Abstract
Background
Three-dimensional (3D) printing is a new technology capable of producing patient-specific 3D cardiac models.
Methods
A cross-sectional survey of pediatric cardiologists was conducted. Members of the Canadian Pediatric Cardiology Association and Congenital Cardiac Interventional Study Consortium were invited to participate. A questionnaire was distributed using Research Electronic Data Capture between May and September 2019. Results were analyzed using descriptive statistics, Fisher exact test, and odds ratio.
Results
A total of 71 pediatric cardiologists responded. Some 85% (60/71) agreed that patient-specific 3D printed cardiac models are a beneficial tool in treating children with congenital heart disease (CHD); 80% of those (48/60) believe 3D models facilitate communication with colleagues; 49% (35/71) of respondents had access to 3D printing technology; and 77% (27/35) of those were using models for clinical care. Access differed according to geographic location (P = 0.004). Of respondents, Americans were 5.5 times more likely (confidence interval, 1.6-19.2) than Canadians to have access to 3D printing technology. The primary reason for lack of access was financial barriers (50%, 18/36). In clinical practice, surgical planning is the primary use of models (96%, 26/27), followed by interventional catheterization planning (52%, 14/27). Double outlet right ventricle was the most commonly modelled lesion (70%, 19/27).
Conclusion
3D printing is a new technology that is beneficial in the care of children with CHD. Access to 3D printing varies by geographic location. In pediatric cardiology, 3D models are primarily used for procedural planning for CHD lesions with complex 3D spatial relationships.
Résumé
Contexte
L’impression en trois dimensions (3D) est une nouvelle technologie permettant de produire des modèles cardiaques 3D sur mesure pour chaque patient.
Méthodologie
Une enquête transversale a été menée auprès de cardiologues-pédiatres. Les membres de l’Association canadienne de cardiologie pédiatrique et du Congenital Cardiac Interventional Study Consortium ont été invités à y participer. À cette fin, un questionnaire a été diffusé au moyen de l’outil REDCap (Research Electronic Data Capture) de mai à septembre 2019. Les résultats ont été analysés au moyen de techniques de statistique descriptive, du test exact de Fisher et du rapport de cotes.
Résultats
Au total, 71 cardiologues-pédiatres ont répondu au questionnaire. Environ 85 % (60/71) des répondants convenaient que les modèles cardiaques personnalisés à chaque patient et produits par impression 3D sont utiles pour traiter les enfants atteints d’une cardiopathie congénitale; de ce nombre, 80 % (48/60) estimaient que les modèles 3D facilitent la communication entre collègues; 49 % (35/71) avaient accès à la technologie d’impression 3D et, parmi eux, 77 % (27/35) se servaient de modèles pour prodiguer des soins cliniques. L’accès variait selon l’emplacement géographique (p = 0,004). Parmi les répondants, les médecins situés aux États-Unis étaient 5,5 fois plus susceptibles (intervalle de confiance : 1,6-19,2) que les médecins canadiens d’avoir accès à la technologie d’impression 3D. Les ressources financières constituaient le principal obstacle à l’accès à cette technologie (50 %, 18/36). Dans la pratique clinique, les modèles sont surtout utilisés pour planifier les interventions chirurgicales (96 %, 26/27) et le cathétérisme interventionnel (52 %, 14/27). Le ventricule droit à double issue était particulièrement modélisé (70 %, 19/27).
Conclusion
L’impression 3D est une nouvelle technologie utile pour soigner les enfants présentant une cardiopathie congénitale. L’accès à cette technologie varie selon l’emplacement géographique. En cardiologie-pédiatrie, les modèles 3D sont surtout utilisés pour planifier les interventions relatives à des cardiopathies congénitales complexes sur le plan tridimensionnel.
The medical application of 3-dimensional (3D) printing technology is a rapidly developing field for children with congenital heart disease (CHD).1 The use of 3D printed cardiac models has been reported in a wide variety of settings, including patient and family education,2,3 medical education,4, 5, 6, 7, 8, 9, 10, 11, 12 preprocedural planning,13, 14, 15, 16 and procedural simulation.17,18 CHD is heterogeneous and patient specific, and varied with respect to 3D spatial relationship between structures. The models can convey intricate and nuanced information about the 3D spatial relationship between cardiac structures that may not be well appreciated in conventional 2-dimensional imaging modalities. This enhanced 3D spatial information can have implications on procedural decision making and, in turn, patient outcomes.
We sought to determine the current spectrum of use of 3D printed cardiac models in CHD. We also sought to determine access to 3D printing technology for pediatric cardiologists.
Materials and Methods
Study design and population
This was a cross-sectional survey targeting pediatric cardiologists who treat patients with CHD. Questionnaires were disseminated and responses were collected between May and September 2019. A voluntary response sampling methodology was used. Members of the Canadian Pediatric Cardiology Association (CPCA) and Congenital Cardiac Interventional Study Consortium (CCISC) were eligible to participate. To recruit participants, an email including study rationale, invitation to participate, and a link to the online questionnaire was sent to the members of CPCA and CCISC via their respective email list serves. Questionnaires were distributed using Research Electronic Data Capture software hosted at BC Children’s Hospital. Research Electronic Data Capture is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources.19,20 This study was reviewed and approved by the BC Children’s Hospital research ethics board. By completing the questionnaire, respondents acknowledged they were giving consent to participate in the research. All responses were received anonymously.
Questionnaire
The questionnaire contained 25 items that enquired about physician’s access to 3D printing technology, experience using 3D printed cardiac models, and opinions on best uses of patient specific 3D printed cardiac models. Before dissemination to the CPCA and CCISC, the questionnaire was distributed to pediatric cardiologists and pediatric cardiology research staff at BC Children’s Hospital to test content and branching logic. Results were analyzed using descriptive statistics, Fisher exact test, and odds ratio.
Results
Demographics
A total of 71 pediatric cardiologists responded to the questionnaire. A total of 47 responses were collected from CPCA members and 24 responses from CCISC members. This represents a response rate of 57% and 10% for CPCA and CCISC, respectively. Respondents were located internationally, in 5 of the 7 continents. For illustration purposes, the quantity of respondents
relative to the respondents’ broad geographic location were mapped (ArcMapTM, v. 10.6; Esri Inc, CA) (Supplemental Fig. S1). The majority (93%) practiced in North America, specifically in Canada or the United States.
Pediatric cardiologists think 3D models are beneficial for children with CHD
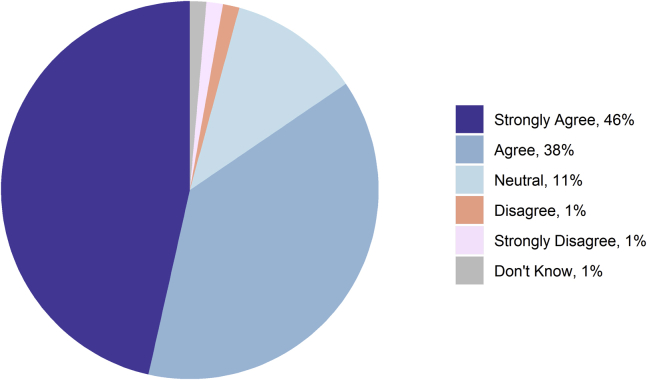
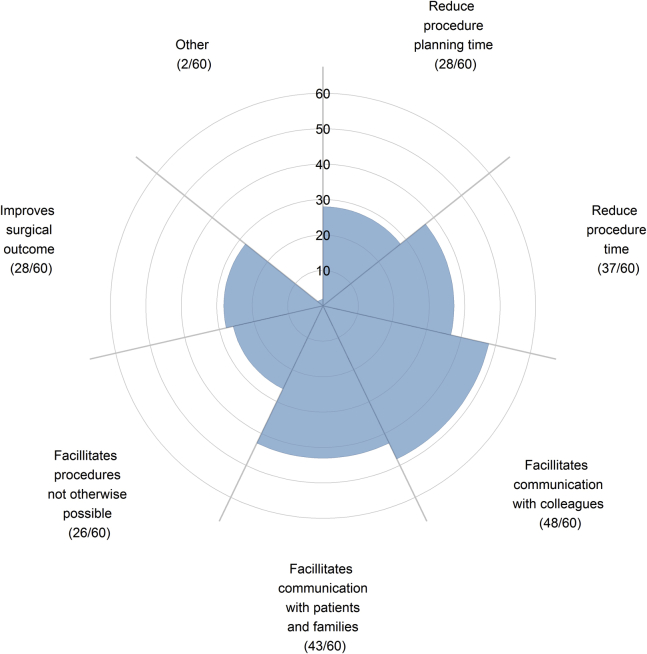
Some 85% (60/71) agreed or strongly agreed that patient-specific 3D cardiac models are or can be a beneficial tool in treating patients with CHD (Fig. 1). Of those who believed 3D models are beneficial tools, the leading perceived benefits of the 3D models were that they facilitated communication with colleagues (80.0%, 48/60) or with patients and their families (72%, 43/60) (Fig. 2). Some 3% of (2/71) respondents disagreed or strongly disagreed that 3D models were a beneficial tool. Respondents who disagreed were able to provide justification in an open text box. One respondent justified that “3D models often cannot accurately depict valve attachments, which can be an important consideration in the clinical management of a CHD patient.”
Figure 1.
Response to the statement “Patient-specific 3-dimensional (3D) cardiac models are or can be a beneficial tool in treating children with congenital heart disease (CHD).”
Figure 2.
Respondents who agreed or strongly agreed that 3D models were beneficial tool in treating children with CHD were surveyed on their opinion of the perceived benefit of 3D models.
Access to 3D printing is presently limited
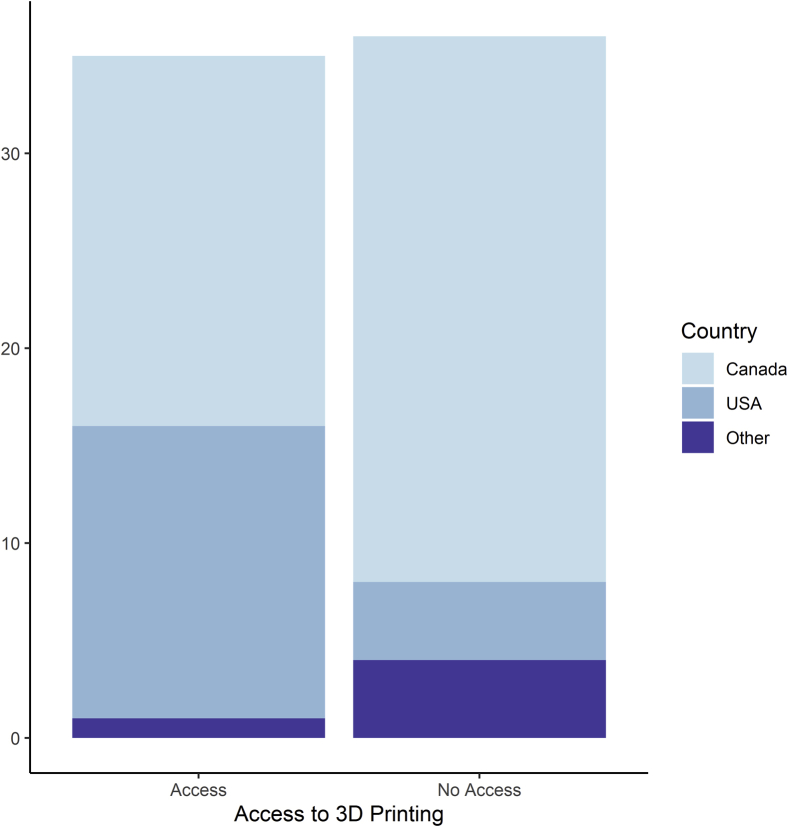
Some 49% (35/36) of respondents reported that they had access to 3D printing technology at their institution. Access to 3D printing technology was not evenly distributed between geographic location of respondents (Fig. 3). There was a significant difference in access to 3D printing technology based on the location of the respondent (P = 0.004). Of those who responded, pediatric cardiologists from the United States were 5.5 times more likely (95% confidence interval, 1.6-19.2) to have access to 3D printing technology compared with Canadian pediatric cardiologists.
Figure 3.
Access to 3D printing technology by country.
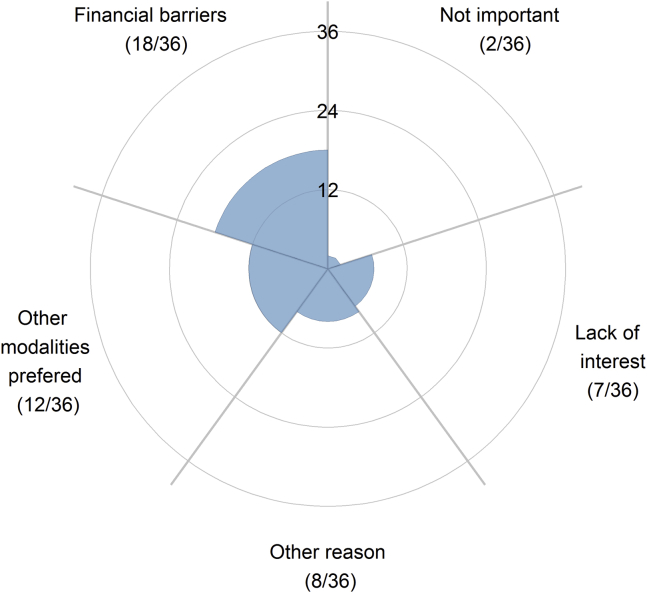
The primary reasons for respondents lacking access to 3D printing technology were financial barriers (50.0%, 18/36) and preference for standard 3D imaging modalities (33%, 12/36), such as 3D echocardiography (Fig. 4). A minority of respondents without access to 3D printing believed that the technology is not important (6%, 2/36) or that there was lack of interest at their institution (19%, 7/36). Some 22% (8/36) of respondents faced other reasons for lack of access to 3D printing technology. Half of these respondents (4/8) elaborated that they practiced in nonsurgical centers and that 3D printing is not important in nonsurgical centers; 25.0% (2/8) responded that their institution was in the process of starting a 3D printing program.
Figure 4.
Respondents without access to 3D printing technology were surveyed on the reason for lack of access.
Of respondents with access to the technology, 77% (27/35) report that they have used the technology in the treatment of patients with CHD. Most respondents who are using 3D models (96%, 26/27) report the primary use is in the conduct of clinical care as opposed to in the context of research protocols (4%, 1/27). Some 82% (22/27) of respondents using 3D printing technology began printing within the last 4 years. The annual volume of 3D printed models produced varied by institution. The median reported volume was 5 models per year (interquartile range, 3-10).
Reported uses
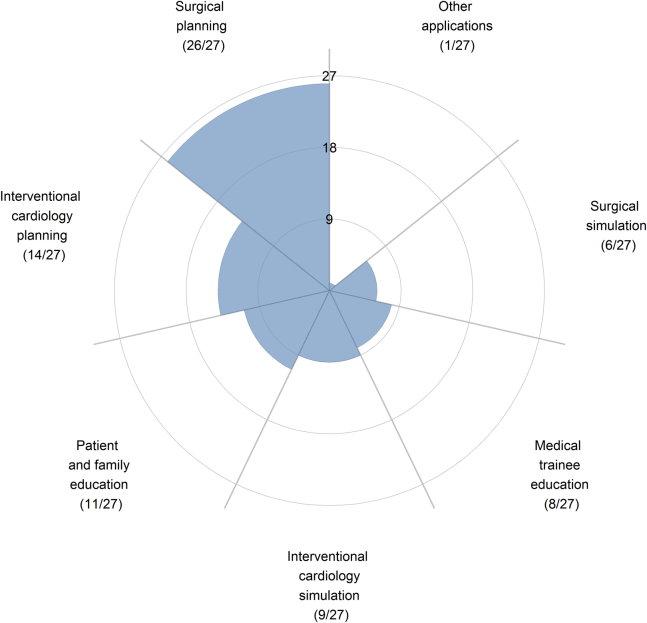
In clinical practice, the primary reported use of 3D printed cardiac models was for procedural planning. Almost all (96%, 26/27) respondents who are using 3D printing technology have used models for surgical planning, and approximately half (52%, 14/27) have reported using the models for interventional cardiology planning. Less than one third of respondents used 3D printed cardiac models for educational purposes, and less than one quarter of respondents of respondents used models for surgical simulation (Fig. 5).
Figure 5.
Respondents who have access to and use 3D printing were surveyed on how they use the technology in clinical practice.
Reported lesions
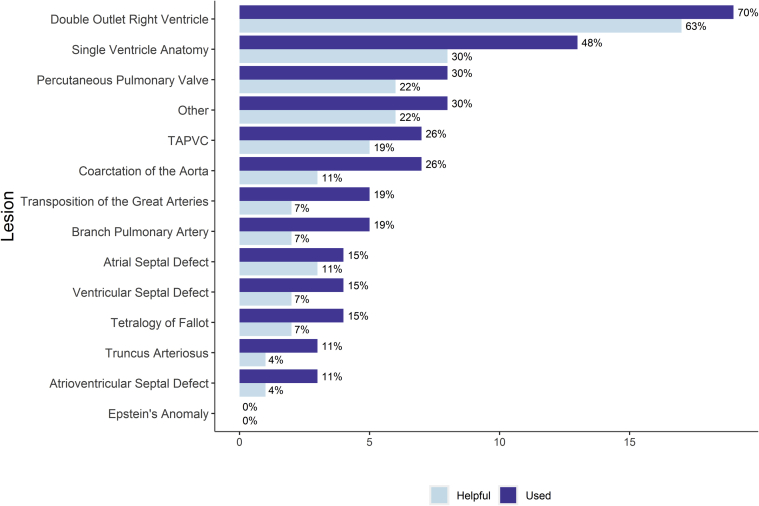
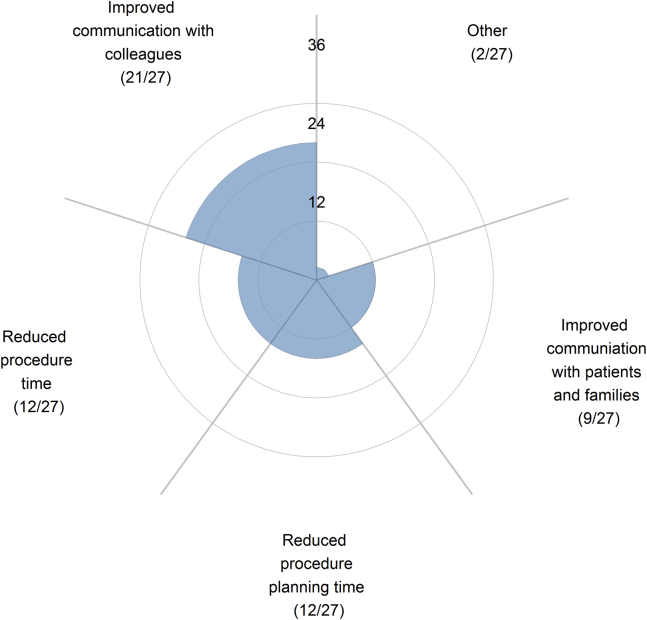
The most common lesion for which 3D models were used was double-outlet right ventricle (DORV) (70%, 19/27), followed by single-ventricle anatomy (48%, 13/27) (Fig. 6). These were also the lesions that pediatric cardiologists found the model most helpful compared with standard of care. Some 90% (17/19) of respondents who had used models for DORV found the 3D models to be helpful. Use of 3D models for other lesions is more sporadic. Compared with standard of care, most respondents who have used 3D printed models (78%, 21/27) reported that the models were most helpful because they improved communication with colleagues (Fig. 7). Some 44% (12/27) of respondents reported that the 3D models were helpful in that they reduced procedure or procedure planning time.
Figure 6.
Cardiac lesions modeled with 3D printing. Dark bars indicate lesions that respondents have used a 3D model to represent. Light bars indicate the lesions that respondents found 3D models were most helpful.
Figure 7.
Respondents who use 3D printing technology were surveyed on how the 3D printed cardiac models are beneficial as a tool in the treatment of CHD compared with standard of care.
Discussion
Access to 3D printing is limited
Among physicians who do have access to 3D printing technology, the majority report they were using the technology in the treatment of children with CHD. Of pediatric cardiologists without access to the technology, only a minority claim the reason for lack of access is because they do not believe the technology is important. These findings indicate that there is acceptance of the technology among pediatric cardiologists and suggests that the limited access is more related to barriers to access than acceptance of technology. The primary barrier to access 3D printing technology was financial. The financial cost of 3D printing is related to the cost of 3D printing machinery and associated operating costs, including disposable equipment, maintenance, and hiring skilled personnel. The capital cost of a 3D printer and associated segmentation software is variable and related to the quality of the printer and software. Printers can range in cost from a few thousand dollars for entry-level printers to several hundred thousand Canadian dollars for high-fidelity printers that can print in a range of materials and colors and have axial resolution up to 10 to 15 μm. The cost of segmentation software is also highly varied from free for open access software to $17,500 Canadian dollars/year for a professional license (Materialise Mimics Innovation Suite).21 The segmentation process of converting clinical images to STL files, which can be read by the 3D printer, is a labor-intensive and time-consuming one that involves expertise from skilled personnel. These skilled personnel require a highly specialized skill set, including the ability to understand segmentation and manage a 3D printer, and knowledge of anatomy. Because medical 3D printing is an emerging field, the number of people with this skill set is limited. Over time, it might be expected that more people are trained in this skill set and personnel costs might decrease. A 2016 systematic review of segmentation methods used for 3D printing cardiovascular systems found that a majority of published studies used manual or semi-automatic segmentation methods over fully automatic segmentation methods.1 As seen with other electronic technologies, as 3D printing technology develops over time, the price of the 3D printers is expected to decrease.22 The cost of segmentation may decrease with development of fully automatic segmentation software,1 which in conjunction with lower printer costs may lead to greater access to 3D printing technology in the field of pediatric cardiology.
An alternative or complementary approach to constructing physical 3D models is to create virtual models using the same source data. There are a range of software options from open access (e.g., Horos; Horos Project) to proprietary subscription-based services. The use of virtual 3D models may be a more cost-effective alternative for centres that do not have an established 3D printing facility.23 However, the use of such software does not alleviate one of the most significant resources limitations: the need for skilled personnel who have expertise in performing segmentation required during the construction of 3D datasets. These virtual 3D models cannot be physically held and may give the user less precise impressions of depth and proximity between structures.24 The utility of virtual vs physical models may vary from user to user. Ultimately, the clinical utility of the models will depend on the proceduralists and those contributing to making clinical decisions based on the models.
Access to 3D printing technology varied by geographical location
Respondents from the United States were significantly more likely to have access to 3D printing technology over their Canadian counterparts. Discrepancy in access to the technology may be related to funding structures in each of the countries. We speculate that in Canada, access is limited to centers where grant or donor funding can be secured to support the development of such a program. In the context of the market-based healthcare system in the United States, a 3D printing program may offer an institution a competitive advantage over others that do not offer 3D printing. Thus, funding for a 3D printing program may be more likely to come from hospital or institutional administration.25 A similar trend in early adoption of healthcare technology between Canada and the United States was seen when magnetic resonance imaging (MRI) was introduced to clinical care in the 1980s. In the late 1980s, the United States had approximately 8 times more MRI units per capita than Canada.26 The combination of capital equipment and skilled human resources needed to initiate an MRI facility is similar to a 3D printing program.27
In clinical practice, the primary use of 3D cardiac models is for procedural planning of complex CHD
Our study found that in practice, the most common use for 3D models of CHD lesions is for procedural planning, specifically for surgical planning. Further to this, 11% of respondents without access to 3D printing technology self-identified that they thought the technology was only necessary in surgical centers. This indicates that among pediatric cardiologists there may be a perception that the most beneficial use of 3D models is in surgical planning, in contrast to other uses in education, communication, or simulation. Of note, 1 respondent commented that 3D models cannot define valves and the subvalvar apparatus, which is a limitation. Although this is an important point, clinicians can combine 3D models with data from echocardiography to provide a more comprehensive preoperative understanding of valve structure and function and its impact on adjacent structures that will be the focus of interventions.
Respondents predominantly reported that this technology is particularly useful in planning procedures where an in-depth understanding of ventriculoarterial relationships is crucial. Models are most commonly constructed for DORV. DORV is a complex CHD and encompasses a wide spectrum of anatomic arrangements, whereby both great vessels may entirely or predominantly arise from the morphologic right ventricle.28 Classification is based on the 3D spatial relationship between the ventricular septal defect and the great arteries.28, 29, 30 These patient-specific factors are tremendously important when determining the optimal therapeutic approach in children with DORV. There are multiple surgical approaches to treat DORV, and surgical decision-making for primary or staged repair is highly influenced by patient’s subclassification and 3D anatomic arrangement.31 The high rate of use of models for DORV may indicate that pediatric cardiologists find 3D models helpful to fully elucidate the intricate spatial relationship between the interventricular communication and great arteries. This technology helps facilitate communication of these critical relationships with cardiac surgeons.
3D models may be underused in the context of medical education
3D printing technology has the potential to be used for multiple medical educational initiatives. In the literature, it has been reported that 3D printed cardiac models have successfully been used to facilitate teaching of multiple CHD lesions, including pulmonic stenosis, atrial septal defect, coarctation of the aorta, d-transposition of the great arteries, hypoplastic left heart syndrome,7 tetralogy of Fallot,7,32 ventricular septal defects,11 and vascular rings and slings,8 for medical students and residents. Other potential uses of 3D printed CHD models include use for distributed medical education in rural locations and to preserve libraries of cardiac specimens that are subject to decay over time.33 However, in our survey we found that less than 30% of respondents indicate they were using 3D models for medical education purposes. This suggests that 3D printing technology may be underused in real-world practice for uses other than procedural planning.
3D models facilitate communication with colleagues
We found that the majority of respondents who think 3D models are beneficial perceive the benefit to be from facilitating communication with colleagues. This was also the greatest reported benefit associated with 3D models compared with standard of care, of respondents who have used 3D printed cardiac models in the care of children with CHD. These results echo findings from Olivieri et al.,34 who investigated the impact of using patient-specific 3D cardiac models during hand-off after congenital cardiac surgery. In their study, healthcare providers rated using 3D models as more effective than standard verbal hand-off. These findings indicate that the main benefit of 3D models may be in improving communication among healthcare providers. However, quantifying how well models improve communication among healthcare providers compared with standard care is subjective and challenging to measure.
Limitations
This study is limited by the voluntary response sampling methodology. Because we used a convenience sampling method, there is potential for a sampling bias. Only pediatric cardiologists registered with CPCA or CCISC were invited to participate, and therefore not all pediatric cardiologists who treat children with CHD were contacted. There is also the potential for response bias, that is, recipients of the survey with a vested interest in 3D printing would be more likely to respond. However, we received approximately equal numbers of responses from participants with and without access to the technology.
Conclusions
3D printing is a new technology that has been readily adopted by cardiologists who treat children with CHD. The majority of pediatric cardiologists surveyed think that patient-specific 3D printed models are a beneficial tool in the treatment of children that can be used to facilitate communication with colleagues and aid in surgical planning. However, access to 3D printing is limited and varies by geographic location. Respondents from the United States were significantly more likely than their Canadian counterparts to have access to the technology. In clinical practice, 3D models are primarily used for procedural planning for CHD lesions with complex 3D spatial relationships.
Acknowledgements
The authors thank Mike Irvine for assistance with the figures.
Funding Sources
Caroline F. Illmann was funded by a Graduate Studentship from the BC Children’s Hospital Research Institute (#6553).
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See editorial by Anderson and Gupta, pages192–194of this issue.
Ethics Statement: The research reported has adhered to the relevant ethical guidelines.
See page 212 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.01.008.
Supplementary Material
References
- 1.Byrne N., Velasco Forte M., Tandon A. A systematic review of image segmentation methodology, used in the additive manufacture of patient-specific 3D printed models of the cardiovascular system. JRSM Cardiovasc Dis. 2016;5 doi: 10.1177/2048004016645467. 2048004016645467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biglino G., Koniordou D., Gasparini M. Piloting the use of patient-specific cardiac models as a novel tool to facilitate communication during clinical consultations. Pediatr Cardiol. 2017;38:813–818. doi: 10.1007/s00246-017-1586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biglino G., Capelli C., Leaver L.-K. Involving patients, families and medical staff in the evaluation of 3D printing models of congenital heart disease. Commun Med. 2015;12:157–169. doi: 10.1558/cam.28455. [DOI] [PubMed] [Google Scholar]
- 4.White S.C., Sedler J., Jones T.W., Seckeler M. Utility of three-dimensional models in resident education on simple and complex intracardiac congenital heart defects. Congenit Heart Dis. 2018;13:1045–1049. doi: 10.1111/chd.12673. [DOI] [PubMed] [Google Scholar]
- 5.Su W., Xiao Y., He S., Huang P., Deng X. Three-dimensional printing models in congenital heart disease education for medical students: a controlled comparative study. BMC Med Educ. 2018;18:178. doi: 10.1186/s12909-018-1293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarris G.E., Polimenakos A.C. Three-dimensional modeling in congenital and structural heart perioperative care and education: a path in evolution. Pediatr Cardiol. 2017;38:883–885. doi: 10.1007/s00246-017-1614-9. [DOI] [PubMed] [Google Scholar]
- 7.Smerling J., Marboe C.C., Lefkowitch J.H. Utility of 3D printed cardiac models for medical student education in congenital heart disease: across a spectrum of disease severity. Pediatr Cardiol. 2019:1–8. doi: 10.1007/s00246-019-02146-8. [DOI] [PubMed] [Google Scholar]
- 8.Jones T.W., Seckeler M.D. Use of 3D models of vascular rings and slings to improve resident education. Congenit Heart Dis. 2017;12:578–582. doi: 10.1111/chd.12486. [DOI] [PubMed] [Google Scholar]
- 9.Loke Y.-H., Harahsheh A.S., Krieger A., Olivieri L.J. Usage of 3D models of tetralogy of Fallot for medical education: impact on learning congenital heart disease. BMC Med Educ. 2017;17 doi: 10.1186/s12909-017-0889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim K.H.A., Loo Z.Y., Goldie S.J., Adams J.W., Mcmenamin P.G. Use of 3D printed models in medical education: a randomized control trial comparing 3D prints versus cadaveric materials for learning external cardiac anatomy. Anat Sci Educ. 2016;9:213–221. doi: 10.1002/ase.1573. [DOI] [PubMed] [Google Scholar]
- 11.Costello J.P., Olivieri L.J., Krieger A. Utilizing three-dimensional printing technology to assess the feasibility of high-fidelity synthetic ventricular septal defect models for simulation in medical education. World J Pediatr Congenit Heart Surg. 2014;5:421–426. doi: 10.1177/2150135114528721. [DOI] [PubMed] [Google Scholar]
- 12.Yoo S.-J., Spray T., Austin E.H. Hands-on surgical training of congenital heart surgery using 3-dimensional print models. J Thorac Cardiovasc Surg. 2017;153:1530–1540. doi: 10.1016/j.jtcvs.2016.12.054. [DOI] [PubMed] [Google Scholar]
- 13.Bhatla P., Tretter J.T., Ludomirsky A. Utility and scope of rapid prototyping in patients with complex muscular ventricular septal defects or double-outlet right ventricle: does it alter management decisions? Pediatr Cardiol. 2017;38:103–114. doi: 10.1007/s00246-016-1489-1. [DOI] [PubMed] [Google Scholar]
- 14.Valverde I., Gomez G., Gonzalez A. Three-dimensional patient-specific cardiac model for surgical planning in Nikaidoh procedure. Cardiol Young. 2015;25:698–704. doi: 10.1017/S1047951114000742. [DOI] [PubMed] [Google Scholar]
- 15.Valverde I., Gomez-Ciriza G., Hussain T. Three-dimensional printed models for surgical planning of complex congenital heart defects: an international multicentre study. Eur J Cardiothorac Surg. 2017;52:1139–1148. doi: 10.1093/ejcts/ezx208. [DOI] [PubMed] [Google Scholar]
- 16.Riesenkampff E., Rietdorf U., Wolf I. The practical clinical value of three-dimensional models of complex congenitally malformed hearts. J Thorac Cardiovasc Surg. 2009;138:571–580. doi: 10.1016/j.jtcvs.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Valverde I., Gomez G., Coserria J.F. 3D printed models for planning endovascular stenting in transverse aortic arch hypoplasia. Catheter Cardiovasc Interv. 2015;85:1006–1012. doi: 10.1002/ccd.25810. [DOI] [PubMed] [Google Scholar]
- 18.Shirakawa T., Koyama Y., Mizoguchi H., Yoshitatsu M. Morphological analysis and preoperative simulation of a double-chambered right ventricle using 3-dimensional printing technology. Interact Cardiovasc Thorac Surg. 2016;22:688–690. doi: 10.1093/icvts/ivw009. [DOI] [PubMed] [Google Scholar]
- 19.Harris P.A., Taylor R., Minor B.L. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris P.A., Taylor R., Thielke R. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valding B., Zrounba H., Martinerie S., May L., Broome M. Should you buy a three-dimensional printer? A study of an orbital fracture. J Craniofac Surg. 2018:1. doi: 10.1097/SCS.0000000000005048. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Bureau of Labor Statistics Long-term price trends for computers, TVs, and related items. U.S Bureau of Labor Statistics. https://www.bls.gov/opub/ted/2015/long-term-price-trends-for-computers-tvs-and-related-items.htm Available at: Published 2015. Accessed October 3, 2019.
- 23.Gupta S.K., Spicer D.E., Anderson R.H. A new low-cost method of virtual cardiac dissection of computed tomographic datasets. Ann Pediatr Cardiol. 2019;12:110–116. doi: 10.4103/apc.APC_167_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valverde I. Three-dimensional printed cardiac models: applications in the field of medical education, cardiovascular surgery, and structural heart interventions. Rev Española Cardiol (English Ed) 2017;70:282–291. doi: 10.1016/j.rec.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Urbach D.R., Croxford R., MacCallum N.L., Stukel T.A. How are volume–outcome associations related to models of health care funding and delivery? A comparison of the United States and Canada. World J Surg. 2005;29:1230–1233. doi: 10.1007/s00268-005-7994-7. [DOI] [PubMed] [Google Scholar]
- 26.Rublee D.A. Medical technology in Canada, Germany, and the United States: an update. Health Aff. 1994;13:113–117. doi: 10.1377/hlthaff.13.4.113. [DOI] [PubMed] [Google Scholar]
- 27.Baker L.C. Managed care and technology adoption in health care: evidence from magnetic resonance imaging. J Health Econ. 2001;20:395–421. doi: 10.1016/s0167-6296(01)00072-8. [DOI] [PubMed] [Google Scholar]
- 28.Walters H.L., Mavroudis C., Tchervenkov C.I. Congenital Heart Surgery Nomenclature and Database Project: double outlet right ventricle. Ann Thorac Surg. 2000;69:249–263. doi: 10.1016/s0003-4975(99)01247-3. [DOI] [PubMed] [Google Scholar]
- 29.Lev M., Bharati S., Meng C.C. A concept of double-outlet right ventricle. J Thorac Cardiovasc Surg. 1972;64:271–281. [PubMed] [Google Scholar]
- 30.Ebadi A., Spicer D.E., Backer C.L., Fricker F.J., Anderson R.H. Double-outlet right ventricle revisited. J Thorac Cardiovasc Surg. 2017;154:598–604. doi: 10.1016/j.jtcvs.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 31.Oladunjoye O., Piekarski B., Baird C. Repair of double outlet right ventricle: midterm outcomes. J Thorac Cardiovasc Surg. 2020;159:254–264. doi: 10.1016/j.jtcvs.2019.06.120. [DOI] [PubMed] [Google Scholar]
- 32.Loke Y.-H., Harahsheh A.S., Krieger A., Olivieri L.J. Usage of 3D models of tetralogy of Fallot for medical education: impact on learning congenital heart disease. BMC Med Educ. 2017;17:54. doi: 10.1186/s12909-017-0889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiraly L., Kiraly B., Szigeti K., Tamas C.Z. Virtual museum of congenital heart defects: digitization and establishment of a database for cardiac specimens. Quant Imaging Med Surg. 2019;9:115–126. doi: 10.21037/qims.2018.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olivieri L.J., Su L., Hynes C.F. “Just-In-Time” simulation training using 3-D printed cardiac models after congenital cardiac surgery. World J Pediatr Congenit Heart Surg. 2016;7:164–168. doi: 10.1177/2150135115623961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.