Abstract
Aims
The aim of the study was to investigate the association between type-2 diabetes mellitus, other underlying diseases and obesity with the outcomes of critically ill Covid-19 patients in Greece.
Methods
In this retrospective observational multi-centre study, data and outcomes of 90 RNA 2109-nCoV confirmed critically ill patients from 8 hospitals throughout Greece, were analysed. All reported information stand through April 13th 2020.
Results
The median age of the patients was 65.5 (IQR 56–73), majority were male (80%) and obesity was present in 34.4% of patients most prevalent to younger than 55 years. Hypertension was the prevailing comorbidity (50%), followed by cardiovascular diseases (21.1%) and type-2 diabetes (18.9%). At admission, common symptoms duration had a median of 8 (IQR 5–11) days. A 13.3% of the patients were discharged, 53.4% were still in the ICUs and 28.9% deceased who were hospitalised for fewer days than the survivors [6 (IQR 3–9) vs. 9 (IQR 7–14.5) respectively]. Aging was not a risk factor but diabetes deteriorates the outcomes. Obesity poses a suggestive burden as it was more notable in deceased versus survivors.
Conclusions
Type 2 diabetes and obesity may have contributed to disease severity and mortality in COVID-19 critically ill patients in Greece.
Keywords: Greece, Type-2 diabetes, Obesity, Covid-19, Intensive Care Unit, Critically ill
1. Introduction
In December 2019, many cases of pneumonia without a defined causative factor were reported in Wuhan China, with some patients rapidly developing respiratory distress syndrome or acute respiratory failure [1]. On Jan 7th, a novel coronavirus was identified by the Chinese Center for Disease Control and Prevention (CCDC) from the throat swab sample of a patient and was subsequently named 2019-nCoV by WHO [2]. The 2019-nCoV infection caused clusters of severe respiratory illness similar to severe acute respiratory syndrome. Most patients were men and a sizable proportion suffered from underlying conditions [3].
The first Covid-19 case in Greece was diagnosed on February 26th and on March 23rd with 695 confirmed cases and 17 death, a nation-wide restriction on freedom of movement was enforced. On March 8th, the first patient with confirmed pneumonia by 2019-nCoV was admitted in an Intensive Care Unit (ICU) due to pulmonary failure. As of April 13th, the total Covid-19 cases in Greece were 2.145, 25.8% of patients were infected due to travel exposure and 40% of patients were infected due to close contact with diagnosed cases. The death toll was in total 99 patients, while 73 patients were supported with mechanical ventilation in critical condition and 16 patients had been discharged from the ICUs [4].
Development of severe acute respiratory syndrome is seen more often in elderly patients with Covid-19 pneumonia and with underlying chronic diseases such as hypertension, cardiovascular diseases, type-2 diabetes, chronic obstructive pulmonary disease or malignancies [5]. There is evidence of increased incidence and severity of Covid-19 in patients with type-2 diabetes, with higher risk for ICU admission and considerable mortality [6]. In addition, obesity has been associated with disease severity and National guidelines recommend that patients with obesity and especially those with severe obesity should take extra measures to avoid Covid-19 contamination [7] . A major problem of the coronavirus pandemic is the considerable burden imposed on the National Health System worldwide due to the hyperacute outbreak and the proportional increase of patients requiring ICU support in an extremely limited period of time. Hence, outcomes may vary according to the burden of the disease in each country. In contrast to the USA and other European Countries, Greece has been among the Countries with the lower number of cases and deaths and thus information regarding the outcomes of critically ill patients may be of interest.
The aim of this retrospective observational study, was to investigate the association between type-2 diabetes mellitus, other underlying diseases and obesity with the outcomes in Greek patients admitted to ICUs for respiratory failure caused by Covid-19 pneumonia. The relation of aging with demographics, comorbidities, clinical characteristics, need for invasive mechanical ventilation and outcomes was also evaluated.
2. Research design and methods
2.1. Study population and data collection
In this retrospective observational multi-centre study, 90 patients with laboratory-confirmed SARS-CoV-2 pneumonia (positive result on reverse-transcriptase-polymerase-chain-reaction [RT-PCR] assay of nasopharyngeal swab) [Method in Supplementary Appendix] who were hospitalised in ICUs of 8 hospitals in Greece from March 10th to April 13th 2020 were included. All these hospitals were designated by the Greek Ministry of Health as Covid-19 Reference Hospitals for the care of patients with SARS-CoV-2 pneumonia. In Table 1 all collaborating hospitals are referred, with the number of their patients that contributed to the analysis. Different regions of Greece are represented and thus the results are Nationwide and empowered, because they are not mirrored only to a certain area of Greece
Table 1.
Participating hospitals.
| Hospital | City | Region | N (%) |
|---|---|---|---|
| University Hospital “ATTIKON” | Athens Metropolitan Area | Attica | 13 (14.4) |
| “EVANGELISMOS” General Hospital | Athens Metropolitan Area | Attica | 25 (27.8) |
| “THRIASSION” General Hospital | Athens Metropolitan Area | Attica | 8 (8.9) |
| “AHEPA” University Hospital | Thessaloniki | Macedonia | 19 (21.1) |
| “G. PAPANIKOLAOU” General Hospital | Thessaloniki | Macedonia | 10 (11.1) |
| “AGIOS DIMITRIOS” General Hospital | Thessaloniki | Macedonia | 5 (5.6) |
| Patras University Hospital | Patra | Peloponnese | 8 (8.9) |
| “KOUTLIBANEIO” General Hospital | Larissa | Thessaly | 2 (2.2) |
The study was approved by the Institutional Ethics Board and the Scientific Council of the coordinating “Attikon” University Hospital. Due to the retrospective nature of the study and the anonymity in reviewing patients’ data, informed consent from individual patients or their legal guardians was waived.
Data were extracted from patients’ medical records and through direct communication with their attending health care providers. Data was collected for age, gender, BMI and underlying health conditions, such as hypertension, cardiovascular disorders, pulmonary diseases, kidney failure, type-2 diabetes or malignancy. Monitored information included: common symptoms onset and duration prior to hospital admission, smoking habits, vital signs (fever, heart rate and respiration rate), laboratory values (blood routine test, arterial blood gas analysis and blood chemistry with troponine levels) and chest radiography – CT findings. For the severity of the patient condition requiring intensive care the ratio of partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2) on admission and three (3) days later was used. Additionally we collected data regarding the proportion of patients that required mechanical ventilation, the length of stay in ICU, the number of patients who died while under observation and treatment, those who were discharged from the Units and those who were still in ICUs. No imputation was made for any missing data. Diabetes was defined from the patients' medical history and type-1 patients were excluded from the study. Decisions regarding admission, management and intubation per patient, were made by the individual referring physician. The defined outcomes of the study, were the burden of diabetes and obesity in critically ill Covid-19 patients. All reported information stand for hospitalization through April 13th 2020.
2.2. Statistical analysis
Sample size was equal to the number of patients treated during the study period in the collaborating hospitals. Descriptive statistical analysis was performed for all study data. Continuous variables were summarized through the use of medians (25–75th percentiles) and categorical variables were displayed as frequency tables (N, %). Standard statistical tests were used to check univariate associations between categorical (Fisher's exact tests or Pearson chi-square test [8], [9]) or categorical and continuous variables (Two-sample Wilcoxon rank-sum test, T-test Kruskal-Wallis equality-of-populations rank test [10], [11], [12]). Shapiro-Wilk W test was performed to assess the normality for each continuous variable combined with graphical methods [13]. Box plots and histograms were used for graphical presentation of differences and trends between variables that have been observed. Age and BMI were summarized and tested both in continuous and categorical forms. Lab variables were presented only in continuous forms. Results with p-value of < 0.05 were considered statistically significant, and results with 0.05 ≤ p ≤ 0.10 suggestive. Multiple imputation methods for missing data were not used. All statistical analyses were performed using STATA/SE 16.1 software (Copyright 1985–2019; StataCorp LP, College Station, Texas, USA).
3. Results
3.1. Demographics
The analysis included 90 critically ill patients with a median age of 65.5 (IQR 56–73). Since age has been shown to be associated with outcomes [14], [15], our cohort was divided into Group A with 21 patients (23.3%) younger or equal to 55 years, Group B with 24 patients (26.7%) aged 56 to 65 and Group C with 45 patients (50%) older than 66 years. The demographic and clinical characteristics of patients according to their age at baseline are presented in Table 2 .
Table 2.
Demographic and clinical characteristics of patients according to the age at baseline.
| Parameters | Group A N = 21 (23.3%) | Group B N = 24 (26.7%) | Group C N = 45 (50%) | p-value |
|---|---|---|---|---|
| Age (years) | ≤55 | 56–65 | ≥66 | |
| Median (25–75th percentile) | ||||
| BMI (kg/m2) | 30.8 (28–35.1) | 29.4 (26.5–32.9) | 27.7 (26–29.3) | 0.003* |
| Duration of symptoms before intubation (days) | 8 (6–11) | 6 (4–10) | 8 (5–11) | 0.381 |
| LOS (days) | 7 (5–12) | 8.5 (7–11.5) | 9 (6–13) | 0.548 |
| Lowest PaO₂:FiO₂ ratio on day 1 after admission | 99 (87–130) | 100 (72–100) | 100 (74–108) | 0.681 |
| Lowest PaO₂:FiO₂ ratio on day 3 after admission | 120 (73–152) | 80 (60–102) | 136 (95–167) | 0.003* |
| PaO₂:FiO₂ ratio improvement | −6 (–23–17) | −10 (–40–0) | 24 (0–66) | <0.001* |
| Lab tests | ||||
| White cell count (x109/L) | 14.1 (11.6–15.6) | 15.3 (11–23.2) | 12.5 (9.2–18.4) | 0.496 |
| Lymphocytopenia (1.0) | 0.08 (0.03–0.1) | 0.06 (0.03–0.1) | 0.06 (0.04–0.09) | 0.501 |
| Arterial lactate (mmol/L) | 1.8 (1.5–2.7) | 2.4 (1.4–3.2) | 2.1 (1.7–2.8) | 0.717 |
| ALT (μKat/L) | 1.2 (0.8–3.5) | 1.4 (0.9–3) | 1 (0.6–1.5) | 0.396 |
| AST (μKat/L) | 0.9 (0.6–1.6) | 1.4 (1.1–2.4) | 0.9 (0.5–1.4) | 0.064* |
| Bilirubin total (μmol/L) | 17.3 (9.1–41.2) | 20 (11.3–48.8) | 16.1 (8.6–31.5) | 0.615 |
| Troponine (μg/L) | 15.5 (3.7–96.5) | 31 (16.5–52) | 24 (5.6–83) | 0.651 |
| Platelet count (x109/L) | 267.5 (179–439) | 209.5 (117–433) | 182 (134–273) | 0.269 |
| N (%) | ||||
| Gender | 0.74 | |||
| Female | 4 (19.1) | 6 (25) | 8 (17.8) | |
| Male | 17 (80.9) | 18 (75) | 37 (82.2) | |
| BMI (kg/m2) | 0.109 | |||
| ≤26 | 2 (9.5) | 5 (20.8) | 15 (33.3) | |
| >26 | 19 (90.5) | 19 (79.2) | 30 (66.7) | |
| BMI (kg/m2) | 0.024* | |||
| ≤25 | 2 (9.5) | 3 (12.5) | 8 (17.8) | |
| 25–30 | 7 (33.3) | 11 (45.8) | 29 (64.4) | |
| >30 | 12 (57.2) | 10 (41.7) | 8 (17.8) | |
| Admission | 0.636 | |||
| Directly to ICU | 2 (9.5) | 3 (12.5) | 3 (6.7) | |
| Inpatient prior to wards | 19 (90.5) | 21 (87.5) | 42 (93.3) | |
| Comorbidities | ||||
| COPD | 2 (9.5) | 0 (0) | 6 (13.3) | 0.169 |
| Cancer | 1 (4.8) | 0 (0) | 6 (13.3) | 0.143 |
| Chronic Kidney disease | 0 (0) | 1 (4.2) | 3 (6.7) | 0.805 |
| Cardiovascular | 0 (0) | 3 (12.5) | 16 (35.6) | 0.001* |
| Diabetes | 2 (9.5) | 4 (16.7) | 11 (24.4) | 0.387 |
| Hypertension | 7 (33.3) | 11 (45.8) | 27 (60) | 0.116 |
| Asthma | 1 (4.8) | 1 (4.2) | 1 (2.2) | 0.798 |
| Number of comorbidities/patient | 0.004* | |||
| 0 | 13 (61.9) | 8 (33.3) | 6 (13.3) | |
| 1 | 5 (23.8) | 12 (50) | 20 (44.5) | |
| 2 | 1 (4.8) | 4 (16.7) | 10 (22.2) | |
| 3 | 2 (9.5) | 0 (0) | 7 (15.6) | |
| 4 | 0 (0) | 0 (0) | 1 (2.2) | |
| 5 | 0 (0) | 0 (0) | 1 (2.2) | |
| Number of comorbidities/patient | 0.022* | |||
| 0+1 | 18 (85.7) | 20 (83.3) | 26 (57.8) | |
| ≥2 | 3 (14.3) | 4 (16.7) | 19 (42.2) | |
| Number of comorbidities/patient | 0.001* | |||
| 0 | 13 (61.9) | 8 (33.3) | 6 (13.3) | |
| ≥1 | 8 (38.1) | 16 (66.7) | 39 (86.7) | |
| Smoking | 0.781 | |||
| No | 16 (76.2) | 16 (66.7) | 32 (71.1) | |
| Yes | 5 (23.8) | 8 (33.3) | 13.(28.9) | |
| Obesity | 0.009* | |||
| No | 9 (42.9) | 14 (58.3) | 36 (80) | |
| Yes | 12 (57.1) | 10 (41.7) | 9 (20) | |
| Signs and symptoms | ||||
| Temperature >38C | 0.066* | |||
| No | 3 (14.3) | 5 (20.8) | 18 (40) | |
| Yes | 18 (85.7) | 19 (79.2) | 27 (60) | |
| Heart rate>100 beats per min | 0.782 | |||
| No | 3 (21.4) | 6 (33.3) | 13 (33.3) | |
| Yes | 11 (78.6) | 12 (66.7) | 26 (66.7) | |
| Missing | ||||
| Respiratory rate ≥20 beats/min | 0.307 | |||
| No | 0 (0) | 2 (8.3) | 1 (2.2) | |
| Yes | 21 (100) | 22 (91.7) | 44 (97.8) | |
| Lowest PaO₂:FiO₂ ratio on day 1 after admission | 0.459 | |||
| Mild | 0 (0) | 1 (5.6) | 1 (2.6) | |
| Moderate | 6 (42.9) | 3 (16.7) | 11 (28.2) | |
| Severe | 8 (57.1) | 14 (77.8) | 27 (69.2) | |
| Missing | ||||
| Lowest PaO₂:FiO₂ ratio on day 3 after admission | 0.113 | |||
| Mild | 0 (0) | 0 (0) | 3 (7.7) | |
| Moderate | 8 (61.5) | 5 (27.8) | 21 (53.9) | |
| Severe | 5 (38.5) | 13 (72.2) | 15 (38.5) | |
| missing | ||||
| CT-scan or Chest X-ray | >0.999 | |||
| No | 0 (0) | 0 (0) | 1 (2.6) | |
| Yes | 14 (100) | 18 (100) | 38 (97.4) | |
| Missing | ||||
| Pleural effusions | 0.013* | |||
| No | 12 (85.7) | 7 (38.9) | 28 (71.8) | |
| Yes | 2 (14.3) | 11 (61.1) | 11 (28.2) | |
| Missing | ||||
| Mechanical ventilation | 0.636 | |||
| No | 2 (9.5) | 3 (12.5) | 3 (6.7) | |
| Yes | 19 (90.5) | 21 (87.5) | 42 (93.3) | |
| Outcome | 0.902 | |||
| Death in ICU | 7 (35) | 8 (34.8) | 11 (25.6) | |
| Discharged | 3 (15) | 3 (13) | 6 (14) | |
| Still in ICU | 10 (50) | 12 (52.2) | 26 (60.4) | |
Values are expressed as median (25–75th percentile) or number %.
Body-Mass Index (BMI), COPD = Chronic Obstructive Pulmonary Disease, ICU = Intensive Care Unit.
PaO₂:FiO₂ ratio of Arterial Oxygen Tension (PaO₂)/to the Fraction of inspired Oxygen, LOS: Length Of Stay (days).
*p < 0.05 is considered as statistical significant 0.05 < p < 0.10 is considered as suggestive. Statistical analysis: ANOVA.
Kruskal-Wallis equality-of-populations rank test, Fisher’ s exact test, Pearson chi-square test.
From the total population, 80% were male with similar distribution across all age groups, namely 80.9% for Group A, 75% for Group B and 82.2% for Group C.
Median Body Mass Index (BMI) was 28 kg/m2 (IQ 26.1–32), while Group A had a significantly higher level of BMI with a median value of 30.8 (IQR 28–35.1), in comparison to 29.4 (IQR 26.5–32.9) and 27.7 (IQR 26–29.3) kg/m2 in Group B and C respectively (p = 0.003).
3.2. Medical history
Twenty-seven patients (30%) had no any underlying disease. Arterial hypertension was the most prevalent comorbidity affecting 45 patients (50%), followed by 19 patients with cardiovascular diseases (21.1%), 17 with type-2 diabetes (18.9%), 8 with COPD (8.9%), 7 with malignancies (7.8%), 4 with chronic kidney disease (4.4%) and 3 with asthma (3.3%). Thirty-seven patients presented with only one comorbidity (41.1%), 15 with two (16.7%), 9 with three (10%), 1 with four (1.1%) and 1 with 5 (1.1%). More patients in Group C had at least one comorbidity (86.7%) compared to Group B (66.67%) and Group A (38.1%) (p = 0.001). The same Group was also associated with higher frequency of>2 underlying diseases (Table 2, p = 0.022). Specific comorbitidies were equally distributed across all age groups with the exception of cardiovascular disorders (CVD) which were with higher prevalence in Group C (Table 2, p = 0.001). However, the presence of CVD was not related to early death, since the frequency of CVD was similar between the deceased (15.4%) and the survivors (23.3%) (Table 3 ). The demographic and clinical characteristics of our study population in relation to the outcomes are presented in Table 3.
Table 3.
Demographic and clinical characteristics of 90 patients at baseline and correlations to death.
| Parameters | Total | Discharged/still in ICU | Non-Survivors | p-value |
|
|---|---|---|---|---|---|
| N = 90 (100%) |
N = 60 (66.7%) | N = 26 (28.9%) | |||
| N (%) | Median (25–75th percentile) | ||||
| Age (years) | 90 (100) | 65.5 (56–73) | 66 (56.5–74) | 65 (53–70) | 0.325 |
| BMI (kg/m2) | 90 (100) | 28 (26.1–32) | 27.7 (26.1–30.6) | 28.7 (27.1–32.6) | 0.231 |
| Duration of symptoms before intubation (days) | 90 (100) | 8 (5–11) | 8 (5–12) | 6.5 (4–10) | 0.043* |
| LOS (days) | 70 (77.8) | 8 (5–13) | 9 (7–14.5) | 6 (3–9) | 0.002* |
| Lowest PaO₂:FiO₂ ratio on day 1 after admission | 71 (78.8) | 100 (75–110) | 100 (73.5–109) | 100 (78–100) | 0.905 |
| Lowest PaO₂:FiO₂ ratio on day after admission 3 | 70 (77.8) | 109 (70–157) | 115 (71.5–163) | 98.5 (65–125) | 0.271 |
| PaO₂:FiO₂ ratio improvement | 70 (77.8) | 0 (−10 to 42) | 6 (−3.5 to 56.5) | 0 (−23 to 22) | 0.127 |
| Lab tests | |||||
| White cell count (x109/L) | 70 (77.8) | 13.7 (10.3–18.9) | 13.1 (10.4–17.9) | 17 (11–26) | 0.083* |
| Lymphocytopenia (1.0) | 71 (78.9) | 0.06 (0.03–0.1) | 0.07 (0.03–0.1) | 0.06 (0.03–0.09) | 0.495 |
| Arterial lactate (mmol/L) | 71 (78.9) | 2.1 (1.6–2.8) | 2.1 (1.7–2.6) | 2.8 (1.6–4.5) | 0.054* |
| ALT (μKat/L) | 71 (78.9) | 1.1 (0.7–2.4) | 1.1 (0.7–2.1) | 1.2 (0.7–4.2) | 0.626 |
| AST (μKat/L) | 71 (78.9) | 1 (0.7–1.8) | 1.1 (0.6–2) | 1 (0.7–3.2) | 0.983 |
| Bilirubin total (μmol/L) | 71 (78.9) | 17.8 (8.7–38) | 18.3 (8.7–35) | 11.3 (7.2–46.2) | 0.531 |
| Troponine (μg/L) | 65 (72.2) | 24 (8–67) | 21 (8–57) | 39 (10–243) | 0.153 |
| Platelet count (x109/L) | 71 (78.9) | 191 (134–344) | 183 (133.5–351.5) | 220 (163–275) | 0.781 |
| N(%) | |||||
| Gender | 0.922 | ||||
| Female | 18 (20) | 11 (18.3) | 5 (19.2) | ||
| Male | 72 (80) | 49 (81.7) | 21 (80.8) | ||
| Admission | 0.693 | ||||
| Directly to ICU | 8 (8.9) | 5 (8.3) | 3 (11.5) | ||
| Inpatient prior to wards | 82 (91.1) | 55 (91.7) | 23 (88.5) | ||
| Number of comorbidities | 0.711 | ||||
| 0 | 27 (30) | 19 (31.6) | 8 (30.9) | ||
| 1 | 37 (41.1) | 22 (36.7) | 11 (42.4) | ||
| 2 | 15 (16.7) | 12 (20) | 3 (11.5) | ||
| 3 | 9 (10) | 6 (10) | 3 (11.5) | ||
| 4 | 1 (1.1) | 1 (1.7) | 0 (3.8) | ||
| 5 | 1 (1.1) | 0 (0) | 1 (3.9) | ||
| Number of comorbidities | 0.66 | ||||
| 0+1 | 64 (71.1) | 41 (68.3) | 19 (73.1) | ||
| ≥2 | 26 (28.9) | 19 (31.7) | 7 (26.9) | ||
| Number of comorbidities | 0.934 | ||||
| 0 | 27 (30) | 19 (31.7) | 8 (30.8) | ||
| ≥1 | 63 (70) | 41 (68.3) | 18 (69.2) | ||
| Comorbidities | >0.999 | ||||
| COPD | 8 (8.9) | 5 (8.3) | 2 (7.7) | ||
| Cancer | 7 (7.8) | 6 (10) | 1 (3.9) | 0.67 | |
| Chronic Kidney disease | 4 (4.4) | 1 (1.7) | 3 (11.5) | 0.081* | |
| Cardiovascular | 19 (21.1) | 14 (23.3) | 4 (15.4) | 0.566 | |
| Diabetes | 17 (18.9) | 8 (13.3) | 8 (30.8) | 0.056* | |
| Hypertension | 45 (50) | 32 (53.3) | 12 (46.2) | 0.541 | |
| Asthma | 3 (3.3) | 2 (3.3) | 1 (3.9) | >0.999 | |
| Smoking | 0.274 | ||||
| No | 64 (71.1) | 44 (73.3) | 16 (61.5) | ||
| Yes | 26 (28.9) | 16 (26.7) | 10 (38.5) | ||
| Obesity | 0.077* | ||||
| No | 59 (65.6) | 44 (73.3) | 14 (53.8) | ||
| Yes | 31 (34.4) | 16 (26.7) | 12 (46.2) | ||
| Age subcategories | 0.643 | ||||
| ≤55 | 21 (23.3) | 13 (21.7) | 7 (26.9) | ||
| 56–65 | 24 (26.7) | 15 (25) | 8 (30.8) | ||
| ≥66 | 45 (50) | 32 (53.3) | 11 (42.3) | ||
| Signs and symptoms before intubation | |||||
| Temperature >38C | 0.184 | ||||
| No | 26 (28.9) | 19 (31.7) | 4 (15.4) | ||
| Yes | 64 (71.1) | 41 (68.3) | 22 (84.6) | ||
| Heart rate>100 beats/min | 0.23 | ||||
| No | 22 (24.4) | 16 (33.3) | 3 (15.8) | ||
| Yes | 49 (54.5) | 32 (66.7) | 16 (84.2) | ||
| Missing | 19 (21.1) | ||||
| Respiratory rate ≥20 breats/min | >0.999 | ||||
| No | 3 (3.3) | 1 (1.7) | 0 (0) | ||
| Yes | 87 (96.7) | 59 (98.3) | 26 (100) | ||
| CT-sacnor Chest XrayN | >0.999 | ||||
| No | 1 (1.1) | 1 (2.1) | 0 (0) | ||
| Yes | 70 (77.8) | 47 (97.9) | 19 (100) | ||
| Missing | 19 (21.1) | ||||
| Pleural effusions | 0.385 | ||||
| No | 47 (52.2) | 30 (62.5) | 14 (73.7) | ||
| Yes | 24 (26.7) | 18 (37.5) | 5 (26.3) | ||
| Missing | 19 (21.1) | ||||
| Invasive mechanical ventilation | 0.099* | ||||
| No | 8 (8.9) | 8 (13.3) | 0 (0) | ||
| Yes | 82 (91.1) | 52 (86.7) | 26 (100) | ||
Values are expressed as median (25–75th percentile) or number %.
Body-Mass Index (BMI), COPD = Chronic Obstructive Pulmonary Disease, ICU = Intensive Care Unit.
PaO₂:FiO₂ ratio of Arterial Oxygen Tension (PaO₂)/to the Fraction of inspired Oxygen, LOS: Length Of Stay (days).
*p < 0.05 is considered as statistical significant 0.05 < p < 0.10 is considered as suggestive. Statistical analysis: ANOVA, Kruskal-Wallis equality-of-populations rank test, Fisher’ s exact test, Pearson chi-square test.
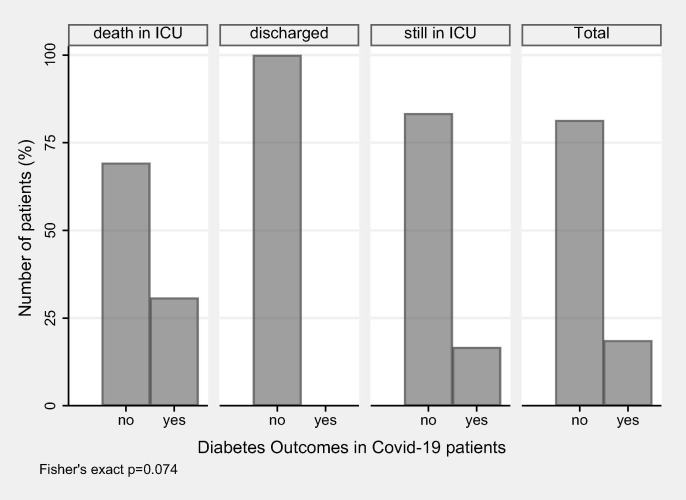
Type-2 diabetes (DM) was present equally in all 3 age study groups with 2 individuals (9.5%) in Group A, 4 (16.7%) in Group B and 11 (24.4%) in Group C (p = 0.387). DM was present only among deceased patients (30.8%) and those still in ICU (16.7%), while none of the discharged patient had DM (p = 0.074) (Fig. 1 ). A stronger trend was observed when the comparison included diseased patients (30.8%) vs. survivors (13.3%) (p = 0.056).
Fig. 1.
Diabetes outcomes in Covid-19 patients.
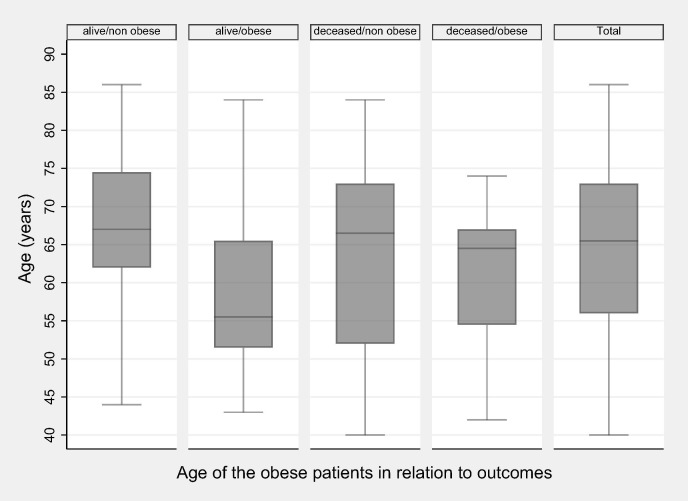
Obesity (BMI > 30) was present in 31 cases (34.4%): 12 patients (57.1%) in Group A, 10 (41.7%) in Group B and 9 (20%) in Group C (p = 0.009). Overweight patients with a BMI between 25 and 30 kg/m2 were present mainly in Group C with 29 patients (64.4%), while there were only 11 (45.8%) in Group B and 7 (33.3%) in Group A. Differences within the study groups were statistically significant (p = 0.024). Obesity was more frequent (46.2%) among deceased than among survivors (26.7%) (p = 0.077). The median age of the living patients without obesity is 67 (IQR 62–74.5) and it is statistically significantly higher than those with obesity 55.5 (IQR 51.5–65.5) (p = 0.007). The deceased patients median age is 66.5 (IQR 52–73) in non-obese & 64.5 (IQR 54.5–67) in obese (p = 0.505) (Fig. 2 ).
Fig. 2.
Age of the obese patients in relation to outcomes.
3.3. Clinical characteristics
Only 8 patients (8.9%) were admitted directly to ICUs, while the median duration of the common symptoms before their appearance in the Emergency Rooms was 8 (IQR 5–11) days, with similar distribution in all age study groups (p = 0.381). However, the reported symptoms lasted fewer days in the deceased patients (6.5 [IQR 4–10]) than in the survivors (8 [IQR 5–12]) (p = 0.043). Vital signs at admission in ICUs, was fever ≥ 38 °C for 64 patients (71.1%), heart rate ≥ 100 beats/sec for 49 (54.5%) and respiratory rate with ≥ 20 breaths per minute in 87 patients (96.7%).
3.4. Laboratory and radiologic findings
The laboratory and radiologic findings of the patients upon admission in ICU are shown in Table 2. Laboratory data for 19 patients from one collaborating center was not submitted. However besides a suggestive trend of higher arterial lactate levels in non-survivors there was not found any other differences for the baseline values of all laboratory parameters measured (Table 3).
Abnormal findings with bilateral pulmonary opacities were detected in all but one patient and pleural effusions were seen in 24 (26.7%). Pleural effusion was more frequently found evident in Groups B (n = 11, 61.1%) and C (n = 11, 28.2%) in comparison to Group A (n = 2, 14.3%) (p < 0.013).
3.5. Respiratory failure data
From the 90 (100%) patients, 82 (91.1%) underwent mechanical ventilation with similar distribution across age groups: 19 (90.5%) in Group A, 21 (87.5%) in Group B and 42 (93.3%) in Group C (p = 0.636). The median lowest ratio of PaO 2/FiO 2 on the 1st day of admission in the ICU was 99 (IQR 87–130) for Group A, 100 (IQR 72–100) for Group B and 100 (IQR 74–108) for Group C. Accordingly, in the 3rd day of hospitalization in the ICU the median ratio value reached was 109 (IQR 70–157): 120 (IQR 73–152) for Group A, 80 (IQR 60–102) for Group B and 136 (IQR 95–167)) for Group C. Patients in Group C showed improved respiratory function after 3 days of hospitalization with a median ratio value of 24 (IQR 0–66) in comparison to Group A with a negative −6 (IQR −23 to 17) and group B with −10 (IQR −40 to 0) (p < 0.001).
3.6. Outcomes
Twelve (12) patients (13.3%) were discharged, 26 died (28.9%) and 48 (53.4%) remained in the ICUs. Outcome data from 4 patients is missing. Distribution of deaths was: Group A 7 (35%), Group B 8 (34.8%) and Group C 11 (25.6%) (p = 0.643). Therefore, in our study, there is no evidence that age is a factor which adversely complicates the health outcomes of a patient in a critical care status from Covid-19.
The median days of hospitalization was 8 (IQR 5–13), which was similar in all three study groups, namely 7 (IQR 5–12) in Group A, 8.5 (IQR 7–11.5) in Group B and 9 (IQR 6–13) in Group C. However, deceased population was hospitalised for a shorter period than survivors 6 (IQR 3–9) days vs. 9 (IQR 7–14.5) (p = 0.002) (Fig. S1).
4. Discussion
In this observational retrospective study, the collaborating hospitals, cited from North to South Greece in different major urban locations, provided information for the disease course of patients hospitalized in their ICUs and thus is representative of cases diagnosed and treated Nationwide. The median age [65.5 (IQR 56–73) years] and the gender propensity of our patients (mostly men) are similar to critically ill Covid-19 patients in neighboring countries or in the USA [5], [14]. Our findings identified that the duration of reported known symptoms [15] with a median duration of 8 days prior to admission and hospitalization was shorter in non-survivors vs survivors (p = 0.043, p = 0.002 respectively).
The vast majority of our patients needed mechanical ventilation upon ICU admission because of severe to moderate acute respiratory distress syndrome as indicated from the very low ratio of PaO 2/FiO 2 [16]. In contrast to reports from Italy [5], statistically significant improvement of the ratio was seen only to older than 66 years old patients and although half of all patients were older, the mortality between age study groups was similar. Most studies demonstrate that mortality is significantly lower in younger ages (<65 years), probably because cardiovascular and metabolic disorders are very common in older patients and may influence the true impact of aging to prognosis [17]. Our patients over 65 years had statistically significant higher number of comorbidities than the younger ones (p = 0.001) without however any increased rate of mortality though. The patients in this study meet the rates of comorbidities for the general population in Greece [18].
Obesity is associated with a negative role in respiratory function [19], [20] and the elderly patients in our study had a lesser degree of obesity. Literature suggests that obese patients are presented with more severe Covid-19 infection [21], [22]. Obesity in our patients was relatively high, particularly in the younger group (p = 0.009) and with a suggestive relation to increased mortality (p = 0.077). Furthermore, the age of the survivors without obesity was higher than the obese survivors (p = 0.007).
Undoubtedly, diabetes mellitus is now recognized as one of the most common commorbidities in Covid-19 patients [14] and since many diabetic patients are obese this leads to a twofold increase in the incidence of requiring intensive care [23]. In our study a suggestive association of diabetes (p = 0.056) with mortality has been noted, as it was present more frequently in non-survivors (30.8%) vs survivors (13.3%). It is also noteworthy that none of the 12 discharged patients had diabetes, while it was present in 30.8% of the deceased. Even more the number of the non survivors with diabetes was significantly higher regardless the age study groups they belong (p = 0.038) (Fig. S2). Thus, people with diabetes appear to develop with an increased risk a more severe Covid-19 infection, but the mechanisms and the underlying molecular pathophysiology remains still uncertain.
A systematic review from China demonstrated that patients suffering from previous CVD may face a greater risk of developing severe condition [24]. Conversely, our results indicate that the presence of CVD was not related to early death, since the frequency of CVD was similar between non-survivors (15.4%) and survivors (23.3%), but was clearly and significantly present to our population of the elder patients (p = 0.001).
Nevertheless, our study has some limitations. We evaluated data extracted retrospectively from patients' medical files, not all laboratory tests were done in all patients and the patient study size was small. The survivors group might not reflect the true case fatality rate, because we are not aware of the progress of the disease for every single patient who remained hospitalized at the time when data were locked. Even more we did not proceed to a complete multivariate sub-group analysis with all confounding factors mainly because the small sample size and thus the significance of our results would be then very weak with a wide spread in confidence intervals. Certainly, a more thorough assessment of comorbitidies, may help establish a risk stratification of patients with Covid-19 upon hospital admission and thus future studies are needed.
Acknowledgments
Acknowledgements
The authors thank the staff who carried out the extensive clinical and nursing work in the Intensive Care Units where the study patients were hospitalized.
Author’s contribution
DG and HP conceptualized and designed the study, wrote the paper and gave approval of the final version to be submitted. TK analyzed the data and performed statistical analysis. KA, JE, ME, TM, KG, GE, PC, KN, PS, SM, GC, VD, MN, SE, VG, AE, KA, ZT, KP, AA and BA collected clinical information, reviewed the manuscript and gave final approval of the final version to be submitted.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2020.108331.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Yang Xiaobo, Yu Yuan, Xu Jiqian, Shu Huaqing, Xia Jia'an, Liu Hong, Wu Yongran, Zhang Lu, Yu Zhui, Fang Minghao, Yu Ting, Wang Yaxin, Pan Shangwen, Zou Xiaojing, Yuan Shiying, Shang You. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Resp Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. Jan 11, 2020. https://www.who.int/internal-publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection- is-suspected [accessed Jan 20, 2020].
- 3.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–550. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Current state of Covid-19 outbreak in Greece and timeline of key containment events https://eody.gov.gr/en/current-state-of-covid-19-outbreak-in-greece-and-timeline-of-key-containment-events/Press Releases 04.03.2020 https://eody.gov.gr/0413_briefing_covid19/.
- 5.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roncon Loris, Zuin Marco, Rigatelli Gianluca, Zuliani Giovanni. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127:104354. doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malavazos Alexis Elias, Corsi Romanelli Massimiliano Marco, Bandera Francesco, Iacobellis Gianluca. Targeting the adipose tissue in COVID‐19. Obesity. 2020;28(7):1178–1179. doi: 10.1002/oby.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher R.A. 5th ed. Oliver & Boyd; Edinburgh: 1934. Statistical methods for research workers. [Google Scholar]
- 9.Pearson K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Philos Mag Series 5, 1900;50(302):157–75.
- 10.Wilcoxon F. Individual comparisons by ranking methods. Biometrics 1945;1: 80–3. Scheffé Henry. The analysis of variance. New York: Wiley; 1959.
- 11.“The Probable Error of a Mean”. Biometrika 1908;6(1): 1–25. doi:10.1093/biomet/6.1.1. hdl: 10338. dmlcz/143545.
- 12.Welch B.L. The generalization of ‘student's' problem when several different population variances are involved. Biometrika. 1947;34:28–35. doi: 10.1093/biomet/34.1-2.28. [DOI] [PubMed] [Google Scholar]
- 13.Royston P. A simple method for evaluating the Shapiro-Francia W' test for non-normality. Statistician. 1983;32:297–300. [Google Scholar]
- 14.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically Ill patients in the Seattle Region - Case Series. N Engl J Med. 2020(Mar). doi: 10.1056/NEJMoa2004500 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 15.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The ARDS Definition Task Force. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA. 2012;307(23):2526–33. doi:10.1001/jama.2012.5669. [DOI] [PubMed]
- 17.Liu Y., Mao B., Liang S., et al. Association between ages and clinical characteristics and outcomes of coronavirus disease 2019. Eur Respir J. 2020 doi: 10.1183/13993003.01112-2020. [DOI] [Google Scholar]
- 18.Magriplis E., Dimakopoulos I., Karageorgou D., et al. Aims, design and preliminary findings of the Hellenic National Nutrition and Health Survey (HNNHS) BMC Med Res Methodol. 2019;19(1):37. doi: 10.1186/s12874-018-0655-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bercault N., Boulain T., Kuteifan K., et al. Obesity-related excess mortality rate in an adult intensive care unit: a risk-adjusted matched cohort study. Crit Care Med. 2004;32(4):998–1003. doi: 10.1097/01.ccm.0000119422.93413.08. [DOI] [PubMed] [Google Scholar]
- 20.Watson R.A., Pride N.B., Thomas E.L., et al. Reduction of total lung capacity in obese men: comparison of total intrathoracic and gas volumes. J Appl Physiol. 2010;108:1605–1612. doi: 10.1152/japplphysiol.01267.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonnet A., Chetboun M., Poissy J., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;(Apr) doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qingxian C, Fengjuan C, Fang L et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China (3/13/2020). Available at SSRN: https://ssrn.com/abstract=3556658 or Doi: 10.2139/ssrn.3556658. [DOI] [PubMed]
- 23.Li B., Yang J., Zhao F., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020 doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morra ME, Van Thanh L, Kamel MG, et al. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and meta‐analysis. Rev Med Virol 2018;28(3):e1977. doi: 10.1002/rmv.1977 [Epub 2018 Apr 17]. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.