Abstract
Purpose
The coronavirus disease-2019 (COVID-19) pandemic has affected healthcare systems across the nation. The purpose of this study is to gauge the early effects of the COVID-19 pandemic on head and neck oncology and reconstructive surgery (HNORS) practice and evaluate their practice patterns especially ones that might be impacted by COVID-19 and compare them to the current literature.
Methods
This study is a cross-sectional study that surveyed fellowship-trained oral and maxillofacial surgeons in HNORS. This cohort of surgeons was contacted via a generated email list of surgeons enrolled in the American Association of Oral and Maxillofacial Surgeons pathology special interest group. An electronic survey contained 16 questions to assess the COVID-19 effect on HNORS practice and capture their practice patterns from mid-March to mid-April 2020. Statistical analysis was performed to analyze counts, percentages, and response rates.
Results
We had a 60% response rate (39 of 64); 72% of our responders worked at academic institutions, 18% marked themselves as hybrid academic/private practice, and only 10% were considered hospital-based surgeons. Only 8% of the survey respondents were requested to pause head and neck cancer surgery, whereas 24% were requested to pause free flap surgery during the pandemic. Fifty-five percent agreed that the head and neck and reconstructive surgery should be conducted during a pandemic. Finally, 45% thought that two weeks was a reasonable delay for head and neck cancer cases, whereas 29% thought they should not be delayed for any amount of time. Regarding practice patterns, microvascular reconstruction was the favored method (100%). Respondents generally admitted patients to an intensive care unit postoperatively (92%) and were kept on a ventilator (53%).
Conclusion
The COVID-19 pandemic had a small impact on the surgical treatment of patients with head and neck oncology. Most HNORS surgeons are practicing in accordance with recently published literature.
The global health organization announced coronavirus disease-2019 (COVID-19) as a pandemic, something reflected by the continuous surge in the number of infected patients in the United States and worldwide. It is expected that only 20% of the population infected by the virus will need medical services. When we dissect the COVID-19–infected population further, calculated estimates are that 15% will have a severe illness and 5% will have a critical illness with a mortality rate ranging between 0.25 and 3%. Higher fatality is expected in the population older than 80 years of age and patients with underlining medical comorbidities. There are 5,198 community hospitals in the United States with 792,417 beds, 3532 emergency departments, and 96,500 intensive care unit (ICU) beds along with 209 federal-funded hospitals. Overwhelming healthcare systems across the country can result from the inability to flatten the COVID-19–infection curve over time. This translates into a shortage of ICU beds, ventilators, availability of medical staff, doctors and nurses, personal protective equipment (PPE), and strain to the healthcare system to provide diagnostic and therapeutic services.1 Oral and maxillofacial surgeons (OMSs), otolaryngologists, dentists, ophthalmologists, and anesthesiologists are considered high-risk service lines. This is clear from the rising number of articles, professional communications, and organization recommendations such as the American Head and Neck Society, American Association of Oral and Maxillofacial Surgeons (AAOMS), and American College of Surgeons. Working in the head and neck region is considered a high risk for exposure to and contracting the virus. Providers manipulating this unique anatomic region might risk infecting other patients, healthcare providers, and their own families. This study will survey OMSs who treat head and neck oncology and perform reconstructive surgery regarding the impact of the COVID-19 pandemic on their surgical practice during mid-March to mid-April 2020. We anticipate that the COVID-19 pandemic will impact the surgical practice of OMSs performing head and neck oncology and reconstructive surgery (HNORS). We also aim to compare current HNORS practice patterns, especially the ones that might be impacted by COVID-19, with the current published literature.
Materials and Methods
In our institution, surveys are exempted from the need to obtain approval from an institutional research board. For this cross-sectional study, we designed an anonymous survey for fellowship-trained OMSs in HNORS. This cohort of surgeons was contacted via a generated email list of surgeons enrolled in the AAOMS pathology special interest group. The participants were contacted electronically via an email message forwarded by the authors.
Confidentiality and protection of personal information of the participants were maintained. The email contained a brief introduction of the investigators and explained the purpose of the survey. A hyperlink to the electronic survey was provided with instructions on how to take the survey in that email. The hyperlink contained in the document opened directly to the page of the survey. In addition, a QR code was directly sent with the email to the survey to allow a smooth response from smartphones, tablets, personal computers, and laptops. The online survey was designed and made available using Microsoft Forms. This platform allowed participants to access and complete the survey without having to create a personal account and without entering any personal information. The account on the website was created and is maintained by the investigators, and once the participants choose to take the survey, a link included in the email message directly opens only the survey of interest and allows for immediate access. After answering the survey, the participants were asked to click on the “submit” box; their responses were saved and uploaded. This method provided confidentiality and simplicity. Participants were contacted twice via email with a two-week time frame between the first and second email communications.
The survey consisted of a 16-item questionnaire aiming to evaluate the impact of COVID-19 on clinical practice and their usual practice patterns of HNORS. These questions are listed in Table 1 . Questions 1 to 6, 9 to 12, 14, and 16 were multiple-choice questions. Only question 12 allowed multiple answers to be selected. The first two questions asked the participants about their clinical experience in managing head and neck cancer and the setting of their practice; the third question down to the ninth question asked if the participants practiced head and neck reconstruction, their preferred reconstructive modality, utilization of “two-team approach,” the postoperative setting they transferred patients with free flap to, their decision to perform a tracheostomy on patients with free flap, and finally, if they kept patients wih free flap on a ventilator or were extubated at the end of the procedure. Questions 10 to16 asked about COVID-19 and its impact on HNORS practice and factors leading to that impact. The selection criteria to participate in the study were 1) a member of the AAOMS/pathology special interest group, 2) completion of a HNORS fellowship, and 3) practicing (OMS)surgeon. The exclusion criteria were 1) failure to properly complete the survey entirely and 2) current HNORS fellows. Statistical analysis was performed to analyze counts and percentages and response rate.
Table 1.
Questions of the Survey
| 1.How long have you been managing head and neck cancer |
| ◦ Less than 5 yr |
| ◦ More than 5 - less than 10 yr |
| ◦ More than 10 yr |
| 2.How would you consider your practice? |
| ◦ Academic |
| ◦ Private practice |
| ◦ Hospital based |
| ◦ Academic and private practice |
| 3.Do you perform head and neck reconstruction? |
| ◦ Yes |
| ◦ No |
| 4.If you do perform head and neck reconstruction? what would be your preferred method of reconstruction |
| ◦ Microvascular tissue transfer |
| ◦ Regional flaps |
| ◦ Local flaps |
| 5.Do you usually perform head and neck cancer cases with a two-team approach? |
| ◦ Yes |
| ◦ No |
| ◦ Maybe |
| 6.Where do you generally transfer your free flaps patients? |
| ◦ ICU |
| ◦ Step-down head and neck unit |
| ◦ Regular floor |
| 7.On what percentage of your free flap patients do you perform a tracheostomy on? |
| 8.what makes you decide to perform a tracheostomy on your free flap patient? |
| 9.Do you keep your free flaps patients in an ICU setting on a ventilator or you extubate them at the end of the procedure? |
| ◦ Keep the patient on the vent |
| ◦ Extubate them at the end of the procedure |
| 10.After COVID-19 been announced as a Pandemic, did your healthcare system, university, state medical or dental board ask you to suspend doing head and neck oncology cases? |
| ◦ Yes |
| ◦ No |
| 11.After COVID-19 been announced as a pandemic, did your healthcare system, university, and state medical or dental board ask you to suspend doing head and neck reconstruction using free flaps? |
| ◦ Yes |
| ◦ No |
| 12.What the factors might contribute to making this decision? you can select multiple answers |
| ◦ Local policies (state, hospital, medical or dental board) |
| ◦ Surgeon own decision |
| ◦ Blood bank shortage |
| ◦ Number of ventilators at your hospital |
| ◦ Personal protective equipment availability |
| ◦ Medical comorbidities of the patient |
| ◦ Cancer stage |
| ◦ Other |
| 13.If you select other please elaborate |
| 14.In a pandemic situation, do you think head and neck oncology and microvascular reconstructive surgery should be performed? |
| ◦ Yes |
| ◦ No |
| ◦ Maybe |
| 15.If you answered maybe, please specify? |
| 16.If you think these cases need to be delayed, what is a reasonable amount of time to delay these cases? |
| ◦ 2 wk |
| ◦ 4 wk to 2 mo |
| ◦ Until the pandemic situation is downgraded or cleared |
| ◦ They should never be delayed |
Results
General Information
Overall, 39 of 64 responded to the survey with a response rate of 60%; 72% of our responders worked at an academic institution, 18% marked themselves as hybrid academic/private practice, and only 10% were considered hospital-based surgeons. Of the participants of this study, 43% had less than five years' experience in managing head and neck oncology, 38% had more than five years but less than ten years, and finally, 20% had more than ten years of experience.
The Impact of COVID-19 on HNORS
Only 8% of the survey responders were asked to pause head and neck oncology cases; however, 23% were asked to pause free flap reconstruction during the COVID-19 pandemic. Contributing circumstances to this situation included local policies and PPE; both were tied as the first reason. Fifty-five percent agreed that head and neck and reconstructive surgery should be conducted during a pandemic. Finally, 45% thought that two weeks is a reasonable time to delay head and neck oncology cases, whereas 29% thought these cases should never be delayed.
Head and Neck Reconstruction and Head and Neck Practice Patterns
Ninety-seven percent of our responders performed head and neck reconstruction, and there was a recognized agreement that microvascular tissue transfer was the favored method of reconstruction. Ninety-two percent kept their patients in an ICU environment, whereas only 8% transferred them to a specialized step-down unit. In the ICU setting, 51% kept the patient on a ventilator whereas 47% extubated these patients at the end of the procedure. A simultaneous two-team approach was implemented in 82%. Tracheostomy is performed at an average of 64% of free flap cases among the survey respondents. Twenty-nine percent of respondents performed a tracheostomy in >90% of free flap cases, whereas 24% performed a tracheostomy in limited cases (<30% of the time).
Discussion
The purpose of this study was to determine the impact of the COVID-19 pandemic on OMSs performing HNORS. We anticipated that the COVID-19 pandemic would have an impact on their surgical practice. We also compared current practice patterns, especially those that might be impacted by COVID-19, with published literature. Most respondents thought that HNORS should be performed in a pandemic situation. The results did not confirm our assumption that the COVID-19 pandemic impacted HNORS clinical practice patterns. Finally, OMSs are practicing in accordance with recently published literature and guidelines.
Impact of COVID-19 on HNORS
Fortunately, only 8% of our responders were asked to delay head and neck cancer treatment; however, this unprecedented situation made surgeons face many ethical dilemmas: first, “is delaying head and neck cancer treatment” acceptable during the COVID-19 pandemic? second, ethical dilemma “if your institution/hospital allowed head and neck cancer cases to be performed but did not allow microvascular reconstructive surgery, should we still perform ablative procedures without a proper reconstruction? This situation becomes more complicated when before COVID, these patients were informed that microvascular reconstruction is the ideal reconstruction modality as it allows complex reconstruction of more than one subunit, can withstand the adverse impact from radiation therapy, can offer immediate dental implant placement, and allow these patients to start adjuvant therapy within the recommended time frame of 6 to 8 weeks after oncologic ablation. Ultimately, the final decision rests with the patient who is under psychological distress from a cancer diagnosis while preparing to start a journey toward cure of their head and neck cancer.
Compare Free Flaps With Other Reconstructive Flaps
The superiority of free microvascular tissue transfer (FMTT) to pedicled regional flaps and local flaps has been debated in the literature but is considered the gold standard for reconstructing head and neck defects arising from complex oncologic ablative procedures.2, 3, 4, 5 Abouyrad et al2 conducted a literature review of free tissue transfer management and outcomes and concluded that FMTT remains highly successful despite the lack of consensus regarding the care of patients receiving FMTT.There are many reasons FMTT is considered the gold standard for head and neck reconstruction; first, these defects arising from head and neck ablation are complex, and they tend to involve multiple anatomic subunits and have proximity to vital structures, and the ultimate goal is to restore preablative form and function. Success rates of FMTT vary in the literature, reaching 95 to 97% irrespective of the setting of FMTT being performed at, such as academic institutions or a community hospital.6, 7, 8 The selection process of the ideal reconstructive option for head and neck ablative defects depends on multiple variables, including available local resources. Gabrysz-Forget et al,9 in a systematic review, found no statistical significance in terms of hospital length of stay between free flap and pedicled flap groups10; however, the results were variable when FMTT was explicitly compared with the submental island or supraclavicular flaps. In this comparison, FMTT length of stay was longer. Comparing FMTT with pectorals myocutanous flaps (PMCF), FMTT tended to have a shorter length of stay. Complications reported with PMCF ranged between 16 and 63%, whereas complications arising from FMTT is between 14 and 30%.10 It is essential to mention that there are many published descriptions of PMCF as a “workhorse” or “the flap that stood the test of time.”11, 12, 13, 14 However, it is essential not to confuse these terms with the “gold standard” that is often tagged to FMTT when it comes to head and neck oncologic reconstruction.15 Tackling head and neck cancer reconstruction with a one-size-fits-all approach is rarely optimal, and a pandemic should not be an excuse to apply old or outdated concepts.3 This was well reflected in our survey, as all respondents selected FMTT as their preferred method of reconstruction Figure 1 .
Figure 1.
Preferred method of reconstruction.
FMTT allows predictable reconstruction of large and complex head and neck defects and tends to tolerate the adverse effects of radiation therapy. Besides, FMTT has a lower incidence of postoperative infection, dehiscence, and partial or complete flap necrosis.9 In some ablative defects such as near-total mandibular composite defects, through-and-through buccal defects, or defects involving the temporomandibular joint local flap such as pedicled flap reconstruction cannot offer a comparable result while laying a solid foundation for dental rehabilitation with dental implants.
Prolonged Operative Time
FMTT comes with shortcomings and might stress the healthcare system in a pandemic scenario such as COVID-19. These cases tend to have longer operative time (6 to 12 hours) with an average of eight hours.16 , 17 Kim et al.18 defined prolonged operative time as surgery lasting longer than 5 hours. Prolonged operative time is an independent risk factor for postoperative complications. Adapting data from general surgery literature, the rate of infection-related complications increased by 2.5% for every 30 minutes in the operating room, and this was true for thromboembolic complications and even free flap failure rates. These complications might increase the need to return to the operating room and increase the overall hospital length of stay by 6%. In the COVID-19 pandemic, this adds further strain to hospital systems with an already increased demand for ventilators. Prolonged operative time is linked to prolonged general anesthesia and intubation duration, which are risk factors for postoperative pneumonia or ventilator-related lung injury. This risk persists even if a surgical airway is performed.18, 19, 20 Many theories offer explanations for the positive correlation between operative time and surgical infections. Amplified exposure to airborne pathogens elongated trauma resulting from prolonged surgical manipulation and finally increased foot traffic in the operating room by the surgical team and axillary staff. Regarding COVID-19 specifically, we know that the virus targets mainly the lower respiratory tract, something that hypothetically might be amplified if a patient is infected with the virus after prolonged operating time. Many centers realized the adverse effects of prolonged operative times in head and neck cancer and reconstructive surgery. These centers adopted various approaches to reduce that time. They adapted a two-team approach to reduce operative time that now needs to be weighed against the shortage of PPE and exposure risk to an increased number of surgeons and assistants participating in high-risk procedures. Eighty-two percent of respondents performed reconstruction surgery with a two-team approach. One strategy to reduce operative time is computer-assisted surgery/planning including surgical planning of the osseous ablative resection margins, osseous donor site cutting guides, and three-dimensional printing modeling with the option of custom-made plates.21 , 22
Tracheostomy
Head and neck ablative or reconstructive procedures can be associated with a higher risk of upper airway obstruction. Therefore, many surgeons elect to perform a tracheostomy to eliminate this risk. Obstruction of the airway may be associated with an oversized, bulky reconstruction or specific anatomical sites, including the mandible, floor of the mouth, and tongue. In addition, bilateral neck dissection has an increased risk of airway obstruction. Tracheostomy has been found to increase the cost of care and lengthen the operative time by 30 to 45 minutes. Specific to head and neck surgery, airway protection with continued intubation rather than tracheostomy has demonstrated its safety in many studies. This is in addition to other advantages, including reduced lower respiratory tract infections and faster return to speech and oral intake. Another advantage of this practice is the improved cost effectiveness by decreasing operative time, ICU stay, and overall hospital stay to discharge. Tracheostomy should be performed in very select cases. In light of the COVID-19 pandemic, tracheostomy is on the list of high-risk, aerosol-generating procedures; recent literature has recommended that a multidisciplinary discussion of the risk-benefit profile should take place. Surgeons performing tracheostomy in light of COVID-19 should have a plan in place to minimize exposure risk. Open surgical tracheostomy is associated with a decreased likelihood of aerosol generation compared with a percutaneous tracheostomy procedure and is preferred at this time. During surgery, paralysis can eliminate the risk of patient movement and coughing during the procedure. Minimizing diathermy, maintaining a bloodless field, and pausing ventilation during insertion of the tracheostomy tube are recommended. Placing the inflated cuff of the endotracheal tube below the tracheostomy site can minimize the duration of apnea and maintain a seal to minimize aerosol. Keeping patients with head and neck cancer intubated is the preferred approach. A potential disadvantage of keeping patients intubated in light of the COVID-19 pandemic is the need to remain on a ventilator for at least one day whether in the ICU setting or the postanesthesia recovery unit; however, this should be balanced with the known and very high risk of aerosol generation postoperatively in the patient with tracheostomy through nebulizer treatments, suctioning requirements, coughing, and excess sputum/secretion production.23 , 24
Factors that Might Impact HNORS
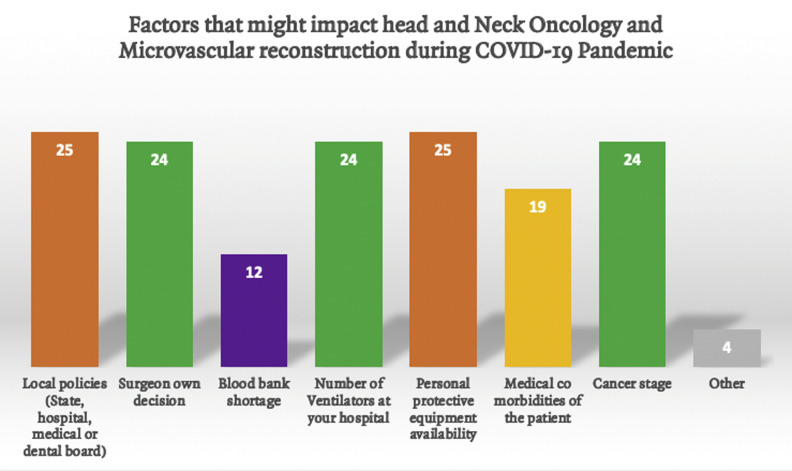
Survey recipients were asked what might cause healthcare systems, universities, and medical or dental boards to suspend head and neck oncology and microvascular reconstruction procedures. Tied as the main reason that might lead to suspension of these cases were local policies and availability of PPE, followed by cancer stage, surgeon decision, and the number of ventilators as the second most common reason. Surgeons thought medical comorbidities were less critical, with blood bank shortage least important Figure 2 .
Figure 2.
Factors that might impact head and neck oncology and reconstruction.COVID-19.
Local Policies and PPE
Similar to many other surgical specialties, OMSs regularly receive guidance from the Centers for Disease Control and Prevention (CDC) about the COVID-19 pandemic. Most recent CDC updates, recommendations, and guidelines are easily accessible (https://www.cdc.g1ov/coronavirus/2019-ncov/index.html). National associations and societies including the AAOMS,24 American Dental Association, American Head and Neck Society (AHNS),25 and American College of Surgeons have published their guidelines.26 Some of these guidelines are based on shared experiences from other countries dealing with the COVID-19 pandemic and were recognized as “hot spots.” Some of this literature comes from China, Italy, and Iran, as well as updated guidelines from the CDC. All of these organizations agreed on the importance of delaying elective, nonurgent admissions and procedures, some of them used the terminology “only time-sensitive or emergent care.” This strategy aims to decrease the anticipated overwhelming stress on the healthcare system without compromising patient care. AAOMS and AHNS recommendations agreed on the importance of COVID-19 testing for surgical patients undergoing a high-risk procedure in terms of COVID-19 spread. An article from Canada defined these procedures as any procedure that might create aerosol or manipulate the eyes, nose, oral cavity, upper and lower airways, and the gastrointestinal tract.27 Focusing on HNORS, this domain is considered high risk as per the recommendations mentioned earlier, as most of these procedures have a high risk for viral spread to the operating surgeon and his/her team. With that in mind, Stanford School of Medicine published their guidelines on head and neck surgery. They recommended that urgent cases (including certain cancer cases) should be treated within 30 days. They recommend that patients should have preoperative testing for COVID-19. If the test is positive, all the personnel in the operating room need to be wearing a powered air-purified respirator (PAPR) until further data are available, and if PAPRs are not available, then N95 masks, with face shields, are required on top of the surgical PPE, which is in line with the CDC recommendations.28 With these recommendations in mind, most hospitals formed operating room committees to review cases and determine the urgency of each case while keeping resources such as anesthesia team availability, ventilators availability for patients without COVID, availability of operating rooms (especially rooms with negative pressure), presence of proper PPE including Powered Air Purifying Respirators (PAPRs)/Controlled Air Purifying Respirators or even N95 masks, and individual state health departments policies.
Preoperative testing capability for COVID-19 is critical because the CDC reserved the ultimate decision for “state and local health departments and individual clinicians” to formulate their own protocol. Some department heads or service leads might recommend delaying these cases, again guided by available resources from beds, operating room supplies, PPE, and operating room staff. Before the COVID-19 pandemic, PAPRs were not discussed frequently in the surgical literature, but recently, many publications have started recommending them for high-risk procedures, especially when surgeons could not verify the COVID-19 status of the patient. PAPRs do have higher protection than N95 masks, and this protection factor might depend on the airflow setting in the PAPR system.27 , 28 Other practical advantages of PAPRs include no need for fit testing, ability to use with facial hair, and finally comfort of use. However, if PAPRs are not available, then N95 masks should be used along with protective goggles and a face shield. During the COVID-19 pandemic, many hospitals experienced a shortage of PPE, which had a negative effect on hospitals and surgeons.
Evidence Regarding Postoperative ICU Admission and Keeping Patients on a Ventilator
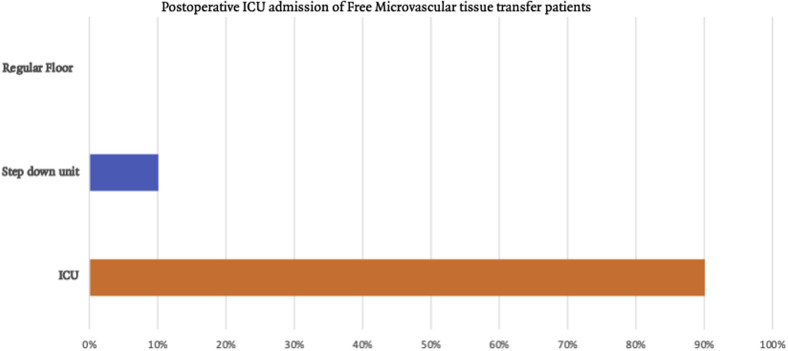
Among free flap surgeons, 88.9% of surgeons admit patients with head and neck oncology with FMTT to the ICU for an average of 2.4 to 3 days,29 , 30 slightly less than that of our survey (90%) Figure 3 . An ICU stay is proposed to provide many advantages, high nurse: patient ratios ranging from 1:1 to 1:2/per patient, constant flap monitoring of flap color, trigger, pinprick, and doppler assessment to detect early signs of vascular compromise something that occurs between 5 and 10%. In the ICU, patients may have sedation and acute postsurgical pain control, respiratory ventilation, and critical tracheostomy care. It might be anecdotal, but surgeons prefer to minimize patient movement and, therefore, flap movement, in the first 24 to 48 hours. Despite this frequent practice of minimizing patients' movement, there is no evidence to show superior outcomes. Finally, the ICU may have the tools to provide steady blood pressure and, in return, more consistent flap perfusion. Many centers are creating momentum to change this practice of sedating patients with flap while keeping them on a ventilator as it has been associated with increased weaning time and increased risk for ventilation-induced pneumonia. Other adverse events that might occur from sedation include hypotension, especially with propofol-based ICU sedation. Another factor to consider in ICU stay is the significant financial burden added to the overall care of patients with head and neck cancer with FMTT. This practice pattern might be tough to offer to patients during a pandemic such as COVID-19 owing to the increased need for ventilators and ICU care. According to John Hopkins School of Public Health, acute care hospitals in the United States have 62,000 full-function ventilators, with 98,000 necessary ventilators and additional 8,900 others in the CDC Strategic National Stockpile. The same organization estimates the 2.4 to 21 million Americans will require hospitalization. While this remains a continually changing situation, extrapolating from the published Italian data, 25% of the hospitalized patients required ventilation support.31 What makes a shortage of ventilators different from other medical supplies is that ventilators might be necessary during a limited window in which the patient's life might be saved. The ventilation situation is also affected by local and healthcare guidelines. A good example is that New York developed guidelines with the concept of “saving most lives” defined by the likelihood of this patient surviving this short-term medical distress, a set of guidelines designed in 2015.32 This preparation is shared among other states. Level I evidence from a prospective, double arm, 1:1 randomized controlled trial of patients with head neck flap randomized to ICU versus specialized ward/step-down unit has been collected. Flap monitoring protocols were shared among the two groups. In this cited study, the authors did not find any significant differences between the two groups regarding medical comorbidities, prior radiation therapy treatment or chemotherapy, ischemia time, blood loss, transfusion, or postoperative antibiotics use. In this randomized controlled trial, the primary outcome was the length of stay, which did not show any statistical differences between the two groups. It should be noted that in this cited study, patients who were assigned to the step-down unit/specialized ward group who needed postoperatively to be transferred to the ICU for impending flap failure, medical disease–like sepsis, delirium, or after a second surgery were still statistically analyzed as a stepdown/specialized unit.33 These findings support other retrospective studies that compared ICU postoperative stay with the step-down unit and were integrated into many clinical care pathways but frequently demonstrate similar or better endpoints in the step-down unit group.29 , 34
Figure 3.
Postoperative ICU admission of free microvascular tissue transfer patients.
Cancer Stage
Mortality rates from head and neck squamous cell carcinoma (HNSCC) are > 50%, accounting for 375,000 mortalities. The American Joint Committee on Cancer indicated that tumor stage is the most critical prognostic factor for HNSCC in which we use the TNM staging system to come up with the clinical and pathological definitive stage.35 Although this opinion is shared by most head and neck surgeons, it does not take into account the wide variability of head and neck cancers such as location/subsite, cancer-related risk factors, and overall patient prognosis. Extrapolating from an article published about Oropharyngeal cancer, and despite its small sample size of 13 patients, Waaijer el al36 observed that with a median scanning interval of 35 days, there was an increase in the radiographic tumor volume around 22 cm3. A similar oral cavity cancer–specific study with a more generous sample size of 38 patients showed a 62% increase in the primary tumor volume and a median increase of 46% of lymph node metastasis. The time intervals were classified into three time intervals. A less-than-two-week period correlated with 33% tumor volume increase, 2 to 4 weeks' time period a 68% significant increase in tumor volume, and finally more than four weeks a 70% increase in tumor volume.37 It is estimated that 17% of patients with HNSCC will progress in stage between the diagnostic workup and treatment. The Dutch head and neck working group stated that 80% of patients with biopsy-proven head and neck cancer should initiate cancer treatment within 30 days interval from their initial visit, something that is widely adopted in Europe. In the United States, delaying surgery for two weeks during COVID-19 might be a reasonable approach guided by center-specific factors. The AHNS suggested this delay, and 38% of survey respondents selected this duration as a reasonable time frame to delay these cases; however, 19% suggested delaying these cases 4 weeks to 2 months, something the literature before COVID-19 did not recommend owing to poor overall prognosis. Thirty-eight percent of surgeons in our survey thought that these cases should not be delayed at all, something that might be hard to implement, particularly with lack of evidence regarding operating room set up during the COVID-19 pandemic, lack of PAPRs, and the shortage of N95 masks and other PPE supplies. It is clear from this survey that delaying head and neck oncology surgical procedures was not widely adapted, which meant these cohort of patients received their treatment in a timely fashion, preventing cancer progression, eliminating the need for reconstructive surgery that are might be needed with advanced and very advanced disease. Hopefully, this approach of not delaying cancer care improves overall survival.
Blood Product Transfusion
Blood shortage during the COVID-19 pandemic is something that is shared among healthcare systems across the board, something that might have a higher impact on smaller community hospitals. Regarding HNORS, blood product transfusion does not impact flap survival and is linked to increased perioperative complications, such as surgical site infection after controlling factors such as age, preoperative hemoglobin (Hgb) and albumin, and cancer stage38; other studies suggested that blood transfusion might increase the risk of cancer recurrence owing to transfusion-related immune modulation, which remains very controversial among cancer surgeons. Many surgeons in light of these findings tend to restrict blood transfusion (allogeneic blood transfusion) and reported blood transfusion remains 12 to 84%39; however, transfusion cutoffs are different (7 g/dl to 10 g/dl).40 A range that might be influenced by the surgeon's preference or criteria is set by the anesthesia team. One of the most common hematological findings we encounter in patients with head and neck cancer is iron-deficiency anemia. This finding might be caused by preoperative alcohol abuse, a common risk factor in patients with head and neck cancer. Other causes attributing to iron-deficiency anemia include dysphagia secondary to pain, bleeding or mechanical limitation arising from oral cavity cancer, and finally poor preoperative nutritional status, something that is usually screened with albumin and prealbumin values and should be optimized preoperatively. Microvascular surgeons tolerate drops in Hgb, which happens postoperatively attributed to hemodilution augmented with postoperative anticoagulation protocols of the surgeon's choice. What complicates the surgeon's decision to transfuse is that anemia is linked with delayed recovery, poor wound healing, and overall fatigue that might interfere with postsurgical mobilization.18 , 41 Alternative options include preoperative autologous blood donation, recombinant erythropoietin, and acute normovolemic hemodilution. Shah at el39 found that women, low body mass index, advanced tumor stage, preoperative Hgb levels, and if osseous FMTT performed were five significant independent predictors for perioperative blood transfusion. Limitations with preoperative autologous blood donation and recombinant erythropoietin are increased cost and lack of national adoption, something that might complicate the decision for performing head and neck cancer and FMTT during a pandemic where the surgeon's clinical judgment is key.
Limitations to our survey include selection bias and insufficient response rate. Practice patterns are impacted by a continually evolving novel pandemic, and a survey at a specific time point will not capture this continued evolution in inpatient care. Future studies should be performed in a retrospective fashion to determine practice pattern changes over time and ideally at a time point near the nadir of this epidemic.
Conclusion
Head and neck oncology and reconstructive surgeons are faced with the same stressful situation other surgeons are facing during this COVID-19 pandemic. The COVID-19 pandemic had a small impact on surgical treatment of patients with head and neck cancer. Most HNOR surgeons are practicing in accordance with recently published literature.
Footnotes
Conflict of Interest Disclosures: None of the authors have any relevant financial relationship(s) with a commercial interest.
References
- 1.Emanuel E.J., Persad G., Upshur R. Fair Allocation of Scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382:2049. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 2.Abouyared M., Katz A.P., Ein L. Controversies in free tissue transfer for head and neck cancer: A review of the literature. Head Neck. 2019;41:3457. doi: 10.1002/hed.25853. [DOI] [PubMed] [Google Scholar]
- 3.Kucur C., Durmus K., Uysal I.O. Management of complications and compromised free flaps following major head and neck surgery. Eur Arch Otorhinolaryngol. 2015;273(1):209. doi: 10.1007/s00405-014-3489-1. [DOI] [PubMed] [Google Scholar]
- 4.Farquhar D.R., Masood M.M., Pappa A.K. Predictors of adverse outcomes in free flap reconstruction: A Single-institution experience. Otolaryngol Head Neck Surg. 2018;159:973. doi: 10.1177/0194599818787801. [DOI] [PubMed] [Google Scholar]
- 5.de Vicente J.C., Rodríguez-Santamarta T., Rosado P. Survival after free flap reconstruction in patients with advanced oral squamous cell carcinoma. J Oral Maxillofacial Surg. 2012;70:453. doi: 10.1016/j.joms.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal E., Carroll W., Dobbs M. Simplifying head and neck microvascular reconstruction. Head Neck. 2004;26:930. doi: 10.1002/hed.20076. [DOI] [PubMed] [Google Scholar]
- 7.Gusenoff J.A., Vega S.J., Jiang S. Free tissue transfer: Comparison of outcomes between university hospitals and community hospitals. Plast Reconstr Surg. 2006;118:671. doi: 10.1097/01.prs.0000233203.84078.6b. [DOI] [PubMed] [Google Scholar]
- 8.Patel U.A., Lin A.C. Flap outcomes when training residents in microvascular anastomosis in the head and neck. Am J Otolaryngol. 2013;34:407. doi: 10.1016/j.amjoto.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Gabrysz-Forget F., Tabet P., Rahal A. Free versus pedicled flaps for reconstruction of head and neck cancer defects: A systematic review. J Otolaryngol Head Neck Surg. 2019;48:13. doi: 10.1186/s40463-019-0334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim B., Kaleem A., Zaid W. Case Reports of two Unusual donor site complications of the Pectoralis major myocutaneous flap and literature review. J Oral Maxillofac Surg. 2016;74:1504.e1. doi: 10.1016/j.joms.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Asamura S., Kakizaki H., Mori K. The Pectoralis major myocutaneous pedicled flap Revisited. Surg Sci. 2013;4:380. [Google Scholar]
- 12.Patel K., Lyu D.J.-H., Kademani D. Pectoralis major myocutaneous flap. Oral Maxillofac Surg Clin North Am. 2014;26:421. doi: 10.1016/j.coms.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Castelli M.L., Pecorari G., Succo G. Pectoralis major myocutaneous flap: Analysis of complications in difficult patients. Eur Arch Otorhinolaryngol. 2001;258:542. doi: 10.1007/s004050100389. [DOI] [PubMed] [Google Scholar]
- 14.Carlson E.R. Pectoralis major myocutaneous flap. Oral Maxillofacial Surg Clin NA. 2003;15:565. doi: 10.1016/S1042-3699(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman G.R., Islam S., Eisenberg R.L. Microvascular reconstruction of the mouth, Jaws, and face: Experience of an Australian oral and maxillofacial surgery Unit. J Oral Maxillofacial Surg. 2012;70:e371. doi: 10.1016/j.joms.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi N.P., Trivedi P., Trivedi H. Microvascular free flap reconstruction for head and neck cancer in a resource-constrained environment in rural India. Indian J Plast Surg. 2013;46:82. doi: 10.4103/0970-0358.113715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindeborg M.M., Puram S.V., Sethi R.K.V. Predictive factors for prolonged operative time in head and neck patients undergoing free flap reconstruction. Am J Otolaryngol. 2020;41:102392. doi: 10.1016/j.amjoto.2020.102392. [DOI] [PubMed] [Google Scholar]
- 18.Kim B., Ver Halen J., Mlodinow A., Kim J. Intraoperative transfusion of Packed red blood cells in microvascular free tissue transfer patients: Assessment of 30-day morbidity using the NSQIP Dataset. J Reconstr Microsurg. 2014;30:103. doi: 10.1055/s-0033-1357275. [DOI] [PubMed] [Google Scholar]
- 19.Cheng C.-D., Lin W.-L., Chen Y.-W., Cherng C.-H. Effects of lung protective ventilation on postoperative pulmonary outcomes for prolonged oral cancer combined with free flap surgery. Medicine. 2020;99:e18999. doi: 10.1097/MD.0000000000018999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-Bustamante A., Frendl G., Sprung J. Postoperative pulmonary complications, early mortality, and hospital stay following Noncardiothoracic surgery: A Multicenter study by the perioperative Research Network investigators. JAMA Surg. 2017;152:157. doi: 10.1001/jamasurg.2016.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanasono M.M., Skoracki R.J. Computer-assisted design and rapid prototype modeling in microvascular mandible reconstruction. The Laryngoscope. 2012;123:597. doi: 10.1002/lary.23717. [DOI] [PubMed] [Google Scholar]
- 22.Toto J.M., Chang E.I., Agag R. Improved operative efficiency of free fibula flap mandible reconstruction with patient-specific, computer-guided preoperative planning. Head Neck. 2015;37:1660. doi: 10.1002/hed.23815. [DOI] [PubMed] [Google Scholar]
- 23.McGrath B.A., Brenner M.J., Respiratory SWTL . Elsevier; 2020. Tracheostomy in the COVID-19 Era: Global and Multidisciplinary Guidance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nannini V.L. Member Alert: Di_cult times; di_cult decisions. Always advocating for OMSs [Internet]. AAOMS; 2020 [cited 2020 Mar 24]. pp. 002203452091424–6. https://www.aaoms.org/news/covid-19-updates#14 Available from: Accessed August 3, 2020.
- 25.AHNS COVID-19 Bulletin - American Head & Neck Society. 2020. pp. 1–3. [Google Scholar]
- 26.Surgeons ACO . 2020 Mar 19. COVID-19: Guidance for triage of non-Emergent surgical procedures; pp. 1–2. [Google Scholar]
- 27.Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568. doi: 10.1007/s12630-020-01591-x. Directives concre`tes a` l’intention des e ´quipes de soins intensifs et d’anesthe ´siologie prenant soin de patients atteints du coronavirus 2019-nCoV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel Z.M., Hwang P.H., Nayak J.V. COVID-19-Information Stanford university school of MedicineDepartments of Otolaryngology-H&N surgery and Neurosurgery [Internet] 2020. https://www.enttoday.org/article/otolaryngologists-may-contract-covid-19-during-surgery/ Available from: Accessed August 3, 2020.
- 29.Morse E., Henderson C., Carafeno T. A clinical care pathway to reduce ICU usage in head and neck microvascular reconstruction. Otolaryngol Head Neck Surg. 2019;160:783. doi: 10.1177/0194599818782404. [DOI] [PubMed] [Google Scholar]
- 30.Abo Sharkh H., Madathil S., Al-Ghamdi O. A Comprehensive clinical care pathway for microvascular maxillofacial reconstructive surgery. J Oral Maxillofac Surg. 2019;77:2347. doi: 10.1016/j.joms.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Truog R.D., Mitchell C., Daley G.Q. The toughest triage — Allocating ventilators in a pandemic. N Engl J Med. 2020;382:1973. doi: 10.1056/NEJMp2005689. [DOI] [PubMed] [Google Scholar]
- 32.Health NYSDO . New York State Task Force on Life and the Law; Albany: 2016. Ventilator Allocation Guidelines; pp. 1–272. [Google Scholar]
- 33.Cervenka B., Olinde L., Gould E. Use of a non-ICU specialty ward for immediate post-operative management of head and neck free flaps; a randomized controlled trial. Oral Oncol Elsevier. 2019;99:104464. doi: 10.1016/j.oraloncology.2019.104464. [DOI] [PubMed] [Google Scholar]
- 34.Dautremont J.F., Rudmik L.R., Yeung J. Cost-effectiveness analysis of a postoperative clinical care pathway in head and neck surgery with microvascular reconstruction. J Otolaryngol Head Neck Surg. 2013;42:59. doi: 10.1186/1916-0216-42-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amin M.B., Edge S., Greene F.L. Springer; 2016. AJCC Cancer Staging Manual; p. 1. [Google Scholar]
- 36.Waaijer A., Terhaard C.H.J., Dehnad H. Waiting times for radiotherapy: Consequences of volume increase for the TCP in oropharyngeal carcinoma. Radiother Oncol. 2003;66:271. doi: 10.1016/s0167-8140(03)00036-7. [DOI] [PubMed] [Google Scholar]
- 37.Wildt J., Bundgaard T., Bentzen S.M. Delay in the diagnosis of oral squamous cell carcinoma. Clin Otolaryngol Allied Sci. 1995;20:21. doi: 10.1111/j.1365-2273.1995.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 38.Dort J.C., Farwell D.G., Findlay M. Optimal perioperative care in major head and neck cancer surgery with free flap reconstruction. JAMA Otolaryngol Head Neck Surg. 2017;143:292. doi: 10.1001/jamaoto.2016.2981. [DOI] [PubMed] [Google Scholar]
- 39.Shah M.D., Goldstein D.P., McCluskey S.A. Blood transfusion prediction in patients undergoing major head and neck surgery with free-flap reconstruction. Arch Otolaryngol Head Neck Surg. 2010;136:1199. doi: 10.1001/archoto.2010.202. [DOI] [PubMed] [Google Scholar]
- 40.Rossmiller S.R., Cannady S.B., Ghanem T.A., Wax M.K. Transfusion criteria in free flap surgery. Otolaryngol Head Neck Surg. 2010;142:359. doi: 10.1016/j.otohns.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 41.Rogers S.N., Horisk K., Groom P., Lowe D. Management of anaemia and blood in patients having neck dissections or free flaps for head and neck cancer. Br J Oral Maxillofacial Surg. 2019;57:543. doi: 10.1016/j.bjoms.2019.05.001. [DOI] [PubMed] [Google Scholar]