In this issue of the Canadian Journal of Cardiology Open, Illmann et al.,1 from BC Children’s Hospital in Vancouver, report their results of a survey carried out with the Canadian Pediatric Cardiology Association and the Congenital Cardiac Interventional Study Consortium. As such, it is a relatively small study, but the results probably reflect the current “state of play” with regard to the appreciation of the burgeoning technique. For example, the authors found that those working in centers in the United States were more than 5 times more likely to have access to the necessary technology than those working in Canada. Financial constraints were identified as the barrier to access for half of those responding to the particular question on that topic. These results regarding the financial burden almost certainly represent the situation in other continents, particularly for those still working in the so-called Third World. This is of relevance when the whole world is facing the problem of increasing costs of health care.
The conclusions reached on the basis of the questionnaire are certainly of importance. As the authors discuss, the use of such printed models greatly facilitates communication, not only between medical practitioners but also with parents, and often with the patients themselves. And, as they also comment, the technique is currently underused in the field of medical education. The authors also comment regarding an alternative approach of producing virtual dissection, which is the one we favor. This is the option to create virtual models from the 3-dimensional datasets and to interrogate them using freely available open-source software, such as Horos, and to view them on a personal computer.2
Although we are in agreement with Illmann et al.1 about the underuse of printed models, we would challenge their suggestion that the use of open-source software does not alleviate one of the most significant limitations, namely, the need for skilled personnel with expertise in “performing segmentation.”1 Although this may be true when creating virtual models using segmentation software such as Materialize Mimics, no such “segmentation” is required for virtual dissection.2,3 Instead, virtual dissection is no more than a modification of the commonly performed volume-rendering technique. It can be achieved relatively easily by simply altering the opacities of colour maps using the 16-bit Colour Look Up Table editor, which is embedded within the software.2 Further, the ease of producing reconstructions in clinically relevant views makes virtual dissection an ideal technique to guide planning ahead of surgical or interventional procedures. The images thus produced provide all necessary morphological details. For example, they serve to emphasize the difference between the channel described as the “ventricular septal defect” in the setting of tetralogy of Fallot (Fig. 1) and the channel usually described in this fashion when both arterial trunks arise exclusively from the right ventricle, this being the lesion termed “double outlet right ventricle” (Fig. 2). As the reconstructions show, it would be a disaster if the surgeon closed the so-called ventricular septal defect in the setting of double outlet right ventricle. This is because the channel is, in reality, the outflow tract for the morphologically left ventricle (Fig. 2). It is better described simply as an interventricular communication.4 As shown in Figure 2, such virtual dissection also clearly demonstrates the relationship of the interventricular communication and the proposed site of surgical closure. Our own experience also shows how easy it is, using virtual dissection, to reveal the presence of the pectinate muscles within the morphologically right atrium and to demonstrate the difference in their arrangement in the morphologically left atrium (Fig. 3). As discussed, the problems of making these distinctions in the clinical setting have been held by some pediatric cardiologists to represent a caveat in the determination of atrial arrangement.
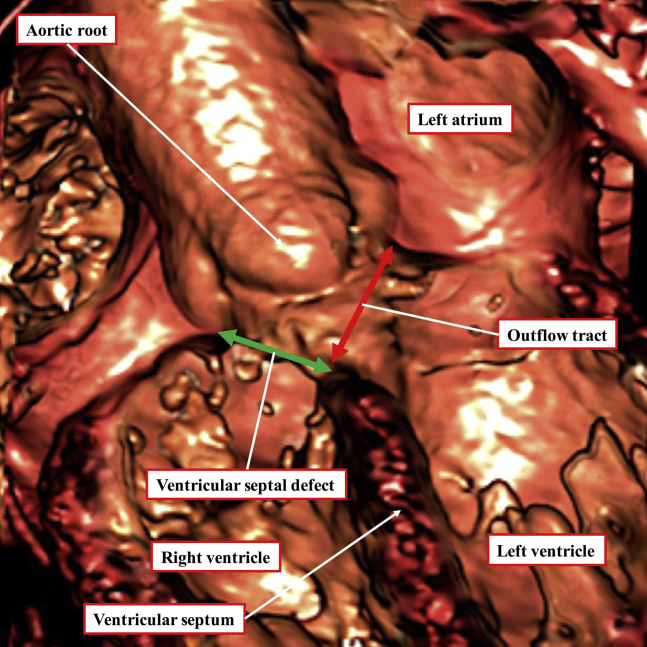
Figure 1.
The virtual dissection is made from a computed tomographic dataset obtained from an adolescent boy with tetralogy of Fallot. The image is equivalent to fluoroscopic left anterior oblique projection with cranial angulation. It shows a large subaortic ventricular septal defect, with the aortic root overriding the crest of the ventricular septum. Surgical repair will entail closure of the defect at its right ventricular margin (green double-headed arrow), thus connecting the aorta to the left ventricle. The left ventricular margin of the defect (red double-headed arrow) is the outflow tract for the left ventricle. This can never be closed unless an alternative route is provided for left ventricular ejection.
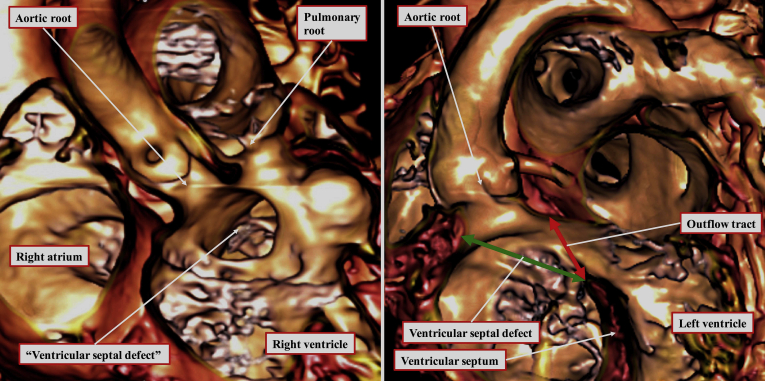
Figure 2.
The virtual dissection of a computed tomographic dataset from a 20-month-old girl with double outlet right ventricle shows the morphology of the interventricular communication in anteroposterior (left) and left anterior oblique (right) projections. The defect in the ventricular septum opens to the right ventricle directly beneath the aortic root. The area (green double-headed arrow) around which the surgeon will create a tunnel to reconnect the aortic root with the left ventricle is analogous to the ventricular septal defect in the setting of tetralogy of Fallot (Fig. 1). The area usually currently described as the “ventricular septal defect” is, in reality, the outlet for the morphologically left ventricle (red double-headed arrow). Closure of this area would obviously represent a surgical disaster. It is better described simply as an interventricular communication.
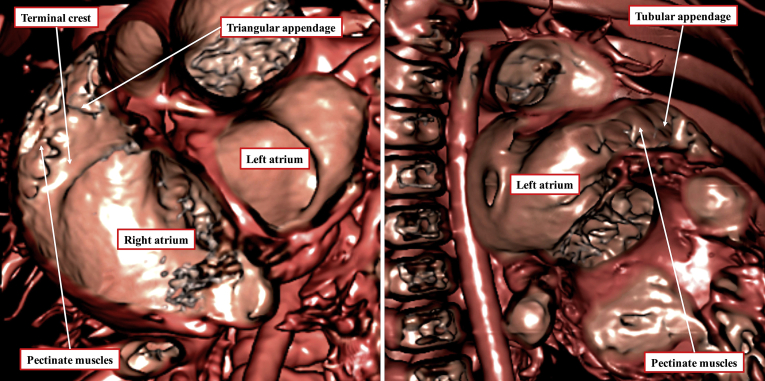
Figure 3.
Additional virtual dissection of the dataset obtained from the patient shown in Figure 2 shows the extent of pectinate muscles in the morphologically right atrial chamber (left) and the morphologically left atrial chamber (right). The differences in the extent of the pectinate muscles serve to demonstrate the morphology of the chambers. Note that the terminal crest is also well visualised on virtual dissection of the morphologically right atrium.
The results of the questionnaire, nonetheless, surely indicate that the technique of 3-dimensional printing will become increasingly popular. The costs of the printing itself will presumably diminish with its increasing use. We remain to be convinced, however, that the 3-dimensional printing of the models represents a major advance over the ability to interrogate the virtual datasets on the computer screen, a procedure that incurs no additional cost.3 Although printing the model is required in some cases, much can be achieved simply by using the virtual model. We should also take note that the increasingly rapid development of other visualization techniques, such as holography, could well overtake all these currently existing technologies.5
Funding Sources
No funding was received by the authors in respect to the information contained with the editorial.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See article by Illmann et al., pages207–213of this issue.
Ethics Statement: The research reported adheres to all relevant ethical guidelines.
See page 194 for disclosure information.
References
- 1.Illmann C.F., Hosking M., Harris K.C. Utility and access to 3D printing in the context of congenital heart disease: an international physician survey study. Can J Cardiol. 2020;2:207–213. doi: 10.1016/j.cjco.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta S.K., Spicer D.E., Anderson R.H. A new low-cost method of virtual cardiac dissection of computed tomographic datasets. Ann Pediatr Cardiol. 2019;12:110–116. doi: 10.4103/apc.APC_167_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S.K., Anderson R.H. Virtual dissection: an alternative to surface- rendered virtual three-dimensional cardiac model. Ann Pediatr Cardiol. 2020;13:102–103. doi: 10.4103/apc.APC_127_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebadi A., Spicer D.E., Backer C.L., Fricker F.J., Anderson R.H. Double-outlet right ventricle revisited. J Thorac Cardiovasc Surg. 2017;154:598–604. doi: 10.1016/j.jtcvs.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 5.Brun H., Bugge R.A.B., Sutherland L.K.R. Mixed reality holograms for heart surgery planning: first user experience in congenital heart disease. Eur Heart J Cardiovasc Imaging. 2019;20:883–888. doi: 10.1093/ehjci/jey184. [DOI] [PubMed] [Google Scholar]