Abstract
Coronaviruses are enveloped positive-strand RNA viruses belonging to family Coronaviridae and order Nidovirales which cause infections in birds and mammals. Among the human coronaviruses, highly pathogenic ones are Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome coronavirus (MERS-CoV) which have been implicated in severe respiratory syndrome in humans. There are no approved antiviral drugs or vaccines for the treatment of human CoV infection to date. The recent outbreak of new coronavirus pandemic, coronavirus disease 2019 (COVID-19) has caused a high mortality rate and infections around the world which necessitates the need for the discovery of novel anti-coronaviral drugs. Among the coronaviruses proteins, 3C-like protease (3CLpro) is an important drug target against coronaviral infection as the auto-cleavage process catalysed by the enzyme is crucial for viral maturation and replication. The present work is aimed at the identification of suitable lead molecules for the inhibition of 3CLpro enzyme via a computational screening of the Food and Drug Administration (FDA) approved antiviral drugs and phytochemicals. Based on binding energies and molecular interaction studies, we shortlisted five lead molecules (both FDA approved drugs and phytochemicals) for each enzyme targets (SARS-CoV-2 3CLpro, SARS-CoV 3CLpro and MERS-CoV 3CLpro). The lead molecules showed higher binding affinity compared to the standard inhibitors and exhibited favourable hydrophobic interactions and a good number of hydrogen bonds with their respective targets. A few promising leads with dual inhibition potential were identified among FDA approved antiviral drugs which include DB13879 (Glecaprevir), DB09102 (Daclatasvir), molecule DB09297 (Paritaprevir) and DB01072 (Atazanavir). Among the phytochemicals, 11,646,359 (Vincapusine), 120,716 (Alloyohimbine) and 10,308,017 (Gummadiol) showed triple inhibition potential against all the three targets and 102,004,710 (18-Hydroxy-3-epi-alpha-yohimbine) exhibited dual inhibition potential. Hence, the proposed lead molecules from our findings can be further investigated through in vitro and in vivo studies to develop into potential drug candidates against human coronaviral infections.
Keywords: Coronaviruses, 3C-like protease, SARS-CoV, MERS-CoV, Molecular docking, Virtual screening, FDA approved drugs, Antiviral drugs, Phytochemicals
1. Introduction
Coronaviruses belong to the Coronavirinae subfamily, family Coronaviridae and order Nidovirales. The subfamily members based on genomic structure and phylogenetic study can be classified under four genera — Alpha-, Beta-, Gamma- and Delta-coronavirus (Cui et al., 2019). The first two genera cause infections in only mammals while birds and mammals are commonly infected by Gamma- and Delta-coronaviruses (Woo et al., 2012). While Alpha-coronaviruses and Beta-coronaviruses are known to cause gastroenteritis in animals, in humans, they commonly cause respiratory distress (Cui et al., 2019). There are four human coronaviruses such as HCoV-229E, HKU1, HCoV-NL63 and HCoV-OC43 which induce mild upper respiratory infections and two highly pathogenic ones, such as Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) and Middle East Respiratory Syndrome coronavirus (MERS-CoV) implicated in severe respiratory syndrome in humans (Forni et al., 2017, Su et al., 2016). All human coronaviruses are reported to have animal origins based on the current sequence studies, for example, SARS-CoV, MERS-CoV, HCoV-229E and HCoV-NL63 have been originated in bats while HKU1 and HCoV-OC43 are probably linked to rodents (Forni et al., 2017, Su et al., 2016). The intermediate hosts such as domestic animals may play a significant role in facilitating the easy transfer of viruses from natural hosts to humans (Cui et al., 2019). Furthermore, domestic animals themselves are susceptible to bat-borne or closely related coronavirus diseases (Lacroix et al., 2017, Simas et al., 2015). At present, 7 of 11 species of Alpha-coronavirus specified by the International Committee on Taxonomy of Viruses (ICTV) and 4 of 9 species of Beta-coronavirus have been reported only in bats. Consequently, bats are probably the main natural reservoirs of Alpha- and Beta-coronaviruses (Woo et al., 2012).
Coronaviruses are enveloped viruses of about 80–120 nm in diameter, with round and often pleiomorphic virions. They contain positive-strand RNA, with the largest genome (~30 kb) known till date (Lai, 2001). A helical capsid found within the viral membrane is composed of genomic RNA complexed with the basic nucleocapsid (N) protein. All coronaviruses display at least three membrane viral proteins. This includes type I glycoprotein, spike (S) protein which forms peplomers on the surface and gives a characteristic crown-like appearance, the membrane (M) protein and a small membrane protein, an envelope protein (E). All coronaviruses have a similar genomic structure (Weiss and Navas-Martin, 2005). The replicase gene located within 5′ region approximately 20–22 kb encodes several enzymatic activities. The gene products of the replicase are encoded within two very large open reading frames, ORFs 1a and 1b, which are translated by a frameshift mechanism into two large polypeptides, pp1a and pp1ab (Gorbalenya, 2001, Lee et al., 1991). With the help of S protein, coronaviruses bind to their specific host cellular receptors. On gaining entry into the cell, pp1a and pp1ab are translated from the viral genome RNA, ORFs 1a and 1b (Bredenbeek et al., 1990, Brian and Baric, 2005). The ORF1a encodes a picornavirus 3C-like protease (3CLpro) and one or two papain-like proteases (PLpro or PLP). These proteases catalyze the processing of viral pp1a and pp1ab into the mature replicase proteins (Lee et al., 1991, Ziebuhr et al., 2001). The enzymes such as RNA-dependent RNA polymerase (RdRp), a helicase (1 1 6) and others are encoded in ORF 1b and processed from pp1ab (Gorbalenya, 2001). The metabolism of coronavirus RNA and disruption of host cell processes are believed as a result of the catalytic activities of various enzymes (Ziebuhr, 2005).
As aforementioned, SARS- and MERS-CoVs genome harbours two ORFs: ORF1a and ORF1b wherein ORF1a encodes two cysteine proteases viz; a papain-like protease (PLpro) and a 3C-like protease (3CLpro) also known as main protease (Mpro). While PLpro manages cleavage on the first three cutting sites of its polyprotein, 3CLpro is responsible for cleavage at other eleven positions causing the release of sixteen non-structural proteins (nsp) (Jo et al., 2020). The crystal structures of SARS- and MERS-CoVs 3CLpro reveal the presence of three structural domains in each monomer wherein domains I and II has a characteristic chymotrypsin-like fold with a catalytic cysteine and are linked to a third C-terminal domain by a long loop (Needle et al., 2015). Therefore, 3CLpro is an important drug target against coronaviral infection as the auto-cleavage process is indispensable for viral maturation and replication (Jo et al., 2020).
The recent outbreak of new coronavirus pandemic or coronavirus disease 2019 (COVID-19) has caused high mortality rate and infections around the world (Wu et al., 2020, Zhou et al., 2020) warrants the need for the discovery of new effective antiviral therapeutics against coronaviral infections. There are no approved antiviral drugs or vaccines for the treatment of human CoV infection to date, though many candidate therapeutics have been investigated in pre-clinical studies (Abd El-Aziz and Stockand, 2020, Dhama et al., 2020, Graham et al., 2013, Lundstrom, 2020, Padron-Regalado, 2020). Although many attempts have been previously made by workers to identify specific inhibitors for 3CLpro enzymes, a few studies have been done to target all the three coronavirus protease enzymes (SARS-CoV-2 3CLpro, SARS-CoV 3CLpro and MERS-CoV 3CLpro) using small molecules. In this study we aimed at finding suitable lead molecules for the inhibition of 3CLpro enzymes through virtual screening of two chemical datasets viz; Food and Drug Administration (FDA) approved antiviral drugs and selected phytochemicals. We have proposed five lead molecules as potential inhibitors for each enzyme targets. These lead molecules could be further investigated for developing as drugs against anti-coronaviral infection.
2. Materials and methods
2.1. Selection and retrieval of phytochemicals and FDA approved drugs
A total of 263 phytochemicals and 75 FDA approved antiviral drugs were retrieved from the database of Indian Plants, Phytochemistry And Therapeutics (IMPPAT) (Mohanraj et al., 2018) and DrugBank database (Wishart et al., 2008) respectively. The three-dimensional structure of the molecules was downloaded in SDF format and the molecules whose only two-dimensional structures were available, were converted into the three-dimensional form using OpenBabel software version 2.4.1 (O’Boyle et al., 2011) and optimized using the Merck molecular force field (MMFF94) (Halgren, 1996).
2.2. Screening of drug-like compounds:
The drug-like compounds from the phytochemicals set were filtered based on Lipinski’s rule of five (Lipinski, 2004), Veber’s rule (Veber et al., 2002) and Adsorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) physicochemical parameters. The physicochemical properties of the compounds were evaluated using DataWarrior program version 5.0 (Sander et al., 2015).
2.3. Protein-preparation
The high resolution three dimensional X-ray crystal structures of the enzyme target: SARS-CoV-2 3CLpro, SARS-CoV 3CLpro and MERS-CoV 3CLpro were retrieved from protein data bank (PDB) (http://www.rcsb.org/) using their accession IDs 6Y2F, 3TNT and 5WKK at a resolution of 1.95 Å, 1.59 Å and 1.55 Å respectively. The heteroatoms including ions, cocrystal ligands (O6K, G85 and AW4 corresponding to PDB IDs: 6Y2F, 3TNT and 5WKK respectively) and water molecules were removed. Hydrogen atoms and Kolmann charges were added to the protein using AutoDockTools 1.5.6 (Morris et al., 2009) and the proteins were converted into PDBQT format.
2.4. Ligand preparation
The selected compounds were prepared for docking using AutoDockTools 1.5.6 (Morris et al., 2009). Hydrogen atoms and Gasteiger charges were added to the selected compounds and the torsions were defined for each compound. The structures were saved in PDBQT format.
2.5. Molecular docking study
The binding affinity of each selected compound along with the control with the three enzyme targets was determined using molecular docking approach. The binding sites for the docking were defined by placing a grid box of suitable dimensions centred at each cocrystallized ligand (Table 1 ). Autodock Vina was used for carrying out molecular docking, which performs docking calculations based on sophisticated gradient optimization method (Trott and Olson, 2010). The binding poses were clustered and ranked in the order of their binding affinities. The molecular interactions (hydrogen bonds and hydrophobic interactions between the target proteins and compounds were studied using LigPlot + version 1.4.5 (Laskowski and Swindells, 2011).
Table 1.
The grid box parameters considered for molecular docking studies.
| Enzyme targets | AutoDock Vina Search Space |
||
|---|---|---|---|
| Center | Dimensions (Å) | Exhaustiveness | |
| SARS-CoV-2 3CLpro | x: 10.9372, y: −2.0146, z: 18.2692 | 25 × 25 × 25 | 8 |
| SARS-CoV 3CLpro | x: 25.1486, y: 44.1145, z: −5.6121 | 25 × 25 × 25 | 8 |
| MERS-CoV 3CLpro | x: −21.9860, y: 25.6036, z: 4.0045 | 25 × 25 × 25 | 8 |
3. Results and discussion
3.1. Virtual screening of drug-like compounds:
A set of 75 FDA approved antiviral drugs and 263 phytochemicals belonging to different classes such as prenol lipids, flavonoids, indoles and derivatives, alkaloids, lignans, organooxygen compounds etc. were used for the present study. Since the FDA approved drugs have already undergone the preclinical and clinical trials and tested safe in patients, the drugs were not tested again using in silico drug-like filters. While plant-derived compounds are much safer to use with fewer adverse effects, we subjected them into virtual screening protocol to reduce the drug-attrition rate. We used the rule of five (ROF) and Veber’s rule filters to test the oral bioavailability of the compounds. According to ROF, a compound is considered to be orally bioactive if their physicochemical properties lie within the safe limits (molecular weight ≤ 500 Da, hydrogen bond donors ≤ 5, hydrogen bond acceptors ≤ 10, and an octanol–water partition coefficient log P ≤ 5) (Lipinski, 2004). Veber's rule states that a good oral bioavailable compound possesses number of rotatable bonds ≤ 10 and topological polar surface area ≤ 140 Å2 (Veber et al., 2002). Further, the molecules were also tested for in silico toxicity studies. Out of 263 phytochemicals, 46 molecules were found to be orally bioactive, non-tumorigenic, non-mutagenic, non-irritant and without any side effects on reproductive health. Thus, these 46 phytochemicals and 75 FDA approved drugs were tested further for their inhibitory potential against the three enzyme targets (Table 2, Table 3 ).
Table 2.
List of FDA approved antiviral drugs selected for molecular docking studies.
| Drugs | DrugBank ID | Therapy |
|---|---|---|
| Ombitasvir | DB09296 | Chronic Hepatitis C |
| Elbasvir | DB11574 | Chronic Hepatitis C |
| Sofosbuvir | DB08934 | Chronic Hepatitis C |
| Ledipasvir | DB09027 | Chronic Hepatitis C |
| Famciclovir | DB00426 | Herpes virus infections |
| Simeprevir | DB06290 | Chronic hepatitis C virus |
| Lopinavir | DB01601 | Human immunodeficiency virus type 1 (HIV-1) infection. |
| Tecovirimat | DB12020 | Smallpox |
| Oseltamivir | DB00198 | Influenza viruses A and B infections |
| Baloxavir marboxil | DB13997 | Influenza A and influenza B infections |
| Didanosine | DB00900 | HIV infection |
| Bictegravir | DB11799 | HIV-1 and HIV-2 infection |
| Adefovir dipivoxil | DB00718 | Hepatitis B |
| Zalcitabine | DB00943 | HIV infection |
| Emtricitabine | DB00879 | HIV-1 infection |
| Zidovudine | DB00495 | HIV infection |
| Darunavir | DB01264 | HIV-1 infection |
| Nevirapine | DB00238 | HIV-1 infection and AIDS. |
| Valganciclovir | DB01610 | Cytomegalovirus infections |
| Nelfinavir | DB00220 | HIV infection |
| Foscarnet | DB00529 | cytomegalovirus retinitis, HIV infection |
| Boceprevir | DB08873 | Chronic Hepatitis C |
| Inosine pranobex | DB13156 | Viral infection |
| Dolutegravir | DB08930 | HIV-1 infection |
| Abacavir | DB01048 | HIV infection and AIDS. |
| Edoxudine | DB13421 | Herpes simplex virus type 1 and 2 infection |
| Ribavirin | DB00811 | Hepatitis C and viral hemorrhagic fevers |
| Elvitegravir | DB09101 | HIV-1 infection |
| Amantadine | DB00915 | Influenza A infection |
| Vidarabine | DB00194 | Herpes viruses, the vaccinia virus and varicella zoster virus infection |
| Daclatasvir | DB09102 | Hepatitis C Virus (HCV) infection |
| Tenofovir alafenamide | DB09299 | Chronic hepatitis B and HIV-1 infection |
| Ritonavir | DB00503 | HIV infection |
| Trifluridine | DB00432 | Keratoconjunctivitis and recurrent epithelial keratitis |
| Zanamivir | DB00558 | Influenza A and B virus infection |
| Acyclovir | DB00787 | Herpes simplex, Varicella zoster, herpes zoster infection |
| Ganciclovir | DB01004 | AIDS-associated cytomegalovirus infections. |
| Entecavir | DB00442 | Hepatitis B infection |
| Raltegravir | DB06817 | HIV infection |
| Doravirine | DB12301 | HIV-1 Infection |
| Pibrentasvir | DB13878 | Hepatitis C virus (HCV) infection |
| Fosamprenavir | DB01319 | HIV infection |
| Glecaprevir | DB13879 | HCV infection |
| Tipranavir | DB00932 | HIV infection |
| Etravirine | DB06414 | HIV-1 infection |
| Amprenavir | DB00701 | HIV infection. |
| Letermovir | DB12070 | Cytomegalovirus (CMV) infection |
| Favipiravir | DB12466 | Influenza |
| Idoxuridine | DB00249 | Herpes simplex virus (HSV) infection |
| Rimantadine | DB00478 | Influenza. |
| Tromantadine | DB13288 | Herpes zoster and simplex virus infection |
| Telaprevir | DB05521 | Chronic Hepatitis C |
| Dasabuvir | DB09183 | Chronic Hepatitis C |
| Grazoprevir | DB11575 | Chronic Hepatitis C |
| Docosanol | DB00632 | HSV infection |
| Penciclovir | DB00299 | HSV infections |
| Velpatasvir | DB11613 | chronic Hepatitis C |
| Tenofovir disoproxil | DB00300 | HIV infection and Hepatitis B |
| Cidofovir | DB00369 | CMV retinitis |
| Voxilaprevir | DB12026 | Chronic Hepatitis C |
| Asunaprevir | DB11586 | HCV infection |
| Valaciclovir | DB00577 | Hepatitis, HIV, and cytomegalovirus infection |
| Efavirenz | DB00625 | HIV-1 infection |
| Peramivir | DB06614 | Influenza A/B. |
| Brivudine | DB03312 | Herpes zoster. |
| Telbivudine | DB01265 | Hepatitis B virus infection |
| Maraviroc | DB04835 | HIV infection |
| Stavudine | DB00649 | HIV infection |
| Paritaprevir | DB09297 | Chronic Hepatitis C |
| Indinavir | DB00224 | HIV infection |
| Lamivudine | DB00709 | HIV-1 and hepatitis B virus (HBV) infection |
| Atazanavir | DB01072 | HIV infection |
| Rilpivirine | DB08864 | HIV-1 infection |
| Delavirdine | DB00705 | HIV-1.infection |
| Saquinavir | DB01232 | HIV-1 and HIV-2 infection |
Table 3.
List of phytochemicals selected for the docking studies (MW: molecular weight; cLogP: octanol–water partition coefficient; cLogS: aqueous solubility at 25° and pH = 7.5; HBA: hydrogen bond acceptor; HBD: hydrogen bond donor; TPSA (Å2): Topological polar surface area; RB: rotatable bonds).
| Molecule Name | CASID/CHEMSPIDER/CID | Class | MW | cLogP | cLogS | HBA | HBD | TPSA | RB | Druglikeness |
|---|---|---|---|---|---|---|---|---|---|---|
| Heterophylloidine | 78174–97-7 | Prenol lipids | 383.486 | 2.2205 | −3.41 | 5 | 0 | 63.68 | 2 | 2.7882 |
| Arjunolone | 82178–34-5 | Flavonoids | 284.266 | 2.6114 | −3.17 | 5 | 2 | 75.99 | 2 | 0.40331 |
| Rosicine | 95690–65-6 | Indoles and derivatives | 324.379 | 0.8137 | −3.091 | 5 | 1 | 54.1 | 2 | 2.2389 |
| Asparagamine A | 156798–15-1 | Organooxygen compounds | 385.458 | 2.1085 | −3.634 | 6 | 0 | 57.23 | 3 | 0.7051 |
| Piscrocin B | 752225–57-3 | Heteroaromatic compounds | 198.173 | −0.1119 | −1.262 | 5 | 3 | 90.9 | 2 | 0.20569 |
| 6-Acetylheteratisine | 10,246,449 | Quinolidines | 433.543 | 1.1579 | −3.186 | 7 | 1 | 85.3 | 4 | 4.6497 |
| Gummadiol | 10,308,017 | Furanoid lignans | 386.355 | 2.0507 | −3.752 | 8 | 2 | 95.84 | 2 | 0.1606 |
| Vidolicine | 28,288,759 | Indoles and derivatives | 352.433 | 1.5661 | −3.47 | 5 | 1 | 54.1 | 3 | 2.5754 |
| 19-Hydroxy-11-methoxytabersonine | 57,619,488 | Plumeran-type alkaloids | 382.458 | 1.3822 | −3.246 | 6 | 2 | 71.03 | 4 | 0.90699 |
| Boldine | 10,154 | Aporphines | 327.379 | 2.7882 | −3.129 | 5 | 2 | 62.16 | 2 | 4.6712 |
| Indoline | 10,328 | Indoles and derivatives | 119.166 | 1.3351 | −2.025 | 1 | 1 | 12.03 | 0 | 0.19917 |
| Tubotaiwine | 100,004 | Strychnos alkaloids | 324.423 | 2.3452 | −3.568 | 4 | 1 | 41.57 | 3 | 1.5804 |
| Cinchonidine | 101,744 | Cinchona alkaloids | 294.397 | 2.6804 | −3.079 | 3 | 1 | 36.36 | 3 | 0.88095 |
| Tryptoline | 107,838 | Indoles and derivatives | 172.23 | 1.2188 | −2.39 | 2 | 2 | 27.82 | 0 | 1.1795 |
| Alloyohimbine | 120,716 | Yohimbine alkaloids | 354.448 | 2.3512 | −3.065 | 5 | 2 | 65.56 | 2 | 1.5035 |
| Cuscohygrine | 1,201,543 | Alkaloids and derivatives | 224.347 | 1.2932 | −1.22 | 3 | 0 | 23.55 | 4 | 4.2839 |
| Sebiferine | 10,405,046 | Phenanthrenes and derivatives | 341.406 | 1.9462 | −2.753 | 5 | 0 | 48 | 3 | 5.7459 |
| Condylocarpine | 10,914,255 | Strychnos alkaloids | 322.407 | 2.2523 | −3.304 | 4 | 1 | 41.57 | 2 | 0.29114 |
| 19,20-Dihydroakuammicine | 11,023,792 | Alkaloids and derivatives | 324.423 | 2.3452 | −3.568 | 4 | 1 | 41.57 | 3 | 2.1615 |
| Lochnericine | 11,382,599 | Aspidospermatan-type alkaloids | 352.433 | 1.5996 | −3.538 | 5 | 1 | 54.1 | 3 | 2.3885 |
| 3-Isoajmalicine | 11,416,867 | Yohimbine alkaloids | 352.433 | 2.2674 | −3.141 | 5 | 1 | 54.56 | 2 | 2.6043 |
| Vindolidin | 11,618,751 | Plumeran-type alkaloids | 426.511 | 1.3936 | −3.098 | 7 | 1 | 79.31 | 5 | 3.2845 |
| Vincapusine | 11,646,359 | Alkaloids and derivatives | 368.432 | 2.6409 | −2.695 | 6 | 1 | 63.93 | 3 | 2.2856 |
| Epibubbialine | 11,830,997 | Azaspirodecane derivatives | 221.255 | −0.1874 | −1.424 | 4 | 1 | 49.77 | 0 | 1.6204 |
| Vindoline | 11,953,805 | Plumeran-type alkaloids | 456.537 | 1.3236 | −3.116 | 8 | 1 | 88.54 | 6 | 3.2845 |
| 1,2-Dihydrovomilenine | 11,953,964 | Ajmaline-sarpagine alkaloids | 352.433 | 1.8244 | −3.654 | 5 | 2 | 61.8 | 2 | 1.1872 |
| Pericyclivine | 11,969,544 | Macroline alkaloids | 322.407 | 2.8635 | −3.038 | 4 | 1 | 45.33 | 2 | 0.20237 |
| Lycoctonine | 11,972,492 | Prenol lipids | 467.601 | −0.1645 | −1.824 | 8 | 3 | 100.85 | 6 | 0.56009 |
| Cathanneine | 12,302,545 | Aspidospermatan-type alkaloids | 426.511 | 1.3713 | −3.408 | 7 | 0 | 68.31 | 5 | 2.5051 |
| Anahygrine | 12,306,778 | Alkaloids and derivatives | 224.347 | 1.3823 | −1.852 | 3 | 1 | 32.34 | 4 | 3.0573 |
| Tabernaemontanin | 12,309,360 | Vobasan alkaloids | 354.448 | 2.6197 | −3.678 | 5 | 1 | 62.4 | 3 | 3.1533 |
| 4-Methoxynorsecurinine | 101,091,319 | Pyrrolizidines | 233.266 | −0.0349 | −1.324 | 4 | 0 | 38.77 | 1 | 1.5563 |
| Akuammicine | 101,281,350 | Strychnos alkaloids | 322.407 | 2.2523 | −3.304 | 4 | 1 | 41.57 | 2 | 0.87991 |
| Heterophyllisine | 101,289,617 | Quinolidines | 375.507 | 1.5254 | −3.175 | 5 | 1 | 59 | 2 | 4.4531 |
| Germacranolide | 101,616,641 | Prenol lipids | 266.336 | 2.1659 | −2.371 | 4 | 2 | 66.76 | 0 | 1.5629 |
| Isoajmaline | 101,624,670 | Ajmaline-sarpagine alkaloids | 326.438 | 1.791 | −3.484 | 4 | 2 | 46.94 | 1 | 3.4513 |
| Hetidine | 101,685,340 | Prenol lipids | 357.448 | 0.8838 | −2.601 | 5 | 2 | 77.84 | 0 | 2.8009 |
| Catharosine | 101,686,461 | Plumeran-type alkaloids | 384.474 | 0.909 | −2.688 | 6 | 2 | 73.24 | 3 | 3.3742 |
| Fluorocarpamine | 101,688,177 | Carboxylic acids and derivatives | 339.414 | 1.8706 | −3.095 | 5 | 1 | 58.64 | 2 | 0.97774 |
| Ajmalicidine | 101,927,009 | Indoles and derivatives | 370.447 | 3.0403 | −3.438 | 6 | 1 | 63.93 | 2 | 2.2403 |
| Hetisinone | 101,930,090 | Prenol lipids | 327.423 | 1.0959 | −2.903 | 4 | 2 | 60.77 | 0 | 0.86256 |
| Rhazimol | 101,986,486 | Corynanthean-type alkaloids | 338.406 | 0.2205 | −2.55 | 5 | 2 | 73.13 | 2 | 2.6104 |
| 18-Hydroxy-3-epi-alpha-yohimbine | 102,004,710 | Yohimbine alkaloids | 370.447 | 1.4991 | −2.666 | 6 | 3 | 85.79 | 2 | 2.3334 |
| Sarpagine | 102,090,391 | Macroline alkaloids | 310.396 | 2.4395 | −2.632 | 4 | 3 | 59.49 | 1 | 2.0345 |
| Velbanamine | 102,399,433 | Indoles and derivatives | 298.428 | 3.3453 | −3.206 | 3 | 2 | 39.26 | 1 | 3.5116 |
| Catharosine | 2564–23-0 | Plumeran-type alkaloids | 384.474 | 0.909 | −2.688 | 6 | 2 | 73.24 | 3 | 3.3742 |
3.2. Top ranked lead molecules for SARS-CoV-2 3CLpro from a set of phytochemicals and FDA approved drugs:
The top five leads-102004710 (18-Hydroxy-3-epi-alpha-yohimbine), 120,716 (Alloyohimbine), 10,308,017 (Gummadiol), 156798–15-1 (Asparagamine A) and 11,646,359 (Vincapusine) for SARS-CoV-2 3CLpro obtained using molecular docking studies of phytochemicals showed binding energies of −8.1 kcal/mol, −8.0 kcal/mol, −7.8 kcal/mol, −7.6 kcal/mol and −7.5 kcal/mol respectively. Molecular docking of FDA approved antiviral drugs yielded top 5 lead molecules-DB06290 (Simeprevir), DB09027 (Ledipasvir), DB09297 (Paritaprevir), DB13879 (Glecaprevir) and DB09102 (Daclatasvir) which showed binding energies of −9.7 kcal/mol, −9.3 kcal/mol, −9.3 kcal/mol, −9.3 kcal/mol and −9.2 kcal/mol respectively. The control α-ketoamide 13a inhibitor (O6K) displayed binding energy of −7.2 kcal/mol. All the lead compounds showed stable interactions with the target through a good number of hydrogen bonds as well as hydrophobic interactions except for 156798–15-1 (Asparagamine A) which exhibited only hydrophobic interactions. Interestingly, compared to the phytochemicals the FDA-approved antiviral drugs showed higher binding affinities to the target. Further, the lead molecules-10308017 (Gummadiol), 11,646,359 (Vincapusine) and DB13879 (Glecaprevir) showed the potential antiviral activity through hydrogen bond interactions with either His41 or Cys145, both of the residues constitute the catalytic dyad of SARS-CoV-2 3CLpro enzyme.
3.3. Top ranked lead molecules for SARS-CoV 3CLpro from a set of phytochemicals and FDA approved drugs:
Among the phytochemicals, top 5 leads-120716 (Alloyohimbine), 10,308,017 (Gummadiol), 11,646,359 (Vincapusine), 82178-34-5 (Arjunolone), 102,004,710 (18-Hydroxy-3-epi-alpha-yohimbine) showed binding energies of −9.0 kcal/mol, −8.4 kcal/mol, −8.3 kcal/mol, −8.1 kcal/mol and −8.0 kcal/mol respectively. Using molecular docking of FDA approved antiviral drugs, top 5 leads-DB13879 (Glecaprevir), DB13878 (Pibrentasvir), DB01072 (Atazanavir), DB09102 (Daclatasvir) and DB11574 (Elbasvir) were shortlisted which displayed binding energies of −9.7 kcal/mol, −9.3 kcal/mol, −9.2 kcal/mol, −9.2 kcal/mol and −8.8 kcal/mol respectively. The molecular binding between these lead compounds and the target is strengthened by a good number of hydrogen bonds and hydrophobic interactions. The leads which displayed hydrogen bond interactions with the catalytic residues-His41 and Cys145 include DB13879 (Glecaprevir), DB11574 (Elbasvir), 10,308,017 (Gummadiol) and 102,004,710 (18-Hydroxy-3-epi-alpha-yohimbine). The control, SG85 inhibitor (G85) showed binding energy of −8.0 kcal/mol with the enzyme target.
3.4. Top ranked lead molecules for MERS-CoV 3CLpro from a set of phytochemicals and FDA approved drugs:
Few lead compounds were also identified for MERS-CoV 3CLpro using molecular docking of phytochemicals and the binding energies of top 5 leads-11646359 (Vincapusine), 120,716 (Alloyohimbine), 10,308,017 (Gummadiol), 11,969,544 (Pericyclivine) and 28,288,759 (Vidolicine) were −9.8 kcal/mol, −8.6 kcal/mol, −8.4 kcal/mol, −8.4 kcal/mol and −8.3 kcal/mol respectively. Among, the FDA approved antiviral drugs, the top 5 leads-DB01072 (Atazanavir), DB06817 (Raltegravir), DB09296 (Ombitasvir), DB08864 (Rilpivirine) and DB09297 (Paritaprevir) scored binding energies of −9.1 kcal/mol, −9.1 kcal/mol, −9.0 kcal.mol, −8.7 kcal/mol and −8.7 kcal/mol respectively. The control, GC813 inhibitor (AW4) showed binding energy of −8.0 kcal/mol. All the lead compounds established both a good number of hydrogen bonds as well as hydrophobic interactions with the target except 28,288,759 which exhibited only hydrophobic interactions. The lead molecules-DB06817 (Raltegravir), 120,716 (Alloyohimbine) and 11,969,544 (Pericyclivine) established hydrogen bond interactions with either His41 or Cys148 or both (catalytic dyad) which may explain their mode of inhibition against the enzyme target.
3.5. Common lead molecules as potential dual or triple inhibitors of the enzyme targets
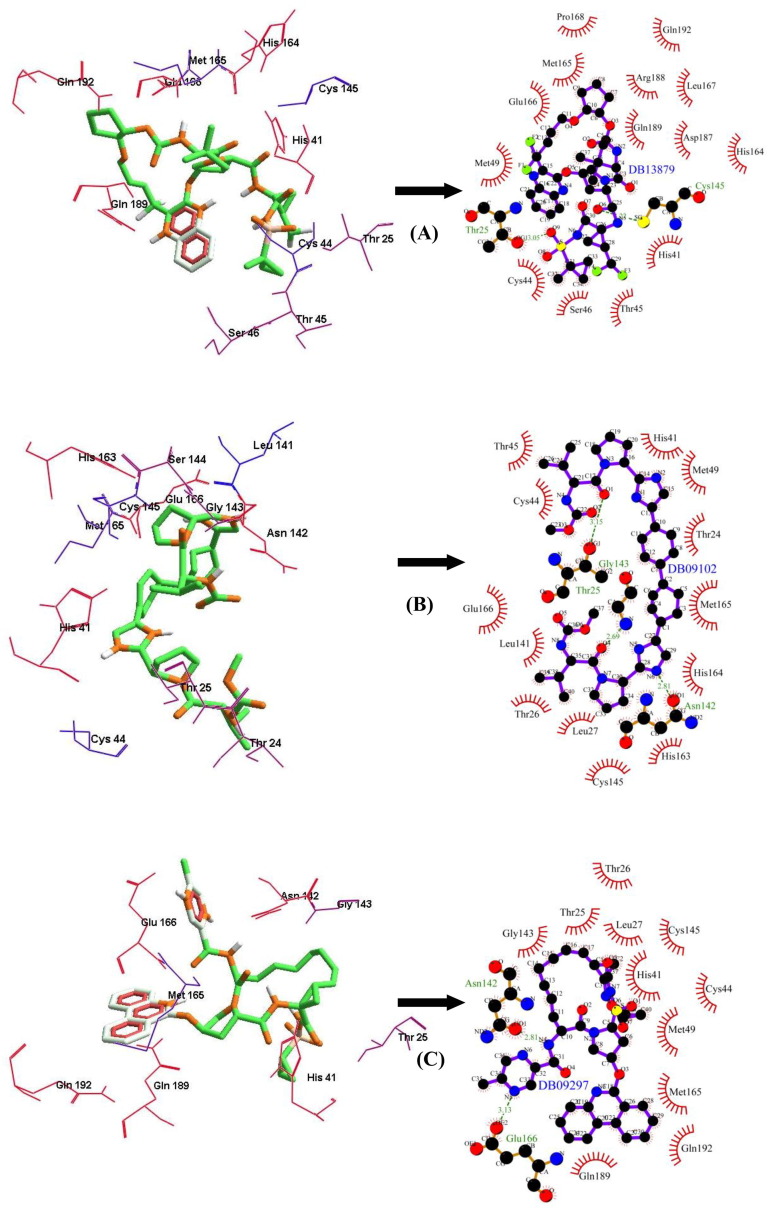
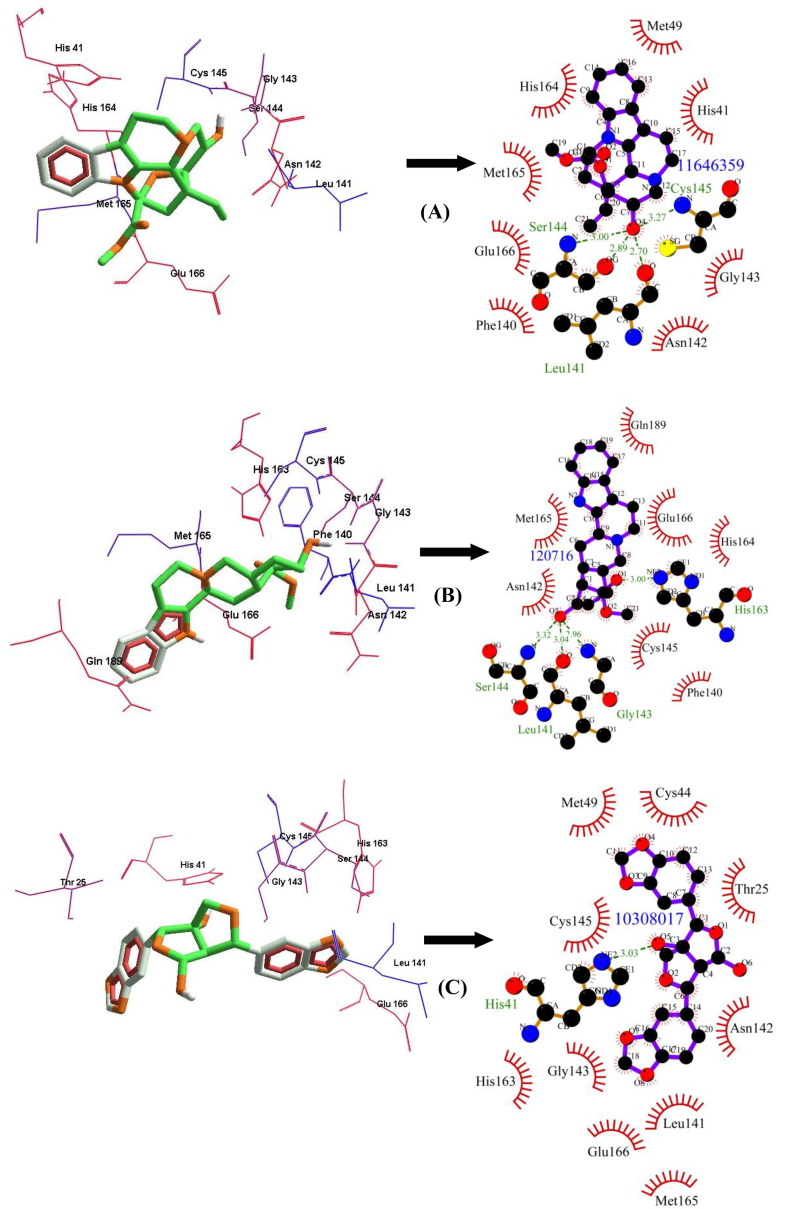
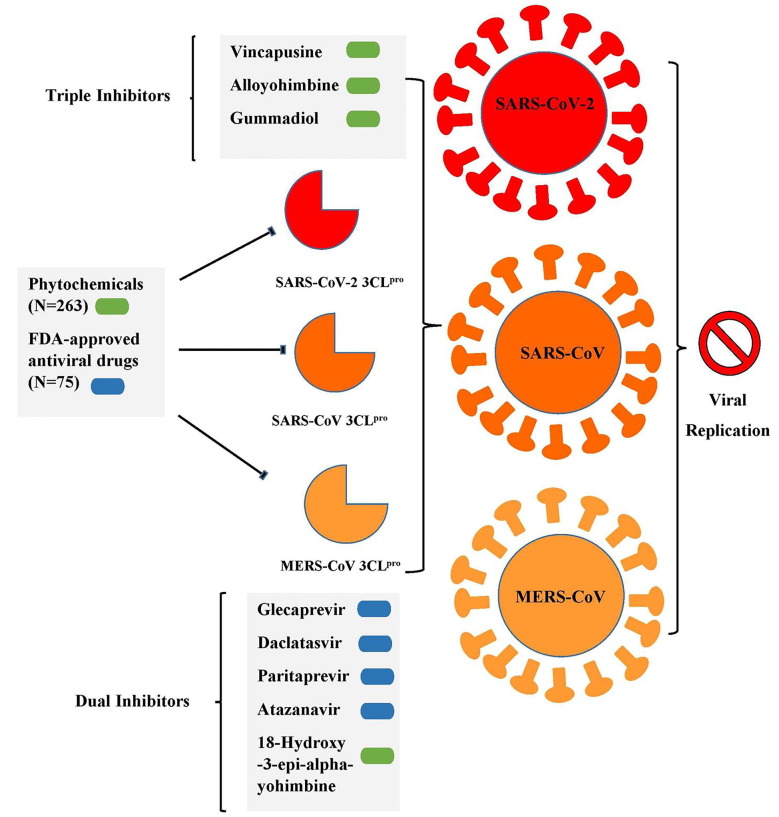
Among the FDA approved antiviral drugs, we found that DB13879 (Glecaprevir) and DB09102 (Daclatasvir) can be potential leads for dual inhibition of SARS-CoV-2 3CLpro (Fig. 1 A, B) and SARS-CoV 3CLpro as they were common top 5 leads. The lead molecule DB09297 (Paritaprevir) can be explored as a dual inhibitor of SARS-CoV-2 3CLpro (Fig. 1C) and MERS-CoV 3CLpro and DB01072 (Atazanavir) can be used as an inhibitor for dual inhibition of SARS-CoV 3CLpro and MERS-CoV 3CLpro. While Glecaprevir, Daclatasvir and Paritaprevir have been used against chronic hepatitis C (For the Study of the Liver (KASL, K.A., others, 2018, Hézode, 2018), Atazanavir (HIV-1 protease inhibitor) is primarily used for the treatment of HIV infection (Eckhardt and Gulick, 2017). Interestingly, we found that the phytochemicals 11,646,359 (Vincapusine), 120,716 (Alloyohimbine) and 10,308,017 (Gummadiol) were common top 5 leads among the three targets and therefore, these phytochemicals can be used as triple inhibitors of SARS-CoV-2 3CLpro (Fig. 2 A–C), SARS-CoV 3CLpro and MERS-CoV 3CLpro. The phytochemical 102,004,710 (18-Hydroxy-3-epi-alpha-yohimbine) was identified to be a potential dual inhibitor of SARS-CoV-2 3CLpro and SARS-CoV 3CLpro. Vinacapusine is a β-amino alcohol-type alkaloid extracted from leaves of Catharanthus pusillus, a traditional medicinal plant of India believed to possess oncolytic properties (Khare, 2007). Gummadiol belongs to the class of Furanoid lignans which can be extracted from Gmelina arborea (Anjaneyulu et al., 1975, Pathala et al., 2015). Alloyohimbine and 18-Hydroxy-3-epi-alpha-yohimbine are alkaloids which are isomeric forms of yohimbine, an alkaloid extracted from the bark of the tree Pausinystalia yohimbe and has been traditionally used for the treatment of sexual disorders (Anadón et al., 2016). However, the bioactivity of these compounds against viral infections have not been reported till date to the best of our knowledge and therefore these are novel phytochemical leads which could be further explored against coronavirus infections in humans. The key findings from the present study has been illustrated with Fig. 3 .
Fig. 1.
The binding poses and LigPlot + results showing molecular interaction between SARS-CoV-2 3CLpro and lead molecules-(FDA approved antiviral drugs) (A) DB13879 (Glecaprevir) (B) DB09102 (Daclatasvir) (C) DB09297 (Paritaprevir). The hydrophobic interacting residues are indicated by red arcs with spikes and the green dashed lines with the bond distance correspond to hydrogen bonds.
Fig. 2.
The binding poses and LigPlot + results showing molecular interaction between SARS-CoV-2 3CLpro and lead molecules-(Phytochemicals) (A) 11,646,359 (Vincapusine) (B) 120,716 (Alloyohimbine) (C) 10,308,017 (Gummadiol). The hydrophobic interacting residues are indicated by red arcs with spikes and the green dashed lines with the bond distance correspond to hydrogen bonds.
Fig. 3.
A graphical summary illustrating the key findings from the present study.
4. Conclusion
The present work is an in silico attempt to propose lead molecules as potential inhibitors of coronavirus 3CLpro enzyme. Our study unravels new chemical entities from a repertoire of phytochemicals and FDA approved drugs that could be repurposed for treatment of coronavirus infection in humans. The leads suggested from this study could offer new candidate molecules in the drug discovery pipeline for the treatment and management of the disease. The current work is limited by small datasets and therefore, it would be worth exploring new chemical databases with big ligand sets for virtual screening procedure for identification of novel inhibitors against the target enzyme. A combined molecular docking and molecular dynamics simulation approach could be envisaged which would further provide useful mechanistic insights into the binding modes of inhibitions at the atomic level. Further research work is necessary to establish the inhibitory activity of the identified FDA approved lead molecules against the coronavirus 3CLpro enzyme through in vitro and in vivo experiments.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP-2019/154), King Saud University, Riyadh, Saudi Arabia. J. Lee thanks to Chungnam National University, Daejeon, Republic of Korea for the funding support. The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Aziz T.M., Stockand J.D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2)-an update on the status. Infect. Genet. Evol. 2020;83 doi: 10.1016/j.meegid.2020.104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anadón, A., Martínez-Larrañaga, M.R., Ares, I., Martínez, M.A., 2016. Chapter 60 - Interactions between Nutraceuticals/Nutrients and Therapeutic Drugs, in: Gupta, R.C. (Ed.), Nutraceuticals. Academic Press, Boston, pp. 855–874. https://doi.org/https://doi.org/10.1016/B978-0-12-802147-7.00060-7.
- Anjaneyulu A.S.R., Rao A.M., Rao V.K., Row L.R., Pelter A., Ward R.S. The structure of gummadiol-a lignan hemi-acetal. Tetrahedron Lett. 1975;16:1803–1806. [Google Scholar]
- Bredenbeek P.J., Pachuk C.J., Noten A.F.H., Charité J., Luytjes W., Weiss S.R., Spaan W.J.M. The primary structure and expression of the second open reading frame of the polymerase gene of the coronavirus MHV-A59; a highly conserved polymerase is expressed by an efficient ribosomal frameshifting mechanism. Nucleic Acids Res. 1990;18:1825–1832. doi: 10.1093/nar/18.7.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian, D.A., Baric, R.S., 2005. Coronavirus genome structure and replication, in: Coronavirus Replication and Reverse Genetics. Springer, pp. 1–30. [DOI] [PMC free article] [PubMed]
- Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccin. Immunother. 2020:1–7. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt, B.J., Gulick, R.M., 2017. 152 - Drugs for HIV Infection, in: Cohen, J., Powderly, W.G., Opal, S.M. (Eds.), Infectious Diseases (Fourth Edition). Elsevier, pp. 1293-1308.e2. https://doi.org/https://doi.org/10.1016/B978-0-7020-6285-8.00152-0.
- For the Study of the Liver (KASL, K.A., others, 2018. 2017 KASL clinical practice guidelines management of hepatitis C: Treatment of chronic hepatitis C. Clin. Mol. Hepatol. 24, 169-229. [DOI] [PMC free article] [PubMed]
- Forni D., Cagliani R., Clerici M., Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017;25:35–48. doi: 10.1016/j.tim.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya, A.E., 2001. Big nidovirus genome, in: The Nidoviruses. Springer, pp. 1–17.
- Graham R.L., Donaldson E.F., Baric R.S. A decade after SARS: strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 2013;11:836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996;17:490–519. doi: 10.1002/(SICI)1096-987X(199604)17:5/6<490::AID-JCC1>3.0.CO;2-P. [DOI] [Google Scholar]
- Hézode C. Treatment of hepatitis C: results in real life. Liver Int. 2018;38:21–27. doi: 10.1111/liv.13638. [DOI] [PubMed] [Google Scholar]
- Jo S., Kim S., Shin D.H., Kim M.-S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare, C.P., 2007. Vinca pusilla Murr., in: Khare, C.P. (Ed.), Indian Medicinal Plants: An Illustrated Dictionary. Springer New York, New York, NY, p. 1. https://doi.org/10.1007/978-0-387-70638-2_1743.
- Lacroix A., Duong V., Hul V., San S., Davun H., Omaliss K., Chea S., Hassanin A., Theppangna W., Silithammavong S., et al. Genetic diversity of coronaviruses in bats in Lao PDR and Cambodia. Infect. Genet. Evol. 2017;48:10–18. doi: 10.1016/j.meegid.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, M.M.C., 2001. Coronaviridae: the viruses and their replication. In Fields Virology, Fourth Edition (Knipe DM, Howley PM, eds), Lippincott Williams and Wilkins, Philadelphia, pp. 1163–1185.
- Laskowski R.A., Swindells M.B. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- Lee H.-J., Shieh C.-K., Gorbalenya A.E., Koonin E.V., La Monica N., Tuler J., Bagdzhadzhyan A., Lai M.M.C. The complete sequence (22 kilobases) of murine coronavirus gene 1 encoding the putative proteases and RNA polymerase. Virology. 1991;180:567–582. doi: 10.1016/0042-6822(91)90071-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski C.A. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov. Today. Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Lundstrom K. Coronavirus Pandemic—Therapy and Vaccines. Biomedicines. 2020;8:109. doi: 10.3390/biomedicines8050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanraj K., Karthikeyan B.S., Vivek-Ananth R.P., Chand R.P.B., Aparna S.R., Mangalapandi P., Samal A. IMPPAT: A curated database of I ndian M edicinal P lants, P hytochemistry A nd T herapeutics. Sci. Rep. 2018;8:1–17. doi: 10.1038/s41598-018-22631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needle D., Lountos G.T., Waugh D.S. Structures of the Middle East respiratory syndrome coronavirus 3C-like protease reveal insights into substrate specificity. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015;71:1102–1111. doi: 10.1107/S1399004715003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Boyle N.M., Banck M., James C.A., Morley C., Vandermeersch T., Hutchison G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padron-Regalado E. Vaccines for SARS-CoV-2: lessons from other coronavirus strains. Infect. Dis. Ther. 2020;9:255–274. doi: 10.1007/s40121-020-00300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathala D., Harini A., Hegde P.L. A review on gambhari (Gmelina arborea Roxb.) J. Pharmacogn. Phytochem. 2015;4:127–132. [Google Scholar]
- Sander T., Freyss J., von Korff M., Rufener C. DataWarrior: an open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015;55:460–473. doi: 10.1021/ci500588j. [DOI] [PubMed] [Google Scholar]
- Simas P.V.M., de Souza Barnabé A.C., Durães-Carvalho R., de Lima Neto D.F., Caserta L.C., Artacho L., Jacomassa F.A.F., Martini M.C., dos Santos M.M.A.B., Felippe P.A.N., et al. Bat coronavirus in Brazil related to appalachian ridge and Porcine epidemic diarrhea viruses. Emerg. Infect. Dis. 2015;21:729–731. doi: 10.3201/eid2104.141783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veber D.F., Johnson S.R., Cheng H.-Y., Smith B.R., Ward K.W., Kopple K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D.S., Knox C., Guo A.C., Cheng D., Shrivastava S., Tzur D., Gautam B., Hassanali M. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36:D901–D906. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C.Y., Lau S.K.P., Lam C.S.F., Lau C.C.Y., Tsang A.K.L., Lau J.H.N., Bai R., Teng J.L.L., Tsang C.C.C., Wang M., et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavi. J. Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr, J., 2005. The coronavirus replicase, in: Coronavirus Replication and Reverse Genetics. Springer, pp. 57–94.
- Ziebuhr J., Thiel V., Gorbalenya A.E. The autocatalytic release of a putative RNA virus transcription factor from its polyprotein precursor involves two paralogous papain-like proteases that cleave the same peptide bond. J. Biol. Chem. 2001;276:33220–33232. doi: 10.1074/jbc.M104097200. [DOI] [PMC free article] [PubMed] [Google Scholar]