Highlights
-
•
First report that demonstrates in-vitro protocorm-like-body (PLB) cultures habituation towards illumination source.
-
•
Discovery of several anticancer bioactive compounds in PLB.
-
•
High intensity (16.9 μmol/s) green light supplementation promotes the accumulation of total phenolic content.
-
•
PLB serves as excellent source for the acquisition of anticancer phytochemicals due to its high proliferation capacity.
Abbreviations: GCMS, Gas chromatography-mass spectrometry; PLB, Protocorm-like bodies; DDMP, 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl; LED, Light-emitting diode; GSH, Reduced glutathione; NF-KB, Nuclear factor kappa B; PAL, Phenylalanine ammonia-lyase; ROS, Reactive oxygen species
Keywords: Protocorm-like-body, Bioactive compounds, Phenolics, Habituation, Light-emitting diodes
Abstract
Gas-chromatography-mass-spectrometry revealed the presence of various bioactive compounds with anticancer properties in protocorm-like-body (PLB) cultures of a Dendrobium hybrid orchid (Dendrobium Enopi x Dendrobium Pink Lady). Pre-illumination of red fluorescent light lessened the stimulating effects of light-emitting diodes (LEDs) on secondary metabolites production among in vitro PLB cultures, possibly due to habituation. The highest flavonoid content of 16.79 μmol/ g of fresh weight (FW) was achieved under blue-red (1:1) LED for PLBs pre-treated with white LED for more than 3 subculture cycles. Phenolics content significantly reduced as PLBs pre-cultured under red fluorescent light for 2 subculture cycles were exposed to LED illuminations, where far red LED resulted in the lowest total phenolic content (18.85 μmol/ g FW). High intensity green LED (16.9 μmol/s) enhanced the accumulation of phenolics while amino acids such as L-leucine, glycine and proline exhibited no significant stimulating effect for secondary metabolites production.
1. Introduction
Plants serve as an excellent natural source for the acquisition of bioactive compounds. These phytochemicals possess a wide array of biological activities and confer plants the ability to survive under adverse environmental conditions. Plant secondary metabolites have been widely utilized, particularly in pharmaceutical, for the production of antibiotics, immuno-modulators and antitumor drugs [1]. Secondary metabolites found within plants can be grouped into 5 major groups, namely non-ribosomal polypeptides, fatty acid-derived compounds and polyketides, terpenoids, steroids, alkaloids as well as enzyme cofactor [2]. Plants are the major producers for secondary metabolites which contribute 80 % of bioactive compounds production [3], along with the other organisms such as microbes and marine organisms.
The hybridization of two hybrid orchids, Dendrobium Enopi and Dendrobium Pink Lady, produces a new hybrid which is valued for the ornamental value it possesses. Aside from that, it is worthwhile to further explore the phytochemicals contained within this new hybrid, as Dendrobium itself is one of the largest genera in orchid family which has been proven to exhibit medicinal properties. Usage of many Dendrobium orchids as folk medicines can be back-dated to more than 2 300 years ago [4]. In China, Dendrobium orchids are extensively cultivated mainly for their medicinal values and to meet the herbal medicine market demand [5]. Dendrobium orchid such as Dendrobium Nobile displays anti-aging and antimicrobial properties. It functions as analgesic [6] and has been used for treating menstrual pain and hyperglycemia [5]. Besides, several other Dendrobium orchids also exhibit various health benefits to human, for instances, Dendrobium denneanum which has been used as anti-inflammatory agent [7]; Dendrobium huoshanense which possesses hepatoprotective effects [8]; and the anti-diabetic effect exhibited by Dendrobium officinale [9].
In plant tissue culture, the source of illumination such as cool fluorescent white light often consists of full visible light spectrum (400−700 nm). However, plants might develop specific physiological and/or biochemical responses towards only a certain light spectrum of narrow range. Thereby, light emitting diode (LED) is useful in this scenario as it allows the radiation of narrow light spectrum in order to meet the requirements of plants for their growth and development. The energy needed by electrons to overcome the semiconductor band gap within a LED determines the colour of the emitted light. This feature permits the adjustment of visible light spectrum to elicit growth and biochemical changes in plants.
Amino acids play a pivotal role as signalling molecules especially in stress-induced plant defense mechanisms. Moreover, amino acids are also essential for the biosynthesis of various secondary metabolites such as alkaloids, wherein amino acids serve as the building blocks and account for the biosynthesis of 12 000 known nitrogen-containing alkaloids [10]. Several other secondary metabolites, for instance, glucosinolates, are generated via biosynthetic pathways with amino acids as the precursors [11]. Hence, it is of great importance to explore the capacity of amino acids in elevating bioactive compounds accumulation.
Present study aims to identify the phytochemicals present within protocorm-like bodies (PLBs) of a new orchid hybrid which retains the potential to serve as an alternate source for the acquisition of bioactive compounds with medicinal values. LEDs are engaged in present study as the illumination system to elicit secondary metabolites production during PLBs proliferation stage. Amino acids are applied exogenously in conjunction with optimized LED irradiation to sustain the production of secondary metabolites.
2. Materials and methods
2.1. Source of PLB cultures
Thirty-day-old PLBs were aseptically excised into clumps and cultured with half-strength Murashige and Skoog (MS) solid media (pH 5.80) without hormone supplement. The cultures were maintained at 24 °C ± 2 and humidity of 51 ± 2 % under illumination of cool white LED light (17.0 μmol/s) with photoperiod of 16 h for 30 days.
2.2. Extraction for bioactive compounds from PLBs
Thirty-day-old PLBs were weighed at 6 g and excised into shattered form. The extraction was started off by using 95 % ethanol as the extraction solvent [12]. PLBs were treated with 45 mL of 95 % ethanol and agitated at 120 rpm by using orbital shaker for 24 h at room temperature. The mixture was centrifuged at 120 rpm for 10 min, after which the supernatant will be collected. Ethanolic extract was left in 60 °C water bath and allowed to completely evaporate prior to adding 45 mL of fresh 95 % ethanol. The steps from agitation and evaporation were repeated for subsequent 48 and 72 h. The extracted solution was subjected to gas chromatography-mass spectrometry (GCMS) for further analysis.
2.3. Gas chromatography-mass spectrometry (GCMS) analysis
The extracted compound mixture was subjected to 0.45 μm membrane filter prior to GCMS analysis. The mixture was injected into GCMS instrument (Agilent Technologies 7890A) and carried by inert helium gas with a flow rate of 1 mL/min. Sample was pressurized at 8.2 psi. Capillary column (30 m x250 μm) with a film thickness of 0.25 μm was engaged in the GCMS. Stationary phase engaged in GCMS was 5 %-phenyl-methylpolysiloxane (HP-5 ms). GCMS conditions were programmed as indicated in the work of Gomathi et al. [13] with slight modifications. The initial temperature was set at 60 °C and held for 15 min, which increased gradually at the rate of 6 °C per minute up to 280 °C and subsequently held for 5 min.
2.4. Effect of LEDs on PLBs pre-cultured with different conditions
Thirty-day-old in vitro PLBs maintained for one, two subculture cycles under red fluorescent light with intensity of 25.0 μmol/s (30 days per cycle) or more than 3 cycles under cool white LED illumination (17.0 μmol/s) were subjected under the illumination of 6 different LED spectra with similar intensity (15−20 μmol/s), namely cool white light (400−700 nm); far red (peak at 730 nm); green (white spectrum with increased green portion with peak at 530 nm); blue (peak at 440 nm); red (peak at 660 nm) and blue-red (peak at 440 and 660 nm respectively). PLBs were aseptically excised into clumps of 0.30 g and nourished with half-strength MS basal media (pH 5.80) supplemented with 2 % sucrose while 3.75 g/L of Gelrite was incorporated as the gelling agent. Three replicates were prepared for each treatment and maintained under different LEDs for 30 days with photoperiod of 16 h, after which the fresh PLBs were subjected to secondary metabolite analyses. All the cultures were maintained at temperature of 24 ± 2 °C and humidity of 51 ± 2 %. Three biological replicates, each with three technical replicates were selected for total phenolic, total flavonoid and 2,2-diphenyl-1-picrylhydrazyl (DPPH) analyses.
2.5. Effects of LEDs with varying intensities
Optimized pre-culture condition for PLBs secondary metabolites accumulation under different LED spectra was determined from previous experiment. PLBs exposed to cool white LED (17.0 μmol/s) for more than 3 subculture cycles were selected as the explants. Thirty-day-old PLBs were aseptically incised into clumps of 0.30 g and illuminated with 6 different LED lights with wavelengths identical with abovementioned spectra. Different intensity of each LED light: cool white light (4.63 μmol/s; 5.18 μmol/s; 17.0 μmol/s); far red (1.11 μmol/s; 9.12 μmol/s; 20.8 μmol/s); green (0.77 μmol/s; 6.15 μmol/s; 16.9 μmol/s); blue (0.91 μmol/s; 6.72 μmol/s; 15.7 μmol/s); red (1.26 μmol/s; 15.40 μmol/s; 29.30 μmol/s) and blue-red (2.01 μmol/s; 20.30 μmol/s; 44.80 μmol/s), were engaged as the illumination sources. Cultures were maintained at temperature of 24 ± 2 °C and humidity of 51 ± 2 %.
2.6. Effect of amino acids on secondary metabolites accumulation
Thirty-day-old PLBs maintained under cool white LED illumination (17.0 μmol/s) for more than 3 subculture cycles were aseptically excised into clumps of 0.30 g and cultured with half-strength MS basal media. Different amino acid supplements which included L-leucine (25 and 50 mg/L), glycine (25, 50, 75 and 100 mg/L) and proline (25, 50, 75 and 100 mg/L) were incorporated into the media to study their respective effect on secondary metabolites accumulation as well as antioxidant capacity of PLB cultures. Media pH were adjusted to be within the range of 5.75–5.80 with 0.1 M of HCl or NaOH, prior to autoclaving under 1.05 kg/cm2 and 121 °C for 15 min (Tomy High Pressure Steam Sterilizer ES-315, Japan). All the cultures were illuminated with high intensity green LED (16.9 μmol/s) light, which has been optimized from previous experiment, with photoperiod of 16 h for 30 days before subjected to secondary metabolite analyses.
2.7. Secondary metabolites extraction
PLBs were weighed in 0.10 g and homogenized with 0.6 mL of 80 % (v/v) analytical reagent grade acetone by using pre-cooled mortar and pestle. The suspension was kept overnight in 1.5 mL centrifuge vial under darkness at 4 °C [14]. The extract solution was subjected to total flavonoid, total phenolic content and total antioxidant activity analyses.
2.8. Total phenolic content evaluation
A volume of 200 μL acetone extract was incorporated into 500 μL of 10 % (v/v) Folin-Ciocalteu’s reagent and 500 μL of sterile distilled water. The mixture was vortexed to ensure the solutions were thoroughly mixed. Subsequently, the mixture was allowed to stand for 6 min prior to adding in 800 μL of 7.5 % (w/v) sodium carbonate [15]. The mixture was vortexed once again and incubated in dark for 30 min under room temperature before taking the absorbance reading at 765 nm by using UV–vis microplate spectrophotometer (Thermo Scientific™ Multiskan™ GO). Total phenolic content (μmol/g FW) was quantified by applying the equation obtained from the standard curve. Standard curve was established by engaging gallic acid serial dilutions (0−100 mg/L) to undergo the described protocols as the acetone extract at the absorbance of 765 nm.
2.9. Total flavonoid content evaluation
Acetone extract (100 μL) was incorporated into 100 μL of reagent solution containing 40 μL of 10 % aluminium chloride hexahydrate, 40 μL of 1 M potassium acetate, 600 of μL HPLC grade methanol and 1 120 μL of sterile distilled water [16]. The mixture was vortexed to ensure the solutions were thoroughly mixed. Subsequently, the mixture was incubated in dark for 30 min under room temperature. The absorbance reading was taken at 415 nm by using UV–vis microplate spectrophotometer (Thermo Scientific™ Multiskan™ GO). Total flavonoid content (μmol/g FW) was quantified by applying the equation obtained from the standard curve. Standard curve was established by engaging quercetin serial dilutions (0−100 mg/L) to undergo the described protocols as the acetone extract at the absorbance of 415 nm.
2.10. Evaluation of total antioxidant activity through DPPH
Total antioxidant activity of the extract was evaluated according to pre-described assay [17] with slight modifications. A volume of 100 μL acetone extract was incorporated into 100 μL of 0.135 mM DPPH dissolved in 80 % (v/v) acetone. The mixture was incubated for 30 min in dark under room temperature to provide ample time for the extract to scavenge free radicals present within DPPH solution. Controls for DPPH assay were prepared by mixing 100 μL of 80 % acetone with 100 μL of 0.135 mM DPPH. Absorbance reading was taken at 517 nm by using UV–vis microplate spectrophotometer (Thermo Scientific™ Multiskan™ GO) at every 2 min intervals up to the time where the absorbance readings reached a plateau. The absorbance reading recorded during the period where it reached stationary was applied into the following equation to ascertain the antioxidant capacity of the extract:
2.11. Statistical analysis
The data collected was analyzed by using IBM Statistical Package for Social Sciences (SPSS) version 24 software. Experiments were conducted in a completely randomized design. Significant differences of secondary metabolites content as well as antioxidant activity between treatments involved in present study were determined by comparing the mean values of 9 replicates. One-way ANOVA was engaged in mean values comparison at significance level of p ≤ 0.05, while two-way ANOVA test was conducted to study the interaction effect between PLBs pre-culture conditions and LED treatments.
3. Results and discussion
3.1. Gas-chromatography-mass-spectrometry (GCMS) analysis
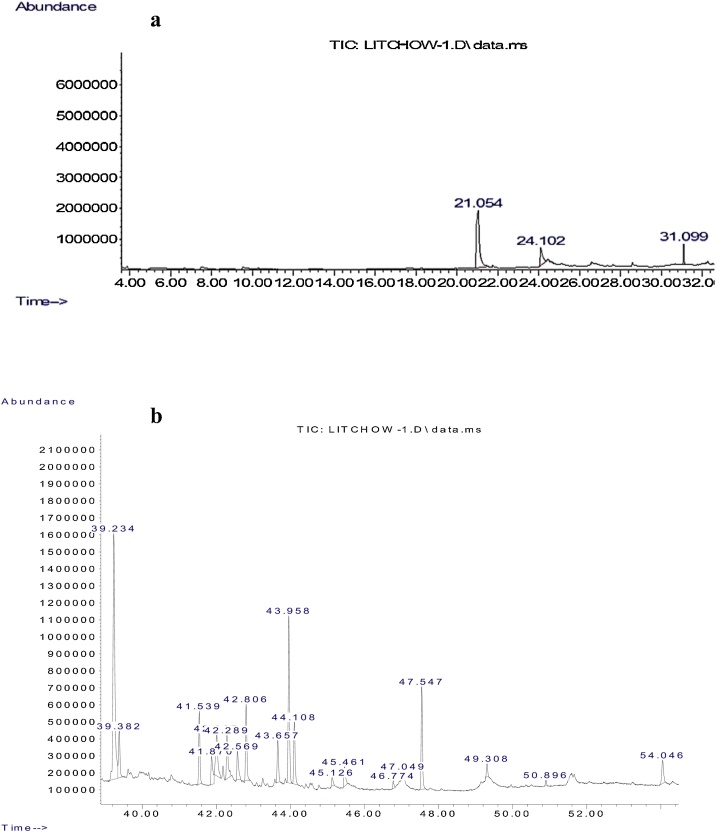
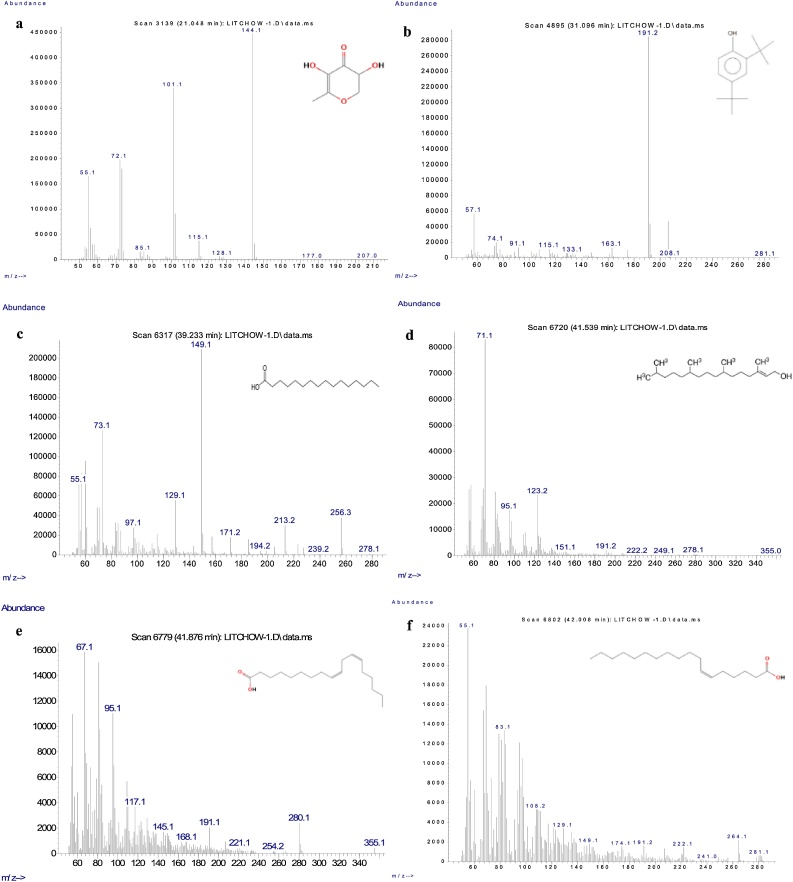
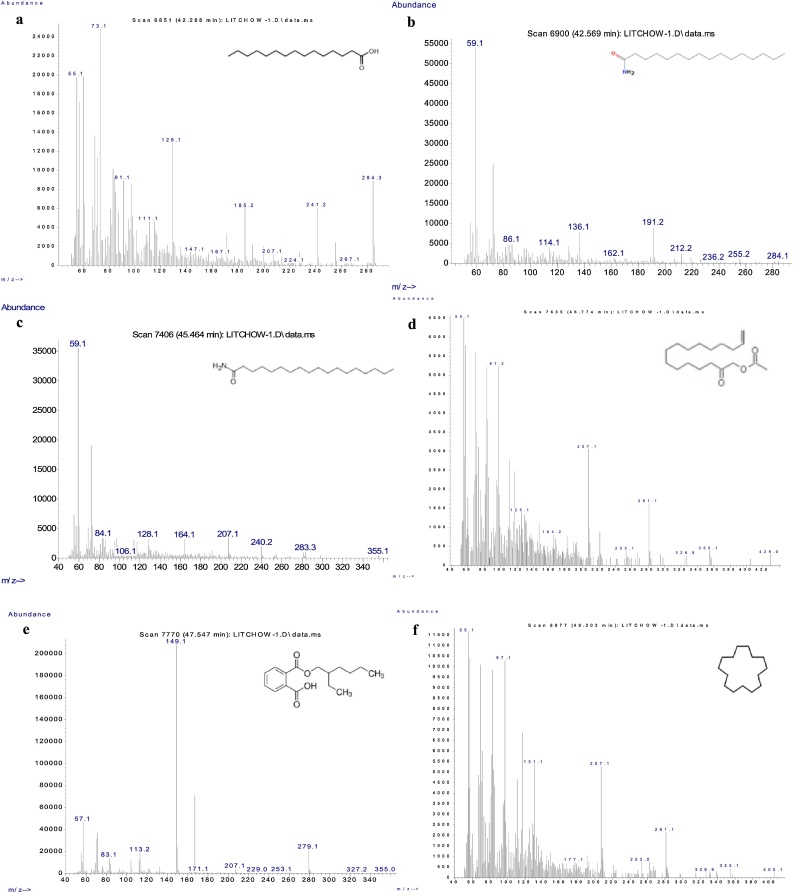
Total of 20 clear and narrow peaks were observed on chromatogram under GCMS profiling (Fig. 1). Compounds with more than 80 % matching percentage with GCMS library data were selected and further discussed (Table 1). Present study revealed the presence of several bioactive compounds within 30-day-old PLB cultures which come from different groups included phenolics, fatty acid, terpene, alkane, amide and esters. Mass spectrum of the selected bioactive compounds (mass/charge) with their respective molecular structures were analyzed by using mass-spectrometry for identification purposes as presented in Fig. 2, Fig. 3.
Fig. 1.
GCMS chromatogram of 30-day-old Dendrobium Enopi x Dendrobium Pink Lady PLBs ethanolic extracts. Chromatogram profile of DDMP with retention time of 21.054 min and phenol, 2,4-bis(1,1-dimethylethyl)- with retention time of 31.099 min (a); Chromatogram profile of other phytochemicals present within PLB cultures (b).
Table 1.
Phytochemical compounds identified to be present within 30-day-old PLBs ethanolic extract followed by GCMS analysis.
| Compounds | Retention Time (min) | Molecular Formula | Molecular Weight | Match Quality |
|---|---|---|---|---|
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 21.054 | C6H8O4 | 144.04 | 83 |
| Benzofuran, 2,3-dihydro- | 24.102 | C8H8O | 120.06 | 72 |
| Phenol, 2,4-bis(1,1-dimethylethyl)- | 31.099 | C14H22O | 206.17 | 96 |
| n-Hexadecanoic acid | 39.234 | C16H32O2 | 256.24 | 86 |
| 2-methyl-2-methoxy-3-hydroxyindan-1-one-3-carboxylate | 39.382 | C13H14O5 | 250.08 | 50 |
| Phytol | 41.539 | C20H40O | 296.31 | 91 |
| 9,12-Octadecadienoic acid (Z,Z) | 41.870 | C18H32O2 | 280.24 | 99 |
| 6-Octadecenoic acid | 42.007 | C18H34O2 | 282.26 | 98 |
| Pentadecanoic acid | 42.289 | C15H30O2 | 242.22 | 83 |
| Hexadecanamide | 42.569 | C16H33NO | 255.26 | 87 |
| 1,2,5,5,6,7-Hexamethylbicyclo[4.1.0]hept-2-en-4-one | 42.806 | C13H20O | 192.15 | 46 |
| 1,4-Methano-1H-indene, octahydro-1,7a-dimethyl-4-(1-methylethenyl)-, [1S(1.alpha.,3a.beta.,4.alpha.,7a.beta.)]- | 43.657 | C15H24 | 204.19 | 30 |
| N-(7-Dimethylamino-4-methylcoumarin-3-yl)maleimide | 43.958 | C16H14N2O4 | 298.10 | 72 |
| Toluene, 4-chloro-2-fluoro-5-nitro- | 44.108 | C7H5ClFNO2 | 189.00 | 35 |
| Octadecanamide | 45.461 | C18H37NO | 283.29 | 97 |
| 13-Tetradecen-1-ol acetate | 46.774 | C16H30O2 | 254.22 | 89 |
| 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester | 47.547 | C16H22O4 | 278.15 | 91 |
| Cyclopentadecane | 49.308 | C15H30 | 210.24 | 93 |
| 1H-Indole, 5-methyl-2-phenyl- | 50.896 | C15H13N | 207.10 | 49 |
| N-Methyl-1-adamantaneacetamide | 54.046 | C13H21NO | 207.16 | 38 |
Fig. 2.
Mass spectrum graphs of identified phytochemical compounds within ethanolic extract of 30-day-old PLBs and their respective chemical structure. 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- (a); Phenol, 2,4-bis(1,1-dimethylethyl)- (b); n-Hexadecanoic acid (c); Phytol (d); 9,12-Octadecadienoic acid (Z,Z) (e); and 6-Octadecenoic acid (f).
Fig. 3.
Mass spectrum graphs of identified phytochemical compounds within ethanolic extract of 30-day-old PLBs and their respective chemical structure. Pentadecanoic acid (a); Hexadecanamide (b); Octadecanamide (c); 13-Tetradecen-1-ol acetate (d); 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester (e); and Cyclopentadecane (f).
Fatty acids such as n-hexadecanoic acid (C16H32O2), 9,12-octadecadienoic acid (C18H32O2), 6-octadecenoic acid (C18H34O2) and pentadecanoic acid (C15H30O2) with retention time of 39.234, 41.870, 42.007 and 42.289 min respectively, were identified in GCMS analysis (Table 1). The presence of flavonoid 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- (C6H8O4) or DDMP in short, and phenol compound 2,4-bis(1,1-dimethylethyl) (C14H22O) were detected in 30-day-old PLB cultures. Besides, nitrogen-bearing phytochemicals such as hexadecanamide (C16H33NO) and octadecanamide (C18H37NO) of amide group as well as 1,2-benzenedicarboxylic acid, mono(2-ethylhexyl) ester (C16H22O4) were identified in GCMS analysis. Terpenoid such as phytol which contains 20 carbons was also found to be present in the PLB cultures with GCMS retention time of 41.539 min.
Various fatty acids such as n-hexadecanoic acid, 9,12-octadecadienoic acid, 6-octadecenoic acid and pentadecanoic acid are present within PLB in vitro cultures in present study. All of which are bioactive compounds with great array of activities. Among these, n-hexadecanoic acid is proven to exhibit anti-inflammatory properties, by providing active bind sites which are compatible to phospholipase enzyme, thereby inhibiting their effect and prevents inflammation [18]. Studies have showed that n-hexadecanoic acid exhibits selective cytotoxicity towards leukemic cells of human [19]. On top of that, anti-inflammatory feature of linoleic acid (9,12-octadecadienoic acid) also helps in relieving rheumatoid arthritis and ocular surface inflammation [20]. Dietary intake of linoleic acid is associated with a decreasing rate of developing coronary heart disease as suggested by Farvid et al. [21]. Owing to its anti-inflammatory effect, linoleic acid has been suggested to possess hepatoprotective activity in rats [22]. The function of linoleic acid in inhibiting the action of enoyl-acyl protein reductase enzyme disrupts the synthesis of fatty acid and exhibits its antibacterial effect [23].
Petroselinic acid (6-octadecenoic acid) is an unsaturated fatty acid with double bond located in between carbon-6 and carbon-7, which is seen as a relatively rare octadecenoic acid in the group. Due to its distinct chemical structure, petroselinic acid permits the production of derivatives which are different from that of other oleic acids. It displays antimicrobial activity [24] and has been widely formulated in cosmetic products as moisturizer, skin care and anti-aging agent [25] by elevating collagen and decorin structural protein levels. Additionally, petroselinic acid functions in inhibiting the formation of arachidonic acid metabolites that serve as the precursors for inflammatory mediators [26]. Pentadecanoic acid is a saturated fatty acid with 15 carbon atoms which possesses antioxidant and antimicrobial properties [27]. This odd-chain fatty acid aids in reducing risk of developing type 2 diabetes and cardiovascular diseases. It has been reported that pentadecanoic acid holds the capacity to undergo partial oxidation for succinyl-coenzyme A (succinyl-coA) production which further replenishes citric acid cycle, and promotes oxidative capacity of mitochondria [28]. Additionally, this compound has being used as lubricant and adhesive agents as well [29].
Phytol, a diterpene in its oxygenated form, is detected in present study. This acyclic diterpene alcohol is best known for the anti-bacterial properties it possesses. Phytol exerts its function as bactericide by inducing cell membrane damage, causing potassium ions leakage and further elevates ROS levels within bacteria. It serves as an effective bactericide against various bacteria such as Staphylococcous aureus, Colletotrichum fragariae, Colletotrichum gloeosporioides, and Colletotrichum accutatum [30]. Previous study found that phytol impairs DNA of Pseudomonas aeruginosa and inhibits the formation of reduced glutathione (GSH) which leads to oxidative stress [31].
DDMP belongs to flavonoids group and retains a strong antioxidant characteristic [32] which makes it an excellent free radicals scavenger. Being the major compound contributing to the anticancer effect of onions, DDMP has been confirmed to be able to induce human colon cancer cells death through apoptosis and ceases cancer cells growth [26]. DDMP expresses its anticancer effect by inhibiting the activation of nuclear factor kappa B (NF-KB), which is a transcription factor associated with various oncogenesis aspects. Apart than that, DDMP also exhibits anti-diabetic property by acting as α-glucosidase inhibitor [33], which in turn reduces postprandial hyperglycemia and prevents the breaking down of starch into its monomers, thereby delaying glucose assimilation.
Phenol-2,4-bis(1,1-dimethylethyl) is an antioxidant compound that alleviates photo-oxidative stress and acts as ultraviolet stabilizer. The antioxidant feature of phenol-2,4-bis(1,1-dimethylethyl) is beneficial in impeding oxidative stress caused by hydrogen peroxide accumulation. This antioxidant role of phenol-2,4-bis-(1,1-dimethylethyl) has been suggested to confer it the anti-neurodegenerative effect for neurological related diseases such as Alzheimer’s disease, as this compound inhibits oxidative stress that is detrimental to neuronal cells [34]. In addition, this particular compound possesses antifungal properties and functions as an effective antifungal agent against various fungi such as Curvularia lunata [35], Aspergillus niger, Fusarium oxysporum and Penicillium chrysogenum [36]. Anticancer effects of phenol- 2,4-bis(1,1-dimethylethyl) have been well-documented in the study of Malek et al. [37], where it is capable of inhibiting the growth of several cancer cells such as nasopharyngeal epidermoid, human hormone-dependent breast, lung and cervical carcinoma cell lines. It ceases their growth by displaying cytotoxity activities towards all the above-mentioned carcinoma cells. The acquisition of this compound from plants is natural and more preferable than their artificially synthetized analogues. Usage of plant-derived phenol-2,4-bis(1,1-dimethylethyl) as antimicrobial agents against phyto-pathogenic microbial is relatively safer and environmental friendly.
1,2-Benzenedicarboxylic acid, mono-(2-ethylhexyl) ester is a bioactive compound which has been proven to exhibit cytotoxicity towards various human cancer cell lines such as hepatocellular liver carcinoma and breast adenocarcinoma [38] while possesses little to none adverse effects on normal and healthy cell lines. The anticancer effect of 1, 2-benzenedicarboxylic acid, mono-(2-ethylhexyl) ester is attributed to its ability in inducing apoptosis of the cancer cells which in turn inhibits the cell growth. The accumulation of this compound has been reported in Centratherum Punctatum Cass. [39] and Hertia cheirifolia [40], both are medicinal plants with anti-inflammatory property.
The presence of other phytochemicals such as hexadecanamide, N-(7-dimethylamino-4-methylcoumarin-3-yl) maleimide (DACM), octadecanamide, 13-tetradecen-1-ol acetate and cyclopentadecane have been detected in the chromatography. Among the identified compounds, hexadecanamide (C16H33NO) is an amide which derives from hexadecanoic acid with anti-inflammatory effect and used as analgesic [41]. DACM (C16H14N2O4) is a fluorescent compound which exhibits its fluorescence only after undergoing chemical reaction with thiols under optimum conditions (pH 7). This compound has been employed as the staining agent in histochemical studies for the detection of sulfhydryl and disulfide groups [42].
Cytotoxic bioactive compounds exert their anticancer effects by interfering with different carcinogenesis stages such as inflammation, proliferation, invasion, angiogenesis and metastasis. Apoptosis is the occurrence of programmed cell death which is often accompanied by distinct morphological features and aided by various energy-dependent biochemical mechanisms [43]. This process is vital as it rapidly eliminates the dysfunctional cells in an irreversible manner and ensures normal cell turnover [44]. Failure to perform normal apoptosis is carcinogenic and also the leading cause of several diseases. For instances, neurodegenerative is an ischemic-related diseases and autoimmune disorders [43]. Hence, the presence of phytochemicals in PLB in vitro cultures such as DDMP, phenol-2,4-bis(1,1-dimethylethyl) and 1,2-benzenedicarboxylic acid, mono-(2-ethylhexyl) ester, serves as an alternate source for the acquisition of anticancer compounds.
3.2. Effect of LEDs on PLBs pre-cultured with different conditions
Present study revealed that PLB pre-culture conditions were significantly associated with their subsequent exposure to LED treatments in influencing secondary metabolites accumulation as well as total antioxidant activity. The interaction between these two independent variables were significant (p < 0.001) for all of the mentioned parameters (Table 2), where F value of 45.934 is recorded for total flavonoid content (F10,141 = 45.934, p < 0.001); 17.422 for total phenolic content (F10,141 = 17.422, p < 0.001); 4.380 for DPPH inhibition rate in percentage (F10,141 = 4.380, p < 0.001).
Table 2.
Two-way ANOVA test of between-subjects effects on the accumulation of total phenolic, total flavonoid and total antioxidant activity.
| Source | Type III Sum of Squares | df | Mean Square | F value | Sig. |
|---|---|---|---|---|---|
| Total flavonoid | |||||
| LED | 286.40 | 5 | 57.28 | 90.31 | 0.000* |
| Pre-culture conditions | 2886.93 | 2 | 1443.46 | 2275.72 | 0.000* |
| LED x Pre-culture conditions | 291.36 | 10 | 29.14 | 45.93 | 0.000* |
| Total phenolic | |||||
| LED | 9981.21 | 5 | 1996.24 | 18.99 | 0.000* |
| Pre-culture conditions | 98632.99 | 2 | 49316.49 | 469.14 | 0.000* |
| LED x Pre-culture conditions | 18314.39 | 10 | 1831.44 | 17.42 | 0.000* |
| DPPH inhibition rate (%) | |||||
| LED | 4751.23 | 5 | 950.25 | 4.30 | 0.001* |
| Pre-culture conditions | 876.03 | 2 | 438.02 | 1.98 | 0.141 |
| LED x Pre-culture conditions | 9671.54 | 10 | 967.15 | 4.38 | 0.000* |
Denoted asterisk (*) indicates significance at p < 0.05.
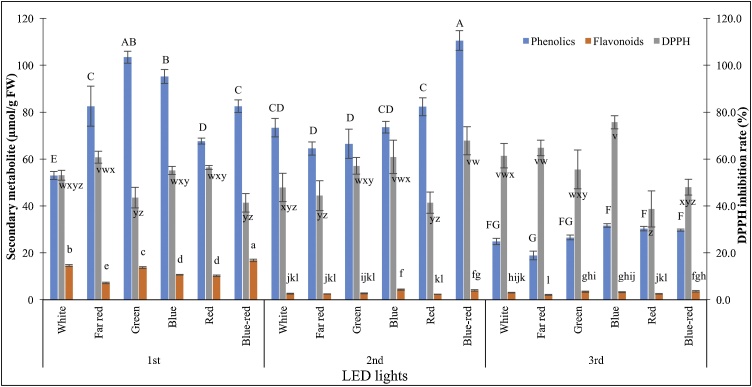
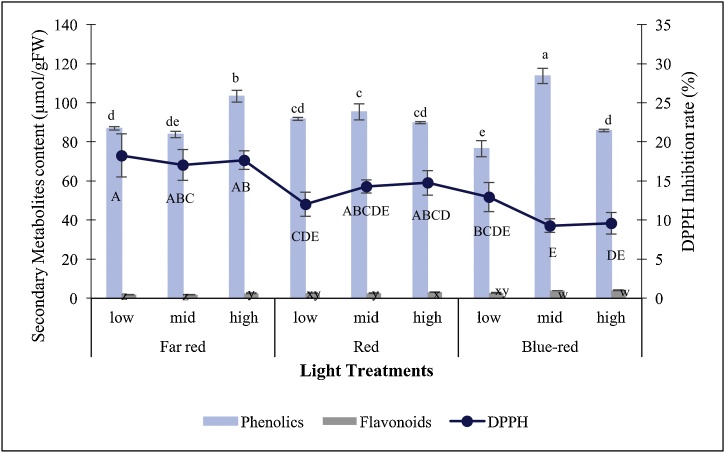
In overall, PLBs pre-illuminated with cool-white LED exhibited significantly higher flavonoid content after being treated with different LED spectra (Fig. 4), as compared with the other two pre-culture conditions. Blue-red LED gave rise to exceptionally high flavonoids content among all the treatments with 16.79 μmol/g FW of flavonoids was recorded. Flavonoids content accumulation declined as pre-culture conditions of PLBs differ.
Fig. 4.
Secondary metabolites accumulation of 30-day-old Dendrobium Enopi x Dendrobium Pink Lady PLBs pre-cultured under different light sources prior to illuminating under LED lights. Different letters indicate statistically significant differences (p < 0.05) using Duncan Multiple Range Test. (1st: > 3 subculture cycles under cool white LED; 2nd: 1 subculture cycle under red fluorescent light; 3rd: 2 subculture cycles under red fluorescent light).
The highest total phenolic content was induced by blue-red LED on PLBs pre-cultured with red fluorescent light for one cycle (Fig. 4) which recorded the accumulation of 110.54 μmol/g FW. Yet there was no significant difference between these particular treatments with that of green LED on PLBs pre-cultured under cool-white LED for more than 3 subculture cycles, which accumulated 103.44 μmol/g FW of total phenolic content. Among the PLBs pre-cultured under cool-white LED for more than three subculture cycles, white LED induced significantly low amount of total phenolic content with only 52.98 μmol/g FW. Present study discovered that total phenolic accumulation capacity of PLBs reduced under LED treatments, after being pre-cultured for two subculture cycles under red fluorescent light. Induction rate of total phenolic was significantly underperformed for all the tested LED spectra as compared with other PLBs pre-culture conditions. The lowest total phenolic content was achieved under far red light treatment with 18.85 μmol/g FW.
Pre-culture conditions of PLB explants exhibited no obvious effect on total antioxidant activity for all the tested LED lights (Fig. 4). The highest total antioxidant activity was recorded within PLBs pre-cultured with red fluorescent light for two subculture cycles which further exposed to blue LED light with 75.68 % of DPPH inhibition rate.
The higher content of total flavonoids and phenolics for PLBs pre-treated with white LED prior to illuminating with various LED spectra could be attributed to the occurrence of habituation of PLBs towards light. Habituation arises whenever in vitro cultures exhibit growth and development responses towards certain exogenously supplied substance, in an independent manner [45]. Plant tissue culture often involves the incorporation of plant growth regulators (PGRs) such as cytokinins and auxins, into the culture media to aid with the growth and development of in vitro cultures. However, upon the passage of time under in vitro conditions, cultures tend to develop habituation towards the exogenous PGRs. Callus cultures is more prone in exhibiting habituation, especially towards cytokinins and auxins [46]. As documented by Pischke et al. [47], callus cultures which often require cytokinin to promote proliferation and shoot organogenesis, possess the tendency to lose their dependence on cytokinin and become habituated over time, probably due to the over-expression of cytokinin receptor CRE 1 gene. Hormone habituation has been well-documented in various previous studies. Yet, development of habituation of PLB cultures towards illumination source is equally noteworthy and needed to be studied in depth.
In present study, the lower content of total flavonoid evaluated under different LED treatments for PLBs pre-treated with red fluorescent light for one or two subculture cycles, is probably due to habituation towards the light source of the PLB stock cultures. Although having a relatively spiky light spectrum profile, red fluorescent light emits light with narrow spectra similar with LEDs. The cultivation of PLB stock cultures under this light source prior to LED treatments allows the cultures to develop defence mechanisms and confers them the ability to cope with the subsequent LED treatments, which might no longer be perceived as a stress factor. Hence, the accumulation of flavonoids, which play a role in protecting plants against biotic and abiotic stresses, has been reduced. On the other hand, white LED-treated PLBs showed an upsurge of flavonoids content after exposure to different LED spectra, as compared with other pre-treatments. Flavonoids are essential for plants as UV stabilizer and scavenge the over-accumulated ROS induced by excessive UV radiation [48]. Besides, flavonoids also aid in plant defence mechanisms by acting as signalling molecules, allelopathic compounds, phytoalexins and detoxifying agents [49]. ROS present in excessive amount due to oxidative stress tends to elevate flavonoids content as well as their distribution within plant. Flavonoids help in such a way that they rapidly eliminate excess ROS, owing to their antioxidant properties. In present study, the sudden change of illumination source from white LED with wide light spectrum (400−700 nm) to different LED lights with narrow light spectra, might have induced photo-oxidative and/or photo-inhibition stress and upraised flavonoids content.
Among the PLBs pre-treated with white LED, blue-red (1:1) LED induced the highest total flavonoid content. Red light plays a role in the combination spectrum of blue and red LEDs. The proportion of red light initiates photo-protective mechanisms of the cultures and promotes biosynthesis of antioxidant compounds [50], at the expense of photosynthetic efficiency. On the other hand, blue light portion in the blue-red LED triggers the accumulation of flavonoids through the activation of chalcone synthesis and dihydroflavonol-4-reductage gene expression [51]. Besides, blue light also aids in enhancing the activity of enzyme such as phenylalanine ammonia-lyase (PAL), which in turn stimulates the accumulation of secondary metabolites through phenyl-propanoid pathways [52]. Phytochromes which are sensitive towards red and far red lights, as well as phototropins in plants which response primarily towards blue light, are believed to play a role in determining the biosynthesis of flavonoids. Chromophores present on photoreceptors confer cultures the ability to convert light signals into biochemical responses which might activate certain enzymes and interaction among proteins within the cultures [53]. Far red light often offsets the effects of red light in stimulating plant developmental responses while the ratio of far red and red light dictates various developmental activities and determines changes in terms of morphological, molecular as well as biochemical [54]. In present study, far red illumination alone without red light proportion resulted in the lowest flavonoids content. It can be deduced that far red light retains adverse effect for flavonoids accumulation. Similar findings have also been reported by Bottomley et al. [55], where far red light offset the effect of red light which elevated the content of kaempferol derivatives in Pisum sativum.
The exceptionally low total phenolic content under white LED treatment for PLBs pre-treated with white LED, suggests that PLBs induce more of these phenolic compounds in order to counteract with the altered illumination sources. The engagement of LEDs as elicitors for secondary metabolites production has been demonstrated in the study conducted by Lian et al. [56]. Narrow light spectra generated by LEDs serve as the abiotic stress factor and trigger the activation of plant defence mechanisms, which in turn leads to the accumulation of secondary metabolites [57]. High phenolics content under green LED could be due to the photo-damaging effect to photosynthetic pigments bring about by green spectrum [58]. The impairment of these essential pigments deprives one of the defence mechanisms against environmental stress, since photosynthetic pigments such as carotenoids, play a role in quenching reactive oxygen species (ROS) and confer plants the protection against photo-inhibition [59]. As a result, excessive ROS accumulation within the cultures activates the antioxidant gene defences, which subsequently stimulates the formation of antioxidants as well as the related enzymes [60]. Production of phenolic compounds is crucial to ensure the survivability of cultures especially under stressful conditions. The antioxidant properties of phenolics help in scavenging ROS which is detrimental to plants by preventing the oxidation of essential cellular components such as lipids, proteins and DNA [61]. Moreover, although green spectrum is not directly involved in plant growth and development, it has been proposed to contribute in reactions which are not directly exposed to light stimulus, for instance, antioxidants metabolism, since this particular spectrum can be transmitted with ease across plant tissues [50].
In present study, PLBs pre-treated with red fluorescent light for one subculture cycle is within the transition stage of habituation, whereby photo-oxidative stress induced by LED illumination is in the intermediate level. In order to compensate with the reduction of flavonoids, phenolics content remains high during this particular stage so as to mitigate plant oxidative stress by scavenging free radicals. The highest phenolics content under blue-red LED could be attributed to the blue light portion. Blue light exert its influence on the activities of PAL, by transforming trans-hydroxycinnamic acids, which inhibit PAL enzyme, to a less inhibitory form, cis-hydroxycinnamic acids, thereby ensuring the biosynthesis of phenolics [62]. In addition, red spectrum in blue-red LED also functions in activating phytochrome B, which further interacts with transcription factor such as vascular plant one-zinc-finger (VOZ) and mediates plant defence mechanisms [63]. Both phenolics and flavonoids of PLBs pre-treated with red fluorescent light for two subculture cycles, showed reduction after exposure to different LED spectra. This further supports the fact that PLB cultures exhibit habituation towards the narrow spectrum of red fluorescent light, wherefore subsequent illumination of various LED spectra might no longer be perceived as abiotic stress factor.
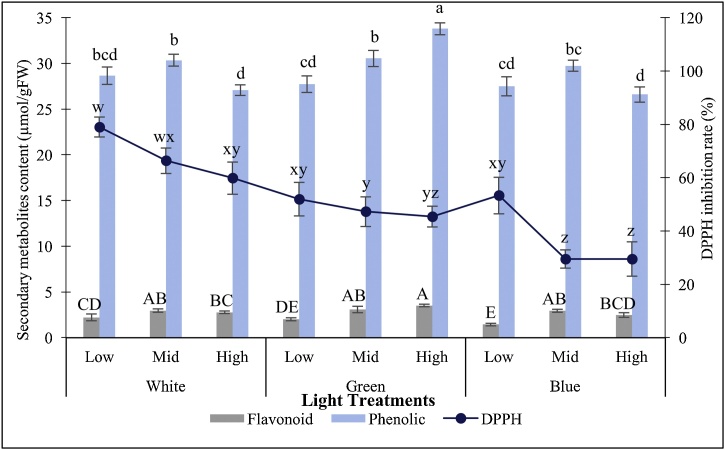
3.3. Effects of LEDs with varying intensities
Comparison among far red, red and blue-red (1:1) LEDs showed the accumulation of flavonoids at its highest content under high intensity (44.80 μmol/s) blue-red LED light treatment, which recorded at 4.10 μmol/g FW (Fig. 5). Generally, high LED intensity regardless of the spectra, stimulated highest flavonoids accumulation for each of the engaged LEDs. Mid-intensity blue-red (1:1) LED induced significantly higher total phenolic content of 113.68 μmol/g FW as compared with the other light treatments (Fig. 5). It was noted that the intensity of 20.30 μmol/s appeared to be the optimum intensity for phenolics induction under blue-red LED, as the phenolics content declined significantly as low or high intensity blue-red LED was employed as the lighting source. For red LED, there were no significant differences among all the tested intensities, where low, mid- and high intensity red LED correspondingly stimulated the accumulation of 91.77, 95.33 and 89.77 μmol/g FW of phenolics content. Overall, PLBs treated with far red LED, exhibited higher antioxidant activity than the others. The highest antioxidant capacity was achieved under low intensity far red LED treatment, with a total of 18.26 % DPPH inhibition rate (Fig. 5), followed by 17.66 % and 17.05 % which correspondingly induced by high and mid-intensity far red LED.
Fig. 5.
Secondary metabolites accumulation of 30-day-old PLBs illuminated with far red, red and blue-red (1:1) LED spectrum of varying intensities. Different letters indicate statistically significant differences (p < 0.05) using Duncan Multiple Range Test.
High intensity green LED (16.9 μmol/s) illumination stimulated the accumulation of 3.52 μmol/g FW of flavonoids content, which was the highest among all the tested treatments (Fig. 6). However it was found that there was no significant difference between this treatment with that of mid-intensity of green (6.15 μmol/s), white (5.18 μmol/s) and blue (6.72 μmol/s) LED which induced 3.09, 2.96 and 2.95 μmol/g FW of flavonoids content respectively. It was noted that high intensity green LED prevailed over other light treatments as it stimulated significantly higher amount of total phenolic content. Total of 33.80 μmol/g FW of total phenolic content which was the highest among the tested treatments was induced by this particular light treatment (Fig. 6). Generally, white LED of any tested intensities gave rise to a higher DPPH inhibition rate (%) than any other treatments (Fig. 6). The highest antioxidant activity, 78.99 % was achieved under low intensity white LED. This value was significantly higher than all the other engaged treatments except for mid-intensity white LED, which recorded an antioxidant activity of 66.39 %. Among the same spectrum, low intensity of each of the spectra was found to induce the highest antioxidant activity.
Fig. 6.
Secondary metabolites accumulation of 30-day-old PLBs illuminated with white, green and blue LED spectrum of varying intensities. Different letters indicate statistically significant differences (p < 0.05) using Duncan Multiple Range Test.
In present study, high intensity green LED prevailed over mid-intensity blue-red (1:1) LED as it resulted in the accumulation of higher total phenolic content (Section 3.2.). Despite total flavonoid content was relatively lower under this light treatment, phenolics production was prioritized due to the discovery of the presence of an essential bioactive compound, phenol-2,4-bis(1,1-dimethylethyl) within the PLBs. Thereby, this illumination was selected for subsequent experiment.
The contents of plant secondary metabolite vary among and within species. The concentrations fluctuate due to several abiotic factors such as light intensity as well as the wavelength [64]. Present study reveals that blue-red (1:1) LED of mid-intensity (20.30 μmol/s) induces high phenolics and flavonoids, suggesting that this intensity is appropriate for the production of secondary metabolites. Low intensity blue-red LED (2.01 μmol/s) might have limited the availability of light photons required for the utilization of phytochromes and phototropins for the conversion of light signals into phytochemicals accumulation. In contrast, high intensity blue-red LED (44.80 μmol/s) provides proper absorption spectrum for photosynthetic pigments. Hence PLB cultures might have prioritized their growth while limited carbon source restricts the production of secondary metabolites [65]. Primary growth always takes precedence over the production of secondary metabolites in plants [66]. Under optimal conditions for primary growth, the available carbon and nitrogen sources are often limited for the production of secondary metabolites, as most of these resources have been allocated to support plant primary growth.
In present study, far red LED stimulates relatively high total antioxidant activity within PLBs. This indicates that far red LED of any intensity, low (1.11 μmol/s), mid- (9.12 μmol/s) or high (20.8 μmol/s), induces photo-oxidative stress within PLB cultures and hence heightens their antioxidant capacity. The reduction of photosynthetic pigments under far red light could be the causal factor for the upsurge of PLBs antioxidant activity under this illumination. Alternative plant defence mechanisms such as non-enzymatic antioxidants which include ascorbic acid and glutathione might play a part in giving rise to a relatively higher antioxidant activity for PLBs under far red illumination, as they play a protective role in impeding plant oxidative stress as ROS scavengers.
Green spectrum is often of non-essential for plant growth and development. However, it has been suggested to possess effects in plant photosynthesis as well as physiology responses towards environmental factors [67]. Present study reveals that increasing light intensity of spectrum consisting of green wavelength elevates phenolic and flavonoid contents. In guard cells, chlorophylls also function as photoreceptors that drive stomatal opening. The spectrum generated by green light possesses little effects on the action spectrum of guard cell chlorophylls, and hence limits stomatal opening [68]. Green spectrum with maximum peak of 540 nm reverses the action of blue light and inhibits stomatal opening [69]. While in a study done by Lurie [70], exposure of broad bean leaves towards green light induces only a slight opening of the stomata. Stomatal closure is often correlated with drought stress which stimulates the accumulation of secondary metabolites as a defence mechanism due to ROS overproduction. Thus, high intensity green light (16.9 μmol/s) illumination in present study might have elevated secondary metabolites of PLB cultures through these means. Furthermore, green light illumination helps in counteracting biotic as well as abiotic stress through the up-regulation of PAL and pathogenesis-related protein 1a genes [71]. The incorporation of green light portion in the blue-red LED spectrum promotes the net photosynthetic rates of lettuce [72]. In present study, green spectrum supplementation in white light could have enhanced the expression of light harvesting chlorophyll a/b-binding protein and PsbA genes, which encodes for D1 protein in photosystem II. Additionally, the further penetration of green light through plant tissues also aids in driving photosynthesis [73]. The promoted photosynthetic rates increase the availability of more carbon sources for the production of carbon-based secondary metabolites, for instance, phenolics.
Low intensity white LED (4.63 μmol/s) provides inadequate light source for the assimilation of PLBs, thereby inducing light stress. Low intensity white illumination exerts its influence in regulating several physiological metabolic processes such as photosynthesis along with antioxidant biosynthesis pathways [74]. Under this scenario, the exposure of PLB cultures to light stress might have altered the biochemical mechanisms by up-regulating the activities of certain antioxidants and/or substances with such effects, which collectively elevates the antioxidant capacity.
3.4. Effect of amino acids on secondary metabolites accumulation
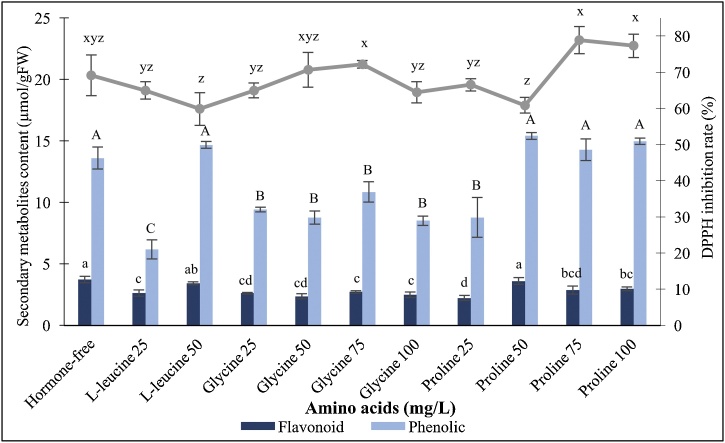
Under high intensity green LED illumination, it was noticed that all the tested amino acids played no significant effects in elevating flavonoids content within PLBs (Fig. 7). The highest recorded total flavonoid content of 3.73 μmol/g FW was induced by half-strength MS basal medium without amino acid supplement. Generally, proline with concentrations higher than 50 mg/L generated higher total phenolic content within PLBs (Fig. 7) whereas glycine with concentrations ranged from 25 to 100 mg/L displayed no stimulating effect in enhancing phenolics content. It was found that 25 mg/L of proline induced the accumulation of 8.78 μmol/g FW of total phenolic content. Further increment of proline concentration to 50, 75 and 100 mg/L significantly enhanced the phenolics content, with 15.41, 14.30 and 14.98 μmol/g FW recorded for each concentration, respectively. Present study revealed that all the tested amino acids possess no prevailing effects on enhancing total antioxidant activity of the PLBs, as all the treatments exhibited no significant differences with that of the control treatment (Fig. 7) which stimulated 69.09 % of DPPH inhibition rate.
Fig. 7.
Effect of different amino acid supplements on secondary metabolites accumulation of 30-day-old PLBs illuminated with high intensity green LED spectrum. Different letters indicate statistically significant differences (p < 0.05) using Duncan Multiple Range Test.
Incorporation of high L-leucine concentration (50 mg/L) stimulates higher phenolics and flavonoids content than low concentration (25 mg/L) does. The assimilated exogenous L-leucine provides an essential source for the yielding of aldehydes. Degradation of L-leucine with the aid of various enzymes such as amino acid decarboxylase, amino acid deaminases and aldehyde synthase, plays a role in the biosynthesis pathways of plant secondary metabolites [75]. In addition, proline supplementation leads to high secondary metabolites production in present study. This can be attributed to the role of proline as signalling molecule during environmental stress conditions. Proline helps in restoring cellular redox balance and activates certain gene expression in plant defence mechanisms. Besides, proline biosynthesis cycle which is coupled with pentose phosphate pathway, generates the formation of erythrose-4-phosphate, a compound which serves as the precursor of shikimate pathway for biosynthesis of secondary metabolites [76]. The capacity of proline in inducing phenolics has been exhibited in the study conducted by Al-Jibouri et al. [77], where supplementation of proline promotes coumarin and eugenol content of Verbascum thapsus L. callus. The accumulation of proline in plants often occurs as a result of exposure to stressful conditions, which include drought, salinity, oxidative stress and UV irradiance [78]. In present study, the administration of exogenously supplied proline acts as antioxidant molecule and aids in diminishing excessive ROS, thereby conferring the PLB cultures a relatively higher antioxidant capacity. The function of proline as antioxidant in quenching singlet oxygen radicals and prevents programmed cell death has been reported in previous study as well [79].
The low concentration of secondary metabolites evaluated within PLBs treated with glycine suggests that the cultures prioritize primary growth instead of secondary metabolites production in present study. The application of exogenous glycine tends to upraise the intake of macronutrients such as nitrogen, potassium and magnesium within plant [80], which subsequently support plant growth and development. This can be due to the affinity of glycine with nutrient elements present within the culture media. The formation of chelates facilitates the uptake and transportation of these nutrients [81].
Nevertheless, supplementary amino acids which include L-leucine, glycine and proline, possess no significant effect in enhancing secondary metabolites accumulation as well as antioxidant activity of PLB cultures, as in comparison with the control treatment. Results suggest that sole illumination of green LED is ample to serve the purpose of stimulating secondary metabolites production due to the capacities of green spectrum in stimulating several biochemical responses as aforementioned.
4. Conclusion
Bioactive compounds which included DDMP, phenol-2,4-bis(1,1-dimethylethyl) and 1,2-benzenedicarboxylic acid, mono-(2-ethylhexyl) ester that possess anticancer cancer properties have been detected in in vitro PLB cultures of Dendrobium Enopi x Dendrobium Pink Lady hybrid orchid. Present study demonstrated that PLB stock cultures pre-cultured under red fluorescent light for one or two subculture cycles showed a reduction in secondary metabolites production after exposure to various LED treatments. This suggests that PLB cultures exhibited habituation towards light after being exposed to illumination source of similar light profile for a prolonged period. At intensity of 16.9 μmol/s, incorporation of green spectrum into white LED stimulated higher phenolics content, while the highest flavonoids content was achieved under mid- (20.30 μmol/s) and high intensity (44.80 μmol/s) blue-red (1:1) LED irradiation. Low intensity of both white (4.63 μmol/s) and far red (1.11 μmol/s) LEDs enhanced total antioxidant activity of the cultures. Nonetheless, amino acid supplements such as L-leucine, glycine and proline (25−100 mg/L) exerted no satisfactory effects in promoting secondary metabolites accumulation.
Author contributions
LCY and SS designed and conducted the experiments, analyzed the data and wrote the manuscript. SS conceptualized and supervised the research. BLC supervised the research. LCY SS performed chemical analysis and validated the chemical data. LCY and SS critically revised and edited the manuscript.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgment
This work was supported by Collaborative Research in Engineering, Science and Technology Centre (CREST) [Grant No: 304/PBIOLOGI/650920-C121]. The authors would also like to thank the School of Biological Sciences and Universiti Sains Malaysia (USM) for supporting this study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00497.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Thirumurugan D., Cholarajan A., Raja S.S., Vijayakumar R. An introductory chapter: secondary metabolites. Second Metab—Sources Appl. 2018:1–21. [Google Scholar]
- 2.McMurry J.E. Cengage Learning; 2014. Organic Chemistry With Biological Applications. [Google Scholar]
- 3.Berdy J. Bioactive microbial metabolites. J. Antibiot. Res. 2005;58(1):1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 4.Cheng J., Dang P.P., Zhao Z., Yuan L.C., Zhou Z.H., Wolf D., Luo Y.B. An assessment of the Chinese medicinal Dendrobium industry: supply, demand and sustainability. J. Ethnopharmacol. 2019;229:81–88. doi: 10.1016/j.jep.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Bulpitt C.J., Li Y., Bulpitt P.F., Wang J. The use of orchids in chinese medicine. J. R. Soc. Med. 2007;100(12):558–563. doi: 10.1258/jrsm.100.12.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong J.M., Goh N.K., Chia L.S., Chia T.F. Recent advances in traditional plant drugs and orchids. Acta Pharmacol. Sin. 2003;24(1):7–21. [PubMed] [Google Scholar]
- 7.Lin Y., Wang F., Yang L.J., Chun Z., Bao J.K., Zhang G.L. Anti-inflammatory phenanthrene derivatives from stems of Dendrobium denneanum. Phytochemistry. 2013;95:242–251. doi: 10.1016/j.phytochem.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Tian C.C., Zha X.Q., Luo J.P. A polysaccharide from Dendrobium huoshanense prevents hepatic inflammatory response caused by carbon tetrachloride. Biotechnol. Biotechnol. Equip. 2015;29(1):132–138. doi: 10.1080/13102818.2014.987514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan L.H., Li X.F., Wang M.N., Zha X.Q., Yang X.F., Liu Z.J. Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. Int. J. Biol. Macromol. 2014;64:420–427. doi: 10.1016/j.ijbiomac.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Hussain M.S., Fareed S., Saba A.M., Rahman A., Ahmad I.Z., Saeed M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioall Sci. 2012;4(1):10–20. doi: 10.4103/0975-7406.92725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halkier B.A., Gershenzon J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- 12.Aja P.M., Ugwu O.P.C., Okoro C.O., Nweke O.L., Ali I.A., Ogbu P.N. Gas chromatographic-mass spectrometric (GC-MS) analysis of ethanol leaf-extract of Vigna unguiculata (cowpea) IJRRPAS. 2016;6(1):1284–1289. [Google Scholar]
- 13.Gomathi D., Kalaiselvi M., Ravikumar G., Devaki K., Uma C. GC-MS analysis of bioactive compounds from the whole plant ethanolic extract of Evolvulus alsinoides (L.) L. J. Food Sci. Technol. 2015;52(2):1212–1217. doi: 10.1007/s13197-013-1105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irshad M., Debnath B., Mitra S., Arafat Y., Li M., Sun Y., Qiu D. Accumulation of anthocyanin in callus cultures of red-pod okra [Abelmoschus esculentus (L.) Hongjiao] in response to light and nitrogen levels. Plant Cell Tiss. Org. 2018;134(1):29–39. [Google Scholar]
- 15.Basma A.A., Zakaria Z., Latha L.Y., Sasidharan S. Antioxidant activity and phytochemical screening of the methanol extracts of Euphorbia hirta L. Asian Pac. J. Trop. Med. 2011;4(5):386–390. doi: 10.1016/S1995-7645(11)60109-0. [DOI] [PubMed] [Google Scholar]
- 16.Al‐Mansoub M.A., Asmawi M.Z., Murugaiyah V. Effect of extraction solvents and plant parts used on the antihyperlipidemic and antioxidant effects of Garcinia atroviridis: a comparative study. J. Sci. Food Agric. 2014;94(8):1552–1558. doi: 10.1002/jsfa.6456. [DOI] [PubMed] [Google Scholar]
- 17.Idamokoro E.M., Masika P.J., Muchenje V. A report on the in vitro antioxidant properties of Vachellia karroo leaf extract: a plant widely grazed by goats in the central eastern cape of south africa. Sustainability. 2017;9(2):164. [Google Scholar]
- 18.Aparna V., Dileep K.V., Mandal P.K., Karthe P., Sadasivan C., Haridas M. Anti‐inflammatory property of n‐hexadecanoic acid: structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012;80(3):434–439. doi: 10.1111/j.1747-0285.2012.01418.x. [DOI] [PubMed] [Google Scholar]
- 19.Harada H., Yamashita U., Kurihara H., Fukushi E., Kawabata J., Kamei Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002;22(5):2587–2590. [PubMed] [Google Scholar]
- 20.Barabbino S., Rolando M., Camicione P., Zanardi S., Cro M., Giuffrida S., Calabria G. Efficacy of systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with inflammatory component. Invest. Ophth. Vis. Sci. 2002;43(13) doi: 10.1097/00003226-200303000-00002. 51-51. [DOI] [PubMed] [Google Scholar]
- 21.Farvid M.S., Ding M., Pan A., Sun Q., Chiuve S.E., Steffen L.M. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130(18):1568–1578. doi: 10.1161/CIRCULATIONAHA.114.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahon K., Das S. Hepatoprotective activity of Ocimum sanctum alcoholic leaf extract against paracetamol-induced liver damage in Albino rats. Pharmacogn. Res. 2011;3(1):13. doi: 10.4103/0974-8490.79110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saikarthik J., Ilango S., Vijayakumar J., Vijayaraghavan R. Phytochemical analysis of methanolic extract of seeds of Mucuna pruriens by gas chromatography mass spectrometry. Int. J. Pharm. Sci. 2017;8(7):2916–2921. [Google Scholar]
- 24.Placek L.L. A review on petroselinic acid and its derivatives. J. Am. Oil Chem. Soc. 1963;40(8):319–329. [Google Scholar]
- 25.Delbeke E.I., Everaert J., Uitterhaegen E., Verweire S., Verlee A., Talou T. Petroselinic acid purification and its use for the fermentation of new sophorolipids. AMB Express. 2016;6(1):28. doi: 10.1186/s13568-016-0199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colworth, A. S. U. R., Colworth, H.H.L.U.R., Colworth, G.M.R.U.R., Colworth, P.J.R.U.R., Colworth, R.A.V.U.R., Colworth, R.J S.U.R., … & BV, C.F.W.L.C.. (1999). European Patent Office Patent No. EP1013178A1.
- 27.Shyamala R., Manikandan R. Determination of bioactive compounds in Ziziphus oenoplia leaves extract using gas chromatography and mass spectroscopic technique. J. Pharmacogn. Phytochem. 2019;8(5):157–160. [Google Scholar]
- 28.Pfeuffer M., Jaudszus A. Pentadecanoic and heptadecanoic acids: multifaceted odd-chain fatty acids. Adv. Nutr. 2016;7(4):730–734. doi: 10.3945/an.115.011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arora S., Meena S. GC-MS Profiling of Ceropegia bulbosaRoxb. var.bulbosa, an endangered plant from Thar Desert, Rajasthan. Innov. Pharm. 2017;6:568–573. [Google Scholar]
- 30.Ryu J., Kwon S.J., Ahn J.W., Jo Y.D., Kim S.H., Jeong S.W. Phytochemicals and antioxidant activity in the kenaf plant (Hibiscus cannabinus L.) J. Plant Biotechnol. 2017;44(2):191–202. [Google Scholar]
- 31.Lee W., Woo E.R., Lee D.G. Phytol has antibacterial property by inducing oxidative stress response in Pseudomonas aeruginosa. Free Radic. Res. 2016;50(12):1309–1318. doi: 10.1080/10715762.2016.1241395. [DOI] [PubMed] [Google Scholar]
- 32.Yu X., Zhao M., Liu F., Zeng S., Hu J. Identification of 2, 3-dihydro-3, 5-dihydroxy-6-methyl-4H-pyran-4-one as a strong antioxidant in glucose–histidine Maillard reaction products. Food Res. Int. 2013;51(1):397–403. [Google Scholar]
- 33.Quan J., Yin X., Jin M., Shen M. Study on the inhibition of alpha-glucosidase by soyasaponins. Zhong yao cai= Zhongyaocai= J. Chin. Med. Mater. 2003;26(9):654–656. [PubMed] [Google Scholar]
- 34.Choi S.J., Kim J.K., Kim H.K., Harris K., Kim C.J., Park G.G. 2, 4-Di-tert-butylphenol from sweet potato protects against oxidative stress in PC12 cells and in mice. J. Med. Food. 2013;16(11):977–983. doi: 10.1089/jmf.2012.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren J., Wang J., Karthikeyan S., Liu H., Cai J. Natural anti-phytopathogenic fungi compound phenol, 2, 4-bis (1, 1-dimethylethyl) from Pseudomonas fluorescens TL-1. Indian J. Biochem. Bio. 2019;56(2):162–168. [Google Scholar]
- 36.Varsha K.K., Devendra L., Shilpa G., Priya S., Pandey A., Nampoothiri K.M. 2, 4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int. J. Food Microbiol. 2015;211:44–50. doi: 10.1016/j.ijfoodmicro.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 37.Malek S.N.A., Shin S.K., Wahab N.A., Yaacob H. Cytotoxic components of Pereskia bleo (Kunth) DC.(Cactaceae) leaves. Molecules. 2009;14(5):1713–1724. doi: 10.3390/molecules14051713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnan K., Mani A., Jasmine S. Cytotoxic activity of bioactive compound 1, 2-benzene dicarboxylic acid, mono 2-ethylhexyl ester extracted from a marine derived Streptomyces sp. VITSJK8. Int. J. Mol. Cell. Med. 2014;3(4):246. [PMC free article] [PubMed] [Google Scholar]
- 39.Sivasubramanian R., Brindha P. In vitro cytotoxic, antioxidant and GC-MS studies on Centratherum punctatum Cass. Int. J. Pharm. Pharm. Sci. 2013;5(3):364–367. [Google Scholar]
- 40.Segueni N., Zellagui A., Boulechfar S., Derouiche K., Rhouati S. Essential oil of Hertia cheirifolia leaves: chemical composition, antibacterial and antioxidant activities. J. Mater. Environ. Sci. 2017;8(2):551–556. [Google Scholar]
- 41.Al-Rubaye A.F., Kaizal A.F., Hameed I.H. Phytochemical screening of methanolic leaves extract of Malva sylvestris. Int. J. Pharmacogn. 2017;9(4):537–552. [Google Scholar]
- 42.Ogawa H., Taneda A., Kanaoka Y., Sekine T. The histochemical distribution of protein bound sulfhydryl groups in human epidermis by the new staining method. J. Histochem. Cytochem. 1979;27(5):942–946. doi: 10.1177/27.5.90070. [DOI] [PubMed] [Google Scholar]
- 43.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Letai A.G. Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nat. Rev.Cancer. 2008;8(2):121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 45.George E.F. Exegetics limited; 1993. Plant Propagation by Tissue Culture. Part 1: the Technology. [Google Scholar]
- 46.Mendoza M.G., Kaeppler H.F. Auxin and sugar effects on callus induction and plant regeneration frequencies from mature embryos of wheat (Triticum aestivum L.) In Vitro Cell. Dev-Pl. 2002;38(1):39–45. [Google Scholar]
- 47.Pischke M.S., Huttlin E.L., Hegeman A.D., Sussman M.R. A transcriptome-based characterization of habituation in plant tissue culture. Plant Physiol. 2006;140(4):1255–1278. doi: 10.1104/pp.105.076059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shirley B.W. Flavonoid biosynthesis:‘new’functions for an ‘old’pathway. Trends Plant Sci. 1996;1(11):377–382. [Google Scholar]
- 49.Panche A., Diwan A., Chandra S. Flavonoids: an overview. J. Nutr. Sci. 2016;5 doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samuolienė G., Sirtautas R., Brazaitytė A., Duchovskis P. LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem. 2012;134(3):1494–1499. doi: 10.1016/j.foodchem.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 51.Cevallos-Casals B.A., Cisneros-Zevallos L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 2010;119(4):1485–1490. [Google Scholar]
- 52.Meng X., Xing T., Wang X. The role of light in the regulation of anthocyanin accumulation in Gerbera hybrida. Plant Growth Regul. 2004;44(3):243. [Google Scholar]
- 53.Kong S.G., Okajima K. Diverse photoreceptors and light responses in plants. Int. J. Plant Res. 2016;129(2):111–114. doi: 10.1007/s10265-016-0792-5. [DOI] [PubMed] [Google Scholar]
- 54.Folta K.M., Maruhnich S.A. Green light: a signal to slow down or stop. J. Exp. Bot. 2007;58(12):3099–3111. doi: 10.1093/jxb/erm130. [DOI] [PubMed] [Google Scholar]
- 55.Bottomley W., Smith H., Galston A. A phytochrome mediated effect of light on the hydroxylation pattern of flavonoids in Pisum sativum var.‘Alaska’. Nature. 1965;207(5003):1311–1312. [Google Scholar]
- 56.Lian T.T., Cha S.Y., Moe M.M., Kim Y.J., Bang K.S. Effects of different colored LEDs on the enhancement of biologically active ingredients in callus cultures of Gynura procumbens (Lour.) Merr. Molecules. 2019;24(23):4336. doi: 10.3390/molecules24234336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naik P.M., Al-Khayri J.M. Abiotic and Biotic Stress in Plants-recent Advances and Future Perspectives. 2016. Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plants; pp. 247–277. [Google Scholar]
- 58.Golovatskaya I., Karnachuk R.A. Role of green light in physiological activity of plants. Russ. J. Plant Physiol. 2015;62(6):727–740. [Google Scholar]
- 59.Loggini B., Scartazza A., Brugnoli E., Navari-Izzo F. Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol. 1999;119(3):1091–1100. doi: 10.1104/pp.119.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Praveena B., Murthy S. Role of photosynthetic pigments in protection against oxidative damege. Int. J. Plant Anim. Environ. Sci. 2013;4(1):167–171. [Google Scholar]
- 61.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Billett E.E., Grayer-Barkmeijer R.J., Johnson C., Harborne J. The effect of blue light on free and esterified phenolic acids in etiolated gherkin tissues. Phytochemistry. 1981;20(6):1259–1263. [Google Scholar]
- 63.Kuo T.C.Y., Chen C.H., Chen S.H., Lu I.H., Chu M.J., Huang L.C. The effect of red light and far-red light conditions on secondary metabolism in agarwood. BMC Plant Biol. 2015;15(1):139. doi: 10.1186/s12870-015-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu C., Sullivan J.H. Reviewing the technical designs for experiments with ultraviolet‐B radiation and impact on photosynthesis, DNA and secondary metabolism. J. Integr. Plant Biol. 2010;52(4):377–387. doi: 10.1111/j.1744-7909.2010.00939.x. [DOI] [PubMed] [Google Scholar]
- 65.Bot J.L., Bénard C., Robin C., Bourgaud F., Adamowicz S. The ‘trade-off’between synthesis of primary and secondary compounds in young tomato leaves is altered by nitrate nutrition: experimental evidence and model consistency. J. Exp. Bot. 2009;60(15):4301–4314. doi: 10.1093/jxb/erp271. [DOI] [PubMed] [Google Scholar]
- 66.Tuomi J., Fagerstrom T., Niemela P. Wiley; New York: 1991. Carbon Allocation, Phenotypic Plasticity, and Induced Defenses. Phytochemical Induction by Herbivores; pp. 85–104. [Google Scholar]
- 67.Smith H.L., McAusland L., Murchie E.H. Don’t ignore the green light: exploring diverse roles in plant processes. J. Exp. Bot. 2017;68(9):2099–2110. doi: 10.1093/jxb/erx098. [DOI] [PubMed] [Google Scholar]
- 68.Zeiger E., Farquhar G.D., Cowan I. Stanford University Press; 1987. Stomatal Function; pp. 195–208. [Google Scholar]
- 69.Kinoshita T., Hayashi Y. New insights into the regulation of stomatal opening by blue light and plasma membrane H+-ATPase. Int. Rev. Cel. Mol. Bio. 2011;289:89–115. doi: 10.1016/B978-0-12-386039-2.00003-1. [DOI] [PubMed] [Google Scholar]
- 70.Lurie S. The effect of wavelength of light on stomatal opening. Planta. 1978;140(3):245–249. doi: 10.1007/BF00390255. [DOI] [PubMed] [Google Scholar]
- 71.Nagendran R., Lee Y.H. Green and red light reduces the disease severity by Pseudomonas cichorii JBC1 in tomato plants via upregulation of defense-related gene expression. Phytopathology. 2015;105(4):412–418. doi: 10.1094/PHYTO-04-14-0108-R. [DOI] [PubMed] [Google Scholar]
- 72.Bian Z., Yang Q., Li T., Cheng R., Barnett Y., Lu C. Study of the beneficial effects of green light on lettuce grown under short‐term continuous red and blue light‐emitting diodes. Physiol. Plant. 2018;164(2):226–240. doi: 10.1111/ppl.12713. [DOI] [PubMed] [Google Scholar]
- 73.Terashima I., Fujita T., Inoue T., Chow W.S., Oguchi R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009;50(4):684–697. doi: 10.1093/pcp/pcp034. [DOI] [PubMed] [Google Scholar]
- 74.Yang B., Tang J., Yu Z., Khare T., Srivastav A., Datir S., Kumar V. Light stress responses and prospects for engineering light stress tolerance in crop plants. J. Plant Growth Regul. 2019;38(4):1489–1506. [Google Scholar]
- 75.Gonda I., Bar E., Portnoy V., Lev S., Burger J., Schaffer A.A. Branched-chain and aromatic amino acid catabolism into aroma volatiles in Cucumis melo L. Fruit. J. Exp. Bot. 2010;61(4):1111–1123. doi: 10.1093/jxb/erp390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shetty K. Role of proline-linked pentose phosphate pathway in biosynthesis of plant phenolics for functional food and environmental applications: a review. Process Biochem. 2004;39(7):789–804. [Google Scholar]
- 77.Al-Jibouri A.M.J., Abed A.S., Ali A.J.A., Majeed D.M. Improvement of phenols production by amino acids in callus cultures of Verbascum thapsus L. Am. J. Plant Sci. 2016;7(1):84–91. [Google Scholar]
- 78.Hayat S., Hayat Q., Alyemeni M.N., Wani A.S., Pichtel J., Ahmad A. Role of proline under changing environments: a review. Plant Signal. Behav. 2012;7(11):1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szabados L., Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15(2):89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Mohammadipour N., Souri M.K. Effects of different levels of glycine in the nutrient solution on the growth, nutrient composition, and antioxidant activity of coriander (Coriandrum sativum L.) Acta Agrobot. 2019;72(1) [Google Scholar]
- 81.Souri M.K., Hatamian M. Aminochelates in plant nutrition: a review. J. Plant Nutr. 2019;42(1):67–78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.