Abstract
Purpose
To describe clinical features of the youngest patient with well-documented HLA-A29-positive birdshot chorioretinopathy (BCR).
Observations
A 17-year-old female presented with poor night vision and floaters. Examination revealed bilateral vitritis, retinal vasculitis, and numerous cream-colored ovoid lesions in the fundus. Fluorescein angiography revealed bilateral optic disc leakage, large vessel leakage and diffuse capillary ferning. There were hundreds of small hypocyanescent spots evenly distributed in the posterior pole of both eyes on indocyanine green angiography. Workup was positive for HLA-A29.2. Systemic immunosuppression with adalimumab 40mg/0.4mL was initiated every two weeks and escalated to weekly dosing. The patient's early age of disease onset prompted evaluation of her parents. The mother's exam was normal and she was HLA-A29 negative. Examination of the father revealed peripapillary choroidal lesions as well as hypocyanescent spots on ICG. HLA-typing revealed the presence of HLA-A29.2.
Conclusions and Importance
BCR rarely occurs in the pediatric population. We present the youngest patient with well-documented BCR in the literature to highlight that this disease deserves consideration even in young patients. Interestingly, choroidal lesions were also found in an asymptomatic parent with HLA-A29.2 positivity.
Keywords: Birdshot chorioretinopathy, Adalimumab, HLA-A29, Indocyanine green angiography, Fluorescein angiography
1. Introduction
Birdshot chorioretinopathy (BCR) is an uncommon form of bilateral chronic posterior uveitis. The prevalence ranges from 0.6 to 2.8% in tertiary referral settings1,2 and typically affects Caucasian women in their third to sixth decade of life.1 BCR is rarely seen in the pediatric population and, to date, all previously reported pediatric cases lack supporting exam findings and ancillary testing results.3,4,5 We describe the youngest patient with BCR confirmed by examination, fluorescein angiography (FA), indocyanine green angiography (ICGA), and HLA typing.
Because of the patient's early age of presentation, the parents were examined for subclinical disease and underwent HLA typing to see if homozygosity played a role in disease onset. This case will also be the first to describe findings in asymptomatic family members.
1.1. Case report
A 17-year-old healthy Caucasian female presented with difficulty seeing at night for three months and intermittent floaters. She was initially evaluated by an optometrist and then a neuro-ophthalmologist and was diagnosed with idiopathic intracranial hypertension (IIH) based on bilateral optic disc edema, a negative MRI brain, normal cerebrospinal fluid studies, and a mildly elevated opening pressure of 31 mmHg on lumbar puncture17 obtained while the patient was agitated and not sedated. Review of systems was positive for headaches and negative for diplopia, pulsatile tinnitus, transient or positional vision loss. The neuro-ophthalmologist also noted retinal lesions on exam, which prompted a retina and then a uveitis consultation.
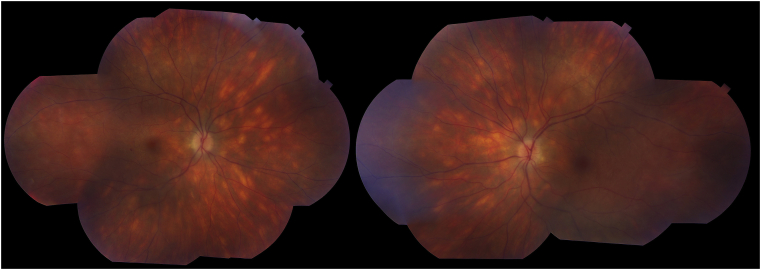
On presentation to the uveitis specialist, best-corrected visual acuity was 20/20 in the right eye and 20/25 in the left eye, pupils were symmetric and there was no relative afferent pupillary defect. Slit-lamp examination demonstrated absence of cells in the anterior chamber, 2+ anterior vitreous cells and trace haze in both eyes. Fundus examination revealed bilateral optic disc edema, vascular sheathing in all quadrants, and numerous and uniform cream-colored lesions in the parapapillary region extending to the mid-periphery (Fig. 1).
Fig. 1.
Fundus montage of the right and left eyes showing multiple, symmetric, evenly distributed yellow ovoid choroidal lesions in the peripapillary area extending to the periphery, appearing to follow the choroidal vasculature. There is also optic disc edema and subtle venous sheathing in all four quadrants. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
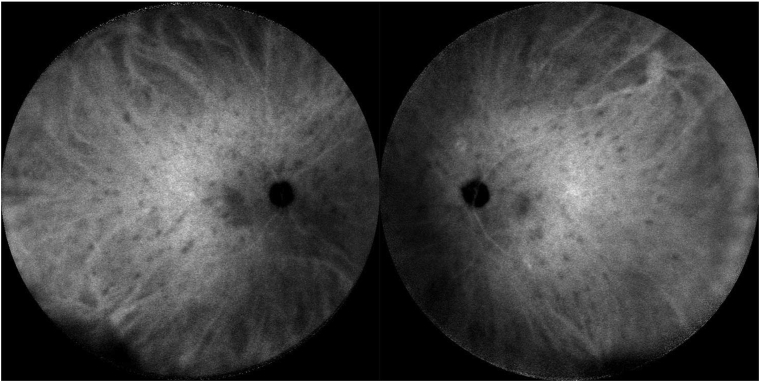
Enhanced depth imaging optical coherence tomography (EDI-OCT) revealed no intraretinal or subretinal fluid in either eye; there was a thickened choroid in the left eye greater than the right. FA showed bilateral optic disc leakage, large vessel leakage, angiographic macular edema, and diffuse capillary ferning consistent with widespread vasculitis (Fig. 2). ICGA was notable for hundreds of small symmetric hypocyanescent spots evenly distributed throughout the fundus of both eyes (Fig. 3).
Fig. 2.
Late-phase fluorescein angiography of both fundi demonstrate leakage of the optic discs, angiographic macular edema, large vessel leakage, and capillary ferning.
Fig. 3.
Indocyanine green angiography of both fundi showing numerous evenly distributed and symmetric hypocyanescent spots.
Serologic studies were negative or normal for QuantiFERON gold, Treponema Pallidum IgG, anti-nuclear cytoplasmic antibodies, and angiotensin converting enzyme. The chest x-ray was normal. HLA typing and subtyping demonstrated the presence of HLA-A29.2.
Based on the history of poor night vision and floaters, clinical exam findings of bilateral low-grade vitritis, vasculitis, numerous symmetric cream-colored ovoid lesions that were hypocyanescent and evenly distributed on ICGA, a negative workup for other causes of posterior uveitis, and HLA-A29.2 positivity, the patient was diagnosed with BCR.
Therapy with adalimumab (ADA) 40mg/0.4mL (Humira, AbbVie, Chicago, IL, USA) every two weeks was initiated. Oral prednisone was avoided because of the pre-existing, but questionable diagnosis of IIH. When the disc edema improved on ADA, the diagnosis of IIH was dismissed and the nerve edema was attributed to BCR. No follow-up lumbar puncture was performed. After 6 months of ADA, repeat FA demonstrated improved large vessel leakage and disc leakage. However, there was still persistent small vessel leakage in the posterior pole bilaterally, and ADA was escalated to a weekly regimen.
The asymptomatic parents had complete ophthalmic examinations. The father's funduscopic exam revealed bilateral peripapillary choroidal lesions. This prompted further workup with FA and ICG imaging. The FA was normal and the late phase ICG demonstrated many small symmetric hypocyanescent choroidal lesions (Fig. 4). HLA typing revealed HLA-A29.2 positivity. Because there was no evidence of inflammation clinically and on FA, the father was not treated. The mother had a normal exam and was negative for HLA-A29.
Fig. 4.
Indocyanine green angiography of the patient's father showing numerous evenly distributed and symmetric hypocyanescent spots throughout the fundus of both eyes.
2. Discussion
This case describes the youngest well-documented patient with BCR and the presence of subclinical disease in a parent.
Birdshot chorioretinopathy is diagnosed clinically based on the following features: bilateral disease, low grade or absent anterior segment inflammation, low grade to moderate vitreous inflammation, and yellow-white choroidal spots known as “birdshot lesions” clustered around the optic nerve and radiating towards the periphery.6 These characteristic ovoid lesions can oftentimes mimic infectious entities such as syphilis and tuberculosis as well as noninfectious entities such as sarcoidosis.7
The typical BCR patient is a Caucasian female in the third to sixth decade of life. In a systematic review of over 500 patients, the mean age of presentation was 53 years.1 Children and adolescents are rarely affected. On literature review, there have been at least seven prior pediatric cases, but these cases have not been well described, lacking exam findings to support the clinical diagnosis. The youngest was a 6-year-old who was briefly mentioned in a systematic review,1 five patients less than 16 years of age were reported in an epidemiologic review of pediatric uveitides in Rome,3 and one 15-year-old was reported in a study of 58 patients (age range 15–70) who underwent HLA-A29 subtyping to assess association of subtype A29.1 or A29.2 with disease.4
HLA-A29 has the strongest genetic association of any uveitic condition,18,19 conferring a relative risk as high as 224.35,8 with subtype 2 present in an estimated 80% of BCR patients.6 Familial cases have been reported among siblings (mean age 47.25, range 31–65 years),9,10 monozygotic twins (age 49 and 64 years),11 and a multigenerational family (age 25 and 44).10 Only the multigenerational family underwent high-resolution DNA typing, and all were heterozygous for the HLA-A29 allele. Both parents of this patient underwent HLA testing to determine if she was homozygous or heterozygous for A29.2. Homozygosity for other HLA types has been associated with earlier onset disease such as HLA-Cw6*0602 for psoriasis12 and HLA-DQA1B1 for celiac disease.13 However, this hypothesis was not supported in our case when the mother tested negative for HLA-A29.
ADA is the only FDA-approved systemic medication for the treatment of non-infectious intermediate, posterior, and panuveitis. The standard dosing (20-40 mg every 14 days) has been effective in controlling ocular inflammation in other pediatric posterior/panuveitides such as Behçet's disease,14 Blau syndrome,15 and Vogt-Koyanagi-Harada disease.16 However, our patient continued to have persistent small vessel leakage in the posterior pole of both eyes, necessitating dose escalation, an off-label regimen, which has been shown to be effective in more than 50% of patients who failed every two week dosing.20
3. Conclusion
This patient represents the youngest case of well-documented BCR in the literature. Although rare, exclusion of the diagnosis of BCR because of age would delay diagnosis and therefore increase risk of long-term vision impairment. Subclinical disease may be present in asymptomatic family members with HLA-A29 positivity. Homozygosity for HLA-A29.2 was not evident in our patient, which suggests that there are other unidentified factors that may have resulted in this very early disease presentation.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures: JL, WMS.
DAG has served as a consultant for Abbvie.
Acknowledgements
None.
References
- 1.Shah K.H., Levinson R.D., Yu F. Birdshot chorioretinopathy. Surv Ophthalmol. 2005;50(6):519–541. doi: 10.1016/j.survophthal.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Grumet P., Kerever S., Gilbert T. Clinical and etiologic characteristics of de novo uveitis in patients aged 60 years and above: experience of a French tertiary center. Graefe’s Arch Clin Exp Ophthalmol. 2019;257(9):1971–1979. doi: 10.1007/s00417-019-04411-1. [DOI] [PubMed] [Google Scholar]
- 3.Pivetti-Pezzi P. Uveitis in children. Eur J Ophthalmol. 1996;6(3):293–298. doi: 10.1177/112067219600600313. [DOI] [PubMed] [Google Scholar]
- 4.LeHoang P., Ozdemir N., Benhamou A. HLA-A29.2 subtype associated with birdshot retinochoroidopathy. Am J Ophthalmol. 1992;113(1):33–35. doi: 10.1016/s0002-9394(14)75749-6. [DOI] [PubMed] [Google Scholar]
- 5.Comander J., Loewenstein J., Sobrin L. Diagnostic testing and disease monitoring in birdshot chorioretinopathy. Semin Ophthalmol. 2011;26(4-5):329–336. doi: 10.3109/08820538.2011.588661. [DOI] [PubMed] [Google Scholar]
- 6.Levinson R.D., Brezin A., Rothova A., Accorinti M., Holland G.N. Research criteria for the diagnosis of birdshot chorioretinopathy: results of an international consensus conference. Am J Ophthalmol. 2006;141(1):185–187. doi: 10.1016/j.ajo.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Sullu Y., Yildiran A., Sullu Y., Helek D., Ozkaya O. Sarcoid uveitis simulating birdshot chorioretinopathy in a child. Retin Cases Brief Rep. 2012;6(1):7–10. doi: 10.1097/ICB.0b013e3181f7455d. [DOI] [PubMed] [Google Scholar]
- 8.Baarsma G.S., Priem H.A., Kijlstra A. Association of birdshot retinochoroidopathy and HLA-A29 antigen. Curr Eye Res. 1990;9(Suppl):63–68. doi: 10.3109/02713689008999422. [DOI] [PubMed] [Google Scholar]
- 9.Trinh L., Bodaghi B., Fardeau C. Clinical features, treatment methods, and evolution of birdshot chorioretinopathy in 5 different families. Am J Ophthalmol. 2009;147(6):1042–1047. doi: 10.1016/j.ajo.2008.12.035. 1047 e1041. [DOI] [PubMed] [Google Scholar]
- 10.Tsui E., Takhar J.S., Joye A., Ahmad T.R., Acharya N.R., Gonzales J.A. High resolution DNA typing of human leukocyte antigen A29 in familial birdshot chorioretinopathy. Ocul Immunol Inflamm. 2019:1–4. doi: 10.1080/09273948.2019.1682173. [DOI] [PubMed] [Google Scholar]
- 11.Fich M., Rosenberg T. Birdshot retinochoroidopathy in monozygotic twins. Acta Ophthalmol. 1992;70(5):693–697. doi: 10.1111/j.1755-3768.1992.tb02155.x. [DOI] [PubMed] [Google Scholar]
- 12.Gudjonsson J.E., Karason A., Antonsdottir A. Psoriasis patients who are homozygous for the HLA-Cw*0602 allele have a 2.5-fold increased risk of developing psoriasis compared with Cw6 heterozygotes. Br J Dermatol. 2003;148(2):233–235. doi: 10.1046/j.1365-2133.2003.05115.x. [DOI] [PubMed] [Google Scholar]
- 13.Zubillaga P., Vidales M.C., Zubillaga I., Ormaechea V., Garcia-Urkia N., Vitoria J.C. HLA-DQA1 and HLA-DQB1 genetic markers and clinical presentation in celiac disease. J Pediatr Gastroenterol Nutr. 2002;34(5):548–554. doi: 10.1097/00005176-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Hiyama T., Harada Y., Doi T., Kiuchi Y. Early administration of adalimumab for paediatric uveitis due to Behcet's disease. Pediatric Rheumatol Online J. 2019;17(1):29. doi: 10.1186/s12969-019-0333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naik A.U., Annamalai R., Biswas J. Uveitis in sporadic Blau syndrome: long-term follow-up of a refractory case treated successfully with adalimumab. Indian J Ophthalmol. 2018;66(10):1483–1485. doi: 10.4103/ijo.IJO_629_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su E., Oza V.S., Latkany P. A case of recalcitrant pediatric Vogt-Koyanagi-Harada disease successfully controlled with adalimumab. J Formos Med Assoc. 2019;118(5):945–950. doi: 10.1016/j.jfma.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Avery R.A. Reference range of cerebrospinal fluid opening pressure in children: historical overview and current data. Neuropediatrics. 2014;45(4):206–211. doi: 10.1055/s-0034-1376202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbort C.P., Jr., Pavesio C., LeHoang P. Why birdshot retinochoroiditis should rather be called 'HLA-A29 uveitis'? British J Ophthalmol. 2017;101(7):851–855. doi: 10.1136/bjophthalmol-2016-309764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wender J.D., Fu A.D., Jumper J.M., McDonald H.R., Johnson R.N., Cunningham E.T. False negative antibody-based HLA-A29 typing in two patients with birdshot chorioretinopathy. British J Ophthalmol. 2008;92(8):1153–1154. doi: 10.1136/bjo.2007.125666. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Koreishi AF, Zumpf KB, Minkus CL, Goldstein DA. Success of Weekly Adalimumab in Refractory Ocular Inflammatory Disease [published online ahead of print April 2020]. Ophthalmology. [DOI] [PubMed]