Dear Editor
Since the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, several neurological manifestations have been reported in these patients, ranging from mild symptoms, such as anosmia, hypogeusia, and headaches, to more severe manifestations, such as acute encephalitis, cerebrovascular stroke, polyneuropathy, and Guillian Barre syndrome (GBS).1 The involvement of the autonomic nervous system (ANS) in SARS-Cov-2 infection has not been reported.
Dysautonomia, defined as the failure or sometimes overactivity of the sympathetic or parasympathetic components of the ANS, has a wide range of clinical manifestations, including labile blood pressure, orthostatic hypotension, impotence, bladder dysfunction, and alterations in bowel functions. It can be acute or chronic and reversible or progressive and has been reported with diverse medical conditions, such as diabetes mellitus, alcoholism, GBS, and Parkinson's disease. Acute dysautonomia has also been reported with viral infections, such as mumps, HIV, hepatitis C virus, Epstein-Barr virus, and Coxsackie B virus.2 Here, we report a case of dysautonomia, secondary to SARS-CoV-2 infection. We will demonstrate this phenomenon by illustrating blood pressure fluctuations and vasopressor requirements throughout 16 days of the patient's ICU stay.
A 72-year-old man was referred from a nursing home for fever and worsening shortness of breath that started a week prior. His-past medical history included diabetes mellitus, hypertension, chronic obstructive pulmonary disease, coronary artery disease, diastolic heart failure, and sick sinus syndrome treated with a permanent pacemaker. He tested positive for COVID-19, and on admission to the ICU, he was hypoxemic with no improvement on high flow oxygen therapy or non-invasive ventilation. On the first hospital day, the patient was intubated and sedated using propofol. Blood cultures and respiratory cultures were drawn. Lower respiratory polymerase chain reaction (PCR) showed Staphylococcus aureus that was mecA negative, and the patient was started on cefazolin for seven days. No growth was shown on cultures later on. Baseline electrocardiogram (ECG) showed a paced rhythm, and transthoracic echocardiogram (TTE) showed a mildly reduced left ventricular ejection fraction (LVEF) of 45–49% and a grade II diastolic dysfunction. This ejection fraction was reduced from the previous TTE done one-month prior which showed a LVEF of 70% with left ventricular hypertrophy. From day one to day 14, prone positioning for 16 h per day was done, and he was sedated and paralyzed using propofol, fentanyl, and rocuronium. He initially had good urine output on furosemide; however, due to progressive renal failure, he required hemodialysis on hospital day 6, and this was continued every other day throughout his hospital stay. On day 13 the patient's troponin trended up, serial ECGs did not show ST-segment elevations or depressions, and repeat TTE showed a mild reduction of LVEF to 40–44% with no regional wall motion abnormalities. COVID myocarditis was suspected, but no cardiac catheter or further imaging studies were done due to the patient's critical condition. On day 14, the patient developed fever, pan-cultures were done which later showed no growth, no antibiotics were initiated, and inflammatory markers continued to trend down. Of note, the patient had a depressed cough and gag reflexes on tracheal suctioning during mechanical ventilation. A week later, with no sustained improvement and the inability to wean him off the ventilator, the patient's family decided to stop ventilatory support and to pursue comfort care.
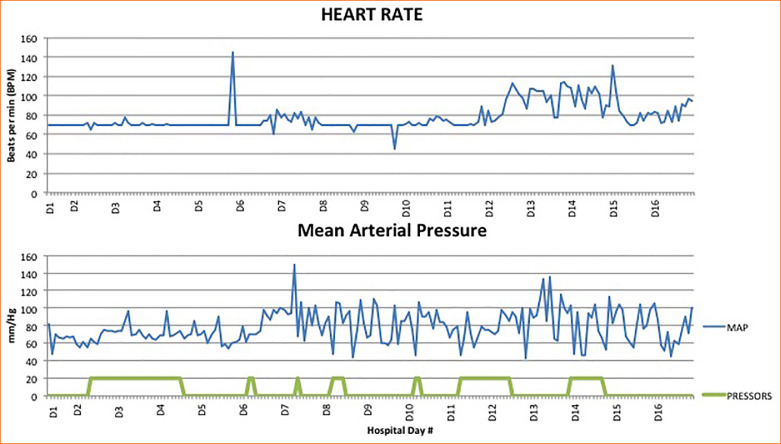
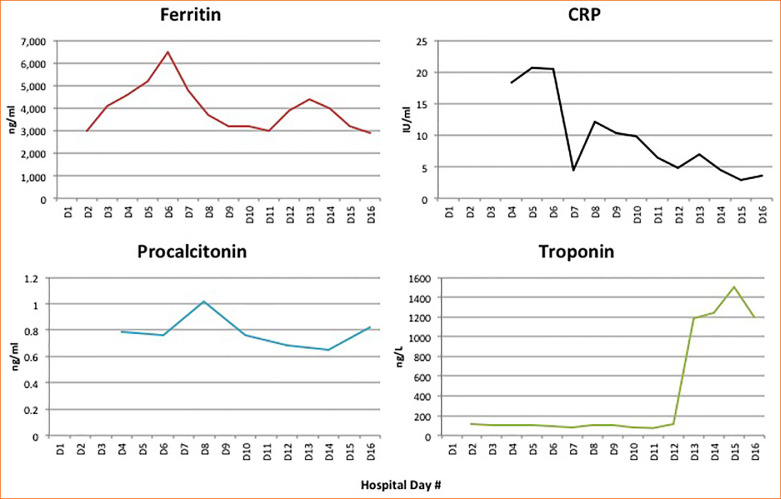
Throughout his hospitalization, the patient was noted to have labile blood pressures, with intermittent, varying vasopressor requirements (norepinephrine and/or vasopressin) (Figure 1 ). These blood pressure fluctuations were noted throughout the day, especially when changing positions from prone to supine or vice versa. Sepsis was ruled out with no growth in cultures, and he had a consistently negative procalcitonin throughout the hospital course and a downward trend in inflammatory markers (Figure 2 ). Efforts were made to maintain a euvolemic state, and his overall fluid balance throughout hospital stay was a positive 1.7 liters, which would not explain hypertensive or hypotensive episodes.
Figure 1.
Heart rate in beats per minute from day 1 through day 16 and the mean blood pressure and millimeters of mercury from day 1 through day 16. The horizontal bars in the mean arterial pressure figure represent periods of time requiring vasopressors.
Figure 2.
Values for ferritin, C-reactive protein (CRP), procalcitonin, and troponin on hospital days 1 through 16.
His-fluctuating blood pressure could be explained by acute dysautonomia through afferent baroreflex failure, a syndrome characterized by highly labile blood pressures with hypertensive crises alternating with hypotensive episodes; orthostatic hypotension is occasionally present. The mechanism involves damage to the afferent baroreceptor pathway, starting from baroreceptors in carotid bodies to the vagal and glossopharyngeal nerve fibers, and finally to the nucleus tractus solitaries (NTS). It can occur secondary to neck irradiation or surgery, and more rarely, in tumors of the NTS.3 , 4 Studies have shown that SARS-COV-2 affects the central nervous system, with a high affinity to the medullary structures, including the ventrolateral medulla and the NTS, as these areas have a strong ACE2 expression.1 We suggest that acute dysautonomia in the form of hemodynamic instability seen with critically ill COVID-19 patients may be explained by afferent baroreflex failure secondary to SARS-COV-2 infection and invasion of the NTS.
It is important to optimize blood pressure management in these patients. Hypotension is not necessarily a sign of septic shock, especially if unsupported by other clinical signs of sepsis, inflammatory markers, and cultures. We recommend maintaining a euvolemic state and avoiding excessive fluid resuscitation during hypotensive episodes with gradual titration of vasopressors to avoid overshooting blood pressures and using short-acting anti-hypertensive medications in hypertensive crisis.
References
- 1.Montalvan V., Lee J., Bueso T. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194 doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathuranath P.S., Duralpandian J., Kishore A. Acute dysautonomia following mumps. Neurol India. 1999;47(2):130. [PubMed] [Google Scholar]
- 3.Biaggioni I., Shibao C.A., Diedrich A. Blood pressure management in afferent baroreflex failure: JACC review topic of the week. J Am Coll Cardiol. 2019;74(23):2939–2947. doi: 10.1016/j.jacc.2019.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufmann H., Norcliffe-Kaufmann L., Palma JA Baroreflex dysfunction. N Engl J Med. 2020;382(2):163–178. doi: 10.1056/NEJMra1509723. [DOI] [PubMed] [Google Scholar]