Abstract
Background
Over the past few decades, nanotechnology has dramatically advanced; from the precise strategies of synthesizing modern nanostructures to methods of entry into the body. Using nanotechnology in diagnosis, drug delivery, determining signaling pathways, and tissue engineering is great hope for the treatment of stroke. The drug-carrying nanoparticles are a way to increase drug absorption through the mouth or nose in treating the stroke.
Objective
In this article, in addition to explaining pros and cons of oral and intra-nasal administration of nanoparticles in the brain ischemia treatment of animal models, the researchers introduce some articles in this field and briefly mentioned their work outcomes.
Methods
A number of relevant published articles 183 were initially collected from three popular databases including PubMed, Google Scholar, and Scopus. The articles not closely related to the main purpose of the present work were removed from the study process. The present data set finally included 125 published articles.
Results
Direct delivery of the drug to the animal brain through the mouth and nose has more therapeutic effects than systemic delivery of drugs. The strategy of adding drugs to the nanoparticles complex can potentially improve the direct delivery of drugs to the CNS.
Conclusion
Despite the limitations of oral and intra-nasal routes, the therapeutic potential of oral and intra-nasal administration of nano-medicines is high in cerebral ischemia treatment.
Keywords: Nanoparticles, stroke, oral, intra-nasal, treatment, CNS, rat
1. Introduction
Neurological disorders are a major cause of mortality [1]. Among nervous disorders, stroke is one of the main causes of death in adults aged 15 years and older. It is also the fourth cause of disease and death in the world. Therefore, the development of potential therapies for preventing ischemic stroke is essential [2-4].
Various barriers in the body can play a vital role in the accumulation or distribution of nanomaterials. The important biological barriers to body defense systems include endothelial dams, cell dams, skin, mucus barriers and Blood-Brain Barrier (BBB). The brain is the most sensitive and complex organ of the body, protected by a very efficient barrier called BBB. This barrier is well suited in protecting the brain against blood contents and its toxic components but the same barrier restricts the drug entry in the brain [5-7]. In these circumstances, Nanoparticles (NPs) are used for drug delivery to the brain. Due to the small size of these particles, they freely move into the blood vessels and enter the brain tissue. These particles are important and new materials having multiple properties which make them very valuable in order to develop drugs in the field of biomedicine in the past two decades [8-12]. The importance of nanoparticles in medical applications is due to the high chemical and biological stability, possibility to combine with both hydrophilic and hydrophobic materials and drugs, high carrying capacity, and the ability to use various routes such as injection, oral and intranasal delivery [13-21]. After entering the body, nanoparticles are distributed in various parts, such as blood cells, liver, spleen, kidneys, intestines, thymus, heart, lungs, and brain. However, the method of nanoparticle entry to the body directly affects cellular absorption, pharmacodynamic properties, and pharmacokinetics. Researches have gained much useful information relating to entering nanoparticles in the cells and their cellular mechanisms. It should also be mentioned that intracellular mechanisms are more complicated. Different types of mechanisms relating to the entrance of nanoparticles in cells include endocytosis, membrane flows, channels or entry through adhesion reactions. The mechanism of interaction between nanoparticles and living systems has particular complexities because they bind and interact with biological agents. Cellular uptake probably results in some changes in the conformation of proteins, membrane interactions and signaling cascades in cells. The small size of nanoparticles increases their interaction with cells and biomolecules. In some cases, low biocompatibility and high toxicity reduce the potentiality of nanoparticle systems in devoting a medical and clinical confirmation to them. On the other hand, the immune system resists against many nanoparticles carrying drugs and exhibits protective reactions such as platelet activation, inflammation, and antibody production. The consequences of these reactions are hypersensitivity, thrombosis, hemolysis, and eventually drug removal [22-24]. However, when nanoparticles are in biological environments especially in vivo, the process will be complicated and choosing a proper way of entering these particles into the body is of paramount importance.
Unfortunately, many diseases that are treated with nanoparticles are chronic and require frequent, long-term treatment which their intravenous infusion will limit their use. Also, in the intravenous injection method, particle size and toxicity are two limiting factors; for example, as the particles size increases, the intensity of inflammation increases. In this method, coarse nanoparticles are trapped by the spleen and liver macrophages, and smaller particles are eliminated by the renal clearance [25-28]. After intravenous or intraperitoneal use, nanoparticles are often found high in the liver and spleen. Also, they are found less in the lungs, kidneys, brain, and heart [29]. Therefore, alternative methods including oral and intra-nasal ones are taken into consideration [30-32].
Oral delivery is the most commonly used drug delivery because it is a non-invasive way for the patient. However, it is difficult to achieve the therapeutic concentration required for some medications due to its low solubility, short-term sustainability and low levels of oral ingestion. In order to solve these problems, the nanoparticles can be used to protect drugs from degradation in the gastrointestinal tract and deliver them to the appropriate place [33-35]. Examples of these particles are polymeric nanoparticles, solid lipid nanoparticles, and nano-crystals, each of which can optimally increase the amount of absorption and the half-life of drugs and their stability in the gastrointestinal tract. The effects of oral administration of these nanoparticles are not size-dependent and don’t show significant changes compared to intravenous injection in pathophysiological levels [30, 31]. On the other hand, with oral administration, the tissue distribution of particles increases [31]. Oral delivery of curcumin loaded solid lipid (high preferential distribution into the brain) [36], rutin-encapsulated chitosan (anti-oxidant) [37], panax notoginsenoside-loaded liposomal vesicles (anti-oxidant activity) [38], resveratrol loaded solid lipid nanoparticles (reducing oxidative stress) [39], and nanoencapsulated quercetin (downregulation of iNOS and caspase-3 activities) [40] is used for the stroke treatment in animal models.
Nose physiology makes it an ideal target for non-invasive delivery of local and systemic drugs, especially for proteins and low solubility in water drugs, which have low oral bioavailability [41]. Since the BBB does not allow the brain to enter many drugs in the bloodstream, researchers have tried to find ways to bypass this barrier. Direct delivery of drugs into the brain has more therapeutic effects than systemic ones. The nasal duct is the fastest and the most direct route to the brain. Through this route, the drug passes from the BBB via non-invasive manner [32, 42]. Nasal mucosa seems to be a target tissue for drug delivery, with many benefits compared to the oral route due to its simple and easy access, high blood flow, large surface, wet environment, porous endothelium and avoiding the first-pass hepatic metabolism [42, 43]. Thus, the strategy of adding drugs to nanoparticles and carry this complex to the olfactory epithelium can potentially improve the direct delivery of drugs to CNS [43]. Curcumin-loaded nanoparticles and rutin-encapsulated chitosan ones (both with antioxidant activity) [37, 44] improve neurobehavioral activity in this path.
Considering the tendency to treat stroke with the help of nanotechnology and intravenous infusion limits, this review introduces all research articles that have been using the oral and intra-nasal pathways for the stroke treatment considering nano-medicine in the animal researches.
2. Oral administration of nanoparticles in stroke treatment
The use of specific systems in the delivery of oral drugs and their possible absorption through the intestinal mucosa have attracted considerable attention in animal researches [45]. However, evaluating the stability of nano-carriers in the gastrointestinal fluid is necessary to predict their suitability for oral administration [46].
The critical parameters in the design of new and effective drug delivery systems for oral use are: (1) their stability related to the digestive fluid. When they are formed of biodegradable materials and their sizes are large enough, the surface area required for enzymatic activity is provided (particle sizes between 10 and 100 nm are not absorbed through the digestive system) [47, 48]; (2) accumulation of particles due to the specific conditions of the gastrointestinal tract leads to a decrease in the ability of particles to interact with the intestinal mucosa [48]. On the other hand, the adhesion properties of nanoparticles have been reported to increase bioavailability and to reduce or minimize irregular adsorption [48].
2.1. Use of Solid Lipid Nanoparticles (SLNs) in Cerebral Ischemia Treatment
SLNs are colloid drug carrier nanoparticles with high ability to affect the Blood-Brain Barrier (BBB) deeply [49, 50]. They are typically in the range of 1 to 1,000 nm and can be easily absorbed by the brain due to their lipophilic nature. Also, the biological nature of SLNs makes them less toxic than other polymeric nanoparticles [50]. SLN is usually stable for up to 3 years, which has a very important advantage over other colloid carriers [51]. These nanoparticles are often recommended as systems carrying drugs to the CNS [52].
SLN has a significant effect on the delivery of medication through intra-nasal and oral routes. Particular drug encapsulation in SLN can lead to (1) overcoming their lower solubility; (2) protecting the body's chemical and physical processes such as digestive process; (3) releasing drug slowly over the time and; (4) conducting the matter for a particular purpose or passing through various biological barriers [52].
2.1.1. Evaluating the Curcumin (CUR)-SLN in the Experimental Pattern of Cerebral Ischemia after oral and Intravenous Administration
Many oral medications which are transmitted to the systemic circulation via the portal vein are subject to metabolism. In this context, improving the bioavailability and therapeutic efficacy of lipid-soluble drugs is possible by incorporating them into SLN. Positive loaded SLNs made of various triglycerides increased the drug's bioavailability by 1.3 to 4.5 times in intra-duodenal administration [53]. Kakkar et al. (2013) have suggested that SLN coated with Tween 80 acts as a carrier to deliver curcumin to the brain through the oral route in rats. CUR is an anti-oxidant and anti-inflammatory molecule that has various applications in the field of neurological disease. CUR is a yellow chemical substance produced by some plants. This material can destroy plaque in the brain. [54-56]. The region below the curves obtained in the blood after oral administration of CUR loaded solid lipid nanoparticle (CUR-SLN) was eight times more than that of free drugs. However, the concentration of CUR–SLN in the brain was 30 times higher than that of free CUR. The ability of CUR-SLN to reach the brain is essentially related to the small size of the CUR-SLN, that helps bypass the first-pass CUR metabolism in the liver. Nonionic surfactants similar to lecithin and Tween 80 used in SLN structure increase their penetration via intestine due to the high tendency between fatty particles and intestinal membrane [52].
Kakkar et al. (2013) evaluated CUR-SLN neural protection potential in bilateral common carotid artery occlusion (BCCAO) induced global brain ischemia in rats [36]. In this study, oral administration of free solubilized CUR (CUR-S) and CUR-SLN (with an average size of 134.6 ± 15.4 nm) at concentrations of 25 and 50 mg/kg, began 5 days before BCCAO and continued for 3 days after stroke induction. Then, behavioral, biochemical and mitochondrial studies were performed in the groups.
The ratio of an Area under Curves (AUC) after 4 hours of oral administration of CUR-SLN in the rats' blood was 8.135 and 16.4 in the brain. Free CUR is affected by metabolic stimulation, resulting in less than 1% oral bioavailability. On intravenous administration, the AUC of CUR-SLN in the brain was 30.82 times higher than the CUR-S; confirming a prolonged circulation of CUR-SLN in both oral and intravenous methods. Treatment with CUR-SLN has significantly increased body weight and returned body temperature to the normal, while free CUR had no effect on these parameters. Ischemia/reperfusion (I/R) injury resulted in increased neurological deficits and decreased muscle strength in the control group’s onset of BCCAO. Free CUR did not show any effect, while the CUR-SLN significantly improved all activities. CUR-SLN-treated animals showed a significant improvement in memory stabilization (there was an improvement of 90% in the cognition of CUR-SLN users) compared to I/R injury and CUR-treated rats. I/R damage caused severe oxidative injury in the brain. Treatment with CUR showed no significant effect on oxidative stress parameters, while CUR-SLN significantly reduced these parameters to the control value. Additionally, I/R damage played a significant role in shuffling the activity of mitochondrial complex enzymes in the rat brain. CUR did not show any effect on I/R damage, while the CUR-SLN significantly improved their performance [36]. In the present study, improved drug levels in the brain may be related to the small size of CUR-SLN that bypass the primary metabolism of curcumin in the liver. Therefore, CUR in the solid-state fatty matrix of SLNs not only protects against digestive enzyme degradation but also achieves a long circulation time, and its clearance is reduced after absorption [36].
2.1.2. Reducing the Mitochondrial Oxidative Stress by Oral Administration of Resveratrol Loaded SLN in BCCAO Stroke Model
Resveratrol (RSV) is an antioxidant compound that protects our body against chronic diseases by removing free radicals [57, 58]. RSV is found in blueberry, grape skin, raisins, white currant, red wine, and peanuts [59-61]. Some researches have shown that RSV has the ability to improve blood vessel contraction and improves blood flow while cleaning the walls of the blood vessels, increasing their diameter, decreasing blood pressure, and preventing Vascular Dementia (VaD). The processes increasing the oxygen supply to the body’s cells prevent creating clots in the blood and reduce the risk of cardiovascular disease by decreasing inflammatory factors and LDL levels [62-64]. Neuroprotective effects of RSV have also been proven; for example, it reduces mitochondrial disorders caused by specific stressors such as brain ischemia [61]. In fact, RSV increases the antioxidant defense system of the brain by up-regulation of mitochondria antioxidant enzymes and reduces the pre-inflammatory cytokines to maintain the brain's homeostasis [65-67]. Although the pharmacokinetic properties of RSV are not desirable because of weak solubility, chemical instability, and rapid metabolism, they reduce its bioavailability [68, 69]. Therefore, the arrival of RSV in the brain is limited. To overcome these limitations, nano-carriers have been developed which are able to protect RSV from metabolism and degradation. Actually, nanotechnology helps to prevent and treat neurodegenerative diseases including oxidative stress by antioxidant delivery [70]. Lipid nanoparticles such as SLNs with highly stable formulations are a suitable system for combining with lipophilic compounds such as RSV [71, 72]. Encapsulation of RSV in SLN (RSV-SLN) not only guarantees its targeting to the brain but also overcomes its fast metabolism and low solubility [39].
A group of researchers studied the positive effects of RSV-SLN in BCCAO induced VaD model on oral administration. In their experiment on rats, drug encapsulation efficiency was 91.25%, and the average particle size was 286 nm. Also, RSV-SLN levels in the brain were 4.5 times higher compared with free RSV treated group [39]. However, in another study, intra-peritoneal administration of RSV-SLN into rats resulted in 6 times higher accumulations of RSV in brain tissue as compared with free RSV treatment group [73].
Oral administration of RSV-SLNs successfully improved cognitive impairment (evaluation of spatial learning and memory formation), significantly reduced the generation of mitochondrial Reactive Oxygen Species (ROS), significantly increased production of antioxidant enzymes and superoxide dismutase activity, and significantly reduced hypoxia-inducible factor 1α (HIF-1α) levels, up-regulation Nuclear factor 2 (Nrf2) and Heme Oxygenase 1 (HO-1) levels in the cortex, hippocampus, striatum, and the cerebellum of rats [39]. Nrf2 is vital for the resistance of neural cells to the oxidative stress caused by stroke. Nrf2 activated by binding to the HO-1 gene and some other genes [74]. ROS as a hydroxyl inhibitor also results in stabilization of HIF-1α. Thus, HIF-1α stability was removed by inhibiting of ROS through the RSV.
2.2. Protective Effects of Core-shell Hybrid Liposomal Vesicles Loaded with Panax Notoginsenoside on Global Cerebral I/R Injury after Oral Administration
Panax Notoginseng (PN) derived from the root of the traditional Chinese herb PN, which has activities such as improving blood circulation, reducing pain, and decreasing the plasma lipid and fibrinogen levels in high-fat rats [75-77]. Panax Notoginsenoside (PNS) is the main active ingredient of PN with anti-inflammatory effects, anti-oxidant activities, synthesis of DNA and protein, modulation emotional responses, vasodilator effects and protective effect of lipopolysaccharide-induced micro-circulation disorders [78-80]. However, PNS is absorbed slightly during oral administration. PNS degradation occurs in the gastric acid environment, intestinal metabolism, and liver elimination. PNS is a water-soluble substance with a high molecular weight which leads to low penetration in the membrane and some types of PNSs can aggregate into the micelles. Such accumulation limits the penetration of PNS through the gut cellular membrane [81, 82]. To overcome these limitations, liposomes are widely regarded as useful carriers for the delivery of PNS due to their unique properties such as biodegradability, biocompatibility, size variation and surface charge [83, 84].
Nanoparticle systems are now widely and successfully used to increase the bioavailability of drugs [85, 86]. But, the use of nanoparticles is limited to delivering water-soluble drugs due to low efficacy in drug encapsulation and quick release of the enclosed drugs [16, 85, 87]. To minimize these negative effects, Zhang et al. (2012) reported a new liposomal system encapsulating mPEG-PLGA-based nanoparticles called core-shell Hybrid Liposomal Vesicles (HLV). They loaded PNS into the HLV (PNS-HLV). This hybrid could increase the trapping efficiency and delay the release of all main components of the PNS to increase its protective effects during oral administration in rats [38]. Also, PNS-loaded liposomes (PNS-LP), PNS-NP, and PNS-solution were prepared [38, 88]. Oral administration of PNS-HLV for 10 days prior to the induction of stroke was able to significantly decrease brain edema and reduce the infarct volume in global cerebral ischemia/reperfusion in comparison to LP, PNS-LP, PNS-NP, and PNS-solution. PNS-HLV bioactivity was also greater than others in oral administration in experimental rats [38]. Accordingly, HLV has a promising future to improve drug bioactivity on oral intake.
2.3. The Therapeutic Efficacy of Nanoencapsulated Quercetin Oral Administration in Combating Ischemia-reperfusion in Young and Aged Rats
Quercetin (QC) is a flavonol that can be found in most fruits, vegetables, leaves, and seeds [89]. This compound has antioxidant, anti-inflammatory, anti-cancer and cardiac-protective effects in humans [90, 91]. It is a very powerful anti-oxidant and is usually the main ingredient of red and orange pigments in various fruits and vegetables [92, 93]. The effectiveness of this substance confirms hypertension, stroke, heart attack, atherosclerosis and blood clotting [94-96].
Since it is necessary to introduce exogenous anti-oxidants as drugs and free radical scavengers in order to combat stroke, QC can be a good candidate. But, QC cannot pass through the BBB which is a serious block in CNS therapeutic purposes. Polymeric nanoparticles are proposed to develop a system that can provide such reservoirs of bioflavonoid anti-oxidants in the brain for the full protection of the neurons against the oxidative onslaught [40].
Ghosh et al. (2013) used polylactide-co-glycolide (PLGA) as an effective carrier of the drug due to its various benefits, namely long half-life, high drug load carrying capacity, proper safety, preventing the destruction process, the ability to cross the BBB, and various routes of administration; such as oral path [40, 97, 98]. Their goal was to investigate the neuroprotective effects of PLGA-nanoparticle encapsulated QC against I/R oxidative stress damage in different areas of the brain caused by the stroke on oral administration. In this research, stroke was induced by Middle Cerebral Artery Occlusion (MCAO) in both young and aged rats. Experimental groups were treated with free QC, empty PLGA nanoparticles and nanoparticulated QC (20-50 nm) through oral gavage, two hours before induction of stroke. The rest of the experimental groups were treated with free QC and nanoparticulated QC until the first and third days of post-operation, respectively via oral administration [40].
The results of Ghosh et al.'s work showed that brain ischemia caused a significant increase in lipid peroxidation, a significant decrease in mitochondrial membrane micro-viscosity, a significant increase in ROS production, also a significant decrease in activity of antioxidant enzymes and tissue osmolality in the hypothalamus, cerebral cortex, cerebellum, and hippocampus of both young and aged rats. In the studies about brain regions, hippocampus seems to be the worst affected area showing iNOS up-regulation and increasing caspase-3 activity by decreasing the number of neurons in hippocampus CA1 and CA3 regions of young and old rats. Oral administration of nanoencapsulated QC resulted in down-regulation of iNOS decreased caspase-3 activities, increased brain tissue osmolality, and increased the number of pyramidal neurons from the hippocampal CA1 and CA3 regions even 72 hours after I/R. Free QC treatment did not significantly improve the mitochondrial membrane micro-viscosity, while nanoparticulated QC made full protection of the mitochondrial membrane in young and aged rats’ brain regions [40]. Given the above evidence, the authors suggested that oral treatment with nanoencapsulated QC might play an important protective role against I/R damages [40].
2.4. Oral Treatment of Nanoparticles Containing Puerarin-HP-β-CD Inclusion in the rat MCAO Model for Improving the Penetration and Bioavailability of Puerarin
Puerarin (PUE) is an isoflavone which was found in herbal medicine and widely used in the treatment of ischemic stroke in China. Previous studies have shown strong antioxidant properties, anti-inflammatory, and anti-apoptotic effects of this drug. However, its low concentration in the brain after oral administration limits the PUE therapeutic efficacy. PUE is available with low water and oil soluble, and poor bioavailability, which severely restricts clinical use. Thus, it is necessary to develop appropriate preparation to help it pass through the BBB [99-102]. Recently, attention is given to the role of polymeric nanoparticles as possible brain-targeting drug systems that can increase the concentration and drug release and reduce environmental toxicity [86, 103]. Hydroxypropyl beta cyclodextrin (HP-β-CD) is often used to increase the water solubility of hydrophobic molecules [104, 105]. Cyclodextrins can also be considered as enhancers of BBB permeability [106, 107].
A group of researchers designed a nanoparticle containing PUE–HP-β-CD inclusion complex (100-300 nm) and assessed its oral treatment effectiveness in drug delivery to investigate the improvement of intercellular communication and neurological changes in the rat MACO model. Their results showed PUE-nanoparticles have significant anti-inflammatory and anti-apoptotic effects and enhanced the penetration of drug across the BBB. Also, the average size of infarction was significantly lower in 3 and 7 days after PUE-nanoparticle treatment than I/R control and free PUE groups; H&E (Haemotoxylin and Eosin) staining showed that the brain penetration of inflammatory cells and the neuronal pyknosis, karyolysis were significantly decreased in PUE-nanoparticle treated rats on days 3 and 7. In addition, I/R rats treated with PUE-nanoparticles significantly improved cortical EEG power, peak, area, frequency, and valley value on days 3 and 7 compared to I/R control and free PUE treated groups [108].
According to the above results, oral treatment of PUE-nanoparticle has therapeutic effects on I/R injury and consequently is precious for clinical purposes.
3. Intra-nasal administration of nanoparticles in the stroke treatment
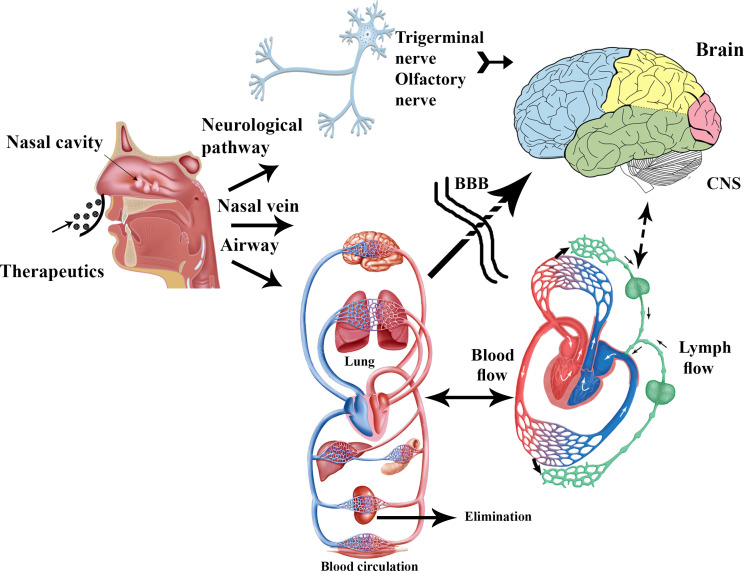
The olfactory neuro-epithelium is the only CNS region that is not protected by the BBB; as a result, it is an exclusive access port for the brain [109]. After nasal administration, the drug can reach the CNS through three main routes: [110] (1) olfactory nerve to the olfactory bulb; (2) trigeminal nerve to the olfactory bulb and brain stem; and (3) the vascular pathway. In the vascular route, the drugs reach into the lungs and then enter into the blood circulation; in this path, drugs must cross the BBB to reach the CNS (Fig. 1) [111, 112].
The potential limitations of nose-to-brain transport include short residence time and irritability mucus reduce transmission by increasing the molecular weight of the drug and access to limited areas of the brain [113]. By considering the large surface area of nose cavity requiring a lower dose of the drug, avoiding the hepatic first-pass metabolism, and high density of vascular blood vessels in the nasal mucosa, the nasal route may be useful for achieving a fast-acting treatment [114].
The nanoparticle-based delivery system has many properties which make them suitable for intra-nasal drug delivery. The properties such as thermodynamic stability increased drug loading, penetration through biological membranes, and bioavailability [115, 116].
3.1. Intra-nasal and Intravenous Delivery of Rutin-encapsulated Chitosan Nanoparticles (RUT-CS-NPs) in the Stroke Treatment
Rutin (RUT) is a flavonoid glycoside that is found in certain herbs and fruits, especially apricots, figs, both black and green tea, plums, cherries, grapes, oranges, and grapefruits [117]. Pharmacological researches have reported positive effects of RUT on many diseases and its therapeutic potential in several diseases including cerebral ischemia and cardiovascular disease. RUT has several properties such as antioxidant, anti-inflammatory, and neuroprotection [117, 118]. In addition, it helps blood circulation and prevents the formation of blood clots [119].
Chitosan (CS) is a biocompatible natural polysaccharide that is often used as a targeted carrier of drugs for the treatment of neurological disorders [120]. CS and its biodegradable products allow them to perform biological functions at the molecular level of the neural cells and BBB permeability, which can use these features in stroke treatments [121]. Some of these features include the high flexibility in surface changes, the ability to bind to different molecules of the ligand, and the creation of stable nano-complexes in physiological conditions [122].
Ahmad et al. (2016) prepared RUT-CS-NPs (92.28 ± 2.96, 199.72 ± 4.48 and 366.85±8.43 nm) via the ionic gelation method for the stroke treatment in rats. RUT-CS-NPs were used for brain-drug delivery as well as monitoring the pharmacokinetics of the drug targeting efficiency and biodistribution after intra-nasal treatment. Their results showed that the particle size has a direct positive relationship with the concentration of CS and pH [37]. This effect may be due to the more accessible binding site of ionic molecules [123]. The first rapid release of the RUT occurred within the first hour (29.95%) and the slow release continued for 24 hours. Nose-to-brain direct transport percentage values of RUT-CS-NPs in comparison to free RUT increased from 29.48 ± 1.05 to 93.00 ± 5.69%, which indicates an increase in brain uptake of RUT-CS-NPs. Similarly, brain targeting efficiency, bioavailability, locomotor activity, and grip strength were significantly improved in intra-nasal administration of RUT-CS-NPs as compared with intravenous administration of brain ischemia animals. Furthermore, significant decreases in infarct size and improvement of behavioral outcomes were observed in the MCAO rat model after intra-nasal administration of RUT-CS-NPs. Overall, RUT-CS-NP is a non-invasive, effective, and safe drug delivery system for the brain ischemia treatment [37].
3.2. Intra-nasal Delivery of PNIPAM Nanoparticles of Curcumin, Demethoxycurcumin, and Bisdemethoxycurcumin on Rats with MCAO
Curcumin (CUR) is a natural polyphenol with antioxidant properties. Studies have shown this substance can repair brain cells and treat nerve disorders [36, 124]. Although the effectiveness of CUR has been tested in a wide range of human diseases but has not been confirmed as a therapeutic agent due to poor bioavailability, low absorption, rapid metabolism, and rapid systemic elimination [125]. To overcome its weak pharmacodynamics and its development for cerebral ischemia treatment, researchers pretreated MCAO rats with Polymeric N-Isopropyl Acrylamide (PNIPAM) nanoparticles of CUR, Bisdemethoxycurcumin (BDMC), and Demethoxycurcumin (DMC) intra-nasally. PNIPAM nanoparticles (CUR, BDMC, and DMC) showed high drug loading, gradual release of the drug, reducing unexpected systemic effects, and bypassing the BBB. Intra-nasal administration of these components well ameliorated behavioral changes and oxidative stress (In general, the antioxidant potential of nano-formulation in this study was CUR > DMC >> BDMC). Also, PNIPAM-CUR significantly prevented I/R injury compared to the other two groups. Therefore, PNIPAM-CUR nanoparticle is a potential neuroprotective agent for stroke treatment [44].
Conclusion
In this paper, some of the strategies for effective drug delivery to the CNS were reviewed. Unfortunately, in the field of nano-therapy in oral and smell pathways have only been carried out in a few studies. In this paper, we tried to outline the results of all of them.
Despite the limitations of oral and intranasal routes, the therapeutic potential of oral and intranasal administration of nano-medicines is higher in cerebral ischemia treatment. The advancement and discovery of new nano-medicine with high selectivity effects help us to make progress in the effective and safe treatment of stroke via these two pathways.
Fig. (1).
Three main routes of nose access to the CNS [110]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Acknowledgements
The authors would like to thank their assistant at Farhangian University Ms. Sanaz Delfan Azari for her helpful comments and also thank the International Journal of Nanomedicine for sincere collaboration using Fig. (1).
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Control CfD 2011.
- 2.Strong K., Mathers C., Bonita R. Preventing stroke: Saving lives around the world. Lancet Neurol. 2007;6(2):182–187. doi: 10.1016/S1474-4422(07)70031-5. [DOI] [PubMed] [Google Scholar]
- 3.Alavian F., Hajizadeh S., Bigdeli M.R., Javan M. The role of protein kinase C in ischemic tolerance induced by hyperoxia in rats with stroke. EXCLI J. 2012;11:188–197. [PMC free article] [PubMed] [Google Scholar]
- 4.Alavian F., Ghiasvand S. Protective effects of jujube extract against permeability of Blood-Brain Barrier (BBB), and the activity of glutathione peroxidase and catalase in stroke model. Majallah-i Danishkadah-i Pizishki-i Isfahan. 2018;36(475):379–385. [Google Scholar]
- 5.Schinkel A.H., Smit J.J., van Tellingen O., et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77(4):491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 6.Misra A., Ganesh S., Shahiwala A., Shah S.P. Drug delivery to the central nervous system: A review. J. Pharm. Pharm. Sci. 2003;6(2):252–273. [PubMed] [Google Scholar]
- 7.Ulbrich K., Hekmatara T., Herbert E., Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the Blood-Brain Barrier (BBB). Eur. J. Pharm. Biopharm. 2009;71(2):251–256. doi: 10.1016/j.ejpb.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Lao F., Chen L., Li W., et al. Fullerene nanoparticles selectively enter oxidation-damaged cerebral microvessel endothelial cells and inhibit JNK-related apoptosis. ACS Nano. 2009;3(11):3358–3368. doi: 10.1021/nn900912n. [DOI] [PubMed] [Google Scholar]
- 9.Kamaly N., Yameen B., Wu J., Farokhzad O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pathak Y. Recent Developments in nanoparticulate drug delivery systems drug delivery nanoparticles formulation and characterization. CRC Press; 2016. pp. 19–33. [Google Scholar]
- 11.Warheit D.B. Nanoparticles. Mater. Today. 2004;7(2):32–35. doi: 10.1016/S1369-7021(04)00081-1. [DOI] [Google Scholar]
- 12.Alavian F. Drug Abuse Treatment through gene manipulation using nanomedicine. Curr. Pharmacogenomics Person. Med. 2018;16(182):1–10. [Google Scholar]
- 13.Petkar KC, Chavhan SS, Agatonovik-Kustrin S, Sawant K. Nanostructured materials in drug and gene delivery: A review of the state of the art. [DOI] [PubMed]
- 14.Prabhu S., Poulose E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012;2(1):32. doi: 10.1186/2228-5326-2-32. [DOI] [Google Scholar]
- 15.Kittelson D.B. Engines and nanoparticles: A review. J. Aerosol Sci. 1998;29(5-6):575–588. doi: 10.1016/S0021-8502(97)10037-4. [DOI] [Google Scholar]
- 16.Mohanraj V., Chen Y. Nanoparticles-a review. Trop. J. Pharm. Res. 2006;5(1):561–573. [Google Scholar]
- 17.Kango S., Kalia S., Celli A., Njuguna J., Habibi Y., Kumar R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites-A review. Prog. Polym. Sci. 2013;38(8):1232–1261. doi: 10.1016/j.progpolymsci.2013.02.003. [DOI] [Google Scholar]
- 18.Khlebtsov N., Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011;40(3):1647–1671. doi: 10.1039/C0CS00018C. [DOI] [PubMed] [Google Scholar]
- 19.Jain P.K., Huang X., El-Sayed I.H., El-Sayed M.A. Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics. 2007;2(3):107–118. doi: 10.1007/s11468-007-9031-1. [DOI] [Google Scholar]
- 20.Wolfram J., Zhu M., Yang Y., et al. Safety of nanoparticles in medicine. Curr. Drug Targets. 2015;16(14):1671–1681. doi: 10.2174/1389450115666140804124808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferro-Flores G., Ocampo-García B.E., Santos-Cuevas C.L., Morales-Avila E., Azorín-Vega E. Multifunctional radiolabeled nanoparticles for targeted therapy. Curr. Med. Chem. 2014;21(1):124–138. doi: 10.2174/09298673113209990218. [DOI] [PubMed] [Google Scholar]
- 22.Jiskoot W., van Schie R.M., Carstens M.G., Schellekens H. Immunological risk of injectable drug delivery systems. Pharm. Res. 2009;26(6):1303–1314. doi: 10.1007/s11095-009-9855-9. [DOI] [PubMed] [Google Scholar]
- 23.Li M., Al-Jamal K.T., Kostarelos K., Reineke J. Physiologically based pharmacokinetic modeling of nanoparticles. ACS Nano. 2010;4(11):6303–6317. doi: 10.1021/nn1018818. [DOI] [PubMed] [Google Scholar]
- 24.Patel J., Patel A. Toxicity of Nanomaterials on the Liver, Kidney, and Spleen. Boca Raton, FL: CRC Press; 2015. [Google Scholar]
- 25.Donaldson K., Tran L., Jimenez L.A., et al. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part. Fibre Toxicol. 2005;2(1):10. doi: 10.1186/1743-8977-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamprecht A., Schäfer U., Lehr C-M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001;18(6):788–793. doi: 10.1023/A:1011032328064. [DOI] [PubMed] [Google Scholar]
- 27.Park M.V., Neigh A.M., Vermeulen J.P., et al. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials. 2011;32(36):9810–9817. doi: 10.1016/j.biomaterials.2011.08.085. [DOI] [PubMed] [Google Scholar]
- 28.Hoet P.H., Brüske-Hohlfeld I., Salata O.V. Nanoparticles - known and unknown health risks. J. Nanobiotechnology. 2004;2(1):12. doi: 10.1186/1477-3155-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kettiger H., Schipanski A., Wick P., Huwyler J. Engineered nanomaterial uptake and tissue distribution: From cell to organism. Int. J. Nanomedicine. 2013;8:3255–3269. doi: 10.2147/IJN.S49770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho M., Cho W-S., Choi M., et al. The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. Toxicol. Lett. 2009;189(3):177–183. doi: 10.1016/j.toxlet.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 31.De Jong W.H., Hagens W.I., Krystek P., Burger M.C., Sips A.J., Geertsma R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29(12):1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 32.Ong W-Y., Shalini S-M., Costantino L. Nose-to-brain drug delivery by nanoparticles in the treatment of neurological disorders. Curr. Med. Chem. 2014;21(37):4247–4256. doi: 10.2174/0929867321666140716103130. [DOI] [PubMed] [Google Scholar]
- 33.Soppimath K.S., Aminabhavi T.M., Kulkarni A.R., Rudzinski W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release. 2001;70(1-2):1–20. doi: 10.1016/S0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 34.Allen T.M., Cullis P.R. Drug delivery systems: Entering the mainstream. Science. 2004;303(5665):1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 35.Ting Y., Jiang Y., Ho C-T., Huang Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods. 2014;7:112–128. doi: 10.1016/j.jff.2013.12.010. [DOI] [Google Scholar]
- 36.Kakkar V., Muppu S.K., Chopra K., Kaur I.P. Curcumin loaded solid lipid nanoparticles: An efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur. J. Pharm. Biopharm. 2013;85(3 Pt A):339–345. doi: 10.1016/j.ejpb.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad N., Ahmad R., Naqvi A.A., et al. Rutin-encapsulated chitosan nanoparticles targeted to the brain in the treatment of cerebral ischemia. Int. J. Biol. Macromol. 2016;91:640–655. doi: 10.1016/j.ijbiomac.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J., Han X., Li X., et al. Core-shell hybrid liposomal vesicles loaded with panax notoginsenoside: Preparation, characterization and protective effects on global cerebral ischemia/reperfusion injury and acute myocardial ischemia in rats. Int. J. Nanomedicine. 2012;7:4299–4310. doi: 10.2147/IJN.S32385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yadav A., Sunkaria A., Singhal N., Sandhir R. Resveratrol loaded solid lipid nanoparticles attenuate mitochondrial oxidative stress in vascular dementia by activating Nrf2/HO-1 pathway. Neurochem. Int. 2018;112:239–254. doi: 10.1016/j.neuint.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh A., Sarkar S., Mandal A.K., Das N. Neuroprotective role of nanoencapsulated quercetin in combating ischemia-reperfusion induced neuronal damage in young and aged rats. PLoS One. 2013;8(4):e57735. doi: 10.1371/journal.pone.0057735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutoh T., Mutoh T., Taki Y., Ishikawa T. Therapeutic potential of natural product-based oral nanomedicines for stroke prevention. J. Med. Food. 2016;19(6):521–527. doi: 10.1089/jmf.2015.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mittal D., Ali A., Md S., Baboota S., Sahni J.K., Ali J. Insights into direct nose to brain delivery: Current status and future perspective. Drug Deliv. 2014;21(2):75–86. doi: 10.3109/10717544.2013.838713. [DOI] [PubMed] [Google Scholar]
- 43.Mistry A., Stolnik S., Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009;379(1):146–157. doi: 10.1016/j.ijpharm.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad N., Umar S., Ashafaq M., et al. A comparative study of PNIPAM nanoparticles of curcumin, demethoxycurcumin, and bisdemethoxycurcumin and their effects on oxidative stress markers in experimental stroke. Protoplasma. 2013;250(6):1327–1338. doi: 10.1007/s00709-013-0516-9. [DOI] [PubMed] [Google Scholar]
- 45.des Rieux A., Fievez V., Garinot M., Schneider Y-J., Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release. 2006;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Shahbazi M-A., Santos H.A. Improving oral absorption via drug-loaded nanocarriers: Absorption mechanisms, intestinal models and rational fabrication. Curr. Drug Metab. 2013;14(1):28–56. doi: 10.2174/138920013804545133. [DOI] [PubMed] [Google Scholar]
- 47.Müller R.H., Rühl D., Runge S.A. Biodegradation of solid lipid nanoparticles as a function of lipase incubation time. Int. J. Pharm. 1996;144(1):115–121. doi: 10.1016/S0378-5173(96)04731-X. [DOI] [Google Scholar]
- 48.Jani P., Halbert G.W., Langridge J., Florence A.T. Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependency. J. Pharm. Pharmacol. 1990;42(12):821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- 49.Mehnert W., Mäder K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012;64:83–101. doi: 10.1016/j.addr.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 50.Kaur I.P., Bhandari R., Bhandari S., Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release. 2008;127(2):97–109. doi: 10.1016/j.jconrel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 51.Freitas C., Müller R.H. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersions. Int. J. Pharm. 1998;168(2):221–229. doi: 10.1016/S0378-5173(98)00092-1. [DOI] [Google Scholar]
- 52.Gastaldi L., Battaglia L., Peira E., et al. Solid lipid nanoparticles as vehicles of drugs to the brain: Current state of the art. Eur. J. Pharm. Biopharm. 2014;87(3):433–444. doi: 10.1016/j.ejpb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 53.Martins S.M., Sarmento B., Nunes C., Lúcio M., Reis S., Ferreira D.C. Brain targeting effect of camptothecin-loaded solid lipid nanoparticles in rat after intravenous administration. Eur. J. Pharm. Biopharm. 2013;85(3 Pt A):488–502. doi: 10.1016/j.ejpb.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 54.Kakkar V., Mishra A.K., Chuttani K., Kaur I.P. Proof of concept studies to confirm the delivery of Curcumin loaded Solid Lipid Nanoparticles (C-SLNs) to brain. Int. J. Pharm. 2013;448(2):354–359. doi: 10.1016/j.ijpharm.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 55.Kakkar V., Kaur I.P. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem. Toxicol. 2011;49(11):2906–2913. doi: 10.1016/j.fct.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Kakkar V., Kaur I.P. Antidepressant activity of Curcumin loaded Solid Lipid Nanoparticles (C-SLNs) in mice. Am J Pharm Res. 2012;2(3):729–736. [Google Scholar]
- 57.Virgili M., Contestabile A. Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine antioxidant agent, trans-resveratrol in rats. Neurosci. Lett. 2000;281(2-3):123–126. doi: 10.1016/S0304-3940(00)00820-X. [DOI] [PubMed] [Google Scholar]
- 58.Gülçin İ. Antioxidant properties of resveratrol: a structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010;11(1):210–218. doi: 10.1016/j.ifset.2009.07.002. [DOI] [Google Scholar]
- 59.Tosun I., Inkaya A.N. Resveratrol as a health and disease benefit agent. Food Rev. Int. 2009;26(1):85–101. doi: 10.1080/87559120802525459. [DOI] [Google Scholar]
- 60.Gupta C., Sharma G., Chan D. 2014. Resveratrol: A chemo-preventative agent with diverse applications. [Google Scholar]
- 61.Jardim F.R., de Rossi F.T., Nascimento M.X., et al. Resveratrol and brain mitochondria: A review. Mol. Neurobiol. 2018;55(3):2085–2101. doi: 10.1007/s12035-017-0448-z. [DOI] [PubMed] [Google Scholar]
- 62.Novakovic A., Gojkovic-Bukarica L., Peric M., et al. The mechanism of endothelium-independent relaxation induced by the wine polyphenol resveratrol in human internal mammary artery. J. Pharmacol. Sci. 2006;101(1):85–90. doi: 10.1254/jphs.FP0050863. [DOI] [PubMed] [Google Scholar]
- 63.Vidavalur R., Otani H., Singal P.K., Maulik N. Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp. Clin. Cardiol. 2006;11(3):217–225. [PMC free article] [PubMed] [Google Scholar]
- 64.Shigematsu S., Ishida S., Hara M., et al. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radic. Biol. Med. 2003;34(7):810–817. doi: 10.1016/S0891-5849(02)01430-2. [DOI] [PubMed] [Google Scholar]
- 65.Chun-Fu W., Jing-Yu Y., Fang W., Xiao-Xiao W. Resveratrol: Botanical origin, pharmacological activity and applications. Chin. J. Nat. Med. 2013;11(1):1–15. [Google Scholar]
- 66.Bellaver B., Souza D.G., Souza D.O., Quincozes-Santos A. Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol. In Vitro. 2014;28(4):479–484. doi: 10.1016/j.tiv.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Shin J.A., Lee H., Lim Y-K., Koh Y., Choi J.H., Park E-M. Therapeutic effects of resveratrol during acute periods following experimental ischemic stroke. J. Neuroimmunol. 2010;227(1-2):93–100. doi: 10.1016/j.jneuroim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 68.Amri A., Chaumeil J.C., Sfar S., Charrueau C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release. 2012;158(2):182–193. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 69.Davidov-Pardo G., McClements D.J. Resveratrol encapsulation: designing delivery systems to overcome solubility, stability and bioavailability issues. Trends Food Sci. Technol. 2014;38(2):88–103. doi: 10.1016/j.tifs.2014.05.003. [DOI] [Google Scholar]
- 70.Sandhir R., Yadav A., Sunkaria A., Singhal N. Nano-antioxidants: An emerging strategy for intervention against neurodegenerative conditions. Neurochem. Int. 2015;89:209–226. doi: 10.1016/j.neuint.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Neves A.R., Queiroz J.F., Reis S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnology. 2016;14(1):27. doi: 10.1186/s12951-016-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blasi P., Giovagnoli S., Schoubben A., Ricci M., Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv. Drug Deliv. Rev. 2007;59(6):454–477. doi: 10.1016/j.addr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Jose S., Anju S.S., Cinu T.A., Aleykutty N.A., Thomas S., Souto E.B. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int. J. Pharm. 2014;474(1-2):6–13. doi: 10.1016/j.ijpharm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 74.Alfieri A., Srivastava S., Siow R.C., Modo M., Fraser P.A., Mann G.E. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J. Physiol. 2011;589(17):4125–4136. doi: 10.1113/jphysiol.2011.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cicero A.F., Vitale G., Savino G., Arletti R. Panax notoginseng (Burk.) effects on fibrinogen and lipid plasma level in rats fed on a high-fat diet. Phytother. Res. 2003;17(2):174–178. doi: 10.1002/ptr.1262. [DOI] [PubMed] [Google Scholar]
- 76.Zhao G-R., Xiang Z-J., Ye T-X., Yuan Y-J., Guo Z-X. Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng. Food Chem. 2006;99(4):767–774. doi: 10.1016/j.foodchem.2005.09.002. [DOI] [Google Scholar]
- 77.Ng T.B. Pharmacological activity of Sanchi ginseng (Panax notoginseng). J. Pharm. Pharmacol. 2006;58(8):1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- 78.Liu J., Wang Y., Qiu L., Yu Y., Wang C. Saponins of Panax notoginseng: Chemistry, cellular targets and therapeutic opportunities in cardiovascular diseases. Expert Opin. Investig. Drugs. 2014;23(4):523–539. doi: 10.1517/13543784.2014.892582. [DOI] [PubMed] [Google Scholar]
- 79.Wang T., Guo R., Zhou G., et al. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: A review. J. Ethnopharmacol. 2016;188:234–258. doi: 10.1016/j.jep.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 80.Duan L., Xiong X., Hu J., Liu Y., Li J., Wang J. Panax notoginseng saponins for treating coronary artery disease: A functional and mechanistic overview. Front. Pharmacol. 2017;8:702. doi: 10.3389/fphar.2017.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim D-H. Chemical Diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J. Ginseng Res. 2012;36(1):1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee J., Lee E., Kim D., Lee J., Yoo J., Koh B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J. Ethnopharmacol. 2009;122(1):143–148. doi: 10.1016/j.jep.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 83.Kim H., Lee J.H., Kim J.E., et al. Micro-/nano-sized delivery systems of ginsenosides for improved systemic bioavailability. J. Ginseng Res. 2018;42(3):361–369. doi: 10.1016/j.jgr.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qiu J., Cai G., Liu X., Ma D. αvβ3 integrin receptor specific peptide modified, salvianolic acid B and panax notoginsenoside loaded nanomedicine for the combination therapy of acute myocardial ischemia. Biomed. Pharmacother. 2017;96:1418–1426. doi: 10.1016/j.biopha.2017.10.086. [DOI] [PubMed] [Google Scholar]
- 85.Kumari A., Yadav S.K., Yadav S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 86.Sahoo S.K., Misra R., Parveen S. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine in Cancer: Pan Stanford. 2017;75(1):73–124. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 87.Bamrungsap S., Zhao Z., Chen T., et al. Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Nanomedicine (Lond.) 2012;7(8):1253–1271. doi: 10.2217/nnm.12.87. [DOI] [PubMed] [Google Scholar]
- 88.Zhang J., Guan P., Wang T., Chang D., Jiang T., Wang S. Freeze-dried liposomes as potential carriers for ocular administration of cytochrome c against selenite cataract formation. J. Pharm. Pharmacol. 2009;61(9):1171–1178. doi: 10.1211/jpp.61.09.0006. [DOI] [PubMed] [Google Scholar]
- 89.Cartea M.E., Francisco M., Soengas P., Velasco P. Phenolic compounds in Brassica vegetables. Molecules. 2010;16(1):251–280. doi: 10.3390/molecules16010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Albini A., Tosetti F., Li V.W., Noonan D.M., Li W.W. Cancer prevention by targeting angiogenesis. Nat. Rev. Clin. Oncol. 2012;9(9):498–509. doi: 10.1038/nrclinonc.2012.120. [DOI] [PubMed] [Google Scholar]
- 91.Guardia T., Rotelli A.E., Juarez A.O., Pelzer L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56(9):683–687. doi: 10.1016/S0014-827X(01)01111-9. [DOI] [PubMed] [Google Scholar]
- 92.Hassimotto N.M.A., Genovese M.I., Lajolo F.M. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J. Agric. Food Chem. 2005;53(8):2928–2935. doi: 10.1021/jf047894h. [DOI] [PubMed] [Google Scholar]
- 93.Boots A.W., Haenen G.R., Bast A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585(2-3):325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 94.Perez-Vizcaino F., Duarte J., Andriantsitohaina R. Endothelial function and cardiovascular disease: Effects of quercetin and wine polyphenols. Free Radic. Res. 2006;40(10):1054–1065. doi: 10.1080/10715760600823128. [DOI] [PubMed] [Google Scholar]
- 95.Formica J.V., Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem. Toxicol. 1995;33(12):1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 96.Hertog M.G., Feskens E.J., Hollman P.C., Katan M.B., Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet. 1993;342(8878):1007–1011. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- 97.Moghimi S.M., Hunter A.C., Murray J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 98.Olivier J-C. Drug transport to brain with targeted nanoparticles. NeuroRx. 2005;2(1):108–119. doi: 10.1602/neurorx.2.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao L.X., Liu A.C., Yu S.W., et al. The permeability of puerarin loaded poly (butylcyanoacrylate) nanoparticles coated with polysorbate 80 on the blood-brain barrier and its protective effect against cerebral ischemia/reperfusion injury. Biol. Pharm. Bull. 2013;36(8):1263–1270. doi: 10.1248/bpb.b12-00769. [DOI] [PubMed] [Google Scholar]
- 100.Zhou F., Wang L., Liu P., et al. Puerarin protects brain tissue against cerebral ischemia/reperfusion injury by inhibiting the inflammatory response. Neural Regen. Res. 2014;9(23):2074–2080. doi: 10.4103/1673-5374.147934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tian F., Xu L-H., Zhao W., Tian L-J., Ji X-L. The optimal therapeutic timing and mechanism of puerarin treatment of spinal cord ischemia-reperfusion injury in rats. J. Ethnopharmacol. 2011;134(3):892–896. doi: 10.1016/j.jep.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 102.Liu G., Liu Z., Yuan S. Recent advances in methods of puerarin biotransformation. Mini Rev. Med. Chem. 2016;16(17):1392–1402. doi: 10.2174/1389557516666160505114456. [DOI] [PubMed] [Google Scholar]
- 103.Lockman P.R., Mumper R.J., Khan M.A., Allen D.D. Nanoparticle technology for drug delivery across the blood-brain barrier. Drug Dev. Ind. Pharm. 2002;28(1):1–13. doi: 10.1081/DDC-120001481. [DOI] [PubMed] [Google Scholar]
- 104.Agüeros M., Ruiz-Gatón L., Vauthier C., et al. Combined hydroxypropyl-β-cyclodextrin and poly(anhydride) nanoparticles improve the oral permeability of paclitaxel. Eur. J. Pharm. Sci. 2009;38(4):405–413. doi: 10.1016/j.ejps.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 105.Loftsson T., Duchêne D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007;329(1-2):1–11. doi: 10.1016/j.ijpharm.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 106.Vecsernyés M., Fenyvesi F., Bácskay I., Deli M.A., Szente L., Fenyvesi É. Cyclodextrins, blood-brain barrier, and treatment of neurological diseases. Arch. Med. Res. 2014;45(8):711–729. doi: 10.1016/j.arcmed.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 107.Gil E.S., Li J., Xiao H., Lowe T.L. Quaternary ammonium β-cyclodextrin nanoparticles for enhancing doxorubicin permeability across the in vitro blood-brain barrier. Biomacromolecules. 2009;10(3):505–516. doi: 10.1021/bm801026k. [DOI] [PubMed] [Google Scholar]
- 108.Tao H.Q., Meng Q., Li M.H., et al. HP-β-CD-PLGA nanoparticles improve the penetration and bioavailability of puerarin and enhance the therapeutic effects on brain ischemia-reperfusion injury in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2013;386(1):61–70. doi: 10.1007/s00210-012-0804-5. [DOI] [PubMed] [Google Scholar]
- 109.Pardeshi C.V., Belgamwar V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013;10(7):957–972. doi: 10.1517/17425247.2013.790887. [DOI] [PubMed] [Google Scholar]
- 110.Lu C-T., Zhao Y-Z., Wong H.L., Cai J., Peng L., Tian X-Q. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomedicine. 2014;9:2241–2257. doi: 10.2147/IJN.S61288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dhuria S.V., Hanson L.R., Frey W.H., II Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010;99(4):1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 112.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000;11(1):1–18. doi: 10.1016/S0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 113.Arora P., Sharma S., Garg S. Permeability issues in nasal drug delivery. Drug Discov. Today. 2002;7(18):967–975. doi: 10.1016/S1359-6446(02)02452-2. [DOI] [PubMed] [Google Scholar]
- 114.Johnson P.H., Quay S.C. Advances in nasal drug delivery through tight junction technology. Expert Opin. Drug Deliv. 2005;2(2):281–298. doi: 10.1517/17425247.2.2.281. [DOI] [PubMed] [Google Scholar]
- 115.Illum L. Nanoparticulate systems for nasal delivery of drugs: A real improvement over simple systems? J. Pharm. Sci. 2007;96(3):473–483. doi: 10.1002/jps.20718. [DOI] [PubMed] [Google Scholar]
- 116.Illum L. Nasal drug delivery-possibilities, problems and solutions. J. Control. Release. 2003;87(1-3):187–198. doi: 10.1016/S0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 117.Enogieru A.B., Haylett W., Hiss D.C., Bardien S., Ekpo O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Longev. 2018;2018:6241017. doi: 10.1155/2018/6241017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Al-Dhabi N.A., Arasu M.V., Park C.H., Park S.U. An up-to-date review of rutin and its biological and pharmacological activities. EXCLI J. 2015;14:59–63. doi: 10.17179/excli2014-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Choi J-H., Kim D-W., Park S-E., et al. Anti-thrombotic effect of rutin isolated from Dendropanax morbifera Leveille. J. Biosci. Bioeng. 2015;120(2):181–186. doi: 10.1016/j.jbiosc.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 120.Park J.H., Saravanakumar G., Kim K., Kwon I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010;62(1):28–41. doi: 10.1016/j.addr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 121.Ding Y., Qiao Y., Wang M., et al. Enhanced neuroprotection of acetyl-11-keto-β-boswellic acid (AKBA)-loaded O-carboxymethyl chitosan nanoparticles through antioxidant and anti-inflammatory pathways. Mol. Neurobiol. 2016;53(6):3842–3853. doi: 10.1007/s12035-015-9333-9. [DOI] [PubMed] [Google Scholar]
- 122.Sarvaiya J., Agrawal Y.K. Chitosan as a suitable nanocarrier material for anti-Alzheimer drug delivery. Int. J. Biol. Macromol. 2015;72:454–465. doi: 10.1016/j.ijbiomac.2014.08.052. [DOI] [PubMed] [Google Scholar]
- 123.Ameeduzzafar A.J., Ali J., Bhatnagar A., Kumar N., Ali A. Chitosan nanoparticles amplify the ocular hypotensive effect of cateolol in rabbits. Int. J. Biol. Macromol. 2014;65:479–491. doi: 10.1016/j.ijbiomac.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 124.Yu G., Liu L., Zhang P., Li Y. Protective effect of Curcumin on chronic cerebral ischemia by altering expression of α-synuclein in 2VO model. Mol. Neurodegener. 2012;7:S33. doi: 10.1186/1750-1326-7-S1-S33. [DOI] [Google Scholar]
- 125.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]