Abstract
Background
Cardiogenic shock (CS) is associated with high mortality. We report on a “Shock Team” approach of combined interdisciplinary expertise for decision making, expedited assessment, and treatment.
Methods
We reviewed 100 patients admitted in CS over 52 months. Patients managed under a Code Shock Team protocol (n = 64, treatment) from 2016 to 2019 were compared with standard care (n = 36, control) from 2015 to 2016. The cohort was predominantly male (78% treatment, 67% control) with a median age of 55 years (interquartile range [IQR], 43-64) for treatment vs 64 years (IQR, 48-69) for control (P = 0.01). New heart failure was more common in the treatment group: 61% vs 36%, P = 0.02. Acute myocardial infarction comprised 13% of patients in CS. There were no significant differences between treatment and control in markers of clinical acuity, including median left ventricular ejection fraction (18% vs 20%), prevalence of moderate-severe right ventricular dysfunction (64% vs 56%), median peak serum lactate (5.3 vs 4.7 mmol/L), acute kidney injury (70% vs 75%), or acute liver injury (50% vs 31%). Inotropes, dialysis, and invasive ventilation were required in 92%, 33%, and 66% of patients, respectively. Temporary mechanical circulatory support was used in 45% of treatment and 28% of control patients (P = 0.08). There were no significant differences in median hospital length of stay (17.5 days), 30-day survival (71%), or survival to hospital discharge (66%). Over 240 days (IQR, 14,847) of median follow-up, survival was 67% for treatment vs 42% for control (hazard ratio, 0.53; 95% confidence interval, 0.28-0.99; P = 0.03).
Conclusion
A multidisciplinary Code Shock Team approach for CS is feasible and may be associated with improved long-term survival.
Résumé
Contexte
Le choc cardiogénique (CC) est associé à une mortalité élevée. Nous décrivons une approche où la prise de décision, l’évaluation rapide des cas et le traitement sont confiés à une « équipe de choc » interdisciplinaire.
Méthodologie
Nous avons examiné les cas de 100 patients hospitalisés en raison d’un CC sur une période de 52 mois. Les patients pris en charge par une équipe interdisciplinaire selon un protocole d’intervention déclenché par un code-choc (n = 64, groupe traité) de 2016 à 2019 ont été comparés à des patients ayant reçu des soins courants (n = 36, groupe témoin) de 2015 à 2016. Les patients de la cohorte étaient majoritairement de sexe masculin (78 % dans le groupe traité, 67 % dans le groupe témoin) et l’âge médian était de 55 ans (intervalle interquartile [IIQ] : 43-64) au sein du groupe traité par rapport à 64 ans (IIQ : 48-69) au sein du groupe témoin (p = 0,01). Les nouveaux cas d’insuffisance cardiaque étaient plus fréquents dans le groupe traité : 61 % vs 36 % (p = 0,02). Les patients hospitalisés en raison d’un CC avaient subi un infarctus aigu du myocarde dans 13 % des cas. Aucune différence significative n’a été relevée entre le groupe traité et le groupe témoin au chapitre des marqueurs d’acuité clinique, y compris la fraction médiane d’éjection ventriculaire gauche (18 % vs 20 %), la prévalence d’une dysfonction modérée ou sévère du ventricule droit (64 % vs 56 %), la concentration maximale médiane de lactate sérique (5,3 vs 4,7 mmol/l), l’insuffisance rénale aiguë (70 % vs 75 %) ou l’insuffisance hépatique aiguë (50 % vs 31 %). L’administration d’inotropes, la dialyse et la ventilation effractive ont été nécessaires chez 92 %, 33 % et 66 % des patients, respectivement. Une assistance circulatoire mécanique temporaire a été utilisée chez 45 % des patients du groupe traité et 28 % des patients du groupe témoin (p = 0,08). Aucune différence significative n’a été notée en ce qui concerne la durée médiane des hospitalisations (17,5 jours), la survie à 30 jours (71 %) ou la survie à la sortie de l’hôpital (66 %). Au cours d’une période de suivi médiane de 240 jours (IIQ : 14 847), le taux de survie était de 67 % dans le groupe traité vs 42 % dans le groupe témoin (rapport des risques instantanés : 0,53; intervalle de confiance à 95 % : 0,28-0,99; p = 0,03).
Conclusion
Dans les cas de CC, l’intervention d’une équipe interdisciplinaire déclenchée par un code-choc est réalisable et pourrait être associée à une amélioration de la survie à long terme.
Cardiogenic shock (CS) is defined as a low cardiac output state with end-organ hypoperfusion.1 The etiology is broad and includes acute myocardial infarction (AMI), acute decompensated heart failure (ADHF) of preexisting cardiomyopathy, fulminant myocarditis, and tachyarrhythmia.1 Clinical presentation is variable ranging from rapid hemodynamic deterioration over hours to a more insidious onset over days. Heterogeneity of etiology, presentation, and clinical trajectory have contributed to difficulties standardizing definitions for diagnosis, leading to delayed recognition, management variability, and uncertain optimal practice. Consequently, despite medical advances, clinical outcomes in CS remain poor with up to 50% in-hospital mortality reported in most series.2,3
An increasing number of institutions are adopting a multidisciplinary team-based strategy for CS and have shown feasibility associated with improved outcomes.4, 5, 6, 7 In 2016, a Code Shock Team approach was implemented at our institution, which uses an emergent “Code” activation similar to other high-acuity, time-sensitive conditions such as ST-elevation myocardial infarction, cardiac arrest, and stroke. The specific aim of this program is to improve patient care in CS by combining interdisciplinary expertise for integrated decision making, early diagnosis, expedited clinical assessment, prompt treatment intervention, close surveillance, and follow-up. We report on our early experience and program outcomes.
Methods
Study design
A retrospective analysis was performed of consecutive patients admitted to the University of Ottawa Heart Institute (Ontario, Canada) coronary care unit (CCU) over a 52-month period between January 2015 and April 2019 in CS who fulfilled prespecified “Code Shock” criteria (detailed next). Patients managed under the Code Shock Team protocol between March 2016 and April 2019 (treatment group) were compared with a similar cohort of patients managed under standard care between January 2015 and March 2016 (historical control group). A prospective registry was maintained for the treatment group. Patients in the control group were identified by screening the Canadian Institute for Health Information registry for patients with a diagnosis of “shock” and through retrospective chart review. Data extracted included demographic information, CS etiology, hemodynamics, laboratory results, treatment, length of stay, and survival. The primary outcome was overall survival over the available duration of follow-up. Secondary outcomes were survival to hospital discharge, 30-day survival, and hospital length of stay. The study protocol was approved by the Ottawa Health Science Network Research Ethics Board.
Code shock selection criteria
The presence of CS formed the inclusion criteria for Code Shock and was defined as (1) reduced cardiac output as evidenced by sustained hypotension (systolic blood pressure < 90 mm Hg or mean arterial pressure < 60 mm Hg for ≥ 30 minutes), single moderate dose inotrope (dobutamine ≥ 5 μg/kg/min or milrinone ≥ 0.25 μg/kg/min), ≥ 2 inotropes or cardiac index < 1.8 L/min; and (2) hypoperfusion as evidenced by oliguria (urine output ≤ 0.5 mL/kg/h), acute kidney injury, acute liver injury, or serum lactate ≥ 2 mmol/L.8,9 Acute kidney injury was defined according to Kidney Disease Improving Global Outcomes criteria as an increase in serum creatinine of ≥ 26.5 μmol/L within 48 hours or > 1.5-fold increase within 7 days.10 Acute liver injury was defined as serum aspartate aminotransferase ≥ 1000 U/L.11,12 Exclusion criteria for Code Shock included (1) not for cardiopulmonary resuscitation or intubation; (2) cardiac arrest > 30 minutes; (3) advanced comorbidities with a life expectancy of < 6 months; (4) septic shock; or (5) active bleeding.
Code Shock protocol
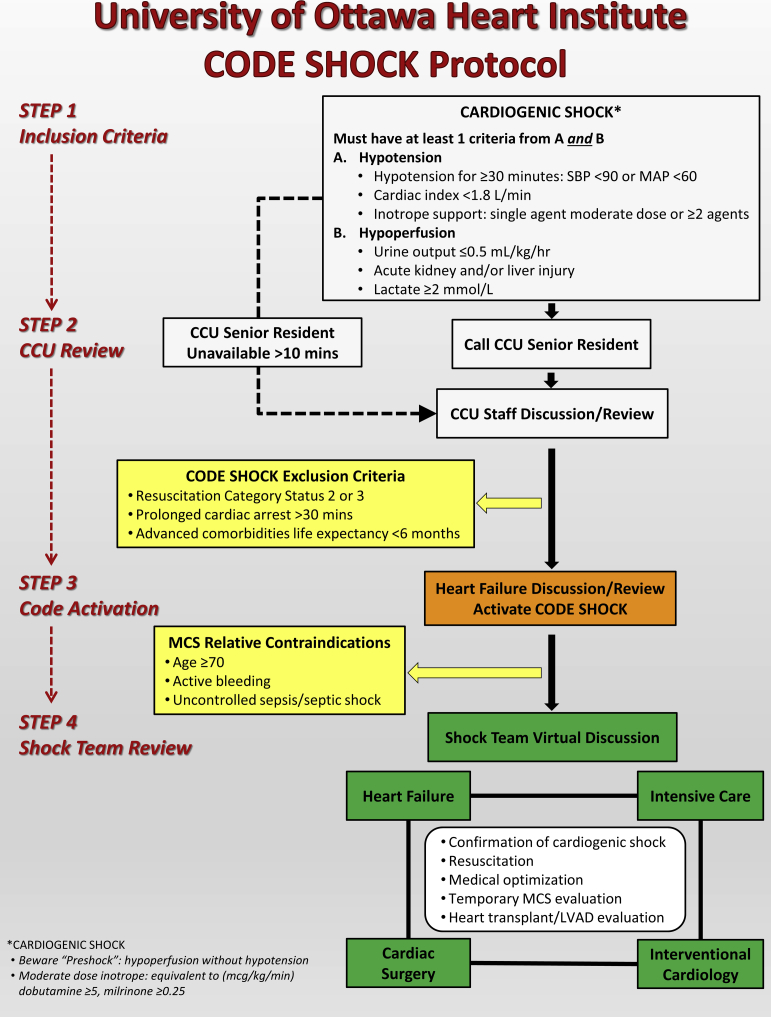
The Code Shock protocol was implemented in March 2016 and revised in January 2018. As shown in Figure 1, the 4-step protocol involves (1) identification of patients in CS; (2) CCU review to confirm fulfilment of Code Shock criteria; (3) advanced heart failure review with subsequent Code Shock activation if deemed appropriate; and (4) Shock Team review for resuscitation, medical optimization, decision-making on temporary mechanical circulatory support (MCS) including candidacy, timing, device choice, and implant strategy, as well as evaluation for durable left ventricular assist device (LVAD) and heart transplantation. A smartphone-based application is used for Code Shock activation and virtual online discussion among the Shock Team. Subsequent bedside rounds were conducted at least daily by the Shock Team. In between these time points, the smartphone-based application is also used by the team to update on the patient’s progress or need for further Shock Team discussion or review. Advanced heart failure coordinates patient care, including determining appropriateness of the initial activation of Code Shock.
Figure 1.
University of Ottawa Heart Institute Code Shock protocol. CCU, coronary care unit; LVAD, left ventricular assist device; MAP, mean arterial pressure; MCS, mechanical circulatory support; SBP, systolic blood pressure.
Statistical analysis
The data were analysed and expressed as mean and standard deviation or median and interquartile range (IQR) for parametric and nonparametric data, respectively. Student t test and Mann–Whitney test were used to analyze between-group differences for continuous variables, and Pearson chi-square testing was used for categorical data. Between-group survival was compared using the Kaplan–Meier method with Mantel-Cox log-rank test. Statistical significance was inferred at a P value < 0.05. Statistical analyses were performed using GraphPad Prism version 8 (GraphPad Software, La Jolla, CA).
Results
Patient characteristics
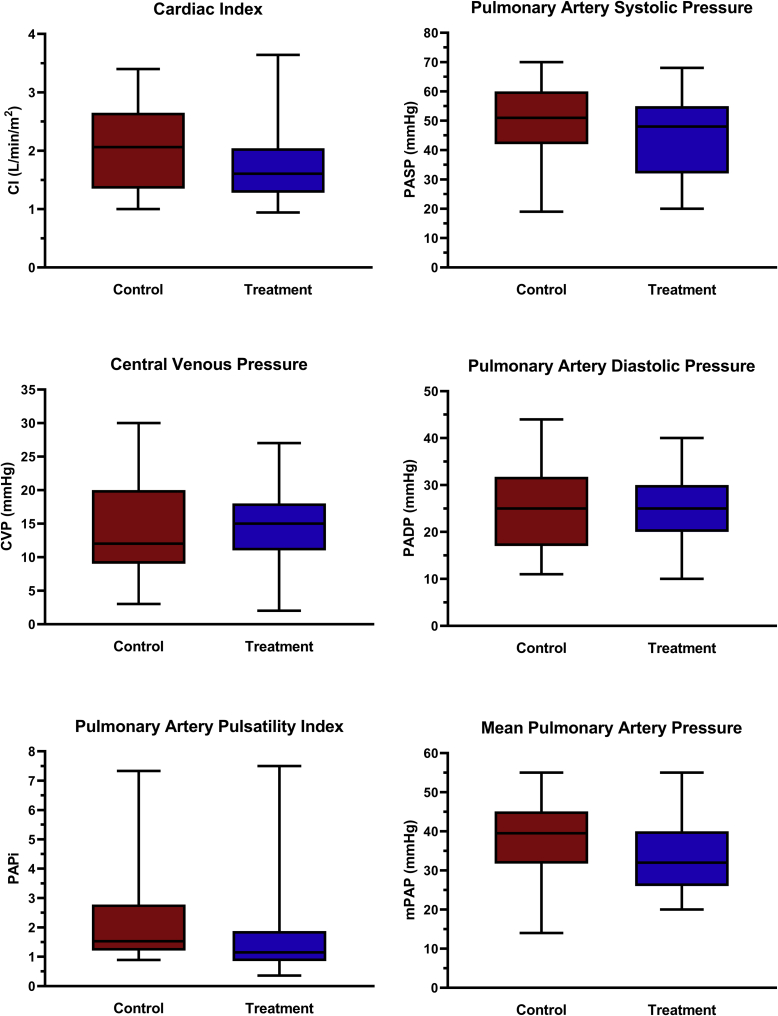
The clinical characteristics of 100 consecutive patients admitted with CS and fulfilling Code Shock criteria included in the study analysis are summarized in Table 1. Approximately three-quarters of patients presented initially to a peripheral institution with a significantly higher proportion of outside hospital transfer for the control (94%) compared with the treatment (63%) group (P < 0.01). The cohort was predominantly male (74%) and significantly younger in the treatment group (median age, 55 years [IQR, 43-64] vs 64 years [IQR, 48-69], P = 0.01). Cardiac arrest (67% in-hospital, 33% out-of-hospital) complicated approximately 1 in 5 patients. There was a higher proportion of patients with a new diagnosis of heart failure in the treatment compared with the control group: 61% vs 36%, P = 0.02. The most common CS etiologies were dilated cardiomyopathy (29%), tachycardia-induced cardiomyopathy (19%), and ischemic cardiomyopathy (16%). The treatment group had a significantly lower proportion of patients with ischemic cardiomyopathy (8% vs 31%) and a higher proportion with acute myocarditis (14% vs 0%). Median left ventricular ejection fraction was 20% (IQR, 13-27), and 58% of patients had moderate to severe right ventricular dysfunction. Serum lactate was elevated, but there were no significant differences in baseline or peak levels between treatment and control groups. Acute kidney injury and liver injury occurred in 72% and 43% of patients, respectively. Invasive pulmonary artery catheter (PAC) hemodynamic monitoring was undertaken in 62% of patients (66% for treatment vs 50% for control, P = 0.13) for a median duration of 4 days (IQR, 2-6). There was a trend to lower median cardiac index (1.6 L/min/m2 for treatment vs 2.1 L/min/m2 for control, P = 0.07) and pulmonary artery pulsatility index (1.1 vs 1.5, P = 0.06) in the treatment compared with control groups, although this did not reach statistical significance (Fig. 2).
Table 1.
Patient demographics
| Treatment n = 64 | Control n = 36 | P value | |
|---|---|---|---|
| Age, y | 55.0 (43.0-63.8) | 63.5 (48.0-68.8) | 0.01 |
| Male | 50 (78) | 24 (67) | 0.21 |
| New heart failure diagnosis | 39 (61) | 13 (36) | 0.02 |
| Cardiac arrest | 13 (20) | 8 (22) | 0.82 |
| Heart failure etiology | |||
| AMI | 7 (11) | 6 (17) | 0.41 |
| Acute myocarditis | 9 (14) | 0 (0) | 0.02 |
| Tachycardia induced | 15 (23) | 4 (11) | 0.19 |
| Dilated cardiomyopathy | 17 (27) | 12 (33) | 0.47 |
| Ischemic cardiomyopathy | 5 (8) | 11 (31) | < 0.01 |
| Other∗ | 11 (17) | 3 (8) | 0.37 |
| Left ventricular ejection fraction, % | 18 (12-25) | 20 (15-30) | 0.13 |
| Moderate-severe right ventricular dysfunction | 39 (64) | 19 (56) | 0.44 |
| Biochemistry | |||
| Baseline lactate, mmol/L | 3.5 (1.9-5.3) | 2.8 (1.9-4.7) | 0.54 |
| Peak lactate, mmol/L | 5.3 (3.1-7.4) | 4.7 (3.0-8.1) | 0.95 |
| Baseline creatinine, μmol/L | 130 (98-179) | 145 (100-274) | 0.25 |
| Peak creatinine, μmol/L | 191 (141-311) | 237 (140-323) | 0.53 |
| Baseline aspartate aminotransferase, U/L | 373 (66-2015) | 115 (33-706) | 0.09 |
| Peak aspartate aminotransferase, U/L | 1147 (99-4926) | 283 (102-2209) | 0.25 |
| Acute kidney injury† | 45 (70) | 27 (75) | 0.62 |
| Acute liver injury‡ | 32 (50) | 11 (31) | 0.06 |
Values are median (interquartile rage) or number (percentage).
AMI, acute myocardial infarction.
Takotsubo cardiomyopathy, tamponade, and sepsis-induced myocardial dysfunction.
Creatinine increase ≥ 26.5 μmol/L within 48 hours or creatinine increase to > 1.5-fold within 7 days.
Aspartate aminotransferase ≥ 1000 U/L.
Figure 2.
Box and whisker plots for hemodynamic indices comparing the treatment (Code Shock) and control (historical) groups. CI, cardiac index; CVP, central venous pressure; mPAP, mean pulmonary artery pressure; PADP, pulmonary artery diastolic pressure; PAPi, pulmonary artery pulsatility index; PASP, pulmonary artery systolic pressure.
Treatment
Table 2 summarizes therapeutic interventions. Median duration of inotrope therapy was 7 days (IQR, 4-12), with 92% of patients treated with a single agent and 46% requiring 2 or more agents. Sixty-six percent of patients required ventilation for a median of 5 days (IQR, 3-8.2) without significant differences between groups. A significantly higher 47% of patients (median 3 days; IQR, 1-19) in the control group needed dialysis compared with 25% (median 4 days; IQR, 1-20) for the treatment group. Of 13 patients with AMI-CS, 92% underwent revascularization (75% percutaneous coronary intervention, 8% coronary artery bypass surgery, and 17% both). Temporary MCS was used in 39% of patients for a median of 4 days (IQR, 3-8). There was a trend toward higher MCS use in the treatment group (45%) compared with the control group (28%) (P = 0.08). An intra-aortic balloon pump (IABP) (67%) and the Impella (Abiomed, Danvers, MA) (49%) were the most common forms of temporary MCS used. There were 18 patients (28%) in the treatment group who received an IABP. All patients had the IABP inserted after admission to the CCU, and 1 in 3 patients had an IABP in situ before Code Shock activation. During the same hospitalization, 16% of patients underwent durable LVAD implantation and 4% received a heart transplantation.
Table 2.
Treatment
| Treatment n = 64 | Control n = 36 | P value | |
|---|---|---|---|
| Inotrope | 60 (94) | 32 (89) | 0.45 |
| Invasive ventilation | 41 (64) | 25 (69) | 0.59 |
| Dialysis | 16 (25) | 17 (47) | 0.02 |
| Temporary MCS | 29 (45) | 10 (28) | 0.08 |
| IABP alone | 10 (34) | 4 (40) | |
| Impella (Abiomed, Danvers, MA) alone | 8 (28) | 1(10) | |
| VA-ECMO alone | 2 (7) | 1(10) | |
| IABP + Impella | 6 (21) | 2 (20) | |
| IABP + VA-ECMO | 1 (3) | 2(20) | |
| Impella + VA-ECMO | 1(3) | 0(0) | |
| IABP + Impella + VA-ECMO | 1(3) | 0(0) | |
| Durable LVAD | 8 (12) | 8 (22) | 0.20 |
| Heart transplantation | 3 (5) | 1 (3) | > 0.99 |
Values are number (percentages).
IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; MCS, mechanical circulatory support; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
Study outcomes
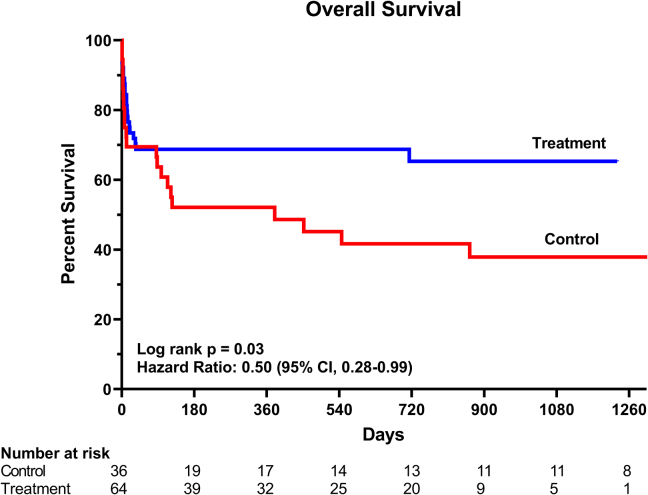
A total of 42 deaths (36 cardiovascular and 6 noncardiovascular; 21 [33%] in treatment and 21 [58%] in control) occurred during a median follow-up of 262 (IQR, 19-780) and 161 days (IQR, 8-1149) for the treatment and control groups, respectively. Cumulative survival was significantly higher in the treatment compared with the control group: hazard ratio, 0.50; 95% CI, 0.28-0.99; P = 0.03 (Fig. 3). There were no significant differences in survival to hospital discharge or 30-day survival: 69% vs 61% and 72% vs 69% for treatment and control groups, respectively (P value nonsignificant for both comparisons). The median length of stay in hospital was 17.5 days (IQR, 9.2-34) including 9.5 days (IQR, 5-20) in the critical care unit and did not differ significantly between study groups. The rate of adverse events including stroke (3%), vascular access complications (11%), bleeding (14%), and infection (21%) did not differ significantly between the treatment and control groups. Of the 66 patients who survived to discharge from hospital, 77% (35 [80%] for treatment and 16 [73%] for control, P = 0.42) had outpatient follow-up at a median of 26 days (IQR, 14-38) to the first clinic visit. Compared with the control group, a significantly higher proportion of patients in the treatment group were followed up by a heart failure specialist (75% vs 50%, P = 0.03).
Figure 3.
Kaplan–Meier survival curves for treatment (Code Shock) and control (historical) groups.
Discussion
This is the first reported Canadian experience of a multidisciplinary team-based strategy for CS using an escalating 4-step Code Shock protocol. The main findings from this analysis are (1) feasibility of a multidisciplinary team approach for the management of CS, (2) improved long-term overall survival without significant differences in short-term 30-day survival or hospital length of stay, and (3) trend toward increased use of temporary MCS.
We demonstrate the feasibility of a multidisciplinary Code Shock Team approach for CS (Fig. 1). As in other reported CS management algorithms, we used established criteria for defining CS.8,9,13,14 However, distinct to other protocols that typically include all-comers with CS or only patients with AMI-CS, we incorporated additional exclusion criteria and the activation of Code Shock with the involvement of the full Shock Team only for selected patients. This balances the benefits of multidisciplinary team care and maintenance of a resource-intensive 24-hour, 7 days per week highly specialized service.15 Our protocol involves a stepwise approach of identification of CS; review by CCU for confirmation of CS; review by advanced heart failure to determine appropriateness for activation of Code Shock; and Code Shock activation with review by the whole team, comprising advanced heart failure, interventional cardiology, cardiac surgery, and intensive care. Similar to other described algorithms, the final management stage is focused on medical optimization, serial assessments of end-organ perfusion, and evaluation for temporary MCS and definitive therapies including LVAD and heart transplantation.5,7 Additionally, the Shock Team rounds daily on all patients to ensure close surveillance and timely decision making on continuation, escalation, or de-escalation of care.
Single-center studies have demonstrated the feasibility of a team-based approach for managing patients with CS and associated improved survival.4, 5, 6, 7,15 The National Cardiogenic Shock Initiative involving 35 centers in the United States recently reported 72% survival to discharge for 171 patients with AMI-CS through routine use of invasive hemodynamics to guide early delivery of temporary MCS.7 Adopting a multidisciplinary Code Shock protocol, we observed no between-group differences in short-term survival to discharge of 30 days, but improved overall survival of 67% over a median follow-up of 240 days (IQR, 14-847). This extended survival benefit was achieved in a very sick patient cohort that included 21% of patients after cardiac arrest, patients with peak median lactate of 5.2 mmol/L, and patients in whom 33% required dialysis and 66% required intubation and ventilation.
Differences in the clinical characteristics of our patient cohort may have contributed to improved long-term outcomes. First, the median age of 55 years in the treatment group was significantly lower than 64 years for the control group. Our patient population (58 years; IQR, 44-66) was also younger than subjects in other studies.5,7,9,16, 17, 18 Older age is a known predictor of mortality due to preexisting comorbidities, end-organ dysfunction, and reduced physiologic reserve to handle hemodynamic insults in CS.5,19 A recent study of 65 patients undergoing temporary MCS for CS demonstrated significantly lower 13% survival rates for patients aged ≥ 65 years compared with 87% of patients aged < 45 years.6 An analysis of the US Nationwide Inpatient Sample Database that included 157,892 patients with AMI-CS also showed higher in-hospital 55% mortality for patients ≥75 years compared with 30% for patients aged < 75 years.16 Second, 61% of patients in the control group had a history of heart failure compared with 36% in the treatment group. This is higher than the 12% to 31% reported prevalence of known heart failure in AMI-CS registries and may predispose to higher short- and long-term mortality.3,7 There were also differences in CS etiology with a higher incidence of reversible conditions such as myocarditis in the treatment group compared with the control group. Ischemic cardiomyopathy was more prevalent in the control group. Studies of patients undergoing temporary MCS have generally shown improved short-term 30-day survival and survival to hospital discharge for patients with nonischemic compared with ischemic cardiomyopathy, potentially due to higher likelihood of recoverability.6,20 Notably, our cohort had a low 13% of patients with AMI-CS. This small patient number limits meaningful comparisons between patients with and without AMI-CS. Historically, however, there is high mortality of up to 50% for AMI-CS.8,9,16,17 Tehrani et al.5 reported an initial 44% 30-day survival in patients with AMI-CS compared with 60% for patients with ADHF. After implementation of a multidisciplinary team-based approach, 30-day survival improved to 82% in the AMI-CS cohort and to 72% in patients with ADHF.5
The Society of Cardiovascular Angiography and Intervention (SCAI) recently proposed a new A to E classification system for CS.13 Increased hospital mortality has been demonstrated for patients in more advanced stages of shock as assessed by this classification.21,22 On the basis of our selected definition for CS, all patients included would be categorized as “classic” (Stage C) or worse under the SCAI classification. Patients “at risk” (Stage A) or “beginning” (Stage B) would not have fulfilled CS criteria and activation of our Code Shock protocol. The SCAI classification system recognizes the dynamic clinical status of patients presenting in CS with potential for reclassification into a different category within the same admission. Thus, timing of assessment (e.g., at first medical contact vs at arrival to a tertiary center vs at admission to the intensive care unit) is likely to affect the prognostic relevance of the SCAI shock classification.
In contrast to most registries that focus on short-term outcomes, our study had a longer duration of follow-up, and we observed higher long-term survival in the treatment group.4, 5, 6, 7,9,20,23 Of patients surviving to discharge, a comparable high 80% of patients in the treatment group and 73% of patients in the control group attended clinic follow-up postdischarge. Despite a similar median 26 days to first follow-up, there were significant differences in the type of postdischarge outpatient follow-up between treatment and control groups. A significantly higher 75% of patients in the treatment group compared with 50% in the control group were followed up by a heart failure specialist. This may account for the improved overall survival in the treatment group despite similar short-term survival to 30 days and hospital discharge. A heart failure provider may have increased access to resources including a nurse for closer monitoring, medication adjustments, and optimal titration of prognostic heart failure therapy.
In our study, 62% of patients underwent hemodynamic monitoring with PAC. Other registries report variable PAC use, ranging from 37% to 92%.4,5,7,24 The use of PAC can differentiate CS from other causes of shock such as sepsis. Furthermore, it can unmask low cardiac output in patients without clinical evidence of hypoperfusion (preshock), assess severity of reduced cardiac output, and accurately assess left- and right-sided filling pressures.13 This information can aid decision-making on therapy such as diuresis, inotropes, vasopressors, or temporary MCS. For the latter, invasive hemodynamics can guide the need for left, right, or biventricular MCS. Although there are no formal guideline recommendations regarding PAC use, the 2017 American Heart Association scientific statement on CS management suggests PAC for diagnosis and management where there is diagnostic uncertainty and in patients unresponsive to initial therapy.1
A recent meta-analysis of randomized studies of temporary MCS (TandemHeart [Pittsburgh, PA], Impella 2.5, Impella CP, and IABP) in 148 patients with AMI-CS failed to show a survival benefit for MCS.18 However, patient selection, device choice, and timing may have affected the findings. More recent prospective registries demonstrate improved survival outcomes with early application of MCS.5,7,25 In our study, there was a trend toward increased use of temporary MCS in the treatment compared with control group. Despite this, there was no difference between treatment and control groups in duration of MCS use and median length of stay in hospital or the critical care unit. The Shock Team approach may have facilitated timely implementation of temporary MCS and appropriate patient selection. The majority of patients who required temporary MCS received an Impella 5.0, inserted via femoral (21%) or axillary (79%) artery cut-down. This approach differs from other studies that predominantly use the Impella CP.5,7 The Impella CP may be implanted in a timelier manner because it is usually placed percutaneously femorally (although transaxillary approaches are also being adopted) but delivers lower cardiac flow rates of 3.5 to 4 L/min. The preferred axillary placement of an Impella 5.0 at our institution enables patient mobilization, is less prone to infection, and can potentially permit a longer duration of support.26 We did not use isolated right ventricular MC and had a relatively low 9% use of venoarterial extracorporeal membrane oxygenation. Both these forms of MCS are associated with poorer outcomes due to increased severity of underlying cardiac disease with associated right ventricular failure, as well as increased management complexity and complications with venoarterial extracorporeal membrane oxygenation.5,27
Study limitations
The limitations of this study include the small sample size, single-center, and retrospective analysis. Although we used identical criteria for CS and Code Shock activation, retrospective identification of the control cohort introduces inherent selection bias and likely accounts for the observed differences in patient characteristics compared with the treatment group. Relevant data related to time from first medical contact to MCS support, which has been proposed as a potentially important clinical quality indicator in CS, were not available, including interhospital patient transfer, presentation to CS diagnosis and Code Shock activation, Shock Team response, and MCS decision to device implant time. Of note, 74% of patients in CS were transferred from another institution, which may have added to time delays. Given the time-sensitive nature of CS, these data are critical to identify opportunities for improvement in delivery of care and outcomes. Information relating to MCS specific adverse events is lacking. However, the increased use of temporary MCS in the treatment group with similar adverse event rates between treatment and control groups is reassuring. Finally, our cohort represents a highly selected patient population with CS. The CCU team may have missed or elected not to refer particular patients with CS. In addition, the proportion of patients with CS who did not fulfil criteria for Code Shock activation is unknown. Although advanced heart failure is involved in the majority of CS cases, our algorithm does not activate the entire Shock Team for all patients to balance resource use. However, the stepwise approach that is adopted may introduce further time delays to decisions and therapies. This may affect the applicability of our results to the general population with CS.
Conclusions
We demonstrate the feasibility in a Canadian setting of a multidisciplinary Code Shock Team approach for the management of patients with CS. This approach may be associated with improved long-term survival. Larger prospective multicenter studies are required to determine the clinical effectiveness of this contemporary approach.
Acknowledgements
The authors thank the University of Ottawa Heart Institute Shock Team, who in addition to the authors include Drs Vincent Chan, Robert Chen, Aun-Yeong Chong, Ross Davies, Sean Dickie, David Glineur, Christopher Glover, Mariana Lamacie, Ryan Mahaffey, Greg Manning, and Brock Wilson.
Funding Sources
This work was funded by a University of Ottawa Heart Institute Division of Cardiology for Patient Safety/Quality grant. Dr Chih is supported by a Heart and Stroke Ontario Clinician Scientist Award.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This research article has adhered to the relevant ethical guidelines.
See page 256 for disclosure information.
References
- 1.van Diepen S., Katz J.N., Albert N.M. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 2.Shah M., Patnaik S., Patel B. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;107:287–303. doi: 10.1007/s00392-017-1182-2. [DOI] [PubMed] [Google Scholar]
- 3.Wayangankar S.A., Bangalore S., McCoy L.A. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the CathPCI, Registry. JACC Cardiovasc Interv. 2016;9:341–351. doi: 10.1016/j.jcin.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Takayama H., Truby L., Koekort M. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant. 2013;32:106–111. doi: 10.1016/j.healun.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Tehrani B.N., Truesdell A.G., Sherwood M.W. Standardized team-based care for cardiogenic shock. J Am Coll Cardiol. 2019;73:1659–1669. doi: 10.1016/j.jacc.2018.12.084. [DOI] [PubMed] [Google Scholar]
- 6.Berg D.D., Sukul D., O'Brien M. Outcomes in patients undergoing percutaneous ventricular assist device implantation for cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2016;5:108–116. doi: 10.1177/2048872615584079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basir M.B., Kapur N.K., Patel K. Improved outcomes associated with the use of shock protocols: updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2019;93:1173–1183. doi: 10.1002/ccd.28307. [DOI] [PubMed] [Google Scholar]
- 8.Hochman J.S., Sleeper L.A., Webb J.G. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 9.Thiele H., Zeymer U., Neumann F.J. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 10.Eknoyan G., Lameire N., Eckardt K., Kasiske B. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 11.Hickman P.E., Potter J.M. Mortality associated with ischaemic hepatitis. Aust N Z J Med. 1990;20:32–34. doi: 10.1111/j.1445-5994.1990.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 12.Tapper E.B., Sengupta N., Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128:1314–1321. doi: 10.1016/j.amjmed.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Baran D.A., Grines C.L., Bailey S. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 14.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 15.Garan A.R., Kirtane A., Takayama H. Redesigning care for patients with acute myocardial infarction complicated by cardiogenic shock: the "Shock Team. JAMA Surg. 2016;151:684–685. doi: 10.1001/jamasurg.2015.5514. [DOI] [PubMed] [Google Scholar]
- 16.Kolte D., Khera S., Aronow W.S. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolte D., Khera S., Dabhadkar K.C. Trends in coronary angiography, revascularization, and outcomes of cardiogenic shock complicating non-ST-elevation myocardial infarction. Am J Cardiol. 2016;117:1–9. doi: 10.1016/j.amjcard.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Thiele H., Jobs A., Ouweneel D.M. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J. 2017;38:3523–3531. doi: 10.1093/eurheartj/ehx363. [DOI] [PubMed] [Google Scholar]
- 19.Strait J.B., Lakatta E.G. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012;8:143–164. doi: 10.1016/j.hfc.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kar B., Gregoric I.D., Basra S.S., Idelchik G.M., Loyalka P. The percutaneous ventricular assist device in severe refractory cardiogenic shock. J Am Coll Cardiol. 2011;57:688–696. doi: 10.1016/j.jacc.2010.08.613. [DOI] [PubMed] [Google Scholar]
- 21.Jentzer J.C., van Diepen S., Barsness G.W. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74:2117–2128. doi: 10.1016/j.jacc.2019.07.077. [DOI] [PubMed] [Google Scholar]
- 22.Jentzer J.C., Baran D.A., van Diepen S. Admission Society for Cardiovascular Angiography and Intervention shock stage stratifies post-discharge mortality risk in cardiac intensive care unit patients. Am Heart J. 2020;219:37–46. doi: 10.1016/j.ahj.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Burkhoff D., Cohen H., Brunckhorst C., O'Neill W.W. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152 doi: 10.1016/j.ahj.2006.05.031. 469.e1-8. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill W.W., Grines C., Schreiber T. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J. 2018;202:33–38. doi: 10.1016/j.ahj.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Basir M.B., Schreiber T.L., Grines C.L. Effect of early initiation of mechanical circulatory support on survival in cardiogenic shock. Am J Cardiol. 2017;119:845–851. doi: 10.1016/j.amjcard.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Truong H.T.D., Hunter G., Lotun K. Insertion of the Impella via the axillary artery for high-risk percutaneous coronary intervention. Cardiovasc Revasc Med. 2018;19:540–544. doi: 10.1016/j.carrev.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Kapur N.K., Paruchuri V., Korabathina R. Effects of a percutaneous mechanical circulatory support device for medically refractory right ventricular failure. J Heart Lung Transplant. 2011;30:1360–1367. doi: 10.1016/j.healun.2011.07.005. [DOI] [PubMed] [Google Scholar]