Abstract
BACKGROUND
Type I Helicobacter pylori (H. pylori) infection causes severe gastric inflammation and is a predisposing factor for gastric carcinogenesis. However, its infection status in stepwise gastric disease progression in this gastric cancer prevalent area has not been evaluated; it is also not known its impact on commonly used epidemiological gastric cancer risk markers such as gastrin-17 (G-17) and pepsinogens (PGs) during clinical practice.
AIM
To explore the prevalence of type I and type II H. pylori infection status and their impact on G-17 and PG levels in clinical practice.
METHODS
Thirty-five hundred and seventy-two hospital admitted patients with upper gastrointestinal symptoms were examined, and 523 patients were enrolled in this study. H. pylori infection was confirmed by both 13C-urea breath test and serological assay. Patients were divided into non-atrophic gastritis (NAG), non-atrophic gastritis with erosion (NAGE), chronic atrophic gastritis (CAG), peptic ulcers (PU) and gastric cancer (GC) groups. Their serological G-17, PG I and PG II values and PG I/PG II ratio were also measured.
RESULTS
A total H. pylori infection rate of 3572 examined patients was 75.9%, the infection rate of 523 enrolled patients was 76.9%, among which type I H. pylori infection accounted for 72.4% (291/402) and type II was 27.6%; 88.4% of GC patients were H. pylori positive, and 84.2% of them were type I infection, only 11.6% of GC patients were H. pylori negative. Infection rates of type I H. pylori in NAG, NAGE, CAG, PU and GC groups were 67.9%, 62.7%, 79.7%, 77.6% and 84.2%, respectively. H. pylori infection resulted in significantly higher G-17 and PG II values and decreased PG I/PG II ratio. Both types of H. pylori induced higher G-17 level, but type I strain infection resulted in an increased PG II level and decreased PG I/PG II ratio in NAG, NAGE and CAG groups over uninfected controls. Overall PG I levels showed no difference among all disease groups and in the presence or absence of H. pylori; in stratified analysis, its level was increased in GC and PU patients in H. pylori and type I H. pylori-positive groups.
CONCLUSION
Type I H. pylori infection is the major form of infection in this geographic region, and a very low percentage (11.6%) of GC patients are not infected by H. pylori. Both types of H. pylori induce an increase in G-17 level, while type I H. pylori is the major strain that affects PG I and PG IIs level and PG I/PG II ratio in stepwise chronic gastric disease. The data provide insights into H. pylori infection status and indicate the necessity and urgency for bacteria eradication and disease prevention in clinical practice.
Keywords: Helicobacter pylori, Chronic gastric diseases, Gastrin-17, Pepsinogen, Gastric cancer
Core tip: Type I and type II Helicobacter pylori (H. pylori) infection status and their impact on gastrin-17 and pepsinogen level in chronic gastric diseases have not been studied in this high gastric cancer risk area. Our results show that type I H. pylori infection is the major form of infection, and a very low percentage (11.6%) of gastric cancer patients are not infected by H. pylori. Both type I and type II H. pylori induce an increase in gastrin-17 level, while type I H. pylori is the major strain that affects pepsinogen (PG) I, PG II level and PG I/PG II ratio in stepwise gastric disease in this geographic area.
INTRODUCTION
Helicobacter pylori (H. pylori) infection is the major cause of chronic gastritis, peptic ulcers, gastric cancer and mucosa-associated lymphoid tissue lymphoma, it is also associated with many extra gastrointestinal diseases[1-3]. H. pylori cytotoxin CagA and VacA are major virulence factors and molecular basis for disease pathogenesis. H. pylori strains that carry cagPAI with CagA-, VacA-positive cause severe gastric inflammation, which contributes to either tissue damage or neoplastic transformation, are high-risk strains of gastric cancer, and the role of CagA protein is critical in these processes[4]. Studies have shown that there is both genotypic and geographic diversity of H. pylori infection, which can trigger different inflammatory processes and result in various degrees of pathological consequences[4-6].
Type I H. pylori expresses CagA and VacA protein; type II strain does not express CagA and VacA[7]. CagA-, VacA-positive strains are the major forms of H. pylori infection in many areas globally, corresponding to their high prevalence in pre-cancerous lesions and gastric cancer incidences[4]. However, their infection status and roles in the stepwise gastric disease progression in this high gastric cancer prevalent area has not been studied[8].
Serological detection of pepsinogen (PG) I, II, PG I/PG II ratio and gastrin-17 (G-17) provide valuable information on the status of gastric mucosa, and they have been used as epidemiological markers for gastric cancer risk investigation[9-12]. Studies have indicated that low concentrations of PG I and PG I/PG II ratios are indicators of gastric atrophy, which are linked with elevated gastric cancer risk[9,10]. However, others have indicated that the results are not consistent and not sensitive enough to replace endoscopy[11,12]. PG I/PG II ratio also should not be used as a biomarker of gastric neoplasia as recommended[1]. It is therefore uncertain if they might be suitable to evaluate stepwise gastric disease progression and development of mucosal precancerous conditions in the presence or absence of H. pylori infection in clinical practice.
In the present study, we investigated the prevalence of type I and type II H. pylori infection in stepwise chronic gastric diseases and the clinical implications. Their impact on G-17 and PGs levels was also evaluated. The results indicated that there is a stepwise increase in type I H. pylori infection rate as disease progress from chronic gastritis to gastric cancer. Both types of H. pylori induce an increase in G-17 level, while type I H. pylori is the major strains that affects PG I, PG II levels and PG I/PG II ratio in chronic gastric diseases in this geographic region. The results provide insight on the subtypes of H. pylori infection status and their impact on G-17 and PGs, which will be helpful to guide H. pylori eradication and application of G-17 and PGs assay in clinical practice.
MATERIALS AND METHODS
Study population
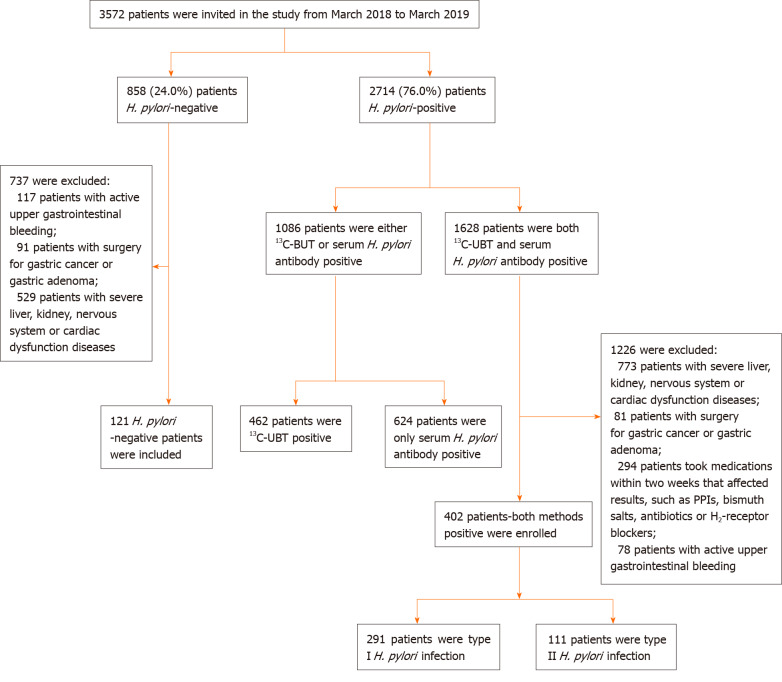
This cross-section study was conducted at the Department of Gastroenterology, People’s Hospital of Zhengzhou University, Zhengzhou, Henan, China. From March 2018 to March 2019, a total of 3572 consecutively ward admitted patients with upper gastrointestinal symptoms were examined. Exclusion criteria were as follows: (1) Taking proton pump inhibitors, bismuth salts, H2-receptor blockers or other medications that could affect test results over the past 2 wk, taking antibiotics over the past 1 mo; (2) Severe concomitant diseases such as liver, kidney, nervous system or cardiac dysfunction; (3) People with active upper gastrointestinal bleeding; (4) Patients with a history of gastrointestinal surgery for gastric cancer, esophageal cancer or gastric adenoma; and (5) People with mental illness or severe neurosis, affecting correct expression or study. The study finally enrolled 523 patients, the flow chart of patient screening is summarized in Figure 1.
Figure 1.
Flowchart of enrolled patients–screening program. H. pylori: Helicobacter pylori.
All 523 enrolled patients were examined by upper-endoscopy to get pathological confirmation. Based on histopathologic types, subjects were categorized into five groups: Non-atrophic gastritis (NAG), non-atrophic gastritis with erosion (NAGE), chronic atrophic gastritis (CAG), peptic ulcers (PU) and gastric cancer (GC). Demographic data of patients including age and gender were recorded, and H. pylori CagA, VacA status, G-17, PG I, II levels and PG I/PG II ratio were analyzed. The research protocol was approved by the Ethics Committee of People’s Hospital of Zhengzhou University (2019-KY-No. 10); informed consents were obtained from all participating patients.
Measurement of H. pylori infection
The status of H. pylori infection was confirmed by both 13C-urea breath test (UBT) and serological H. pylori antibody test, patients were considered not infected when both tests were negative; when patients were either 13C-UBT or serological H. pylori antibody positive, but not both, they were not enrolled to avoid false-positive or -negative results. 13C-UBT was performed after overnight fasting, a baseline breath sample was obtained by blowing gas into a bag container, and a powder capsule containing 50 mg of 13C-urea was given to patients with 80-100 mL water. The second breath sample was collected after 30 min of meditation. Patients were considered H. pylori positive if the difference between baseline sample and 30-min sample exceeded 4.0 arbitrary units by 13C-breath test (HY-IREXC 16 channel; Huayou Mingkang Photoelectric Technology Co., Ltd, Guangzhou, China).
Serological measurements of H. pylori antibody, G-17 and PGs
Five milliliters of fasting venous blood sample was collected from each participant. All samples were centrifuged at 1500 × g for 5 min and analyzed within 2 h of blood collection. Serum anti-H. pylori antibody, G-17, PG I, PG II levels, and PG I/PG II ratio were measured by enzyme-linked immunosorbent assay (ELISA) kit (Helicobacter pylori ELISA kit, Blot Biotech Co., Ltd, Shenzhen, China; PG I, PG II, G-17 ELISA kits, Biohit Biotechnology Co., Ltd. Anhui, China). The procedure followed manufacturers’ instructions; quality control analysis showed that the coefficient of variation in intra-batch and inter-batch sample tests was less than 10%.
Determination of H. pylori positivity from blood samples: (1) Type I H. pylori antibody positive: either or both CagA and VacA bands were present; (2) Type II H. pylori antibody positive: only one of urease (Ure) A and UreB bands or both appeared, no CagA, VacA bands were present; and (3) H. pylori antibody negative: Only control band appeared in the color-developing zone, and no positive zone was observed. Representative H. pylori serological test blot pictures are provided in Supplementary Figure 1.
Endoscopic and histopathological evaluation
Histopathological diagnosis was available in all enrolled 523 patients. Two pieces of biopsy specimen were obtained from the lesion area, antrum and angulus during endoscopic examination. The biopsies were oriented, fixed in formalin, embedded in paraffin blocks and then sectioned and stained with hematoxylin and eosin for histopathological analysis. For histologic sections where there was initial disagreement on histopathologic interpretation, the final results were determined through adjudication among two pathologists and a third pathologist.
Statistical analysis
Data were analyzed using SPSS for Windows Version 22 (Armonk, NY, United States). Continuous variables were described as mean ± standard deviation, while categorical variables were described as percentages or frequencies. All data were tested for normal distribution by Kolmogorov-Smirnov test and homogeneity of variances by Levene’s test. Data of normal distribution and similar variances were tested by Student’s “t” test for two independent samples comparison, and analysis of variance for multiple comparisons among different groups. A comparison of ratios was made by the χ2 test. A P value less than 0.05 was considered statistically significant, which was derived from two-tailed tests. Receiver operating characteristic (ROC) curves were used to calculate the overall diagnostic performance of G-17, PG I, PG II, and PG I/PG II ratio in PU, CAG and GC patients to determine the best cutoff values, sensitivity and specificity.
RESULTS
Overall H. pylori infection status of patients
H. pylori infection status of 3572 patients is presented in Figure 1. Among which, 2714 (76.0%) patients were positive either by UBT test, serological test or both, and 858 (24.0%) patients were negative. Among H. pylori-positive patients, 1226 were excluded due to either surgery, medication or bleeding reasons, and 1086 patients were excluded due to either only 13C-BUT or serum antibody test positive but not both; the final enrolled patient number was 402 with both tests being positive. In 858 H. pylori-negative patients, 737 patients were excluded either due to bleeding, surgery or severe organ diseases; this resulted in only 121 patients being enrolled with both tests being negative. Interestingly, we noticed almost identical H. pylori infection rates when comparing the prior- and post-excluded non-enrolled patients. The infection rates of the final enrolled patients were 76.9% positive and 23.1% negative (Figure 1).
Patient clinical data and H. pylori infection status
Among the 523 enrolled patients, 305 were male and 218 were female, with an average age of 53.4 ± 11.6 years (range from 28 to 79). Their clinical characteristics and H. pylori infection status are presented in Table 1. Patients in 51-60 and 61-80 years age groups had the highest H. pylori infection rates of 78.5% (150/191) and 78.4% (116/148), respectively. The average age of patients in the GC group was significantly higher than that in NAG, NAGE and PU groups (P < 0.05). The mean age of the CAG group was higher than that in the NAG group (P < 0.001). There was no significant difference in H. pylori infection status between male and female gender. H. pylori infection in male and female genders were mainly type I H. pylori strains (54.8%, 56.9% respectively), which were significantly higher than that of type II and H. pylori negative patient groups (range from 20.2%-23.3%). There was a significant male dominance in CAG, PU and GC groups (P < 0.05). Among 77 CAG patients, 48 (62.3%) patients were antrum atrophic gastritis and 29 (33.8%) were corpus atrophic gastritis, and all 43 gastric cancer patients were intestinal type.
Table 1.
Patient clinical data and Helicobacter pylori infection status
| Groups | Total patients, n = 523 | NAG, n = 213 | NAGE, n = 96 | PU, n = 94 | CAG, n = 77 | GC, n = 43 | Hp (+), n = 402 | Type I Hp(+), n = 291 | Type II Hp(+), n = 111 | Hp (-), n = 121 |
| Age in yr | ||||||||||
| mean ± SD | 53.4 ± 11.6 | 50.3 ± 11.3a | 53.7 ± 11.3 | 53.5 ± 13.1 | 58.0 ± 9.4a | 59.6 ± 7.8c | 53.7 ± 11.4 | 53.6 ± 11.3 | 53.9 ± 11.7 | 52.5 ± 12.1 |
| 28-40 | 76 | 57 (26.8%) | 13 (13.5%) | 16 (17.0%) | 4 (5.2%) | 0 | 57 (75.0%) | 43 (56.6%)e | 15 (19.7%) | 18 (23.7%) |
| 41-50 | 108 | 71 (33.3%) | 21 (21.9%) | 17 (18.1%) | 7 (9.1%) | 6 (14.0%) | 78 (72.2%) | 55 (50.9%)e | 23 (21.3%) | 30 (27.8%) |
| 51-60 | 191 | 54 (25.3%) | 36 (37.5%) | 30 (31.9%) | 32 (41.6%) | 21 (48.8%) | 150 (78.5%) | 108 (56.5%)e | 42 (22.0%) | 41 (21.5%) |
| 61-80 | 148 | 31 (14.6%) | 26 (27.1%) | 31 (33.0%) | 34 (44.2%) | 16 (37.2%) | 116 (78.4%) | 85 (57.5%)e | 31 (20.9%) | 32 (21.6%) |
| Gender | ||||||||||
| Male | 305 | 105 (49.3%) | 52 (54.2%) | 68 (70.2%)g | 53 (68.8%)g | 27 (58.3%)g | 234 (76.7%) | 167 (54.8%)i | 67 (22.0%) | 71 (23.3%) |
| Female | 218 | 108 (50.7%) | 44 (45.8%) | 26 (27.7%) | 24 (31.2%) | 16 (37.3%) | 168 (77.1%) | 124 (56.9%)i | 44 (20.2%) | 50 (22.9%) |
Data are presented as n (%), unless otherwise indicted.
P < 0.05, mean age in CAG group was compared with NAG group;
P < 0.05, mean age in GC group was compared with NAG, NAGE groups;
P < 0.05, infection rate of type I H. pylori patients was compared with type II and H. pylori-negative patients in the same age groups;
P < 0.05, percentage in male groups was compared with female groups among PU, CAG, and GC groups;
P < 0.05, infection rate of type I H. pylori patients was compare with type II and Hp-negative patients in the same gender groups. CAG: Chronic atrophic gastritis; GC: Gastric cancer; H. pylori: Helicobacter pylori; NAG: Non-atrophic gastritis; NAGE: Non-atrophic gastritis with erosion; PU: Peptic ulcer; SD: Standard deviation.
Prevalence of Type I and Type II H. pylori infection in stepwise chronic gastric diseases
Total H. pylori infection rate of 523 patients was 76.9% (402/523), of which type I H. pylori infection rate was 72.4% (291/402), and type II infection rate was 27.6% (111/402). Overall, 88.4% of GC patients were H. pylori positive, and 84.2% of them were type I infection, only 11.6% of GC were H. pylori negative. As the disease progressed, H. pylori infection rate was gradually increased in NAG, NAGE, PU, CAG and GC groups; among which H. pylori-positive rate reached the highest level in PU group, accounting for 90.4% (85/94) of the patients. Infection rates of type I H. pylori in NAG, NAGE, PU, CAG and GC groups were 67.9%, 62.7%, 77.6%, 79.7%, 84.2%, respectively; and was significantly higher than the corresponding type II H. pylori groups; and type I H. pylori infection rates were also higher in PU, CAG, and GC groups when compared with the NAG group (Table 2).
Table 2.
Infection rates of type I and type II Helicobacter pylori in stepwise chronic gastric diseases
| Groups | Total | H. pylori (+) | H. pylori (-) | Type I H. pylori (+) | Type II H. pylori (+) |
| NAG | 213 | 140 (65.7) | 73 (34.3) | 95 (67.9)a | 45 (32.1) |
| NAGE | 96 | 75 (78.1)c | 21 (21.9) | 47 (62.7)a | 28 (37.3) |
| PU | 94 | 85 (90.4)c | 9 (9.6) | 66 (77.6)ae | 19 (22.4) |
| CAG | 77 | 64 (83.1)c | 13 (16.9) | 51 (79.7)ae | 13 (20.3) |
| GC | 43 | 38 (88.4)c | 5 (11.6) | 32 (84.2)ae | 6 (15.8) |
| Total | 523 | 402 (76.9) | 121 (23.1) | 291 (72.4) | 111 (27.6) |
Data are presented as n (%).
P < 0.05, when type I H. pylori infection rate was compared with type II H. pylori infection rate in the same disease groups;
P < 0.05, when H. pylori infection rate was compared with the rate of control [non-atrophic gastritis (NAG)] group;
P < 0.05, when type I H. pylori infection rate was compared with the rate of NAG group. CAG: Chronic atrophic gastritis; GC: Gastric cancer; H. pylori : Helicobacter pylori; NAG: Non-atrophic gastritis; NAGE: Non-atrophic gastritis with erosion; PU: Peptic ulcer.
Distribution of H. pylori CagA, VacA, UreA and UreB in stepwise chronic gastric diseases
Positive rates of CagA, VacA, UreA and UreB antibodies are described in Table 3. In 402 infected samples, the percentage of CagA, VacA, UreA and UreB were 70.1%, 61.9%, 70.6% and 98.8%, respectively. CagA antibody was detected in 63.3%, 60.0%, 76.5%, 79.7% and 84.2% in NAG, NAGE, PU, CAG and GC groups, respectively. The positive rates of CagA and VacA were highest in GC group and lowest in NAGE group. UreB antibodies were present in 98.8% of patients with H. pylori infection, and there was no statistical differences between disease groups (P > 0.05).
Table 3.
Distribution of CagA, VacA, UreA and UreB serological antibodies in stepwise chronic gastric disease
| Groups | CagA | VacA | UreA | UreB |
| NAG, n = 140 | 89 (63.6) | 82 (55.6) | 93 (66.4) | 138 (98.6) |
| NAGE, n = 75 | 45 (60.0) | 36 (48.0) | 44 (58.7) | 74 (98.7) |
| PU, n = 85 | 65 (76.5) | 57 (67.0) | 67 (78.8) | 83 (97.6) |
| CAG, n = 64 | 51 (79.7) | 43 (67.2) | 49 (76.6) | 64 (100.0) |
| GC, n = 38 | 32 (84.2) | 28 (73.7) | 31 (81.6) | 38 (100.0) |
| Total, n = 402 | 282 (70.1) | 249 (61.9) | 284 (70.6) | 397 (98.8) |
Data are presented as n (%). CAG: Chronic atrophic gastritis; GC: Gastric cancer; NAG: Non-atrophic gastritis; NAGE: Non-atrophic gastritis with erosion; PU: Peptic ulcer.
Effects of H. pylori infection on overall G-17, PG I, PG II levels and PG I/PG II ratio
Overall serum G-17, PG I, PG II values and PG I/PG II ratio at different H. pylori infection status are shown in Table 4. G-17 and PG II values in H. pylori infected groups were significantly higher and PG I/PG II ratio were lower than those in H. pylori-negative patients (P < 0.05); while PG I level was not different between H. pylori-positive and -negative groups (P > 0.05). There was also no significant difference in PG I levels between type I and II H. pylori-infected patients. G-17 and PG II levels were significantly increased and PG I/PG II ratio were decreased only in patients with type I H. pylori infection when compared with type II and H. pylori-negative patients (P < 0.05).
Table 4.
Effects of Helicobacter pylori infection on gastric gastrin-17, pepsinogen I, pepsinogen II levels and pepsinogen I/II ratios
| H. pylori status | n | G-17, pmol/L | PG I, µg/L | PG II, µg/L | PG I/PG II |
| H. pylori (+) | 402 | 7.9 ± 8.9a | 131.4 ± 86.6 | 17.3 ±13.2a | 8.4 ± 4.8a |
| H. pylori (-) | 121 | 3.6 ± 5.2 | 117.0 ± 76.7 | 10.5 ± 9.2 | 12.8 ± 6.5 |
| Type I H. pylori (+) | 291 | 8.5 ± 9.5ac | 134.2 ± 86.4 | 18.7 ± 13.7ac | 7.7± 4.2ac |
| Type II H. pylori (+) | 111 | 6.1 ± 6.5a | 123.9 ± 86.9 | 13.9 ± 11.0 | 10.5 ± 5.6a |
Data are presented as mean ± standard deviation.
P < 0.05, when compared with H. pylori-negative group;
P < 0.05, when compared between type I and type II H. pylori-infected groups. G-17: Gastrin-17; H. pylori: Helicobacter pylori; PG I: Pepsinogen I; PG I/PG II : Pepsinogen I/II ratio; PG II: Pepsinogen II.
Receiver operating characteristic curves for G-17, PG I, PG II and PG I / PG II in the prediction of CAG, PU and GC patients
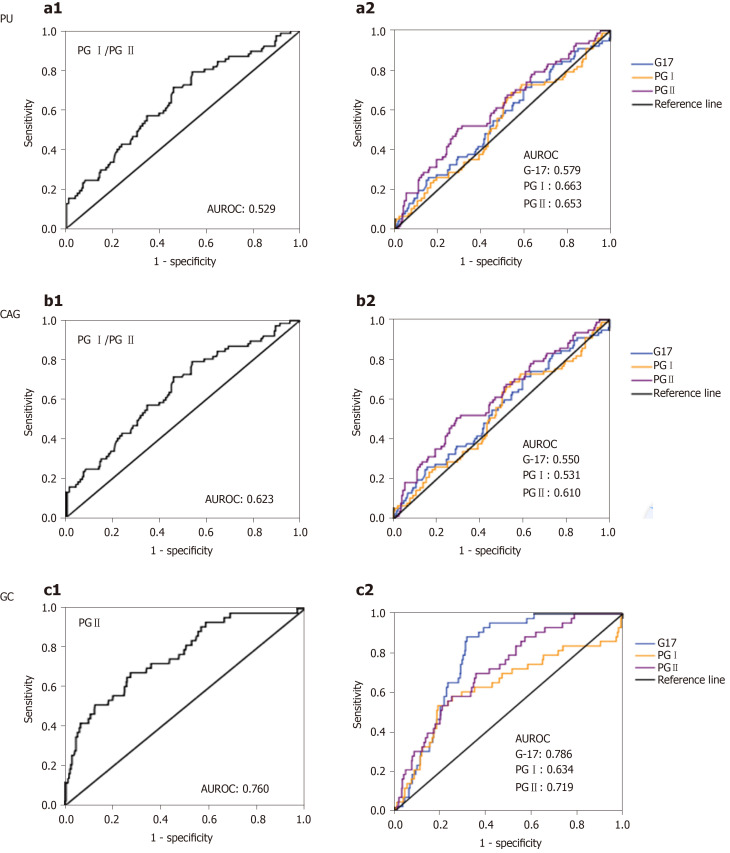
Figure 2 shows ROC curves for G-17, PG I, PG II and PG I/PG II in the prediction of CAG, PU and GC patients. ROC curves for predicting PU based on G-17, PG I, PG II and PG I/PG II ratio are listed in Figure 2 a1-2. Area under the receiver operating characteristic curves (AUROC) were 0.579 (95%confidence interval (CI): 0.514-0.643), 0.663 (95%CI: 0.599-0.729) and 0.653 (95%CI: 0.593-0.713), and 0.529 (95%CI: 0.460-0.598); cut-off values of Youden index were 5.91 pmol/L, 146.20 µg/L, 13.31 µg/L and 9.49, respectively; the sensitivity was 45.7%, 52.1%, 62.8% and 55.3%, respectively; and specificity was 69.4%, 76.9%, 64.5% and 54.0%, respectively.
Figure 2.
Receiver operating characteristic curves for gastrin-17, pepsinogen I, pepsinogen II and pepsinogen I / pepsinogen II in prediction of chronic atrophic gastritis, peptic ulcers and gastric cancer patients. Receiver operating characteristic (ROC) curves of gastrin-17 (G-17), pepsinogen (PG) I, PG II level and PG I/PG II ratio in predicting chronic atrophic gastritis (CAG), peptic ulcers (PU) and gastric cancer (GC) patients. a1-2: ROC curves of PG I/PG II ratio, G-17, PG I and PG II values for predicting PU; b1-2: ROC of PG I/PG II ratio, G-17, PG I and PG II values for predicting CAG; c1-2: ROC of PG I/PG II ratio, G-17, PG I and PG II values for predicting GC. PG: Pepsinogen; PU: Peptic ulcers; AUROC: Area under receiver operating characteristic curve; CAG: Chronic atrophic gastritis; G-17: Gastrin-17; GC: Gastric cancer.
ROC curves for predicting CAG based on G-17, PG I, PG II and PG I/PG II ratio are listed in Figure 2 b1-2. AUROC were 0.550 (95%CI: 0.474-0.626), 0.531 (95%CI: 0.455-0.607), 0.610 (95%CI: 0.535-0.684) and 0.623 (95%CI: 0.552-0.694), respectively; the cut-off values of Youden index were 4.63 pmol/L, 110.7 µg/L, 7.41 µg/L and 9.37, respectively; the sensitivity was 71.4%, 68.8%, 50.5% and 68.8%, respectively; and specificity was 39.4%, 46.0%, 70.9% and 49.8%, respectively.
ROC curves for predicting GC based on G-17, PG I, PG II and PG I/PG II ratio are listed in Figure 2 c1-2, AUROC were 0.786 (95%CI: 0.737-0.834), 0.634 (95%CI: 0.533-0.735), 0.719 (95%CI: 0.646-0.792) and 0.760 (95%CI: 0.684-0.836), respectively; cut-off values of Youden index: 6.21pmol/L, 174.49 µg/L, 14.83 µg/L and 6.47, respectively; the sensitivity was 88.4%, 53.5%, 68.8% and 67.4%, respectively; and specificity was 68.3%, 81.0%, 64.4% and 72.7%, respectively.
Effects of type I and type II H. pylori infection on G-17, PG I, PG II levels and PG I/PG II ratio in gastric diseases
Table 5 shows the stratified disease group analysis results. G-17 levels in NAG, NAGE and GC groups in H. pylori-infected patients were significantly higher than that of H. pylori-negative patients (P < 0.05), and this effect was mostly from type I infection, as type II H. pylori infection and H. pylori-negative group did not show a difference across disease groups. In PU patients, the level of G-17 in type I H. pylori-positive patients was significantly higher than that in type II H. pylori-infected patients (P< 0.05). G-17 levels in the GC group were significantly higher when compared with NAG, NAGE and CAG groups in both H. pylori- and type I H. pylori-infected patients.
Table 5.
Effects of Helicobacter pylori infection on gastric gastrin 17, pepsinogens levels in stepwise chronic gastric diseases
| NAG | NAGE | PU | CAG | GC | |
| G-17 pmol/L level in different groups | |||||
| mean ± SD | 5.6 ± 6.2a | 6.7 ± 8.3a | 9.1 ± 12.2 | 5.1 ± 6.5a | 12.2 ± 7.6 |
| H. pylori (+) | 6.9 ± 6.5ac | 7.5 ± 8.5ac | 9.5 ± 12.6 | 5.2 ± 6.8a | 13.0 ± 7.6c |
| H. pylori (-) | 3.0 ± 4.6 | 3.9 ± 6.6 | 5.5 ± 7.4 | 4.4 ± 5.5 | 6.0 ± 2.5 |
| Type I H. pylori (+) | 7.3 ± 6.7ac | 7.6 ± 8.4ac | 10.9 ± 13.8e | 25.3 ± 7.4a | 13.8 ± 7.9c |
| Type II H. pylori (+) | 6.0 ± 6.2c | 7.3 ± 9.0 | 4.6 ± 4.9 | 4.8 ± 3.2 | 8.9 ± 4.5 |
| PG I µg/L level in different groups | |||||
| mean ± SD | 116.5 ± 72.4ag | 116.0 ± 74.2ag | 168.7 ± 103.7 | 103.1 ± 60.2ag | 168.6 ± 103.5 |
| H. pylori (+) | 117.0 ± 69.8g | 117.5 ± 74.8g | 172.9 ± 105.0 | 101.4 ± 64.4ag | 169.4 ± 104.9 |
| H. pylori (-) | 115.4 ± 77.6 | 110.7 ± 73.6 | 128.8 ± 86.2 | 110.3 ± 64.3 | 162.0 ± 103.7 |
| Type I H. pylori (+) | 118.4 ± 67.5ag | 113.9 ± 62.6ag | 178.1 ± 109.6 | 100.6 ± 65.0ag | 170.8 ± 96.3 |
| Type II H. pylori (+) | 114.0 ± 75.1 | 123.4 ± 92.9 | 154.9 ± 87.2 | 105.9 ± 78.6 | 162.1 ± 154.5 |
| PG II µg/L level in different groups | |||||
| mean ± SD | 13.7 ± 11.2ag | 14.4 ± 12.1a | 19.5 ± 14.3 | 13.8 ± 10.1ag | 24.5 ± 15.9 |
| H. pylori (+) | 15.3 ± 11.3ac | 15.9 ± 13.0ac | 20.4 ± 14.7c | 14.8 ± 11.0ac | 25.1 ± 15.9 |
| H. pylori (-) | 10.5 ± 10.2 | 8.9 ± 5.5 | 10.8 ± 4.8 | 8.7 ± 4.3 | 20.6 ± 16.5 |
| Type I H. pylori (+) | 17.1 ± 12.0ce | 16.5 ± 11.3c | 21.9 ± 16.0ce | 14.7 ± 11.3ac | 25.5 ± 16.3 |
| Type II H. pylori (+) | 11.5 ± 8.6 | 14.9 ± 15.5 | 15.1 ± 6.8 | 15.4 ± 9.6c | 22.8 ± 15.3 |
| PG I/PG II ratios in different groups | |||||
| mean ± SD | 10.6 ± 6.3ai | 9.6 ± 5.4a | 9.7 ± 4.3a | 7.9 ± 4.4a | 5.6 ± 3.8 |
| H. pylori (+) | 9.3 ± 5.6aci | 8.7 ± 4.6ac | 9.5 ± 4.4ai | 6.8 ± 3.3c | 5.1 ± 3.0 |
| H. pylori (-) | 13.2 ± 7.0 | 13.1 ± 6.7 | 11.8 ± 3.7 | 12.8 ± 4.7 | 9.1 ± 7.3 |
| Type I H. pylori (+) | 8.2 ± 4.9ace | 7.8 ± 3.3ace | 9.2 ± 4.2a | 6.5 ± 3.2c | 4.7 ± 2.9c |
| Type II H. pylori (+) | 11.4 ± 6.3 | 10.3 ± 5.9 | 10.7 ± 4.9 | 8.8 ± 3.4c | 7.6 ± 2.1 |
P < 0.05, vs gastric cancer patients;
P < 0.05, vs H. pylori-negative patients in the same disease groups;
P < 0.05, type I and type II H. pylori-positive patients were compared in the same disease groups;
P < 0.05, vs peptic ulcer patients;
P < 0.05, vs chronic atrophic gastritis patients. CAG: Chronic atrophic gastritis; G-17: Gastrin 17; GC: Gastric cancer; H. pylori: Helicobacter pylori; NAG: Non-atrophic gastritis; NAGE: Non-atrophic gastritis with erosion; PU: Peptic ulcer; PG I: Pepsinogen I; PG II: Pepsinogen II; PG I/PG II: Pepsinogen I /pepsinogen II ratio.
PG I levels in type II H. pylori-positive and H. pylori-negative patients showed no difference among all disease groups, while PG I level in GC group was significantly higher when compared with NAG, NAGE and CAG groups only in both type I and H. pylori-positive patients (P < 0.05).
PG II levels in H. pylori- and type I H. pylori-infected patients were significantly higher in NAG, NAGE, PU and CAG groups when compared with H. pylori-negative patients (P < 0.05) except in the GC group, which showed no difference. PG II levels in H. pylori-negative and type II H. pylori-infected patients had no difference among all disease groups. In H. pylori-infected patients, PG II level was significantly higher in the GC group than that in NAG, NAGE and CAG groups. PG II level was also higher in the GC group over the CAG group in type I H. pylori-infected patients.
PG I/PG II ratios of H. pylori- and type I H. pylori-positive patients were lower when compared with H. pylori-negative patients in NAG, NAGE and CAG groups (P < 0.05). Except for the CAG group, PG I/PG II ratios in type II H. pylori-infected groups were not significantly different from those in H. pylori-negative groups (P > 0.05). In type II and H. pylori-negative patients, PG I/PG II ratios showed no difference across different disease groups (P > 0.05); while PG I/PG II ratio of both H. pylori- and type I H. pylori-positive patients was significantly lower in the GC group when compared with the NAG, NAGE and PU groups (P < 0.05) (Table 5).
DISCUSSION
H. pylori cytotoxins CagA and VacA are major virulence factors and pathogenic mechanisms. Virulent strains are associated with increased risk of gastroduodenal disorders, but virulence varied among different strains of H. pylori[4-6,13]. Clinical relevance of type I H. pylori infection in gastric disease and gastric cancer has been very well defined, but the mechanism of type II H. pylori-induced gastric disease needs further exploration. The present results have demonstrated that type I H. pylori is the major form of infection in stepwise gastric diseases in present region, which is a high gastric cancer risk area[8].
Meta-analyses[4,14] have shown that CagA seropositivity are correlated with the occurrence of gastric cancer among H. pylori-infected patients. Sheikh et al[15] examined 201 patients infected by H. pylori and found that cagA gene was detected in 66.7% of the isolates; positive rates of cagA gene in gastric cancer and peptic ulcer patients were 68.2% and 71%, respectively. Kim et al[16] found that H. pylori infection in South Koreans was closely related to highly virulent strains [vacA s1/i1/m1, cagA (+), iceA1 (+), and oipA (+)] and has an intimate association with the progression of peptic ulcer diseases. A recent survey of the high gastric cancer incidence area in Shandong Province, China showed[17] that seroprevalences of CagA, VacA antibodies in 573 H. pylori-infected patients were 83.9%, 38.9%, respectively; suggesting that CagA-positive strains are the dominant form of infection in Chinese population and are associated with progression of gastric mucosal lesions.
Here, we noted a high infection rate of H. pylori in GC patients, where 88.4% of GC are H. pylori positive, 84.2% of them are type I infection, and only 11.6% of GC are H. pylori negative. Such a high rate of H. pylori infection in GC patients has not been reported in this area previously. A Japanese study in 2011 indicated that H. pylori negative gastric cancers are rare in Japan[18], and both intestinal type and diffuse type of gastric cancer are closely related to H. pylori infection[19]. The current results are in line with these results, and indicate an important role of type I H. pylori in the development of upper gastrointestinal diseases and gastric cancer. It is also worth exploring further the mechanism of H. pylori-negative and type II H. pylori-infected GC patients.
PG I is produced by chief cells and mucosal neck cells in the fundic glands, whereas PG II is produced in fundic glands, pyloric glands and Brunner’s glands. Gastric inflammation can lead to increased release of both pepsinogens into the bloodstream, with a greater increase in PG II than in PG I[20]. Other previous studies[8,9,21] have reported that low PG I levels and/or low PG I/PG II ratios are associated with increased risk of gastric cancer. PG I < 70 µg/L and PG I/PG II ratio < 3.0 have been frequently applied as the thresholds for defining population with high-risk of gastric cancer. H. pylori infection causes chronic inflammation of gastric mucosa[22] and affects the secretion of PGs and gastrin, but very few studies have explored the different effects of type I and type II H. pylori on G-17 and PGs levels.
In this study, H. pylori-positive patients have high levels serum PG II and G-17 and lower levels of PG I/PG II ratio when compared with H. pylori-negative patients. The results are in agreement with previous reports[20,23]. Our study further found that type I H. pylori infection result in a significant increase in PG II levels and a marked decline of PG I/PG II ratios when compared with type II H. pylori infection, which is an effect that has not been shown previously. We also noted that PG I levels have no difference in H. pylori-negative and type II H. pylori-positive patients across different disease groups. PG I levels were significantly increased in GC and PU patients in H. pylori- and type I H. pylori-infected group over the NAG, NAGE and CAG group patients. The results differ from previous reports that found low PG I level is associated with GC risk[24], probably due to different analysis methods or patients population involved. Further research is needed to explore the effect of H. pylori on PG I level in different gastric diseases.
Hypergastrinemia is more common in patients with H. pylori-infected gastroduodenal diseases, which may be one cause of precancerous lesions or gastric cancer[25,26]. Gastrin is synthesized and secreted from antral G-cells. H. pylori-associated gastritis tends to raise serum gastrin levels, which is possibly due to hyperplasia of antral G-cells and an acid-suppressive effect of chronic gastritis when the corpus mucosa is involved. Our study shows a significant increase in G-17 in H. pylori-positive patients, which is consistent with previous results[25,26], and we further find that the levels of G-17 in type I H. pylori infected patients are significantly higher than that in type II H. pylori-infected patients (P < 0.05). The response of G-17 to H. pylori appears to be disease stage dependent, as in PU and CAG patients; there is no difference when compared with H. pylori-negative controls. Further studies are required to elucidate its pathogenesis in H. pylori-induced carcinogenesis.
In the current study, as the disease progresses, the levels of G-17, PG I, PG II and PG I/PG II ratios do not show differences in all H. pylori-negative and type II H. pylori-positive groups. In contrast, in type I H. pylori-positive groups, G-17, PG I and PG II levels are significantly higher and PG I/PG II ratios are lower in GC patients when compared with NAG, NAGE and CAG patients. The explanation of the results is probably due to the presence of CagA-, VacA-proteins, which are major H. pylori virulence factors that cause greater epithelial injury and mucosal inflammation, including the release of inflammatory mediators or cytokines that affect epithelial cell homeostasis, therefore resulting in disturbed secretion of PGs and gastrin[27,28].
Application of G-17, PG I, PG II levels and PG I/PG II ratios in gastric cancer epidemiology study have been reported extensively, although their predicative values in stepwise gastric disease in clinical studies have not been very well defined. Previous investigations have generated inconsistent conclusions[9-12], and our results using AUROC analysis (Figure 2) indicated relatively low predictive value range from 0.529-0.786 for PU, CAG and GC patients, and type I H. pylori infection is the major factor that affects their levels as the disease progresses. These data provide insight to evaluate their application during clinical practice and are helpful in explaining the results.
In CAG patients, we also noticed that our PGI level and PG I/PG II ratios are slightly higher when compared with other reports[9-12], and as all the CAG patients have histological confirmation, we therefore consider this effect could be due to patient population- or region-based variations, or might be due to variations from the degrees of atrophy itself. Future studies are required to explain these discrepancies.
Results of this study also show that H. pylori infection rate in our hospitalized patients is 76.9%, which is higher than that in the general population[1,2]. This could be because all patients enrolled in this study have gastrointestinal symptoms, so the infection rate of H. pylori is reasonably higher. One important difference between the current investigation and previous studies is that screening criteria for patients with H. pylori infection is more strict, as patients with only both 13C-BUT and serological H. pylori antibody positive were enrolled. This selection makes our research results more accurate and reliable, allowing for the true status of H. pylori infection and reducing the possibility of false-positives and -negatives. Moreover, the fact that the proportions of H. pylori infection rates prior- and post-patient selections are almost identical indicates that the data analyses are reliable and not biased.
In conclusion, the present study evaluated type I and type II H. pylori infection status in chronic gastric disease and their impact on commonly used gastric cancer risk markers, such as PG I and PG II levels and PG I/PG II ratio in this high gastric cancer risk area. We noted that H. pylori infection rate is high in our gastric cancer patients, and only about 11.6% of gastric cancers were H. pylori negative. Type I H. pylori was the major form of infection. Our results also reveal that the effects of H. pylori on PG I, PG II and PG I/PG II ratio are mostly from type I strain infection and not from type II strain infection. The data provide insight in H. pylori-induced carcinogenesis and will be helpful to guide clinical practice for H. pylori eradication, to explain G-17 and PGs clinical results and for disease prevention.
ARTICLE HIGHLIGHTS
Research background
Type I Helicobacter pylori (H. pylori) is the major form of H. pylori infection in many areas globally. However, its infection status and role in stepwise gastric disease in this gastric cancer prevalent area have not been studied. Its impact on the commonly used gastric cancer risk markers such as gastrin-17 (G-17) and pepsinogens (PGs) is also not known.
Research objectives
We investigated the prevalence of type I and type II H. pylori infection in stepwise gastric diseases and the clinical implications; their impact on G-17 and PGs levels during routine clinical practice were also evaluated.
Research methods
Five hundred and twenty-three hospital admitted patients were enrolled in this study. H. pylori infection was confirmed by both 13C-urea breath test and serological assay. Their serological G-17, PG I, PG II values and PG I/PG II ratio were also measured. Receiver operating characteristic curves were used to calculate the overall diagnostic performance of G-17, PG I, PG II and PG I/PG II ratio in peptic ulcers (PU), chronic atrophic gastritis (CAG) and gastric cancer (GC) patients to determine the best cutoff values, sensitivity and specificity.
Research results
The infection rate of 523 enrolled patients was 76.9%, among which type I H. pylori infection accounted for 72.4%, and type II was 27.6%. Overall, 88.4% of GC patients were H. pylori positive, 84.2% of them were type I infection, and only 11.6% of GC patients were H. pylori negative. H. pylori infection resulted in significantly higher G-17 and PG II values and decreased PG I/PG II ratio. Both types of H. pylori induced higher G-17 level, but type I strain infection resulted in an increased PG II level and decreased PG I/PG II ratio in NAG, NAGE and CAG patients. PG I levels showed no difference among disease groups, and only showed a difference in stratified analysis in GC and PU patients. The diagnostic performance of G-17, PG I, PG II and PG I/PG II ratio in PU, CAG and GC patients indicated relatively low predictive value.
Research conclusions
Type I H. pylori infection is the major form of infection in this geographic region, and a very low percentage (11.6%) of GC patients are not infected by H. pylori. Both types of H. pylori induce an increased G-17 level, while type I H. pylori is the major strain that affects PG I, PG II levels and PG I/PG II ratio in stepwise chronic gastric diseases.
Research perspectives
The results provide insight on H. pylori infection status in hospital admitted patients, and their impact on G-17 and PGs levels, which will be helpful to guide H. pylori eradication and explain G-17 and PGs assay results in clinical practice.
ACKNOWLEDGEMENTS
Authors are grateful to the staff of the Department of Gastroenterology and Hepatology, People’s Hospital of Zhengzhou University for their valuable assistance in this work.
Footnotes
Institutional review board statement: This study was approved by the Ethics Committee of People’s Hospital of Zhengzhou University (ID: 2019-KY-No. 10), Zhengzhou, China.
Conflict-of-interest statement: Authors declare no competing interests.
STROBE statement: The authors have read the STROBE Statement, and the manuscript was prepared and revised according to the STROBE Statement.
Manuscript source: Unsolicited manuscript
Peer-review started: February 24, 2020
First decision: March 27, 2020
Article in press: June 4, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeon CH, Kishikawa H, Link A S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Ma YJ
Contributor Information
Lin Yuan, Department of Gastroenterology and Hepatology, People's Hospital of Zhengzhou University, Henan Province, China; Henan Provincial People’s Hospital, Henan Province, China.
Jun-Bo Zhao, Department of Gastroenterology and Hepatology, People's Hospital of Zhengzhou University, Henan Province, China; Henan Provincial People’s Hospital, Henan Province, China.
Ying-Lei Zhou, Department of Gastroenterology and Hepatology, People's Hospital of Zhengzhou University, Henan Province, China; Henan Provincial People’s Hospital, Henan Province, China; Henan University School of Medicine, Zhengzhou 450003, Henan Province, China.
Ya-Bin Qi, Department of Gastroenterology and Hepatology, People's Hospital of Zhengzhou University, Henan Province, China; Henan Provincial People’s Hospital, Henan Province, China; Henan University School of Medicine, Zhengzhou 450003, Henan Province, China.
Qiong-Ya Guo, Department of Gastroenterology and Hepatology, People's Hospital of Zhengzhou University, Henan Province, China; Henan Provincial People’s Hospital, Henan Province, China; Henan University School of Medicine, Zhengzhou 450003, Henan Province, China.
Hai-Hui Zhang, Department of Gastroenterology and Hepatology, People's Hospital of Zhengzhou University, Henan Province, China; Henan Provincial People’s Hospital, Henan Province, China; Henan University School of Medicine, Zhengzhou 450003, Henan Province, China.
Muhammad Noman Khan, Department of Gastroenterology and Hepatology, People's Hospital of Zhengzhou University, Henan Province, China; Henan Provincial People’s Hospital, Henan Province, China; Henan University School of Medicine, Zhengzhou 450003, Henan Province, China.
Ling Lan, Department of Gastroenterology and Hepatology, People's Hospital of Zhengzhou University, Henan Province, China; Henan Provincial People’s Hospital, Henan Province, China; Henan University School of Medicine, Zhengzhou 450003, Henan Province, China.
Chang-He Jia, Department of Gastroenterology and Hepatology, People's Hospital of Zhengzhou University, Henan Province, China; Henan Provincial People’s Hospital, Henan Province, China; Henan University School of Medicine, Zhengzhou 450003, Henan Province, China.
Yan-Rui Zhang, Department of Gastroenterology and Hepatology, People's Hospital of Zhengzhou University, Henan Province, China; Henan Provincial People’s Hospital, Henan Province, China; Henan University School of Medicine, Zhengzhou 450003, Henan Province, China.
Song-Ze Ding, Department of Gastroenterology and Hepatology, People's Hospital of Zhengzhou University, Henan Province, China; Henan Provincial People’s Hospital, Henan Province, China; Henan University School of Medicine, Zhengzhou 450003, Henan Province, China. dingsongze@hotmail.com.
Data sharing statement
No additional data are available.
References
- 1.Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 2.Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB, Du YQ, Lu NH Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. doi: 10.1111/hel.12475. [DOI] [PubMed] [Google Scholar]
- 3.Banić M, Franceschi F, Babić Z, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection. Helicobacter. 2012;17 Suppl 1:49–55. doi: 10.1111/j.1523-5378.2012.00983.x. [DOI] [PubMed] [Google Scholar]
- 4.Matos JI, de Sousa HA, Marcos-Pinto R, Dinis-Ribeiro M. Helicobacter pylori CagA and VacA genotypes and gastric phenotype: a meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:1431–1441. doi: 10.1097/MEG.0b013e328364b53e. [DOI] [PubMed] [Google Scholar]
- 5.Jones KR, Whitmire JM, Merrell DS. A Tale of Two Toxins: Helicobacter pylori CagA and VacA Modulate Host Pathways that Impact Disease. Front Microbiol. 2010;1:115. doi: 10.3389/fmicb.2010.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rick JR, Goldman M, Semino-Mora C, Liu H, Olsen C, Rueda-Pedraza E, Sullivan C, Dubois A. In situ expression of cagA and risk of gastroduodenal disease in Helicobacter pylori-infected children. J Pediatr Gastroenterol Nutr. 2010;50:167–172. doi: 10.1097/MPG.0b013e3181bab326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiang Z, Censini S, Bayeli PF, Telford JL, Figura N, Rappuoli R, Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995;63:94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Chen Q, Quan P, Zhang M, Zhang S, Guo L, Sun X, Wang C. Cancer incidence and mortality in Henan province, 2012. Chin J Cancer Res. 2016;28:275–285. doi: 10.21147/j.issn.1000-9604.2016.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang TH, Chiu SY, Chen SL, Yen AM, Fann JC, Liu CY, Chou CK, Chiu HM, Shun CT, Wu MS, Lin JT, Lee YC, Chen TH, Lin MW. Serum Pepsinogen as a Predictor for Gastric Cancer Death: A 16-Year Community-based Cohort Study. J Clin Gastroenterol. 2019;53:e186–e193. doi: 10.1097/MCG.0000000000000992. [DOI] [PubMed] [Google Scholar]
- 10.Kudo T, Kakizaki S, Sohara N, Onozato Y, Okamura S, Inui Y, Mori M. Analysis of ABC (D) stratification for screening patients with gastric cancer. World J Gastroenterol. 2011;17:4793–4798. doi: 10.3748/wjg.v17.i43.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNicholl AG, Forné M, Barrio J, De la Coba C, González B, Rivera R, Esteve M, Fernandez-Bañares F, Madrigal B, Gras-Miralles B, Perez-Aisa A, Viver-Pi-Sunyer JM, Bory F, Rosinach M, Loras C, Esteban C, Santolaria S, Gomollon F, Valle J, Gisbert JP Helicobacter pylori Study Group of Asociación Española de Gastroenterología (AEG) Accuracy of GastroPanel for the diagnosis of atrophic gastritis. Eur J Gastroenterol Hepatol. 2014;26:941–948. doi: 10.1097/MEG.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koivusalo AI, Pakarinen MP, Kolho KL. Is GastroPanel serum assay useful in the diagnosis of Helicobacter pylori infection and associated gastritis in children? Diagn Microbiol Infect Dis. 2007;57:35–38. doi: 10.1016/j.diagmicrobio.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Ding SZ, Goldberg JB, Hatakeyama M. Helicobacter pylori infection, oncogenic pathways and epigenetic mechanisms in gastric carcinogenesis. Future Oncol. 2010;6:851–862. doi: 10.2217/fon.10.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiota S, Matsunari O, Watada M, Yamaoka Y. Serum Helicobacter pylori CagA antibody as a biomarker for gastric cancer in east-Asian countries. Future Microbiol. 2010;5:1885–1893. doi: 10.2217/fmb.10.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheikh AF, Yadyad MJ, Goodarzi H, Hashemi SJ, Aslani S, Assarzadegan MA, Ranjbar R. CagA and vacA allelic combination of Helicobacter pylori in gastroduodenal disorders. Microb Pathog. 2018;122:144–150. doi: 10.1016/j.micpath.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Kim N, Nam RH, Suh JH, Chang H, Lee JW, Kim YS, Kim JM, Choi JW, Park JG, Lee YS, Lee DH, Jung HC. Association of polymorphisms in virulence factor of Helicobacter pylori and gastroduodenal diseases in South Korea. J Gastroenterol Hepatol. 2014;29:984–991. doi: 10.1111/jgh.12509. [DOI] [PubMed] [Google Scholar]
- 17.Pan KF, Formichella L, Zhang L, Zhang Y, Ma JL, Li ZX, Liu C, Wang YM, Goettner G, Ulm K, Classen M, You WC, Gerhard M. Helicobacter pylori antibody responses and evolution of precancerous gastric lesions in a Chinese population. Int J Cancer. 2014;134:2118–2125. doi: 10.1002/ijc.28560. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo T, Ito M, Takata S, Tanaka S, Yoshihara M, Chayama K. Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter. 2011;16:415–419. doi: 10.1111/j.1523-5378.2011.00889.x. [DOI] [PubMed] [Google Scholar]
- 19.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 20.Tu H, Sun L, Dong X, Gong Y, Xu Q, Jing J, Yuan Y. Serum anti-Helicobacter pylori immunoglobulin G titer correlates with grade of histological gastritis, mucosal bacterial density, and levels of serum biomarkers. Scand J Gastroenterol. 2014;49:259–266. doi: 10.3109/00365521.2013.869352. [DOI] [PubMed] [Google Scholar]
- 21.Huang YK, Yu JC, Kang WM, Ma ZQ, Ye X, Tian SB, Yan C. Significance of Serum Pepsinogens as a Biomarker for Gastric Cancer and Atrophic Gastritis Screening: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0142080. doi: 10.1371/journal.pone.0142080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adamu MA, Weck MN, Rothenbacher D, Brenner H. Incidence and risk factors for the development of chronic atrophic gastritis: five year follow-up of a population-based cohort study. Int J Cancer. 2011;128:1652–1658. doi: 10.1002/ijc.25476. [DOI] [PubMed] [Google Scholar]
- 23.Shan JH, Bai XJ, Han LL, Yuan Y, Sun XF. Changes with aging in gastric biomarkers levels and in biochemical factors associated with Helicobacter pylori infection in asymptomatic Chinese population. World J Gastroenterol. 2017;23:5945–5953. doi: 10.3748/wjg.v23.i32.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bornschein J, Selgrad M, Wex T, Kuester D, Malfertheiner P. Serological assessment of gastric mucosal atrophy in gastric cancer. BMC Gastroenterol. 2012;12:10. doi: 10.1186/1471-230X-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malfertheiner P. The intriguing relationship of Helicobacter pylori infection and acid secretion in peptic ulcer disease and gastric cancer. Dig Dis. 2011;29:459–464. doi: 10.1159/000332213. [DOI] [PubMed] [Google Scholar]
- 26.Sun L, Tu H, Liu J, Gong Y, Xu Q, Jing J, Dong N, Yuan Y. A comprehensive evaluation of fasting serum gastrin-17 as a predictor of diseased stomach in Chinese population. Scand J Gastroenterol. 2014;49:1164–1172. doi: 10.3109/00365521.2014.950693. [DOI] [PubMed] [Google Scholar]
- 27.Di Mario F, Cavallaro LG, Moussa AM, Caruana P, Merli R, Maini A, Bertolini S, Dal Bó N, Rugge M, Cavestro GM, Aragona G, Plebani M, Franzé A, Nervi G. Usefulness of serum pepsinogens in Helicobacter pylori chronic gastritis: relationship with inflammation, activity, and density of the bacterium. Dig Dis Sci. 2006;51:1791–1795. doi: 10.1007/s10620-006-9206-1. [DOI] [PubMed] [Google Scholar]
- 28.Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15:306–316. doi: 10.1016/j.chom.2014.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.