Abstract
Background
Heart failure is a common medical problem in the world, which has a high prevalence in both developed and developing countries. Today, among the medications used for the heart failure treatment, there are many medications with a positive cardiac contraction effect (positive inotropic such as digital glycosides, adrenergic receptor stimulants, and phosphodiesterase inhibitors), a large number of cardiac diluents (such as Angiotensin-Converting Enzyme (ACE) inhibitor group), and a few other types of drugs whose final effects are still under review. Statins are valuable drugs that are broadly prescribed in hyperlipidemia and cardiovascular patients due to their multiple properties, such as cholesterol reduction, endothelial function improvement, anti-oxidative, anti-inflammatory, neovascularization, and immunomodulatory activities.
Methods
There is evidence that the therapeutic role of statins in HF, due to myocardial hypertrophy, show reduction in cardiomyocyte loss in the apoptosis process, oxidative stress, inflammation, and also the return of neurohormonal imbalance. However, the fact that these drugs have no side-effects has not been confirmed in all studies, as statins prevent the production of particular beneficial and protective factors, such as coenzyme Q10 (CoQ10), while inhibiting the production of specific proteins involved in pathologic mechanisms.
Results
Recently, it has been hypothesized that, despite the positive effects reported, high doses of statins in patients with long-term heart failure lead to progress in heart failure by inhibiting CoQ10 synthesis and intensifying hypertrophy.
Conclusion
Thus, it can be stated that the advantage of using statins depends on factors, such as stroke fraction, and the existence of other standard indications such as atherosclerotic diseases or high Low-Density Lipoprotein-C (LDL-C).
Keywords: Heart failure, cardiovascular diseases, hypertension, cholesterol, enzyme, statin
1. INTRODUCTION
1.1. Cardiovascular Diseases
Cardiovascular diseases are the most prevalent reason for death in most countries across the world, including Iran; and is the most important factor contributing to disability. Now, although the world is witnessing a rapid progress in the diagnosis and treatment of such diseases, more than 30% of patients with heart attacks die, and those who survive, never fully recover; and their lifestyle in the rest of their life continues under the influence of these diseases [1, 2]. Further, these diseases impose massive costs on health systems in societies. Still, cardiovascular diseases are recognized as one of the most preventable noncommunicable diseases of humans [3-5].
1.2. Heart failure
Heart failure is a common medical problem in the world, which has a high prevalence in both developed and developing countries [6, 7]. Approximately, 15 million people in the world and 5.7 million patients with heart failure have been diagnosed in the United States [8, 9]. Furthermore, in Iran, according to statistics issued by the Control Disease Center in 2009, the number of people with heart failure in 18 provinces has been around 3.3 in every 100 population, of which about 2.8 percent were over the age of 50 years and, roughly 0.4% aged between 15 and 45 years. In this report, the average age of mortality has been estimated at 65.7 years and the percentage of years of potential life lost is 1.7 years [10]. Age is deemed an effective factor in the prevalence of heart failure such that the prevalence of heart failure in individuals between 50 and 59 years old is 1%, however, 10% in people above 70 years; the disease frequency doubles after spending 10 years of a person’s life [11, 12].
According to reports, heart failure is the most critical factor in the admittance of patients in the cardiac ward, and about 50% of the patients have to undergo hospitalization in less than six months due to symptom exacerbation. At least one-third of heart failure patients are hospitalized once a year, and this happens to 15 to 20 percent of patients several times a year. The mortality rate in patients with heart failure has been measured to be about 20% after the first year of diagnosis; and over the passage of time, between 10 and 20% of cases die each year. Studies show that the 5-year survival rate of patients, since the onset of symptoms, is less than 50% [13, 14].
2. Pathophysiology of heart failure
The incidence of heart failure is usually associated with underlying cardiovascular diseases. Coronary artery atherosclerosis, high blood pressure and weakened heart muscle in adults, and also cardiomyopathy and valve disease in young people are frequent cardiovascular causes contributing to the disease occurrence. Non-cardiac causes of heart failure include severe anemia, thiamine deficit, and the use of some anti-cancer drugs such as doxorubicin [15-17].
Systolic and diastolic inefficacies are two of the most important mechanisms of this disease. In the Systolic Heart Failure (SHF), the heart has a problem in contractile function; thus, resulting in reduced Stroke Volume (SV) and Cardiac Output (CO), followed by hypotension. These patients have a decrease in Ejection fraction, with an ejection fraction of less than 40% indicating a state of being affected by heart failure. In the Diastolic Heart Failure (DHF), the ventricle wall cannot rest sufficiently; hence, resulting in inadequate ventricular filling during diastole and subsequently decreasing the stroke volume and cardiac output [18-22].
3. Effective Medications on heart failure
Today, among the medications used for the heart failure treatment, there are many medications with a positive cardiac contraction effect (positive inotropic such as digital glycosides, adrenergic receptor stimulants, and phosphodiesterase inhibitors), a large number of cardiac diluents (such as Angiotensin-Converting Enzyme (ACE) inhibitor group), and a few other types of drugs whose final effects are still under review (such as the HMG-CoA reductase enzyme inhibitor group) (Table 1) [23].
Table 1.
Some effective medicines for the treatment of heart failure (HF).
| Drug | Initial Dose | Target Dose* | Side Effects | |
|---|---|---|---|---|
| - | Captopril | 6.25-25 mg TID | 50 mg TID | Hypotension |
| - | Enalapril | 2.5 mg BID | 20 mg BID | SCr/BUN increase |
|
Angiotensin Converting Enzyme Inhibitors |
Lisinopril | 2.5-5 mg daily | 40 mg daily | Hyperkalemia |
| - | Trandolapril | 1 mg daily | 4 mg daily | Cough |
| - | Perindopril | 2 mg daily | 16 mg daily | - |
| - | Bisoprolol | 1.25 mg daily | 10 mg daily | Hypotension |
| Beta Blockers | Carvedilol | 3.125 mg BID | 50 mg BID | First-degree heart block |
| - | Carvedilol CR | 10 mg daily | 80 mg daily | Dizziness |
| - | Metoprolol succinate | 12.5-25 mg daily | 200 mg daily | - |
| - | Candesartan | 4-8 mg daily | 32 mg daily | Hypotension |
| Angiotensin Receptor Blockers | Losartan | 25-50 mg daily | 150 mg daily | SCr/BUN increase |
| - | Valsartan | 20-40 mg BID | 160 mg BID | Hyperkalemia |
| - | Hydralazine | 25-50 mg TID-QID | 300 mg daily | Hypotension Headache |
| Vasodilators | Isosorbide dinitrate(ISD) | 20-30 mg TID-QID | 120 mg daily | Dizziness Asthenia |
| - | Fixed-dose combination | 37.5 mg hydralazine/20 mg ISD TID | 75 mg hydralazine/40 mg ISD TID | Nausea |
| - | Bumetanide | 0.5-1.0 mg daily or | 10 mg daily | Fluid loss |
| Diuretics | Furosemide | 20-40 mg daily or | 600 mg daily | Hyperuricemia |
| - | Torsemide | 10-20 mg daily | 200 mg daily | Cramping/diarrhea |
| - | Ethacrynic acid | 25-50 mg daily | 100 mg BID | Hypotension |
| Positive inotropic | Digoxin | 0.125-0.25 mg daily | 0.25 mg daily | Heart block Arrhythmias Anorexia |
4. Statins
Statins are one of the blood-cholesterol-lowering drugs that reduce the synthesis of cholesterol in the liver by inhibiting the 3-Hydroxy-3-Methylglutaryl Coenzyme (HMG-CoA) reductase enzyme [24]. The enzyme HMG-CoA reductase is involved in the synthesis of cholesterol and catalyzes conversion HMG-CoA to mevalonic acid, a precursor to cholesterol synthesis. Statins increase the expression of LDL receptors at the cell surface, which results in the absorption of LDL into cells. These medications, by increasing the synthesis of HMG-CoA reductase, lead to the preservation and regeneration of cellular VLDL for a short time and temporarily, thereby reducing plasma LDL levels and eventually maintaining the normal state of intracellular cholesterol [24, 25]. Statins can also have a decreasing effect on VLDL by the effect on the secretion of apolipoprotein B [26, 27]. The drugs in the statin family/group all have LDL-lowering effects, which themselves reduce the damage to coronary arteries. However, each has different effects on reducing cholesterol and cardiovascular events [28].
Due to their type of function, these drugs have a vital therapeutic role in cardiovascular atherosclerotic patients and reduce the mortality rate of hypercholesterolemic diseases by about 30% [29]. Statins increase low-density lipoprotein LDL receptors in the liver (Up regulation), thereby lowering the density of blood free cholesterol. Statins reduce cholesterol LDL by 20-60% and triglyceride by 10-40%, with an increase of 5% to 15% in HDL [30-32]. Based on the results of clinical tests, these drugs reduce the risk of coronary artery disease and thus reduce the overall mortality rate of heart patients. Available statistics regarding the matter show their desirable effects on the dramatic reduction, by 42%, in deaths from coronary artery diseases in people with high LDL [33, 34].
Based on the previous studies, there is a direct correlation between taking statins and control of heat shock proteins (HSP or stress proteins) [35]. HSPs are a family of proteins that are produced by cells in response to exposure to stressful conditions; whereas some of them such as HSP90, HSP84, HSP70, HSP27, HSP20, and alpha B crystallin all have been reported as having roles in the cardiovascular diseases [36]. It has been previously reported that HSPs and Heat Shock Factor 1 (HSF1) are mainly the pathogenesis of protection against atherosclerosis [35]. Recently, Forouzanfar et al. (2018) demonstrated that statins upregulated endothelial thrombomodulin by involved NO-dependent dissociation of HSF1 from HSP90, nuclear translocation of HSF1, and activation of specific heat shock elements; whereas statin treatment was associated with a reduction in antibody titers to Hsp-60, -65, and -70. In short, the extensive range of non-lipid-mediated features of statin medicines can be ascribed in part to the modulating effects of these medications on HSPs [37].
Considering the association between Endoplasmic Reticulum (ER) and statins some study are available [38]. ER in eukaryotic cells has numerous vital cellular roles including protein synthesis, protein folding, protein translocation, calcium homoeostasis and lipid biosynthesis [39]. A highly preserved mechanism that results from the cumulation of unfolded or misfolded proteins in the ER is ER stress response [38]. ER stress plays a momentous role in cardiovascular diseases, such as atherosclerosis and chronic heart failure. ER stress has a major function in cardiomyocyte apoptosis during the progression of Heart Failure (HF) in all its stages, with ER stress being increased by hypoxia, oxidative stress, Ang II stimulation and inflammatory factors [38, 39].
In the study conducted by Yuanyuan et al. (2017), it has been proven that statin can effectively suppress the development of AAA, and reduce ER stress, ER stress-associated apoptosis signaling pathways, and inflammatory response [40].
Studies have also shown that one of the beneficial effects of statins in prevention and remedy of cardiac hypertrophy was mediated through their inhibitory effect on TNFα action, via their antioxidant activity (inhibition of NADPH oxidase) and TNFα production (by inhibiting the activation of NF-κB) [41].
5. Statins and beneficial effects in HF
According to Bielecka-Dabrowa A, in patients with dilated cardiomyopathy treated by atorvastatin, the reduction in IL-6 and (N-terminal pro B-NT-pro BNP type brain natriuretic peptide) were observed after 2 months; and, the severity of heart failure in patients was improved 2 and 6 months after starting the treatment according to the classification of The New York Heart Association (NYHA) [42]. A study was conducted aiming to investigate the effect of statins on the mortality rate of individuals with severe ischemic and non-ischemic failure (less than 30% ejection fraction and NYHA IIIB / IV symptoms) among 1153 people. Considering factors such as age, sex, diabetes, the cause of HF, ejection fraction and NYHA class, the use of statins resulted in a 62% reduction in risk of death. Given that this adjustment in essential characteristics such as serum cholesterol levels was not significant, this study considered further efforts to achieve the potential advantages of statins in people with severe heart failure to be necessary [43].
A prospective study scrutinized the relationship between statins and prognosis in patients with ischemic and non-ischemic heart failure and involved with LVEF. Of the 960 patients evaluated throughout 9 years, 532 had ischemic heart failure and received angiotensin inhibitor and β-blockers. During the follow-up, 440 people passed away. Statin therapy was significantly associated with reduced risk of death in both groups of patients with ischemic and non-ischemic origin [44].
The purpose of another study was to review randomized trials by comparing statins with placebo for HF and comparing the effects of different statins. The mortality rate all-cause mortality, mortality rate from cardiovascular disease, hospitalization due to HF exacerbation, were considered drug side effects and changes in LVEF. In this meta-analysis, ten studies with 10192 patients with a follow up of 3 to 47 months randomly undergoing treatment with simvastatin, atorvastatin, and resuvastatin were examined. Although statins did not affect the mortality rate, the hospitalization rate due to the deterioration of HF, declined significantly. Atorvastatin was effective in reducing all-cause mortality rate as well as the hospitalization rate due to HF deterioration. In the treatment by atorvastatin and simvastatin, improvement effects in LVEF were observed; however, these beneficial effects were not observed in patients treated with resuvastatin [45].
In another study, 5011 patients with at least 60 years of age with either systolic IV or II, III NYHA ischemic heart failure, randomly received rosuvastatin 10 mg or placebo each day. In the rosuvastatin consumer group, there was a significant reduction in levels of low-density lipoprotein cholesterol and high-sensitivity C-reactive protein compared to placebo users. There was no difference in cardiovascular mortality rate between the two groups after follow up of 32.8 months. However, the hospitalization rates due to cardiovascular causes in the rosuvastatin user group were 371 lower than placebo users. Also, the rosuvastatin group did not show any excessive effects associated with muscle or other drug side effects [46].
In recent years, a meta-analysis was conducted to compare the effects of lipophilic and hydrophilic statin therapies on cardiac inflammation and function in HF. Factors measured in this study were changes in LVEF, B-type natriuretic peptide (BNP), and inflammation (changes in hsCRP and IL-6). The results of a study of 6214 patients during the follow-up, show better treatment effects of lipophilic statins on cardiac function and inflammation in patients with HF as compared to hydrophilic statins [47].
In a double-blind study of 16 weeks, the crossover impacts of atorvastatin 10 mg/day on the concentration of systemic inflammatory markers in 22 HF patients (including 20 patients with non-ischemic HF) with NYHA II or III class symptoms as well as left ventricular ejection fraction were assessed less than 40%. Treatment with statins reduced the soluble tumor necrosis factor receptor-1 by 8%, the C-reactive protein by 37%, and endothelin-1 by 17%. In patients under treatment, statin has no remarkable impact on other inflammatory markers such as interleukin-6 and brain natriuretic peptides. The overall outcome of this study is that short-term treatment with atorvastatin reduces the levels of different inflammatory markers in HF patients [48].
Considering the use of statins in cardiovascular patients with high risk status, in a study conducted by Preiss et al. (2015) on 14873 high-risk and very high risk patients with various cardiovascular diseases, including acute coronary syndrome (ACS), nonfatal myocardial infarction (MI) treated with statins for reduction LDL-C during the 12 month follow up, the results showed statin therapy leads to significantly reduced in LDL-C in all patients. So that the reduction of LDL-C levels by 1 mmol/L results in a decrease in CVD mortality and in the occurrence of non-fatal myocardial infarction by 20-25% [49].
6. Risks of Statin Use
Although statins, with their distinct functions, have a decreasing impact on LDL cholesterol and subsequently improve the general condition of patients with HF; however, according to some studies, lower levels of cholesterol in those receiving statins ends in undesirable and unpleasant effects for such patients [50, 51].
A study was carried out to assess levels of cholesterol and other lipoproteins in 1134 people with advanced heart failure, characterized by NYHA with symptoms of classes 3 and 4. The results revealed that in patients with lower TC level, factors such as LDL, HDL, Triglycerides (TG), albumin, sodium, LVEF, and cardiac output are significantly lower than others. In this study, it has been shown that, contrary to expectations, the survival rate (the chance to survive) of patients with lower cholesterol level, is lower than that of other patients; it was highlighted that the low cholesterol level is a risk factor for heart failure exacerbation in patients. This study also considers further studies on the potential role of low levels of cholesterol and lipoproteins in the pathophysiology of HF progression to be essential [52].
Lately, a study addressed a low level of High-Density Lipoprotein (HDL) cholesterol as the most critical factor in exacerbation of heart failure. Additionally, this study asserts that levels of HDL, Apolipoprotein (Apo) A-I, triglycerides, TC, and LDL are associated with either onset or worsening of HF. In the research, a rise in HDL cholesterol or ApoA-I is introduced as a desirable factor in the HF treatment [53].
Also, a study was done aiming at examining the relationship between low levels of cholesterol and mortality in 614 patients with non-ischemic systolic heart failure with LVEF less than 40%. The findings showed that in patients with lower serum total cholesterol, hemodynamic symptoms were worse, LVEF levels were low, and symptoms of higher NYHA classes were observed. Among these patients, the risk of untimely death was more notable and the need for transplantation was increased. In this research, Low TC has been named as an essential factor in bringing patients with non-ischemic systolic heart failure close to death [54].
Regarding the beneficial effects of statins, it is reported that that low serum cholesterol is associated with a worse prognosis in HF and that Ubiquinone levels are significantly decreased after statin consumption [55].
Ubiquinone or Ubidecarenone, also known as the coenzyme, CoQ10, in addition to having antioxidative activities, is an essential factor in the transport of mitochondrial electrons and energy supply of myocardium. Some studies have indicated that low concentrations of this coenzyme are associated with the mortality rate of HF patients [56, 57].
However, Deichmann et al. (2010) have demonstrated that although statins inhibit one of the main stages in coenzyme Q10 synthesis and subsequently reduced in serum and muscle tissue coenzyme Q10 levels, a trial of 200 mg of coenzyme Q10 daily should be considered for these patients [58].
In a study, the association between plasma rate and survival in people with chronic HF has been addressed. In this study, it was found that low levels of this coenzyme and plasma Total Cholesterol (TC) in people with CHF may be associated with high mortality rates. The study concludes that CoQ10 deficiency may act as a damaging factor in the long-term prognosis of people with CHF [59].
Another study suggests that statins, along with inhibition of the HMG-CoA reductase enzyme, also block the pathway leading to the production of CoQ10. CoQ10 plays a role in energy transfer in skeletal muscle. In this study, it has been assumed that a decrease in the concentration of this coenzyme causes myopathy due to the use of statins [60].
Extensive studies have been conducted on the antioxidative and anti-inflammatory effects of CoQ10 in different communities to determine their role in controlling and managing heart disease. Statin-Associated Muscle Symptoms (SAMS) emerge following the reduction of this coenzyme when using statins. The Q-SYMBIO test showed that CoQ10 supplementation in patients with heart failure not only improves the ability to function but also significantly decreases cardiovascular events and mortality [61].
Conclusion
Statins are valuable drugs that are broadly prescribed in hyperlipidemia and cardiovascular patients due to their multiple properties, such as cholesterol reduction, endothelial function improvement, anti-oxidative, anti-inflammatory, neovascularization, and immunomodulatory activities. The effects of statins include improving or restoringendothelial function, enhancing the stability of atherosclerotic plaques, normalization of sympathetic outflow, antiproliferative and prevention of platelet aggregation are other functional properties of statins.
There is evidence that the therapeutic role of statins in HF, due to myocardial hypertrophy, show reduction in cardiomyocyte loss in the apoptosis process, oxidative stress, inflammation, and also the return of neurohormonal imbalance. However, the fact that these drugs have no side-effects has not been confirmed in all studies, as statins prevent the production of particular beneficial and protective factors, such as CoQ10, while inhibiting the production of specific proteins involved in pathologic mechanisms. Recently, it has been hypothesized that, despite the positive effects reported, high doses of statins in patients with long-term heart failure lead to progress in heart failure by inhibiting CoQ10 synthesis and intensifying hypertrophy as shown in Table 2 (Fig. 1). Thus, it can be stated that the advantage of using statins depends on factors such as stroke fraction, and the existence of other standard indications such as atherosclerotic diseases or high LDL-C.
Table 2.
Clinical dose and pharmacokinetic effects of some statins drugs.
| Drug |
Daily Dose
(mg) |
Absorption
(%) |
Protein Binding
(%) |
Half Life
(Hour) |
Solubility |
Median
Decrease “LDL” (%) |
Median
Decrease “TG” (%) |
Median Increase “HDL”
(%) |
|---|---|---|---|---|---|---|---|---|
| Lovastatin | 20-80 | 31 | 95 | 2-3 | Lipophilic | 20-40 | 10-19 | 7-10 |
| Simvastatin | 5-80 | 60-85 | 98 | 2-3 | Lipophilic | 28-45 | 4-19 | 5-12 |
| Pravastatin | 10-40 | 35 | 40-50 | 1-3 | Hydrophilic | 20-40 | 9-15 | 12-16 |
| Fluvastatin | 20-80 | 98 | 99 | 0.5-1 | Hydrophilic | 22-24 | 7-12 | 2-4 |
| Atorvastatin | 10-80 | - | 98 | 13-15 | Lipophilic | 30-60 | 26-45 | 5-15 |
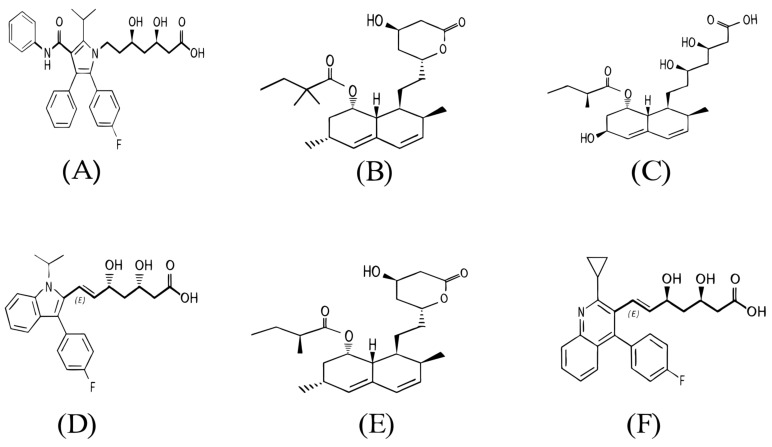
Fig. (1).
Chemical structure of some main statin drugs. A: Atorvastatin; B: Simvastatin; C: Pravastatin; D: Fluvastatin; E: Lovastatin; F: Pitavastatin.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Collins D.R.J., Tompson A.C., Onakpoya I.J., Roberts N., Ward A.M., Heneghan C.J. Global cardiovascular risk assessment in the primary prevention of cardiovascular disease in adults: Systematic review of systematic reviews. BMJ Open. 2017;7(3):e013650. doi: 10.1136/bmjopen-2016-013650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Divya A. A Short review on cardiovascular diseases. Res Rev: J Pharm Toxicol Stud. 2016;4(3):165–170. [Google Scholar]
- 3.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 4.2016.
- 5.Roger V.L., Go A.S., Lloyd-Jones D.M., et al. Executive summary: heart disease and stroke statistics--2012 update: A report from the American Heart Association. Circulation. 2012;125(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 6.Inamdar A.A., Inamdar A.C. Heart failure: Diagnosis, management and utilization. J. Clin. Med. 2016;5(7):62. doi: 10.3390/jcm5070062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dassanayaka S., Jones S.P. Recent developments in heart failure. Circ. Res. 2015;117(7):e58–e63. doi: 10.1161/CIRCRESAHA.115.305765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huffman M.D., Berry J.D., Ning H., et al. Lifetime risk for heart failure among white and black Americans: Cardiovascular lifetime risk pooling project. J. Am. Coll. Cardiol. 2013;61(14):1510–1517. doi: 10.1016/j.jacc.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 10.Rogers C., Bush N. Heart failure: Pathophysiology, diagnosis, medical treatment guidelines, and nursing management. Nurs. Clin. North Am. 2015;50(4):787–799. doi: 10.1016/j.cnur.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Jody RT, Alexander MC. Women with heart failure are at high psychosocial risk: A systematic review of how sex and gender influence heart failure self-care cardiology research and practice. 2011. [DOI] [PMC free article] [PubMed]
- 12.Clark A.M., Freydberg C.N., McAlister F.A., Tsuyuki R.T., Armstrong P.W., Strain L.A. Patient and informal caregivers’ knowledge of heart failure: Necessary but insufficient for effective self-care. Eur. J. Heart Fail. 2009;11(6):617–621. doi: 10.1093/eurjhf/hfp058. [DOI] [PubMed] [Google Scholar]
- 13.Thomas J. Thom the heart Hurst’s volume. 19th ed. MG Graw-hill; 1998. pp. 13–14. [Google Scholar]
- 14.Bello D., Shah N.B., Edep M.E., Tateo I.M., Massie B.M. Self-reported differences between cardiologists and heart failure specialists in the management of chronic heart failure. Am. Heart J. 1999;138(1):100–107. doi: 10.1016/s0002-8703(99)70253-x. [DOI] [PubMed] [Google Scholar]
- 15.Nasri H., Behradmanesh S., Maghsoudi A.R., Ahmadi A., Nasri P., Rafieian-Kopaei M. Efficacy of supplementary vitamin D on improvement of glycemic parameters in patients with type 2 diabetes mellitus; a randomized double blind clinical trial. J. Renal Inj. Prev. 2013;3(1):31–34. doi: 10.12861/jrip.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohamadnejad M., Al-Haddad M.A., Moayyed K.A., Eloubeidi M.A. Biliary fascioliasis diagnosed by EUS. Gastrointest. Endosc. 2016;83(3):658–659. doi: 10.1016/j.gie.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Denham N.C., Pearman C.M., Caldwell J.L., et al. calcium in the pathophysiology of atrial fibrillation and heart failure. Front. Physiol. 2018;9:1380. doi: 10.3389/fphys.2018.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onwuanyi A., Taylor M. Acute decompensated heart failure: Pathophysiology and treatment. Am. J. Cardiol. 2007;99(6B) Suppl. 2:25D–30D. doi: 10.1016/j.amjcard.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Velli-peka H. A review on behalf of the acute heart failure committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Review Eur J Heart Fail. 2017;19:821–836. doi: 10.1002/ejhf.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borlaug B.A. The pathophysiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2014;11(9):507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 21.Lee D.S., Gona P., Vasan R.S., et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: Insights from the Framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119(24):3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmadi A., Mobasheri M., Soori H. Prevalence of major coronary heart disease risk factors in Iran. Int J Epidemiol Res. 2014;1(1):3–6. [Google Scholar]
- 23.Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J. Lipid Res. 1980;21(5):505–517. [PubMed] [Google Scholar]
- 24.Brown M.S., Goldstein J.L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J. Lipid Res. 1980;21(5):505–517. [PubMed] [Google Scholar]
- 25.Kelly J.P., Dunning A., Schulte P.J., et al. Statins and exercise training response in heart failure patients: Insights from HF-action. JACC Heart Fail. 2016;4(8):617–624. doi: 10.1016/j.jchf.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor F., Huffman M.D., Macedo A.F., et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013;2013(1):CD004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonsu K.O., Kadirvelu A., Reidpath D.D. Statins in heart failure: Do we need another trial? Vasc. Health Risk Manag. 2013;9:303–319. doi: 10.2147/VHRM.S44499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang S.Y., Li H., Tang J.J., et al. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nat. Commun. 2018;9(1):5138. doi: 10.1038/s41467-018-07590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delahoy P.J., Magliano D.J., Webb K., Grobler M., Liew D. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: An updated meta-analysis. Clin. Ther. 2009;31(2):236–244. doi: 10.1016/j.clinthera.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Kazi D.S., Penko J.M., Bibbins-Domingo K. Statins for primary prevention of cardiovascular disease: Review of evidence and recommendations for clinical practice. Med. Clin. North Am. 2017;101(4):689–699. doi: 10.1016/j.mcna.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Mahmoudvand H., Sepahvand A., Khatami M., Moayyedkazemi A. Prevalence and associated risk factors of Cystoisospora belli and Cyclospora cayetanensis infection among Iranian patients with colorectal cancer. J. Parasit. Dis. 2019;43(3):402–405. doi: 10.1007/s12639-019-01104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odden M.C., Pletcher M.J., Coxson P.G., et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann. Intern. Med. 2015;162(8):533–541. doi: 10.7326/M14-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logue J. AL-Ghibiwi H, Alamri AA, Preiss D. Systematic review of studies exploring reasons for statin non-adherence and of randomized controlled trials of interventions to improve adherence. Atherosclerosis. 2015;241(1):e52. doi: 10.1016/j.atherosclerosis.2015.04.185. [DOI] [Google Scholar]
- 34.Preiss D., Campbell R.T., Murray H.M., et al. The effect of statin therapy on heart failure events: A collaborative meta-analysis of unpublished data from major randomized trials. 2015. [DOI] [PMC free article] [PubMed]
- 35.Zilaee M., Ferns G.A., Ghayour-Mobarhan M. Heat shock proteins and cardiovascular disease. Adv. Clin. Chem. 2014;64:73–115. doi: 10.1016/B978-0-12-800263-6.00002-1. [DOI] [PubMed] [Google Scholar]
- 36.Willis M.S., Patterson C. Hold me tight: Role of the heat shock protein family of chaperones in cardiac disease. Circulation. 2010;122(17):1740–1751. doi: 10.1161/CIRCULATIONAHA.110.942250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forouzanfar F., Butler A.E., Banach M., Barreto G.E., Sahbekar A. Modulation of heat shock proteins by statins. Pharmacol. Res. 2018;134:134–144. doi: 10.1016/j.phrs.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Qin Y., Wang Y., Liu O., et al. tauroursodeoxycholic acid attenuates angiotensin ii induced abdominal aortic aneurysm formation in apolipoprotein e-deficient mice by inhibiting endoplasmic reticulum stress. Eur. J. Vasc. Endovasc. Surg. 2017;53(3):337–345. doi: 10.1016/j.ejvs.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Wang S., Kaufman R.J. The impact of the unfolded protein response on human disease. J. Cell Biol. 2012;197(7):857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y., Lu G., Sun D., Zuo H., Wang D.W., Yan J. Inhibition of endoplasmic reticulum stress signaling pathway: A new mechanism of statins to suppress the development of abdominal aortic aneurysm. PLoS One. 2017;12(4):e0174821. doi: 10.1371/journal.pone.0174821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mollazadeh H., Atkin S.L., Butler A.E., Ruscica M., Sirtori C.R., Sahebkar A. The effect of statin therapy on endoplasmic reticulum stress. Pharmacol. Res. 2018;137(November):150–158. doi: 10.1016/j.phrs.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Bielecka-Dabrowa A., Goch J.H., Mikhailidis D.P., Rysz J., Maciejewski M., Banach M. the influence of atorvastation on parameters of inflammation and function of the left ventricle in patients with dilated cardiomyopathy. Med. Sci. Monit. 2009;15(12):12. [PubMed] [Google Scholar]
- 43.Mozaffarian D., Nye R., Levy W.C. Statin therapy is associated with lower mortality among patients with severe heart failure. Am. J. Cardiol. 2004;93(9):1124–1129. doi: 10.1016/j.amjcard.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 44.Gastelurrutia P., Lupón J., de Antonio M., et al. Statins in heart failure: The paradox between large randomized clinical trials and real life. Mayo Clin. Proc. 2012;87(6):555–560. doi: 10.1016/j.mayocp.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipinski M.J., Cauthen C.A., Biondi-Zoccai G.G., et al. Meta-analysis of randomized controlled trials of statins versus placebo in patients with heart failure. Am. J. Cardiol. 2009;104(12):1708–1716. doi: 10.1016/j.amjcard.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 46.Kjekshus J., Apetrei E., Barrios V., et al. Rosuvastatin in older patients with systolic heart failure. N. Engl. J. Med. 2007;357(22):2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 47.Bonsu K.O., Reidpath D.D., Kadirvelu A. effects of statin treatment on inflammation and cardiac function in heart failure: An adjusted indirect comparison meta-analysis of randomized trials. Cardiovasc. Ther. 2015;33(6):338–346. doi: 10.1111/1755-5922.12150. [DOI] [PubMed] [Google Scholar]
- 48.Mozaffarian D., Minami E., Letterer R.A., Lawler R.L., McDonald G.B., Levy W.C. The effects of atorvastatin (10 mg) on systemic inflammation in heart failure. Am. J. Cardiol. 2005;96(12):1699–1704. doi: 10.1016/j.amjcard.2005.07.092. [DOI] [PubMed] [Google Scholar]
- 49.Dyrbuś K., Osadnik T., Desperak P., Desperak A., Gąsior M., Banach M. Evaluation of dyslipidaemia and the impact of hypolipidemic therapy on prognosis in high and very high risk patients through the Hyperlipidaemia Therapy in tERtiary Cardiological cEnTer (TERCET) Registry. Pharmacol. Res. 2018;132:204–210. doi: 10.1016/j.phrs.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 50.Krum H., McMurray J.J. Statins and chronic heart failure: Do we need a large-scale outcome trial? J. Am. Coll. Cardiol. 2002;39(10):1567–1573. doi: 10.1016/S0735-1097(02)01827-2. [DOI] [PubMed] [Google Scholar]
- 51.Böhm M., Hjalmarson A., Kjekshus J., Laufs U., McMurray J., van Veldhuisen D.J. Heart failure and statins-why do we need a clinical trial? Z. Kardiol. 2005;94(4):223–230. doi: 10.1007/s00392-005-0210-9. [DOI] [PubMed] [Google Scholar]
- 52.Horwich T.B., Hamilton M.A., Maclellan W.R., Fonarow G.C. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J. Card. Fail. 2002;8(4):216–224. doi: 10.1054/jcaf.2002.0804216. [DOI] [PubMed] [Google Scholar]
- 53.Miura S., Saku K. Effects of statin and lipoprotein metabolism in heart failure. J. Cardiol. 2010;55(3):287–290. doi: 10.1016/j.jjcc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Afsarmanesh N., Horwich T.B., Fonarow G.C. Total cholesterol levels and mortality risk in nonischemic systolic heart failure. Am. Heart J. 2006;152(6):1077–1083. doi: 10.1016/j.ahj.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 55.Tousoulis D., Charakida M., Stefanadi E., Siasos G., Latsios G., Stefanadis C. Statins in heart failure. Beyond the lipid lowering effect. Int. J. Cardiol. 2007;115(2):144–150. doi: 10.1016/j.ijcard.2006.03.094. [DOI] [PubMed] [Google Scholar]
- 56.Rosenfeldt F., Hilton D., Pepe S., Krum H. Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. Biofactors. 2003;18(1-4):91–100. doi: 10.1002/biof.5520180211. [DOI] [PubMed] [Google Scholar]
- 57.Okello E., Jiang X., Mohamed S., Zhao Q., Wang T. Combined statin/coenzyme Q10 as adjunctive treatment of chronic heart failure. Med. Hypotheses. 2009;73(3):306–308. doi: 10.1016/j.mehy.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 58.Deichmann R., Lavie C., Andrews S. Coenzyme q10 and statin-induced mitochondrial dysfunction. Ochsner J. 2010;10(1):16–21. [PMC free article] [PubMed] [Google Scholar]
- 59.Molyneux S.L., Florkowski C.M., George P.M., et al. Coenzyme Q10: An independent predictor of mortality in chronic heart failure. J. Am. Coll. Cardiol. 2008;52(18):1435–1441. doi: 10.1016/j.jacc.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 60.Coenzyme Q. Coenzyme Q10 and statin-related myopathy. Drug Ther. Bull. 2015;53(5):54–56. doi: 10.1136/dtb.2015.5.0325. [DOI] [PubMed] [Google Scholar]
- 61.Ayers J., Cook J., Koenig R.A., Sisson E.M., Dixon D.L. Recent developments in the role of coenzyme Q10 for coronary heart disease: A systematic review. Curr. Atheroscler. Rep. 2018;20(6):29. doi: 10.1007/s11883-018-0730-1. [DOI] [PubMed] [Google Scholar]