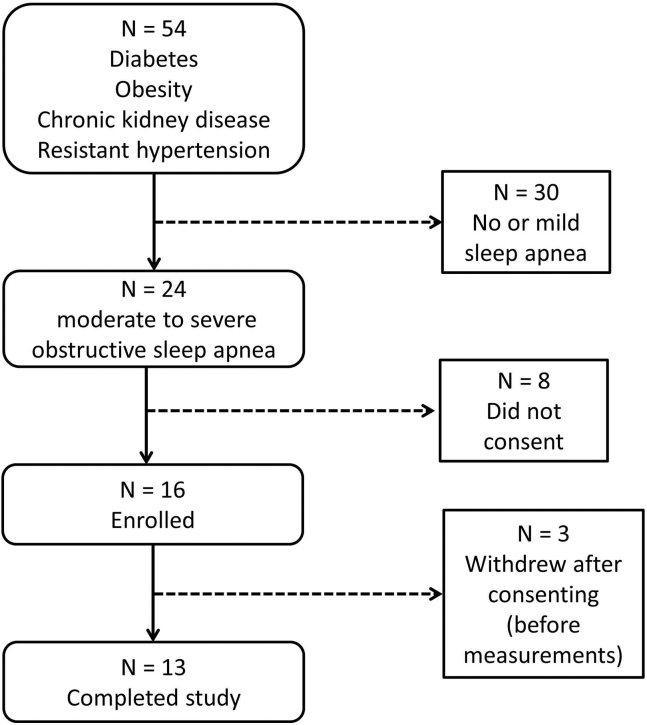
A total of 54 patients meeting inclusion criteria underwent a standard nocturnal polysomnogram. Of these, 24 patients tested positive for OSA expressed as hypopnea/apnea index > 15. Of these 24 patients, 16 consented and were randomized to treatment with therapeutic or subtherapeutic CPAP. Three patients withdrew from the study before undergoing any testing (given the complexity of the study protocol), and 13 patients completed the study as designed (Fig. 1 shows flow). Thus, 6 patients were randomized to sham CPAP and 7 patients to therapeutic CPAP.
Figure 1.
Study flow diagram.
Baseline data
Baseline demographic and clinical data of the13 patients who completed this study are shown in Table 1. Randomized subjects were all middle-aged obese individuals with DM, microalbuminuria, and uncontrolled HTN on more than 3 BP-lowering drugs. They were all men, and all had mild CKD, defined mostly on the basis of albuminuria. The median body mass index was 33 kg/m2. OSA was moderate to severe as evidenced by the high AHI (Table 1). They all had high SA as assessed from MSNA (Table 1). Adherence to CPAP treatment was assessed from downloads by the study coordinator during visits in weeks 2 and 5 after the initiation of therapy with CPAP. The actual range of CPAP use was 5 to 9 hours per night.
Table 1.
Baseline data
| Variable | Value | CPAP subtherapeutic | CPAP therapeutic |
|---|---|---|---|
| Total N | 13 | 6 | 7 |
| Age (y) | 60 (57-68) | 63 (54.5-71) | 59 (58-67) |
| Male sex (N, %) | 13 (100%) | 6 (100%) | 7 (100%) |
| BMI at screening (kg/m2) | 32.8 (30.8-35.05) | 34.2 (32.5-35.7) | 30.9 (28.7-34.9) |
| BP medications (actual) | 4 = 7 patients | 4 = 3 patients | 4 = 4 patients |
| 5 = 3 | 5 = 1 | 5 = 2 | |
| 6 = 2 | 6 = 1 | 6 = 1 | |
| 7 = 1 | 7 = 1 | 7 = 0 | |
| Creatinine (μmol/L) | 87 (76.5-121) | 82 (62.75-111) | 90 (71-152) |
| Creatinine clearance (mL/min) | 80 (55.4-91) | 79 (53-95) | 80 (56-91) |
| ACR (mg/mmol) | 16.8 (4.3-84.9) | 49.1 (6.7-83.2) | 13.1 (4.3-187.3) |
| SBP (mm Hg) | 140 (131-150.5) | 137.5 (127-147.75) | 140 (136-165) |
| DBP (mm Hg) | 73 (66-81.5) | 71 (62-81.5) | 73 (66-85) |
| HR (beats/min) | 55 (51-59) | 55 (51-57.5) | 55 (51-62) |
| Sleep test (AHI/h) | 38.75 (24.63-56.75) | 56 (23-74.5) | 34.5 (24.5-46) |
All data (unless specified otherwise) presented as median (interquartile range). Creatinine clearance was calculated according to Cockroft–Gault formula.
ACR, albumin:creatinine ratio; AHI, apnea-hypopnea index; BMI, body mass index; BP, blood pressure; CPAP, continuous positive airway pressure; DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
Effect of treatment on BP
In patients randomized to therapeutic CPAP, systolic and diastolic BP significantly decreased compared with patients randomized to subtherapeutic CPAP (P = 0.022 and P = 0.012 for systolic and diastolic BP, respectively) (Table 2).
Table 2.
Effect of treatment on BP, SA, renin, and aldosterone
| Variable | CPAP therapeutic |
CPAP subtherapeutic |
P value | ||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| MSNA bursts/min | 47.5 (39-52.3) | 49 (35.4-52.4) | 40.4 (37.6-52.4) | 46 (38.5-53.5) | 0.99 |
| MSNA bursts/100 beats | 82 (70.9-95.3) | 90.3 (69-90.4) | 81.4 (72.4-89) | 86 (76.9-96.5) | 0.99 |
| ABPM SBP awake (mm Hg) | 144 (136-153) | 136 (133.3-144.3) | 138.5 (127.8-146.8) | 154 (152-160) | 0.045 |
| ABPM DBP awake (mm Hg) | 79 (70-88) | 73.5 (71-77.8) | 73 (67.8-80.3) | 83 (72-97) | 0.015 |
| ABPM SBP sleep (mm Hg) | 135 (124-145) | 119 (115.8-134) | 132 (124-138.8) | 146 (137-167) | 0.004 |
| ABPM DBP sleep (mm Hg) | 75 (65-83) | 65 (56.5-73.8) | 68 (63.3-76) | 81 (65-87) | 0.004 |
| ABPM SBP Overall (mm Hg) | 141 (135-150) | 133 (130.3-138.8) | 136.5 (128.8-145) | 153 (148-161) | 0.022 |
| ABPM DBP Overall (mm Hg) | 80 (69-86) | 72.5 (66.5-76.3) | 72 (65.8-79) | 84 (72-94) | 0.012 |
| Heart rate awake (beats/min) | 70 (59-79) | 67 (58-85) | 65 (61-70) | 64.5 (57-77) | 0.83 |
| Heart rate sleep (beats/min) | 66 (45-87) | 62 (50-78) | 58.5 (51-64) | 59.5 (53-70) | 0.62 |
| Heart rate overall (beats/min) | 69 (59-79) | 65 (56-85) | 65 (59-68) | 63 (56-77) | 0.61 |
| Aldosterone (pmol/L) | 178.5 (92.8-260.5) | 118.5 (76.3-152.5) | 98 (82.5-147.3) | 103 (89.8-165.8) | 0.23 |
| Plasma renin activity (ng/mL/h) | 0.55 (0.4-3.33) | 1.05 (0.8-2.2) | 1.25 (0.73-3.8) | 0.4 (0.2-3.7) | 0.85 |
| Norepinephrine (pg/mL) | 341 (266.5-669) | 428 (368.5-794.3) | 432 (368.8-478.8) | 315 (161.5-554) | 0.27 |
| Epinephrine (pg/mL) | 32 (16-33.5) | 30.5 (13-60.8) | 49.5 (10.8-158) | 20 (15-53) | 0.54 |
| BMI (kg/m2) | 30.9 (28.7-34.9) | 30.8 (28.6-34.2) | 34.2 (32.5-35.7) | 34.9 (32.2-36.6) | 0.83 |
All data presented as median (interquartile range). P relates to the effect of treatment (therapeutic vs subtherapeutic CPAP).
ABPM, ambulatory blood pressure monitoring; BMI, body mass index; BP, blood pressure; CPAP, continuous positive airway pressure; DBP, diastolic blood pressure; MSNA, muscle sympathetic nerve activity; SA, sympathetic activity; SBP, systolic blood pressure.
Likewise, treatment with therapeutic CPAP caused significant decreases in systolic and diastolic BP during sleep compared with treatment with subtherapeutic CPAP (P = 0.004 for both systolic and diastolic BP, respectively) (Table 2).
Treatment with therapeutic CPAP also caused significant decreases in awake systolic and diastolic BP compared with treatment with subtherapeutic CPAP (P = 0.045 for systolic BP and P = 0.015 for diastolic BP, respectively) (Table 2).
Effect of treatment on MSNA
There was no difference in the effect of treatment by therapeutic vs subtherapeutic CPAP at 6 weeks on MSNA (Table 2). Patients from both arms showed small, nonsignificant increases in MSNA that are not biologically relevant.
Effect of treatment on plasma norepinephrine, epinephrine, renin, and aldosterone
There were no statistically significant or clinically meaningful changes in plasma renin activity, aldosterone, norepinephrine, and epinephrine in either arm (Table 2).
Effect of treatment on body weight and kidney function
There was no statistically different change in body weight from baseline, with an overall median difference (MD) of 0.2 kg/m2 (IQR, −3.3 to 1.2 kg/m2). Likewise, there were no significant changes in body weight grouped by therapeutic (MD, 0.13 kg/m2; IQR, −0.07 to 1.18) vs subtherapeutic CPAP (MD, 0.28 kg/m2; IQR, −3.3 to 0.49; P value between groups 0.83). The change in kidney function at 6 weeks was not significant (MD, −6.5 μmol/L in subtherapeutic and 0 μmol/L in therapeutic CPAP group, P = 0.45).
Discussion
This study had 2 major findings. In agreement with the effect of CPAP in patients without CKD, therapeutic CPAP caused significant and clinically relevant decreases in awake and sleep BP in obese patients with DM, OSA, CKD, and resistant HTN. Second, in contrast to the effects of CPAP on sympathetic outflow in patients with OSA but without CKD, SA (as assessed from MSNA) remained unchanged by treatment with CPAP in obese patients with DM, CKD, and resistant HTN.
The majority of patients with HTN in the setting of diabetic kidney disease require 3 or more BP-lowering drugs to control BP to the target.32,33 One could argue that this is partially related to the lower target for BP in diabetic patients (<130/80 mm Hg) compared with patients with nondiabetic kidney disease (<140/90 mm Hg) until recently. However, even for the target BP < 140/90 mm Hg, patients with diabetic CKD in clinical trials required, on average, 3 or more BP-lowering drugs to achieve the above target.32,33 Another reason for polypharmacy to achieve BP control in these patients is the possibility of undiagnosed and thus untreated OSA. In the present study, the prevalence of OSA with AHI > 15 was 44%. These results confirm the high prevalence of OSA in patients with resistant HTN, obesity, DM, and CKD previously reported by others.4,5,7
The observed decreases in BP in the group with therapeutic CPAP were biologically meaningful and significant compared with baseline BP readings within the group, as well as compared with changes in the 6-week readings in patients randomized to subtherapeutic CPAP. Changes in BP (as assessed by 24-hour ABPM) in response to treatment with CPAP in patients with resistant HTN differ substantially from study to study ranging from no decreases in BP to significant decreases in systolic BP by 6.5 to 10.0 mm Hg.17, 18, 19, 20 The largest decreases in BP were shown in the trial with the shortest follow-up (8 weeks), and it was the only trial in which patients were randomized to therapeutic CPAP and sham CPAP.18 From this point of view, our results are similar with regard to design and duration. Furthermore, 2 trials included in this meta-analysis that showed no change in BP in response to CPAP also share in common lowest pretreatment BP (129.9 and 129 mm Hg, respectively).19,20 On the other hand, the largest decreases in BP were observed in those with the highest pretreatment BP (148 mm Hg).18 Although this meta-analysis did not demonstrate statistical heterogeneity, from this discussion, there is clearly a significant clinical heterogeneity with regard to the population studied as well as study design among trials on CPAP in patients with OSA and resistant HTN.
In contrast to the decrease in BP by therapeutic CPAP, subtherapeutic CPAP caused a small (but not significant) increase in BP. In the trial by de Oliveira et al.,18 which had a similar design with sham CPAP, systolic BP in participants randomized to sham CPAP showed minimal change (0.7 mm Hg, 95% confidence interval, −5.3 to +6.7 mm Hg).18 Thus, the more pronounced (still nonsignificant) increase in BP in the subtherapeutic arm in our study may be related to the small size of our trial (which we acknowledge as the limiting factor). One could speculate that in some patients, subtherapeutic CPAP could aggravate the severity of OSA (frequency of hypopneic/apneic episodes) or compromise the recovery phase from OSA-caused hypoxemia (by wearing the mask per se). However, we do not have data on the actual number and severity of the apneic/hypopneic episodes in the therapeutic or subtherapeutic group postintervention. Furthermore, because our data and reports by others (after the completion of this trial) indicate that sham CPAP increases BP in patients with OSA, the rationale for sham CPAP in patients with OSA as a placebo or control intervention in clinical trials is clearly no longer acceptable.18
The decreases in BP were not accompanied by changes in SA assessed from MSNA reflecting central sympathetic discharges. Levels of plasma catecholamines that are affected by the rate of the release, uptake, and metabolism also did not show any change. The observed decrease in BP, in the absence of changes in SA, is suggestive of other mechanisms (than just sympathetic hyperactivity) induced by OSA as playing a greater role in HTN in this population. Previous studies on OSA in patients with fluid-retaining states such as chronic heart failure and end-stage kidney disease suggest that intravascular and extravascular volume expansion, and in particular nocturnal rostral shifts of fluids, are contributory to OSA.34, 35, 36, 37, 38 Although we did not observe a statistically significant effect of therapeutic or subtherapeutic CPAP on body weight, which does not eliminate the possibility of subtle, but clinically relevant changes in distribution of body fluid between the groups during the day and night. Indeed, our study shows no changes in renin and aldosterone but lacks a more detailed assessment of intravascular and extravascular volumes, and in particular of central volumes (hemodynamic measurements or humoral parameters such as atrial natriuretic factor) to address whether the observed changes in BP between the groups could be due to the effect of CPAP on these factors.
The persistence of high SA in obese patients with diabetic CKD and OSA after treatment with CPAP indicates activation of central sympathoexcitatory signalling pathways in these patients not affected by “mechanical” treatment of OSA. Indeed, limited data (by small number of studies and small number of studied subjects in these studies) on the effect of renal sympathetic denervation in patients with OSA (but without CKD) suggest that renal denervation decreases the severity of OSA and BP.21,22 These studies lack data on SA and intravascular and extravascular volume, thereby limiting explanation of the observed findings.28,29
Study limitations
This study does have certain limitations. All patients who completed this study were men. This certainly was not an intention, and it may reflect that our inclusion criteria captured a population dominated by men. Because there are many differences in OSA between men and women including more pronounced rostral fluid shift in men compared with women, and in particular in patients with fluid retention states such as chronic heart failure and CKD, one cannot generalize findings from this study to both men and women.39 Fewer patients actually completed the trial than planned. We found that our inclusion criteria were perhaps set up as being unrealistically restrictive, thus having an impact on the size of the pool of eligible patients. Furthermore, as designed, this study was time-consuming for the individual participants, and some of them perceived the procedures, in particular peroneal nerve microneurography, as being partially invasive, further compromising recruitment. As a result, the lack of difference in MSNA could represent a type II error. However, MSNA did not decrease in any individual patient in either group. Additionally, MSNA remained high with more than 80% saturation (bursts/100 beats) as previously reported for patients with CKD.22, 23, 24 Last, the actual BP-lowering effect by CPAP may be an overestimate because subtherapeutic CPAP increased BP. Therefore, we report the absolute changes in BP, as well as the actual changes BP for each group compared with baseline BP.
Conclusions
A decrease in BP by treatment with CPAP in obese patients with DM, CKD, and OSA indicates the contribution of OSA to the severity of HTN in this clinical scenario. This decrease in BP, in the absence of changes in SA, is suggestive of other mechanisms induced by OSA as playing a role in the maintenance of HTN in these patients. A study on the effects of treatment with CPAP combined with renal denervation with detailed assessment of SA and intravascular volume and central hemodynamics would be desirable to further explore the role of sympathetic nervous system and kidneys in the development and maintenance of HTN in patients with OSA and CKD.
Acknowledgements
Drs Ruzicka, Knoll, and Hiremath receive research salary support from the Department of Medicine, University of Ottawa.
Funding Sources
This study was supported by a Research Award provided by the Department of Medicine, University of Ottawa.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: Research reported in this article was approved by the Ottawa Health Sciences Research Ethics Board.
Clinical Trials registration NCT number NCT01875341.
See page 263 for disclosure information.
References
- 1.Ficker J.H., Dertinger S.H., Siegfried W. Obstructive sleep apnoea and diabetes mellitus: the role of cardiovascular autonomic neuropathy. Eur Respir J. 1998;11:14–19. doi: 10.1183/09031936.98.11010014. [DOI] [PubMed] [Google Scholar]
- 2.Senaratna C.V., Perret J.L., Lodge C.J. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Rosas S.E. Sleep apnea in individuals with chronic kidney disease: a wake-up call. Clin J Am Soc Nephrol. 2011;6:954–956. doi: 10.2215/CJN.02840311. [DOI] [PubMed] [Google Scholar]
- 4.Zoccali C., Mallamaci F., Tripepi G. Sleep apnea in renal patients. J Am Soc Nephrol. 2001;12:2854–2859. doi: 10.1681/ASN.V12122854. [DOI] [PubMed] [Google Scholar]
- 5.West S.D., Nicoll D.J., Stradling J.R. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax. 2006;61:945–950. doi: 10.1136/thx.2005.057745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grassi G., Facchini A., Trevano F.Q. Obstructive sleep apnea-dependent and -independent adrenergic activation in obesity. Hypertension. 2005;46:321–325. doi: 10.1161/01.HYP.0000174243.39897.6c. [DOI] [PubMed] [Google Scholar]
- 7.Logan A.G., Perlikowski S.M., Mente A. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Somers V.K., Dyken M.E., Clary M.P., Abboud F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abboud F., Kumar R. Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J Clin Invest. 2014;124:1454–1457. doi: 10.1172/JCI70420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker H.F., Jerrentrup A., Ploch T. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 11.Henderson L.A., Fatouleh R.H., Lundblad L.C., McKenzie D.K., Macefield V.G. Effects of 12 months continuous positive airway pressure on sympathetic activity related brainstem function and structure in obstructive sleep apnea. Front Neurosci. 2016;10:90. doi: 10.3389/fnins.2016.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logan A.G., Tkacova R., Perlikowski S.M. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21:241–247. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- 13.Naughton M.T., Benard D.C., Liu P.P. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152:473–479. doi: 10.1164/ajrccm.152.2.7633695. [DOI] [PubMed] [Google Scholar]
- 14.Tamisier R., Tan C.O., Pepin J.L., Levy P., Taylor J.A. Blood pressure increases in OSA due to maintained neurovascular sympathetic transduction: impact of CPAP. Sleep. 2015;38:1973–1980. doi: 10.5665/sleep.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L., Cao Q., Guo Z., Dai Q. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich) 2016;18:153–158. doi: 10.1111/jch.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Garcia M.A., Capote F., Campos-Rodriguez F. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–2415. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 17.Pedrosa R.P., Drager L.F., de Paula L.K.G. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest. 2013;144:1487–1494. doi: 10.1378/chest.13-0085. [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira A.C., Martinez D., Massierer D. The antihypertensive effect of positive airway pressure on resistant hypertension of patients with obstructive sleep apnea: a randomized, double-blind, clinical trial. Am J Respir Crit Care Med. 2014;190:345–347. doi: 10.1164/rccm.201403-0479LE. [DOI] [PubMed] [Google Scholar]
- 19.Lozano L., Tovar J.L., Sampol G. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28:2161–2168. doi: 10.1097/HJH.0b013e32833b9c63. [DOI] [PubMed] [Google Scholar]
- 20.Muxfeldt E.S., Margallo V., Costa L.M. Effects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: a randomized controlled trial. Hypertension. 2015;65:736–742. doi: 10.1161/HYPERTENSIONAHA.114.04852. [DOI] [PubMed] [Google Scholar]
- 21.Usui K., Bradley T.D., Spaak J. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. JACC. 2005;45:2008–2011. doi: 10.1016/j.jacc.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 22.Converse R.L., Jr., Jacobsen T.N., Toto R.D. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 23.Hausberg M., Kosch M., Harmelink P. Sympathetic nerve activity in end-stage renal disease. Circulation. 2002;106:1974–1979. doi: 10.1161/01.cir.0000034043.16664.96. [DOI] [PubMed] [Google Scholar]
- 24.Klein I.H., Ligtenberg G., Oey P.L., Koomans H.A., Blankestijn P.J. Sympathetic activity is increased in polycystic kidney disease and is associated with hypertension. J Am Soc Nephrol. 2001;12:2427–2433. doi: 10.1681/ASN.V12112427. [DOI] [PubMed] [Google Scholar]
- 25.Cerasola G., Vecchi M., Mule G. Sympathetic activity and blood pressure pattern in autosomal dominant polycystic kidney disease hypertensives. Am J Nephrol. 1998;18:391–398. doi: 10.1159/000013382. [DOI] [PubMed] [Google Scholar]
- 26.Grassi G., Pisano A., Bolignano D. Sympathetic nerve traffic activation in essential hypertension and its correlates: systematic reviews and meta-analyses. Hypertension. 2018;72:483–491. doi: 10.1161/HYPERTENSIONAHA.118.11038. [DOI] [PubMed] [Google Scholar]
- 27.Linz D., Hohl M., Nickel A. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension. 2013;62:767–774. doi: 10.1161/HYPERTENSIONAHA.113.01728. [DOI] [PubMed] [Google Scholar]
- 28.Witkowski A., Prejbisz A., Florczak E. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 29.Zhao M.M., Tan X.X., Ding N., Zhang X.L. [Comparison of efficacy between continuous positive airway pressure and renal artery sympathetic denervation by radiofrequency ablation in obstructive sleep apnea syndrome patients with hypertension] Zhonghua Yi Xue Za Zhi. 2013;93:1234–1237. [PubMed] [Google Scholar]
- 30.Ruzicka M., Floras J.S., McReynolds A.J. Do high doses of AT(1)-receptor blockers attenuate central sympathetic outflow in humans with chronic heart failure? Clin Sci (Lond) 2013;124:589–595. doi: 10.1042/CS20120437. [DOI] [PubMed] [Google Scholar]
- 31.Narkiewicz K., Kato M., Phillips B.G. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–2335. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 32.Brenner B.M., Cooper M.E., de Zeeuw D. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 33.Parving H.H., Lehnert H., Brochner-Mortensen J. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 34.Elias R.M., Bradley T.D., Kasai T., Motwani S.S., Chan C.T. Rostral overnight fluid shift in end-stage renal disease: relationship with obstructive sleep apnea. Nephrol Dial Transplant. 2012;27:1569–1573. doi: 10.1093/ndt/gfr605. [DOI] [PubMed] [Google Scholar]
- 35.Elias R.M., Chan C.T., Paul N. Relationship of pharyngeal water content and jugular volume with severity of obstructive sleep apnea in renal failure. Nephrol Dial Transplant. 2013;28:937–944. doi: 10.1093/ndt/gfs473. [DOI] [PubMed] [Google Scholar]
- 36.Guyenet P.G. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 37.Yumino D., Redolfi S., Ruttanaumpawan P. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation. 2010;121:1598–1605. doi: 10.1161/CIRCULATIONAHA.109.902452. [DOI] [PubMed] [Google Scholar]
- 38.Hanly P.J., Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344:102–107. doi: 10.1056/NEJM200101113440204. [DOI] [PubMed] [Google Scholar]
- 39.White L.H., Bradley T.D. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol. 2013;591:1179–1193. doi: 10.1113/jphysiol.2012.245159. [DOI] [PMC free article] [PubMed] [Google Scholar]