Abstract
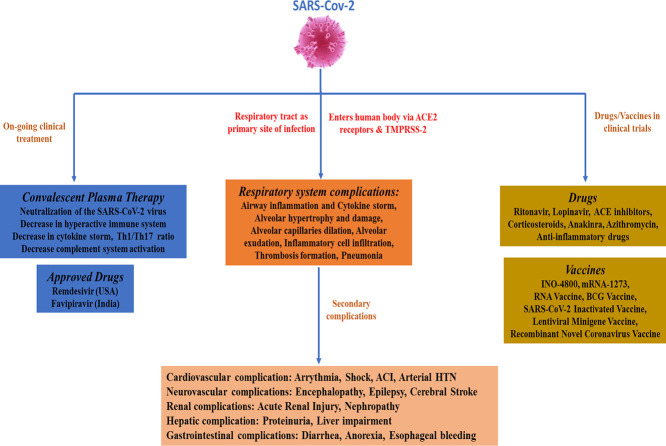
Coronavirus disease 2019 (COVID-19) is an unprecedented disease caused by highly pathogenic SARS-CoV-2 and characterized by extreme respiratory deterrence, pneumonia and immune damage. The phylogenetic analysis demonstrated the sequence similarity of SARS-CoV-2 with other SARS-like bat viruses. The primary source and intermediate host are not yet confirmed, although transmission from human to human is universally confirmed. The new SARS-CoV-2 virus reaches cells via ACE-2 and subsequently down-regulates ACE-2, leaving angiotensin II unbalanced in affected organs primarily in the lungs, heart, brain, and kidneys. As reported recently, numerous secondary complications i.e., neurological, nephrological, cardiovascular, gastrointestinal, immune, and hepatic complications, are associated with COVID-19 infection along with prominent respiratory disease including pneumonia. Extensive research work on recently discovered SARS-CoV-2 is in the pipeline to clarify pathogenic mechanisms, epidemiological features, and identify new drug targets that will lead to the development of successful strategies for prevention and treatment. There are currently no appropriate scientifically approved vaccines/drugs for COVID-19. Nonetheless, few broad-spectrum antiviral drugs, azithromycin were tested against COVID-19 in clinical trials, and finally, FDA approved emergency use of remdesivir in hospitalized COVID-19 patients. Additionally, administration of convalescent plasma obtained from recovered COVID-19 patients to infected COVID-19 patients reduces the viral burden via immunomodulation. This review analysis therefore concentrates primarily on recent discoveries related to COVID-19 pathogenesis along with a full description of the structure, genome, and secondary complication associated with SARS-CoV-2. Finally, a short and brief clinical update has been provided concerning the development of therapeutic medications and vaccines to counter COVID-19.
Abbreviations: ARI, acute renal injury; ANG-I, angiotensin I; ANG-II, angiotensin II; ACE-2, angiotensin-converting enzyme 2; CRI, chronic renal injury; CVD, cardiovascular diseases; CTD, C-terminal domain; ERGIC, endoplasmic reticulum-golgi intermediate compartment; NTD, N-terminal domain; MERS-CoV, middle east respiratory syndrome coronavirus; RBD, receptor-binding domain; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS, transmembrane protease serine protease-2 (TMPRSS-2); 5’-UTR, 5′-untranslated region
Keywords: COVID-19, SARS-CoV-2, Anti-viral drugs, Convalescent plasma, Vaccines
Graphical abstract
1. Introduction
According to the World Health Organization (WHO), viral infections are widespread infectious diseases that cause serious public health concerns, resulting in thousands of deaths worldwide [1]. There have been three zoonotic outbreaks caused by coronaviruses (CoVs) since the beginning of this century, i.e. severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002, middle-east respiratory syndrome coronavirus (MERS-CoV) in 2012, followed by the recent ongoing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2019. [2,3]. Numerous patients with baffling pneumonia secondary to an unknown virus emerged in Wuhan, China in December 2019, and later the virus was identified as a new beta coronavirus and officially recognized as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [4,5]. On 11th February 2020, the WHO named this disease as “COVID-19,” and subsequently, on 11th March 2020, the WHO declared the “COVID-19” a pandemic [6]. Currently, the outbreak has spread globally with approximately 10 million confirmed cases, 0.5 million confirmed deaths, and 5 million total worldwide recoveries as of 30th June 2020. Numerous pieces of evidences suggest that person-to-person transmission is an expected path for spreading of COVID-19 infection; primarily occurs via direct interaction or via microdroplets propagated by coughing or sneezing from an infected COVID-19 individual [[7], [8], [9]]. The clinical symptoms of COVID-19 vary from asymptomatic or symptomatic to clinical conditions marked by respiratory distress to systemic effects of sepsis, shock, and multiple syndromes of organ failure [10]. The typical clinical highlights include headache, fever, sore throat, cough, fatigue, breathlessness [[7], [8], [9], [10]] and have a strong prognosis in general. A considerable number of COVID-19 cases, however, have progressed rapidly to severe forms, especially among older men with underlying diseases. Significant cases may involve dyspnea, acute respiratory distress syndrome, acute heart injury, acute kidney injury, acute liver injury, neurological injury, gastrointestinal injury, immune failure, compromised coagulation and even death [11].
CoVs belong to a large family of single-stranded RNA viruses (+ssRNA) and are broadly classified into four categories, alpha-CoV, beta-CoV, gamma-CoV, and delta-CoV [4,5]. CoVs can infect humans as well as animals and can cause varieties of infections, including respiratory, enteric, renal, and neurological diseases [4]. CoVs occupy a crown-like presence under an electron microscope due to the existence of spike-like glycoproteins on the viral envelope [6]. SARS-CoV-2 belongs to β-coronavirus category, which contains shrouded and non-segmented single-stranded RNA virus [45]. Genome sequencing of SARS-CoV-2 has shown 96.2% overall similarity with a bat CoV RaTG13 and 79.5% uniformity to SARS-CoV [12]. CoVs have been shown to penetrate the host cell via different mechanisms, which includes an endosomal and non-endosomal entry with the help of proteases [13,14]. It is well established now that SARS-CoV-2 makes use of angiotensin-converting enzyme 2 (ACE-2), the similar receptor used by SARS-CoV, to enter or to infect humans.
Along with ACE-2, SARS-CoV-2 has shown to take the help of transmembrane protease serine protease-2 (TMPRSS-2) as the critical protease assisting their entrance into the human cell [15]. This receptor (ACE-2) is widely expressed in pulmonary tissues as well as in some immune cells, including monocytes and macrophages [16]. SARS-CoV-2 virus entry into the human body stimulates the release as well as activation of monocyte, macrophage, and dendritic cell along with the release of cytokines (Interleukins). Further, IL-6 initiates an intensification cascade resulting in cis signaling with activation and differentiation of TH17, lymphocytic changes, and trans-signaling in endothelial cells [[17], [18], [19], [20]]. SARS-CoV-2 infection cause final release and increase in pro-inflammatory cytokines, including interleukins, TNF-α, GCSF, and MCP [21,22]. This augmented systemic cytokine storm seen in SARS-CoV-2 diagnosed patients underwrites the pathophysiology of severe COVID-19 and known as the “cytokine storm”. Extreme COVID-19 patients show strong inflammatory response and transcriptomic RNA-seq reviews of COVID-19 patients showed that several immune pathways and pro-inflammatory cytokines CXCL, CCL2, CXCL2, CCL8, IL33 and CCL3L1 in bronchoalveolar lavage fluid and TNFSF10, CXCL10, IL10, TIMP1, C5, IL18, AREG and NRG1 in peripheral mononuclear cells (PBMC) were induced by SARS-CoV-2; suggesting a sustained inflammation and cytokine storm. Besides this, excessive apoptotic cell death of T cell resulting from flawed stimulation by dysfunction of dendritic cell might be a contributing factor to the COVID-19 immunopathology [22]. Fortunately, 80% of individuals infected with SARS-CoV-2 are asymptomatic or suffer from mild symptoms due to the activation of the body's innate immune system by triggering the body's antiviral defense mechanisms, including natural killer cells and antiviral T cells, and interferon induction. Unfortunately, 20% of SARS-CoV-2-infected individuals are the immune-compromised, aged, patients with underlying health conditions will experience more severe illness characterized by substantial respiratory symptoms leading to acute respiratory distress syndrome (ARDS) and even death. Several epidemiological, genetic, and clinical elements of COVID-19 infection resemble previous SARS-CoV and MERS-CoV infection [23]. Ironically, even after the frequent re-emergence of CoVs infections and after several years of research, we still lack vaccines or therapeutic agents to treat CoV infection, which further climaxes an unmet need to develop effective drugs/vaccines to prevent re-emergence of future CoV epidemics. Here, in this review, we have discussed about etiology, epidemiology, the structure of SARS-CoV-2, as well as the pathogenesis of SARS-CoV-2, induced COVID-19 infection. Besides, a brief dialogue has been made on secondary complications associated with COVID-19. Lastly, we have reviewed current clinical therapy, potential therapeutic intervention followed by drugs and vaccines currently in clinical trials for COVID-19.
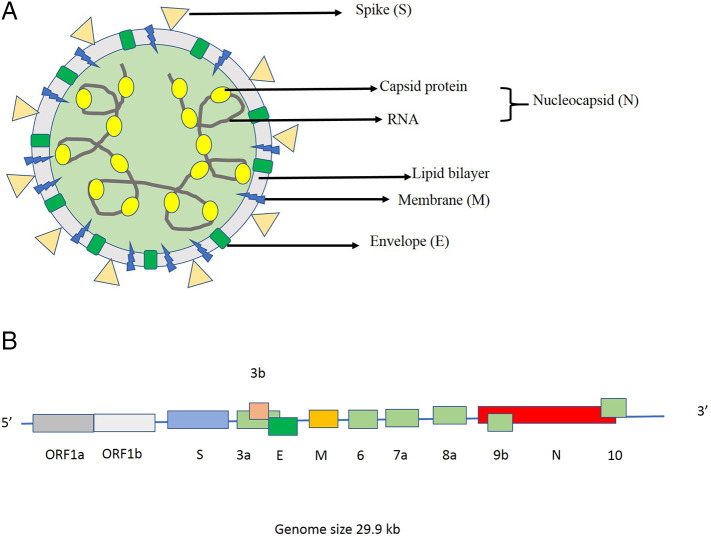
2. Genome and structure of SARS-CoV-19
Based on the taxonomic classification of viruses, SARS-CoV-2 is classified under the order Nidovirales, family Coronaviridae, and genus Betacoronavirus (β-CoV). All the viruses from this genus have a positive-sense, single-stranded RNA besieged by a lipid envelope. A phosphorylated capsid protein shields this single strand of RNA and both RNA & capsid together form a nucleocapsid. This nucleocapsid is hidden within the phospholipid bilayers and coated by spike glycoprotein trimer (S) and probably the hemagglutinin-esterase (HE) protein. Due to spike protein on the lipid envelope, SARS-CoV-2 holds the classic coronavirus structure with crown-like spikes (Fig. 1a). The membrane (M) protein and the envelope (E) protein are sited among the spikes in the envelope. Apart from spike protein, it also expresses other proteins such as helicase, RNA polymerase, papain-like protease, 3-chymotrypsin-like protease, glycoprotein, and some other accessory proteins [24]. As mentioned above, SARS-CoV-2 shows a 96% nucleotide identity with a coronavirus isolated from a bat (BetaCoV RaTG13), suggesting it as a bat origin virus [25]. Two theories have been put forward to clarify the cross-species transmission of this virus. First, there might be an intermediate host between bats and humans. Most likely, it is pangolin as their genome is approximately 90% similar to SARS-CoV-2, indicating two sub-lineages of this virus in the phylogenetic tree. As per the second theory, the rich genetic diversity and frequent recombination ability of SARS-CoV-2 might enhance the possibility for cross-species spread [26]. The 29.9 kb genome of SARS-CoV-2 (GenBank no. MN908947) encodes 9860 amino acids which include the 5′-untranslated region (5’-UTR), replicate open reading frame (orf) 1a/b, structural proteins, other orf such as 3, 6, 7a, 7b, 8 and 9b and the 3′-untranslated region (3’-UTR) (Fig. 1b). The 5’ UTR is 265 nt long, whereas 3’ UTR is 229 nt long. SARS-CoV-2 contains at least ten ORFs, including ORF1a/b, spike (S), envelope (E), membrane (M), nucleocapsid (N), and some other accessory genes, such as ORF3b and OFR8 [27]. Almost two-third of viral RNA houses the first ORFs (ORF1a/b), which are further translated into two big polyproteins i.e. pp1a and pp1ab. These polyproteins are finally processed by different proteases into 16 non-structural proteins (nsp1- nsp16) to produce the replicas-transcriptase complex. Moreover, the other ORFs, present on the other one-third part of the genome encode for four main structural proteins (S, E, M and N) proteins, as well as some accessory proteins with unknown functions [5]. The S, ORF3a, E, M, ORF8 and N genes are 3822, 828, 228, 669, 366 and 1260 nt in length, respectively.
Fig. 1.
(a). Structure of SARS-Cov-2 depicting the structural proteins and nucleocapsid. (b) Structure of single-strand RNA of SARS-Cov-2.
The ~1200 aa long glycoprotein spikes are homo-trimeric type-I viral fusion proteins, which is vital to establish the host tropism via the facilitation of receptor binding and membrane fusion [28]. Each monomer of trimeric S-protein is about 180 kDa and contains a cleavable N-terminal signal peptide, a heavy N-glycosylated ectodomain, a transmembrane region and one cytoplasmic tail with lots of S-acylated C residues. The proteases cleave N-glycosylated ectodomain into two domains: the variable S1 and the more conserved S2 domains (Fig. 2 ). In general, the S1 domain is associated with receptor-binding events, whereas S2 is involved in membrane fusion [25]. Structurally, N- and C- terminal of S1 fold as two separate domains: N-terminal domain (NTD) and C-terminal domain (CTD). The CTD contains a loosely attached receptor-binding domain (RBD) to bind with the host cell receptor i.e. ACE2 promptly (refer article by [27] for detailed information). Homology modeling between SARS-CoV-2 and SARS-CoV has shown that both have a similar RBD formation. The SARS-CoV-2 RBD has a twisted five-stranded antiparallel β sheet (β1, β2, β3, β4 and β7) with small connecting helices and loops forming as the core. Between the β4 and β7 strands in the core, there is an extended insertion containing short β5 and β6 strands, α4 and α5 helices and loops [27].
Fig. 2.
Diagrammatic representation of spike protein showing sites for S1 & S2 subunits, Signal sequence (SS), N-terminal domain (NTD), Subdomain (SD) 1 & 2, Receptor binding domain (RBD), Transmembrane domain (TM), Fusion peptide (FP), Internal Fusion peptide (IFP), Heptad repeats (HR), Central helix (CH), Connector domain (CD) and Cytoplasmic tail (CT).
The smallest structural protein, E protein, is made up of three domains- N terminal (hydrophilic), class IA transmembrane domain (hydrophobic), and C terminal (hydrophilic). They function as viroporins by modifying the host cell membranes and thus facilitates the release of virus from the infected cells. The M protein provides a distinct shape to the viral envelope by interacting with the nucleocapsid and by specific packaging of the viral genome into the virion. Generally, M proteins in different coronaviruses display variable amino acid contents; however, all contain the same 3-D structure with a small N-terminal, three transmembrane domains, and a long C-terminal. The N protein is multi-functional protein as it is involved in complex formation with single-stranded RNA, enabling M-N protein interactions during the packaging of virion particles, and augmenting the transcription process of the viral genome. N protein is also made up of three conserved domains- an N-terminal domain (NTD), a linker region, and a C-terminal domain (CTD). The highly variable NTD interacts electrostatically with the 3′ end of the viral RNA. Similarly, the serine and arginine-rich LKR region also interacts with viral RNA in vitro. However, its main role is in signal transduction, where it acts as an antagonist to attenuate the antiviral responses of the host (for e.g. Interferon, RNAi) [29].
3. Pathogenesis of COVID-19: human-virus interaction with specific focus on ACE-2 receptor
At the molecular level, the virus-human interaction starts with the binding of S-protein to the ACE-2 receptor, followed by the fusion of the viral membrane with the host cell membrane. S-proteins are activated by priming cleavage (between S1 and S2) and activating cleavage (on S2’ site) by one or several host proteases. Depending on the sequence of the S1/S2 cleavage site, the priming cleavage can be done by different host cell proteases, including furin, transmembrane protease serine protease-2 (TMPRSS-2), TMPRSS-4, cathepsins, trypsin, or human airway trypsin-like protease [28]. However, the accessibility of a particular protease in the host cell cannot be a regulating factor for the pathogenicity of SARS-Cov-2, because S-proteins are well-known to modify protease cleavage sites so that different proteases can perform the cleavage of S. This is one of the mechanisms adopted by SARS-Cov-2 to infect and fuse with different host cell membranes [25]. Apart from this, SARS-CoV-2 encodes several proteins to attenuate the innate immune responses, especially the activation of type 1 interferon in host cells, ultimately leading to an enhanced immunopathogenesis. The nsp15 (also known as endoribonuclease EndoU) is vital for restraining the detection of viral RNA by specific cytoplasmic pattern recognition receptors [30].
The NTD of S1 displays a galectin (galactose-binding lectins) structural fold to get attached to the sugar on the surface of the host cell. On the other hand, binding to the ACE-2 is assisted by the CTD of S1. The CTD comprises two subdomains: a central structure made up of five-stranded antiparallel β-sheet and the RBD, which governs the specificity of receptor binding [25]. The extended insertion (between the β4 and β7 strands) of the RBD contains some of the crucial residues required for receptor binding. [31] showed the formation of a hACE2 dimer in the presence of an amino acid transporter B0AT1. Two molecules of CTD are individually attached to this dimeric hACE2-B0AT1 form, with a local resolution of 3.5A° at the interface. The RBD endures hinge-like conformational movements to cover or uncover the elements of receptor binding momentarily to enhance the host-cell interactions [31]. S2 regulates the fusion with the host cell membrane and then insertion of viral RNA [32]. The S2 is made up of the fusion peptide (FP), a cleavage site (S2′), an internal fusion peptide (IFP), and two heptad-repeat domains before the transmembrane domain (TM) (Fig. 2). Since both FP and IFP are essential for the virus entry into the host cell [33], S protein is required to be cleaved by proteases at both priming and activating cleavage sites to release out these peptides [28]. However, there are ambiguous views about the second cleavage at S2’ site. [28] described that the second cleavage occurs after the first furin cleavage at the conserved site sequence (AYT↓M) in S2’. On the other hand, [25] showed that the different proteases could cleave at KR↓SF to generate a downstream fusion peptide (S-F-I-E-D-L-L-F) in the S2′ site. This cleavage is followed by the conformational change induced fusion of membrane, in which newly generated peptide incorporates itself into the host cell membrane [25].
One of the widely studied cell proteases in SARS-Cov-2 is proprotein convertase furin. Coutard et al. [28] observed that the furin-like cleavage site (PRRAR↓SV) is absent in lineage B beta-coronaviruses except in SARS-Cov-2 [26]. The presence of proline in this furin-like cleavage site generates a turn in the polypeptide, which facilitates the addition of O-linked glycans to the amino acid residues flanking the cleavage site, specifically to S673, T678 and S686. The role of these O-linked glycans is not known precisely; however, they could produce a ‘mucin-like domain’ to protect crucial amino acid residues or epitopes on the S-protein. The high expression of furin in the lungs suggests the high pathogenicity of this virus in the human respiratory system [34,35]. Gene expression studies have shown that ACE2 occurs primarily in alveolar epithelial type II cells of lungs, heart, kidney, adipose tissue, and nasal epithelial cells (specifically in goblet cells and ciliated cells). Studies through surface plasmon resonance have proven that the ACE2 binds to the SARS-CoV-2 ectodomain with about 10- to 20-fold higher affinity as compared to SARS-CoV [26]. The crucial K residue at 31 in hACE2 identifies the Q residue at 394 in the RBD region of SARS-CoV-2. Bioinformatical analysis by Wan et al. [36] has predicted that the single N501T mutation in RBD of S-protein might lead to an augmented binding affinity for ACE2. The residues at positions 442, 472, 479, 487, and 491 in S-protein are crucial as these are located at the receptor complex interface with ACE2 [26].
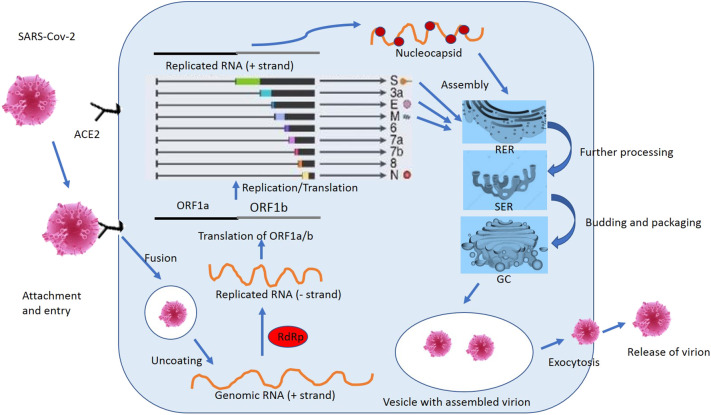
After the fusion of viral lipid bilayer through the endosomal pathway, SARS-Cov-2 injects its RNA into the host cell cytoplasm. Once inside the cytoplasm, it is translated into two polyproteins (pp1a and 1ab) and structural proteins. The polyproteins are further processed and cleaved by different proteases to form a replicase-transcriptase complex. The newly synthesized polymerases produce various sub-genomic mRNAs by the discontinuous transcription, which gets translated into related viral proteins [[17], [18], [19], [20]]. These viral particles are processed futher into the endoplasmic reticulum-golgi intermediate compartment (ERGIC). Here, nsps play an essential role by reorganizing the membranes coming from the ERGIC into the double-membrane vesicles, where viral replication and transcription occur [[17], [18], [19], [20]]. Subsequently, the nucleocapsid is produced by the aggregation of viral RNA and nucleocapsid protein. At last, the virion particles are released out of the host cell by the process of exocytosis or extrusion [32]. (Fig. 3 ).
Fig. 3.
The complete life cycle of SARS-Cov-2 inside the host cell. ACE2: Angiotensin-converting enzyme, RdRp: RNA-dependent RNA polymerase, ORF: Open reading frame, N: Nucleocapsid protein, M: Membrane protein, E: Envelope protein, S: Spike protein, RER: Rough Endoplasmic reticulum, SER: Smooth Endoplasmic reticulum, GC: Golgi apparatus.
4. COVID-19 and secondary manifestations: recent update
4.1. Impact of COVID-19 on cardiovascular system
COVs such as SARS, MERS, and COVID-19, have mainly been associated with the number of cardiovascular complications such as myocarditis, myocardial injury, cardiac shock, cardiac arrhythmia, heart failure [37]. Although. the dominant clinical feature of COVID-19 is considered to be respiratory dysfunction, but some patients showed severe damage to the cardiovascular system. Recent reports suggested that patients with cardiovascular diseases (CVD) have been at increased risk of death [38,39]. In 2006, a study conducted on SARS patients showed that out of 121 patients, 12 patients had cardiovascular complications with tachycardia and hypotension were the most common finding reported besides with the other complications such as bradycardia, transient cardiomegaly [40]. In line with SARS, the latest case reports of COVID-19 reported that up to 50% of patients with a high risk of mortality have chronic CVD. Recently, the clinical report of COVID-19 revealed that 7.2%, 8.7%, and 16.7% of patients with acute cardiac injury, shock, and arrhythmia, respectively, required immediate hospitalization and intensive care [[7], [8], [9]]. A most recent report from China CDC showed that 25% of patients have pre-existing comorbidities like cardiovascular complications and diabetes [41]. A case report on 138 COVID-19 patients reported that 19.6%, 16.7%, 7.2%, 8.7%, and 3.6% of patients developed acute respiratory distress syndrome, arrhythmia, acute cardiac injury, shock, and acute kidney injury, respectively [[7], [8], [9]] (Fig. 4 ). The overall mortality rate remains minimal at 2.3%; though, it bounces to 6% in hypertensive patients, 7.3% in diabetic patients, 10.5% in CVD patients, and 14.8% for very older patients (>80 years of age). The point to be noticed that the mortality rate for CVD (10.5%) is bigger as compared to patients with chronic respiratory disease (6.3%) [41].
Fig. 4.
Summary of secondary complications associated with COVID-19 infection.
CVD may become more severe in the presence of viral infection because of the imbalance between the increased metabolic demand and reduced cardiac reserve. Therefore, patients with coronary artery disease and heart failure have a high mortality rate [42]. ACE-2 was identified as a crucial factor mediating SARS-CoV spike (S) protein's interaction with susceptible host cells. In physiological conditions, angiotensin-converting enzyme (ACE) is a master regulator of the renin-angiotensin system and one of best in target enzymes for the treatment of hypertension. The protease renin converts angiotensinogen to angiotensin I (ANG I), which is consequently transformed to ANG II by ACE. ANG II then unites to the ANG II type 1 receptor (AT1R) to promote inflammation, oxidative stress, and increase in blood pressure.
Further, ANG II is again metabolized to ANG III and ANG IV via various aminopeptidases [43,44]. In addition, ANG I and ANG II are too converted to Ang-(1–7) with the help of endopeptidases and ACE-2, respectively. Lastly, Ang-(1–7) attaches to the Mas receptor (Mas-R) to wield anti-inflammatory and antifibrotic actions, stimulate the release of nitric oxide, and decrease blood pressure [43,44]. ACE-2 has shown a variety of cardioprotective effects, including anti-thrombotic, anti- hypertrophic, anti-fibrotic, and anti-arrhythmic [43,44]. ACE-2 is widely expressed in cardiac tissue, including cardiomyocytes, fibroblasts, and coronary endothelial cells. The number of studies has demonstrated that over-expression of ACE2 can prevent or even reverse the heart failure phenotype, whereas loss of ACE2 can enhance the progression of heart failure [45,46]. Various research groups have documented that SARS-CoV-2 use ACE-2 as a cellular entry receptor [15,39] and the degree of infection caused by SARS-CoV-2 in heart or other cardiovascular tissues (potentially contributes to the myocarditis) is largely unknown. Therefore, it can be concluded that cardiovascular tissues or cells expressing ACE2 are possibly at extreme risk for SARS-CoV-2 infection. In COVID-19 patients with pre-existing CVD, the demise of ACE2 by SARS-CoV-2- via internalization mechanism has been predicted to exacerbate CVD acutely and maybe long term [43,44].
4.2. COVID-19 and neurological complications
Viral contamination can lead to origin of serious damage to the brain and spinal cord, including encephalitis and severe acute demyelinating lesions [47,48]. A recent study (n = 214) concluded that, in addition to systemic and respiratory symptoms, 36.4% (78/214) of total COVID-19 patients develop neurological symptoms [49]. Recently reports from UK and China documented that COVID-19 patients showed convulsions, delirium, altered consciousness, restlessness loss of smell (anosmia), and taste (dysgeusia) due to infection in the brain [[17], [18], [19], [20]]. The neurological symptoms of COVID-19 can be divided into different stages based on the severity like mild (headache, dizziness, disturbances of the state of consciousness) followed by middle (ataxia, epileptic manifestations, and stroke) and severe (hypo-ageusia, hyposmia, neuralgia) [[17], [18], [19], [20]] (Fig. 4). Encephalopathy is one of the prominent symptoms of viral infection, and several reports published that encephalopathy has been seen in patients with COVID-19 as proof of brain infection due to this SARS-CoV-2 [[17], [18], [19], [20]]. Still, there is a question for scientists or neurologist that the brain infection due to COVID-19 play a role in respiratory trouble or vice versa and this regard one report published by Li et al. [[17], [18], [19], [20]] suggested that COVID-19 might infect respiratory center located in the medulla oblongata and pons (brainstem) that cause breathing problem in patients. Recently published reports also suggest some similarities between SARS-CoV and COVID-19 for following the pathway of infection [[17], [18], [19], [20]]. Guillain-Barre syndrome (GBS) associated with COVID-19 was firstly reported in China with manifestations, including leg weakness, and severe fatigue. It has been documented that SARS-CoV-2 has been found in CSF and can infect brain cells and subsequently cause viral encephalitis [47,48,50].
Moreover, another study reported that out of 221 COVID-19 patients in Wuhan, China, 11 patients have established stroke, cerebral thrombosis, and cerebral hemorrhage. If we look back at previously published reports in which SARS-CoV [51] or MERS-COV [52] were administered in mice through nasal routes, which enter the brain through olfactory nerves and spread throughout the brain. Various possible explanations have been reported for SARS-CoV-2 induced CNS damage including entry into the brain through blood circulation, by infecting the sensory and motor nerve endings and some indirect mechanisms like hypoxia or loss of oxygen also cause decrease supply of oxygen in brain tissue cause acute or permanent changes in the brain areas [47,48]. Viruses may penetrate the brain and exacerbate infections by activating neuroinflammation. i.e. excessive activation and release of interleukins, TNF-α and other markers, which ultimately leads to free radical generation, excitotoxicity, and neuron death [47,48]. Nevertheless, there is a need for a deep understanding of the possible link between the brain and respiratory infection by COVID-19, and clinical reports or biopsy studies may conclude that brain infection involved or not in respiratory failure.
4.3. Impact of COVID-19 on renal system
Several published studies on COVID-19 enlightened lungs and the respiratory system as the principal organ/system involved and affected in the disease; meanwhile, manifestations of this disease also involves other organs like the gastrointestinal tract, renal system and hepatic system as observed in coronavirus infected patients [[17], [18], [19], [20]]. Recent clinical reports have documented the negative impact of SARS-CoV-2 on other organs like liver, kidneys and intestine in COVID-19 patients, which can lead to a higher incidence of hepatic or renal injury and even worsening of the health status of patients who are already suffering from hepatic disorders, GIT disorders, ARI (Acute Renal Injury) or CRI (Chronic Renal Injury) and thus results in higher mortality rate [[17], [18], [19], [20]]. It has been reported that ARI and liver dysfunction are the main complications, along with acute respiratory distress syndrome, arrhythmia, acute cardiac injury, shock, and other secondary infections. Studies also revealed that ARI is one of the severe symptoms of COVID-19, generally in the case of critical patients [53].
By this time, through various studies, it is evident that to infect humans, SARS-CoV-2 uses ACE2, which is the same receptor used by SARS-CoV for infecting human cells [15]. A recent study showed that the expression of ACE-2 in kidney cells is similar to that in the lungs, esophagus, small intestine, and colon [[17], [18], [19], [20]]. It has been suggested that the kidneys serve as an essential target organ for SARS-CoV [54] and the SARS-CoV-2 virus [39]. Previously published reports during the outbreak of SARS and MERS-CoV infections claim that ARI was observed in 15% cases with a high mortality rate of 60%–90%. However, in the case of COVID-19, low incidence (3%–9%) of ARI was observed in early reports to date [55]. Recently, a study carried out on 193 patients with COVID-19, between January to February 2020 including 28 pneumonia patients (15 viral pneumonia and 13 mycoplasma pneumonia patients) concluded that proteinuria was observed in 59% cases, hematuria in 44%, elevated levels of blood urea nitrogen in 14%, and high serum creatinine levels in 10% cases, which is significantly worse as compared to the other pneumonia cases (Fig. 4). A univariate Cox regression analysis was carried out and found that all elevated factors were significantly linked with high mortality in COVID-19 patients [[17], [18], [19], [20]]. Also, it has been suggested that COVID-19 patients with developed ARI are at 5.3-times more risk of those without ARI and even much higher than those with comorbid chronic illnesses [[17], [18], [19], [20]]. Similarly, another study was carried out on 701 patients (367 men and 334 women) for the assessment of the prevalence of ARI in COVID-19 patients as well as for the evaluation of the relationship between abnormal kidney function markers and fatality in COVID-19 patients with a median age of 63 years. The study concluded that proteinuria and hematuria were found in 43.9% and 26.7% patients, respectively, whereas high plasma creatinine and blood urea nitrogen was observed in 14.4% and 13.1% patients, respectively at the time of admission to hospitals. In addition, data analysis has revealed that there is a significantly higher risk for in-hospital death of patients with renal complications [53]. Moreover, the same study concluded that the prevalence of renal complications during hospital admission as well as the development of ARI during hospitalization in COVID-19 patients, is high and is associated with high in-hospital mortality [53].
The etiology or pathogenesis of kidney injury in patients with COVID-19 is not precisely clear but might be due to multiple reasons. It may be due to the direct cytopathic effects of SARS-CoV-2 on kidney tissue, as evidenced by the detection of SARS-CoV-2 fragments in blood and urine in patients with COVID-19. Recent data on human tissue RNA-sequencing denoted that ACE-2 expression in kidneys is almost ten times greater as compared to that in the lungs [[17], [18], [19], [20]]. Hence, we can conclude that the renal failure might be raised due to coronavirus entering in the kidney cells through an ACE2-dependent pathway. Other supposed mechanisms may be the immune responses generated by the human body against this virus i.e. specific effector mechanisms of immune system induced by this virus (specific antibody or specific T-cell lymphocyte) may cause damage to the kidneys. One possible mechanism of renal impairment may be due to hypoxia, shock, and rhabdomyolysis exerted by cytokine storm. Also, dehydration resulting from fever or decreased fluid intake has various impacts on the kidney, ranging from the reduction of glomerular filtration rate to ARI. Further, hydration can reverse the condition in case of mild to moderate fluid depletion. However, if ischemia persists, then acute tubular necrosis may occur. These pathways are expected to be responsible for renal impairment in COVID-19. However, further studies are required to investigate other pathways responsible for renal impairment in COVID-19 patients [56]. Renal impairment in COVID-19 (Coronavirus induced-nephropathy) has a complicated and unclear etiology; though, ARI in COVID-19 patients is strongly correlated with a high mortality rate. Therefore, to prevent mortality in such conditions, continuous monitoring of renal function in patients with COVID-19 with caution is necessary irrespective of the past disease history, so that early clinical interventions could be taken timely.
4.4. Impact of COVID-19 on hepatic and gastrointestinal system
The liver is the vital metabolic organ with numerous physiological roles and play a significant function in immunity and detoxification of xenobiotic. The liver is a highly sensitive organ that can be easily influenced by the presence of viruses, bacteria, and other harmful pathogens. In 2003, during the SARS outbreak, SARS-CoV reportedly caused atypical pneumonia and served as a contributing factor in liver impairment in up to 60% of SARS patients [57,58]. Several studies documented that during the initial stage of SARS, there was a mild to moderate rise of ALT and AST levels, decrease in serum albumin, and an increase in serum bilirubin levels in the patients [59,60]. It has been reported that the severity of liver damage was directly proportional to the severity of cases of SARS patients [[59], [60], [61]]. Several studies on SARS patients, reported that the SARS-CoV genome was identified in hepatocytes by RT-PCR technique, which establish a significant relationship between SARS-CoV and hepatic damage [60,62,95]. ACE-2 is responsible for receptor-associated cellular entry of SARS-CoV [13,60]. Endothelial cells of the liver have an abundance of ACE 2. Therefore, it served as a potential target for the cellular entry of SARS-CoV [13]. Significant increase in mitotic cells, eosinophilic debris and balloon-like hepatocytes has been shown in the liver biopsy of patients, indicating a relevance between SARS-CoV and apoptotic liver damage [63]. Several in-vitro studies reported that SARS-CoV-specific protein 7a posses' ability to cause apoptosis in the lung, kidney, and liver through a caspase-dependent mechanism and confers the relative potential of SARS-CoV in liver injury [64].
Coming to the latest studies on COVID-19, it has been reported that 14.8% to 53% COVID-19 cases have abnormal ALT/AST levels, with mildly elevated bilirubin levels during the initial stages of the disease (Fig. 4). The possibility of developing liver injury mainly depends upon the severity of disease in COVID-19 patients, more severe the patient, higher the possibility of liver injury and vice-versa [37,65]. It has been found that endothelial cells in the liver and bile duct express abundant ACE-2 that makes it a potential target for cellular entry of SARS-CoV-2 [66]. However, the ACE 2 expression in endothelial cells of the bile duct is significantly higher as compared to endothelial cells of liver cells. These results from various studies suggested that damage of bile duct cells may be a significant cause for liver impairment in COVID-19 patients, along with the damage of liver cells. Excessive inflammatory cytokines have been observed in endothelial cells of the bile duct and hepatocytes in severe COVID-19 patients [67]. However, still, there is a paucity of data concerning the role of SARS-CoV-2 in liver damage and therefore needs more investigation on these biomarkers whether they are responsible for liver damage or not. Recently, in a postmortem biopsy of COVID-19 patient, a moderate level of microvascular steatosis indicates that either SARS-CoV-2 infection or drug used during treatment was the possible cause for liver injury [68,69].
In addition, several people with COVID-19 had gastrointestinal (GI) symptoms, such as vomiting and nausea, before showing signs and symptoms of fever and lower respiratory tract. A meta-analysis of COVID-19 patients (n = 4243) from 6 countries reported prevalence of all GI symptoms, including loss of appetite, nausea/vomiting, diarrhea, or abdominal pain [70]. Appetite loss (26.8%) was the most common symptom, accompanied by diarrhea (12.5%), nausea/vomiting (10.2%), and stomach pain/discomfort (9.2%) [11,70]. SARS-CoV-2 RNA presence was detected in stools of 15.3% of patients on hospital presentation, including patients without any GI symptoms. In addition, patients with presenting diarrhea had higher stool RNA presence and viral load than patients with no diarrhea. Due to 80% similarity to SARS-CoV, SARS-CoV-2 persuaded GIT infection is not surprising and is specified to be mediated through the ACE2 cell receptors. ACE2 receptors are highly expressed in the small intestine, especially in proximal and distal enterocytes, and the binding affinity of ACE2 receptors determines infectivity. Since ACE2 modulates bowel inflammation, SARS-CoV-2 can disrupt ACE2 ‘s function and lead to diarrhea [70]. Because of the similarity of SARS and COVID-19 infection, currently, various alternatives and therapies which were earlier used for the treatment of SARS such as anti-biotics, anti-viral, and steroidal drugs are widely being used for the treatment of COVID-19 [11,47,48].
4.5. Impact of COVID-19 on diabetic patients, pregnancy, children’ and elderly population
COVID-19 is emerging as a double edge sword for the population suffering from diabetes. Diabetes is a significant risk factor for hospitalization and mortality due to COVID-19 infection. The severity of COVID-19 in with diabetic patients is two-fold higher as compared to mild and severe patients with COVID-19 [[17], [18], [19], [20]]. While, recent reports from china claims that death rates due to COVID-19 virus are three-fold higher in diabetic patients [[17], [18], [19], [20]]. Indeed, it is well established that diabetic population is at high-risk group for viral infection due to hyperglycemia-induced increased oxidative stress as well as synthesis and release of advanced glycation end products and pro-inflammatory cytokines [71]. These processes may constitute the underlying mechanism for higher mortality and morbidity in COVID-19 patients with diabetes. Most notably, diabetes was too considered as a risk factor during previous SARS, MERS and severe influenza A H1N1 pandemic [[72], [73], [74]]. Infection of SARS-CoV-2 in diabetic patient’ perhaps trigger the excess release of hyperglycemic hormones (glucocorticoids and catecholamines), leading to enhanced blood glucose levels [[7], [8], [9]].
In contrast, a retrospective study conducted in the first epicenter of COVID-19 infection (Wuhan) confirmed that about 10% of the COVID-19 patients with T2DM suffered at least one episode of hypoglycemia [75]. Moreover, a recently published meta-analysis of 12 studies depicting data from 2108 Chinese COVID-19 patients concluded that the prevalence of diabetes in COVID-19 patients is 10.3%, which is similar to the diabetic prevalence in healthy population [76]. Therefore, it can be concluded that though pre-existing diabetes worsens the consequences in COVID-19 patients, but the predisposition of the diabetic population to SARS-CoV-2 infection may not be higher as compared to healthy people.
A previous study of SARS-CoV patients without pre-existing diabetes (n = 39) has reported that 20 of the 39 SARS-CoV patients developed diabetes during hospitalization because immunostaining of ACE-2 in the pancreatic tissue suggested that SARS-CoV might have damaged pancreatic islets and caused acute Type I diabetes mellitus like condition [74,76]. Whereas in the case of SARS-COV-2, evidence is still lacking to confirm that pancreatic destruction in COVID-19 patients. The development of a viral infection in diabetic patients renders them harder to treat due to oscillating blood glucose levels and the presence of other secondary diabetic complications. Therefore, it can be hypothesized that the treatment of COVID-19 cum diabetic patients with ACE-2 stimulatory drugs might increase the risk of acquiring more severe and fatal COVID-19. Another component that should be considered is the existence of ACE-2 polymorphisms in Asian populations, which is further linked to diabetes mellitus and hypertension, genetically predisposes towards increased risk of SARS-COV-2 infection [94]. In a nutshell, intake of ACE inhibitors may lead to the upregulation of ACE-2 receptors in diabetic patients, binding of spike (S) glycoprotein to the ACE-2 is a vital step for virus entry into human cells. Additionally, some other notions have been proposed that the immune system is exceedingly compromised in diabetic subjects, which render them harder to combat the virus and likely result in a more extended recovery period. Secondly, the virus can flourish for a longer time in an atmosphere of high blood glucose ([77], https://diabetesvoice.org/en/news/covid-19-and-diabetes/).
Pregnant females are exceptionally predisposed to viral pathogens and pneumonia since a lot of physiologic alterations takes place in the respiratory, cardiac and immune systems (for e.g. diaphragm gets elevated, oxygen demand increased, along with edema of the upper respiratory tract), [78,93]. Earlier, viral pneumonia in pregnant women was shown to be correlated with an increased risk of premature fetal birth, decreased fetal growth and perinatal mortality [93]. Numerous physiological adaptive changes render pregnant women more susceptible to COVID-19 infection than the general population [79]. In this context, a study published in March 2020 revealed the effects of SARS-CoV-2 on pregnant females (n = 38) infected with COVID-19 as well as on their neonates in China. Despite having a limited sample size, the study concluded zero cases of intrauterine transmission of SARS-CoV-2 from moms with COVID-19 to their fetuses/neonates [80]. In April 2020, a study from around 25 different hospitals in China has been published with clinical records of pregnant women (n = 116) with COVID-19 pneumonia between January 20 and March 24, 2020. The median gestational age of all pregnant women was 36 weeks, and common symptoms observed were fever (50.9%,) and cough (28.4%,); whereas 23.3% women were without symptoms. The proof of maternal transmission of SARS-CoV-2 was confirmed in nasopharynx, amniotic fluid and blood of neonates. The authors concluded that the observed clinical symptoms of pregnant women with COVID-19 pneumonia are identical to those of non-pregnant women with COVID-19 pneumonia. Currently, there is a paucity of data to confirm that pregnant women with COVID 19 are more susceptible to severe pneumonia and vertical transmission of SARS-CoV-2 to fetus during the third- trimester of pregnancy [31]. Although these two recent studies are of the same viewpoint, but the maternal-fetal transmission cannot be ruled out. Most recently, a systematic review has been published with data of 172 pregnant women and 162 neonates from 23 studies conducted in different countries to examine the risk or occurrence of maternal-fetal SARS-CoV-2 transmission. The authors have concluded that in pregnant women infected with COVID-19, diabetes and hypertension are among the top associated comorbidities and there is a risk of pre-mature fetal delivery. Moreover, the newborn child to COVID-19 mothers have displayed the occurrence of respiratory distress syndrome as well as pneumonia and some proof of vertical maternal-fetal transmission of SARS-CoV-2 infection [81].
A recent study on pediatric patients with COVID-19 (n = 2143) observed that marginally more boys (56.6%) are influenced in the COVID-19 outbreak as compared to girls (43.4%) but with non-significant gender difference. The median age is seven years, age varies from 1 day to 18 years. This is suggestive of the fact that all ages in childhood are equally susceptible to SARD-CoV-2 [82]. Moreover, the children's COVID-19 cases were less severe as compared to adults' cases, and this is somewhat perplexing. This difference may be associated with exposure time as well as host factors. ACE-2 is a cell receptor for entry of SARS-CoV-2 and is hypothesized that children are less responsive to SARS-CoV-2, because the less maturity and function of ACE 2 in children than that in adults [83]. Additionally, as compared to adults, children are expectedly more prone to upper respiratory infections (for e.g., respiratory syncytial virus in winter), and their blood might have elevated levels of efficient antibodies against viruses [83]. Furthermore, the immune system of children is in the developing phase and might react to pathogens differently in contrast to adults. However, it has been documented that the percentage of severe COVID-19 cases in children is 10.6%, 7.3%, 4.2%, 4.1%, and 3.0% in the age group of ˂1, 1–5, 6–10, 11–15 and > 15 years, respectively. These findings are suggestive of the fact that infants are extra susceptible to COVID-19 infection. Consequently, the precise mechanisms for the discrepancy in clinical symptoms of children and adults remain elusive [82].
A recent study assessed data from eight countries and four USA cities which remain epicenters of the COVID-19 pandemic. The risk of death is 13- to 73-fold higher in elderly individuals (>65 years age) as compared to non-elderly individuals (<65 years age). The age-dependent threat is moderately stronger in European countries in contrast to US cities. The finding disclosed that 5–9% and < one-third of all COVID-19 deaths in European countries and 4 USA cities, respectively, are from non-elderly age groups having <65 years of age. Moreover, the bulk of deaths in this age group take place in the age of sub-group 40–65 that covers 36 to 48% of the total population in the 0–65 years old category. Additionally, the majority of the fatalities in the non-elderly population occur in those COVID-19 patients who have any pre-existing or underlying diseases [84]. Based on current evidences, CVD, hypertension, COPD, asthma, diabetes mellitus, renal failure, hepatic disorder, cancer, and immunocompromised status are some of the pre-existing diseased states in COVID-19 which confer an enhanced threat. Overall, half of the dead victims from COVID-19 in European countries and 4 USA cities are at least 80 years old. Most of these COVID-19 patients were suffering from one of the comorbidities above which result in fatal consequences during the COVID-19 pandemic [84].
5. Current therapy and potential therapeutics currently in development for COVID-19
Currently, there is no clinically available drug or therapeutics endorsed by the U.S. FDA to prevent or contain COVID-19. The on-going clinical management for COVID-19 includes disease prevention (use of some clinically approved anti-viral drugs, hydroxychloroquine, immunosuppressant drugs), and supportive care in hospitals, including oxygen and ventilation support [11,47,48,85]. The quest for efficient treatment is proceeding with numerous investigations ongoing across the world. Recently, the USFDA has authorized the emergency use of remdesivir in severely ill hospitalized patients as this drug has been shown to fasten the recovery rate and is associated with less mortality rate ([86] issues emergency-use-authorization-potential-covid-19-treatment). In addition, Indian authorities (Drug Controller General of India) have approved oral antiviral drug favipiravir to treat mild to moderate COVID-19 [87].The list of repurposed drugs with completed clinical trials and a list of various potential repurposed drugs currently in clinical trials has been summarized in Table 1 and Table 2 , respectively [85,88].
Table 1.
Summary of the completed clinical trials of repurposed drugs for treatment of COVID-19.
| Sr. no | Drug name | Target/rationale for use | Clinical phase (sample size) | Findings | Status | Clinical trial identifier |
|---|---|---|---|---|---|---|
| 1. | Hydroxychloroquine | Block viral entry by inhibiting glycosylation of host receptors, proteolytic processing, and endosomal acidification. Additionally, may provide immunomodulatory effects through inhibition of cytokine production, autophagy, and lysosomal activity in host cell. |
Phase 3 (30) |
No Result Posted for this study | Completed | NCT04261517 |
| 2. | Lopinavir/ritonavir Ribavirin Interferon Beta-1B |
Protease Inhibitor | Phase II (127) |
No Result Posted for this study | Completed | NCT04276688 |
| 3. | Hydroxychloroquine Lopinavir/Ritonavir Interferon Beta-1A Interferon Beta-1B |
Protease Inhibitor |
Phase IV (60) |
No Result Posted for this study |
Completed | NCT04343768 |
| 4. | Baricitinib | Anti-Janus kinase inhibitor (anti-JAK) acting against JAK1 and JAK2 that inhibits JAK1- and JAK2-mediated cytokine release | Phase 2 & Phase 3 (12) |
No Result Posted for this study |
Completed | NCT04358614 |
| 5. | Ganovo Ritonavir Interferon nebulization |
Hepatitis C virus protease inhibitor | Phase IV (11) |
No Result Posted for this study |
Completed | NCT04291729 |
| 6. | Methylprednisolone | Immunomodulatory effect | Phase 2 & Phase 3 (80) |
No Result Posted for this study | Completed | NCT04244591 |
Table 2.
Summary of the potential repurposed drugs currently in clinical trials for treatment of COVID-19.
| Sr. no | Potential drug candidate | Mechanism of action/rationale | Current clinical status | On-going Clinical Trial for COVID-19 in different countries |
|---|---|---|---|---|
| Anti-viral drugs | ||||
| 1. | Baloxavir | Antiviral active against influenza viruses | FDA approved anti-viral medication for treatment of influenza A and influenza B flu in Japan and in the United States | Chinese Clinical Trial Registry: (CHiCTR2000029544), (CHiCTR2000029548) |
| 2. | Favipiravir | Broad-spectrum antiviral drug which cause inhibition of viral RNA-dependent RNA polymerase against various viruses, including coronaviruses | Favipiravir is approved for treatment of influenza in Japan | National Clinical Trial Identifier: (NCT04336904), (NCT04349241), (NCT04336904), (NCT04346628), (NCT04310228), (NCT04319900), (NCT04303299), (NCT04333589), (NCT04336904), (NCT04345419) (NCT04351295), (NCT04349241) Chinese Clinical Trial Registration Number: (ChiCTR2000029544), (ChiCTR2000030113) (ChiCTR2000029548), (ChiCTR2000030894) (ChiCTR2000030987) Japan Clinical Trial Registration Number: (JapicCTI-205,238), (JPRN-jRCTs031190226), (JPRN-jRCTs041190120) |
| 3. | Lopinavir Ritonavir |
In-vitro activity against SARS-CoV-2 in Vero E6 cells; In-vitro activity against SARS-CoV-1 and MERS-CoV; showed protection in animal studies for treatment of MERS-CoV | Lopinavir/Ritonavir is an FDA approved antiretroviral drug of the protease inhibitor class used to treat HIV infection | National Clinical Trial Identifier: (NCT04307693), (NCT04276688), (NCT04328012) |
| 4. | Oseltamivir | Anti-viral active against influenza viruses | Oseltamivir is an FDA approved drug used to treat and prevent influenza A and influenza B | National Clinical Trial Identifier: (NCT04303299), (NCT04261270), (NCT04255017) |
| 5. | Remdesivir (recently approved by FDA as Orphan drug for COVID-19) |
Broad-spectrum anti-viral (including coronaviruses) | Remdesivir is an antiviral drug, act as nucleotide analog, specifically an adenosine analogue, which inserts into viral RNA chains, causing their premature termination. | National Clinical Trial Identifier: (Phase III) (NCT04292899), (NCT04292730), (NCT04280705) (NCT04323761), (NCT04302766) |
| 6. | Umifenovir | Broad-spectrum anti-viral with in-vitro activity against various viruses, including coronaviruses | Umifenovir is an antiviral treatment used for influenza infection used in Russia and China. | National Clinical Trial Identifier: (NCT04252885), (NCT04260594) Chinese Clinical Trial Registration Number: (ChiCTR200030254) |
| Supporting agents | ||||
| 7. | Anakinra | Disease modifying Anti -rheumatic Drug Recombinant human interleukin-1 (IL-1) receptor antagonist; inhibit cytokine release syndrome (CRS) symptoms or cytokine storm in severely ill patients |
Anakinra is clinically approved to manage symptoms of rheumatoid arthritis |
National Clinical Trial Identifier: (NCT04324021), (NCT04356366), (NCT04339712), (NCT04330638) |
| 8. | Ascorbic acid | Antioxidant and cofactor for numerous physiologic reactions; may support host defenses against infection and protect host cells against infection induced oxidative stress | Ascorbic acid is clinically used to prevent and treat scurvy, a disease caused by a lack of vitamin C |
National Clinical Trial Identifier: (NCT04264533) |
| 9. | Combination of Azithromycin and Hydroxychloroquine |
Azithromycin: Antibacterial with some in-vitro activity against some viruses (e.g., influenza A H1N1, Zika), has immunomodulatory and anti-inflammatory effects, including effects on proinflammatory cytokines Hydroxychloroquine: In-vitro activity against various viruses, including coronaviruses, has immunomodulatory activity that theoretically could contribute to an antiinflammatory response in patients with viral infections |
Azithromycin is clinically used as adjunctive therapy to management of certain respiratory conditions (e.g., bronchiectasis, bronchiolitis, cystic fibrosis, COPD exacerbations, ARDS) Hydroxychloroquine is also FDA-approved to treat malaria and autoimmune conditions such as chronic discoid lupus erythematosus, systemic lupus erythematosus in adults, and rheumatoid arthritis |
National Clinical Trial Identifier: (NCT04329832), (NCT04339426), (NCT04334382), (NCT04336332), (NCT04341870), (NCT04332094), (NCT04335552), (NCT04344379), (NCT04344444), (NCT04345861), (NCT04321278), (NCT04322396), (NCT04322123), (NCT04354428), (NCT04341727), (NCT04355052), (NCT04324463) |
| 10. | Colchicine | Exerts broad antiinflammatory and immunomodulatory effects through multiple mechanisms, may combat the hyperinflammatory state of COVID-19 (e.g., cytokine storm) by suppressing proinflammatory cytokines and chemokines | Colchicine is clinically approved medication used to treat gout and Behçet's disease | National Clinical Trial Identifier: (NCT04326790), (NCT04322565), (NCT04328480), (NCT04350320), (NCT04355143) |
| 11. | Corticosteroids | Potent anti-inflammatory and antifibrotic properties; use of corticosteroids may prevent an extended cytokine response and may accelerate resolution of pulmonary and systemic inflammation in pneumonia, may combat cytokine storm | Corticosteroid drugs are clinically used to treat rheumatoid arthritis, lupus, asthma, allergies and many other inflammatory conditions | National Clinical Trial Identifier: (NCT04327401), (NCT04344730), (NCT04348305) |
| 12. | Nitric oxide | Selective pulmonary vasodilator; may be useful in the adjunctive treatment of acute respiratory distress syndrome (ARDS), a potential complication of COVID-19 | National Clinical Trial Identifier: (NCT04306393) |
|
| 13. | Ruxolitinib | Janus kinase (JAK) 1 and 2 inhibitor; 7 may potentially combat cytokine release syndrome (CRS) in severely ill patients Ability to inhibit a variety of proinflammatory cytokines, including interferon, during viral infections such as COVID-19 |
Ruxolitinib is a clinically approved Janus-associated kinase (JAK) inhibitor with potential antineoplastic and immunomodulating activities | National Clinical Trial Identifier: (NCT04331665), (NCT04334044), (NCT04338958) Chinese Clinical Trial Registry (ChiCTR2000029580), (ChiCTR2000030170) |
| 14. | Sarilumab | Recombinant humanized monoclonal antibody specific for the interleukin-6 (IL-6) receptor; may potentially combat cytokine release syndrome (CRS) and pulmonary symptoms in severely ill patients | Sarilumab is an FDA approved human monoclonal antibody against the interleukin-6 receptor used for the treatment of rheumatoid arthritis. | National Clinical Trial Identifier: (NCT04357808), (NCT04315298) |
| 15. | Sirolimus | Immunosuppressive agent (mTOR inhibitor) mTOR complex 1 (mTORC1) is involved in the replication of various viruses, including coronavirus |
Sirolimus, also known as rapamycin, clinically used to coat coronary stents, prevent organ transplant rejection. | National Clinical Trial Identifier: (NCT04341675) |
| 16. | ACE Inhibitors, Angiotensin II Receptor Blockers (ARBs) |
Hypothetical benefit: ACE inhibitors or ARBs may have a protective effect against lung damage or may have paradoxical effect in terms of virus binding Hypothetical harm: Human pathogenic coronaviruses bind to their target cells through angiotensin converting enzyme 2 (ACE2)0.1, 4, 5 Expression of ACE2 may be increased in patients treated with ACE inhibitors or ARBs.1, 4, 8 Increased expression of ACE2 may potentially facilitate COVID-19 infections.1 |
Renin Angiotensin Aldosterone System Inhibitor, clinically approved for treatment of hypertension. | National Clinical Trial Identifier: (NCT04312009) |
| 17. | Anticoagulants (low molecular weight heparin [LMWH], unfractionated heparin [UFH]) | Current evidence indicates that patients with severe COVID-19 may develop a hypercoagulable state. Coagulation abnormalities observed in these patients include thrombotic disseminated intravascular coagulation (DIC), venous thromboembolism, elevated D-dimer levels, high fibrinogen levels, and microvascular thrombosis in the pulmonary vasculature |
Heparin is clinically approved anti-coagulant. | National Clinical Trial Identifier: (NCT04345848) |
6. Convalescent plasma therapy in COVID-19 patients
Plasmapheresis, also known as therapeutic plasma exchange (TPE), has been suggested as an alternative therapy to treat COVID-19 patients. Apheresis is the safest advised technique to acquire plasma, and this technique first involves the collection of blood from COVID-19 survivors, followed by plasma separation from blood cells. The same separated plasma is then infused into the circulation of severely ill COVID-19 patients [89]. In this context, Chinese authorities were the first to have reported success in treating COVID-19 infected patients with contributed plasma from survivors of the illness, the intended benefit being shielding antibodies formed in the blood of the survivors [89]. Currently, convalescent plasma administration to COVID-19 patients has been shown to lessen viral burden and enhance clinical status [90]. The likely mechanisms of action of convalescent plasma in COVID-19 patients may be attributed to the immediate neutralization of the SARS-CoV-2 virus, decrease in the intensity of the hyperactive immune system, decrease in cytokine storm, Th1/Th17 ratio, decrease complement system activation and alteration of a hypercoagulable state [90]. During plasma transfusion, in addition of transfer of neutralizing antibodies, some immunoglobulin G (IgG) and immunoglobulin M (IgM), anti-inflammatory cytokines, anti- clotting factors, defensins, pentraxins are too transferred to COVID-19 patients, which might confer additional health benefits and fasten the recovery [90]. In a nutshell, the transfer of numerous aforementioned blood factors during the transfusion process might account for some inhibition of excessive inflammatory response in COVID-19 patients. Though this technique is not approved by the FDA as a novel therapy, neither can be considered the best treatment option but can be considered as a possible emergency treatment for fulminant COVID-19. In the context of plasma therapy, FDA has released guidance to health care providers and investigators regarding use as well as the study of investigational convalescent plasma (COVID-19 convalescent plasma) [91]. Till date, a total of 97 clinical trials for the use of convalescent plasma in COVID-19 has been registered with ClinicalTrial.gov, and the full list can be accessed at (https://clinicaltrials.gov/ct2/results?term=Convalescent+Plasma+Therapy&cond=COVID19&Search=Apply&age_v=&gndr=&type=&rslt=). A list of a first ten registered clinical trials (three completed) with an infusion of investigational convalescent plasma (from survivors) into the blood of severely ill patients has been summarized in Table 3 .
Table 3.
Summary of the clinical trials of Convalescent Plasma for treatment of COVID-19.
| Sr. no | Plasma intervention/treatment | Sponsor | Clinical phase (sample size) | Status | Clinical trial identifier |
|---|---|---|---|---|---|
| 1. | Convalescent Plasma from Recovered COVID-19 Donors | Hackensack Meridian Health, New Jersey, United States | Phase II (55) |
On-going | NCT04343755 |
| 2. | Convalescent Plasma from Recovered COVID-19 Donors | Erasmus Medical Center, Netherland | Phase II & III (426) |
On-going | NCT04342182 |
| 3. | Convalescent Plasma from Recovered COVID-19 Donors | Cristina Avendaño Solá, Spain | Phase II (278) |
On-going | NCT04345523 |
| 4. | Convalescent Plasma from Recovered COVID-19 Donors | King Fahad Specialist Hospital Dammam, Saudi Arabia |
Phase II (40) |
On-going | NCT04347681 |
| 5. | Convalescent Plasma from Recovered COVID-19 Donors | Centro de Hematología y Medicina Interna, Mexico | Phase II (10) |
On-going | NCT04357106 |
| 6. | Convalescent Plasma from Recovered COVID-19 Donors | University of Chicago, Illinois, United States | Phase I (10) |
On-going | NCT04340050 |
| 7. | Anti-SARS-CoV-2 Inactivated Convalescent Plasma recovered from COVID-19 Donors | Shanghai Public Health Clinical Center, Shanghai, China | Observational Study (15) |
On-going | NCT04292340 |
| 8. | Convalescent Plasma from Recovered COVID-19 Donors | Institute of Liver and Biliary Sciences, New Delhi, India | Phase II (20) |
Completed | NCT04346446 |
| 9. | Convalescent Plasma Therapy on Critically ill Novel Coronavirus (COVID-19) Patients | Alkarkh Health Directorate-Baghdad. Iraq | Interventional study (49) |
Completed | NCT04441424 |
| 10. | Effectiveness of Convalescent Immune Plasma Therapy | Bagcilar Training and Research Hospital, Istanbul, Turkey | Interventional study (60) |
Completed | NCT04442958 |
7. Vaccines currently in clinical trials for COVID-19
The development of a new vaccine to contain COVID-19 infection is conceivably the safest way to end this pandemic. At present, there is no vaccine candidate to prevent COVID-19, but many of investigators are battling to create an effective and safe one [92]. Accumulating evidences indicate that previous research on vaccines for CoV has linked some challenges to the development of a new COVID-19 vaccine, which including: (i) Developing Safe and efficient vaccine: Numerous vaccines have been developed during SARS and MERS outbreak and tested for their efficacy in animals. Nearly all of them enhanced the animals' survival but unsuccessful in combating infection, whereas some of them caused complications, such as lung damage. Therefore, COVID-19 has to be thoroughly tested to ensure its safety for humans, (ii) Deliver enduring defense: A safe and effective COVID-19 vaccine should be able to provide a long-lasting shield against CoV as it has been seen that after infection with CoVs, there may be chances of re-infection with the same virus after months or years, although this is the case with a fraction of people, (iii) Defending elderly population: Older people are highly vulnerable to COVID-19 and typically do not respond to vaccination. Therefore, an exemplar COVID-19 vaccine should work well for this older age group. Keeping in view the aforementioned challenges, several universities, global health care authorities/groups, and vaccine developers are currently partnering to support each other in this crucial time to produce vaccines. A list of COVID-19 vaccines currently undergoing clinical trials has been summarized in Table 4 [92].
Table 4.
Summary of the clinical trials of vaccines for treatment of COVID-19.
| Sr. no | Vaccine intervention/treatment | Sponsor | Clinical phase (sample size) |
Status | Clinical trial identifier |
|---|---|---|---|---|---|
| 1. | INO-4800, a Prophylactic Vaccine; Intradermally | Inovio Pharmaceuticals, USA | Phase I (40) |
On-going | NCT04336410 |
| 2. | 2019-nCoV Vaccine (mRNA-1273) Lipid nanoparticle (LNP) dispersion containing an mRNA that encodes for the prefusion stabilized spike protein 2019-nCoV |
National Institute of Allergy and Infectious Diseases (NIAID), USA | Phase I (45) |
Completed | NCT04283461 |
| 3. | Dose-Confirmation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1273 COVID-19 Vaccine in Adults Aged 18 Years and Older | Moderna TX, Inc. | Phase II (600) |
On-going | NCT04405076 |
| 4. | Study to Describe the Safety, Tolerability, Immunogenicity, and Potential Efficacy of RNA Vaccine Candidates (BNT162a1, BNT162b1, BNT162b2 and BNT162c2) against COVID-19 in Healthy Adults | Biontech SE Pfizer |
Phase I (7600) |
Completed | NCT04368728 |
| 5. | A Trial Investigating the Safety and Effects of Four BNT162 Vaccines Against COVID-2019 in Healthy Adults | Biontech RNA Pharmaceuticals GmbH | Phase1 &II (200) |
On-going | NCT04380701 |
| 6. | Pathogen-specific aAPC; Subcutaneously | Shenzhen Geno-Immune Medical Institute, Guangdong, China | Phase I (100) |
On-going | NCT04299724 |
| 7. | Lentiviral Minigene Vaccine (LV-SMENP) of Covid-19 Coronavirus; Subcutaneously | Shenzhen Geno-Immune Medical Institute, Guangdong, China | Phase I &II (100) |
On-going | NCT04276896 |
| 8. | BCG Vaccine for Covid-19; Intracutaneously | UMC Utrecht, Netherland | Phase III (1500) |
On-going | NCT04328441 |
| 9. | SARS-CoV-2 Inactivated Vaccine | Sinovac Biotech Co., Ltd., Jiangsu, China | Phase I (Medium Dose, 144) Phase II (High Dose, 600) |
On-going | NCT04352608 |
| 10. | BCG Vaccine for Covid-19; Intradermally | Murdoch Children's Research Institute, Victoria, Australia |
Phase III (4170) |
On-going | NCT04327206 |
| 11. | Coronavirus Disease (COVID-19) vaccine ChAdOx1 nCoV-19 |
University of Oxford, United Kingdom | Phase I & II (1090) |
Completed | NCT04324606 |
| 12. | Investigating a Vaccine Against COVID-19 | University of Oxford, United Kingdom | Phase II & III (10260) |
On-going | NCT04400838 |
| 13. | Biological: Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) | CanSino Biologics Inc. | Phase I (108) |
Completed | NCT04313127 |
| 14. | A Phase II Clinical Trial to Evaluate the Recombinant Vaccine for COVID-19 (Adenovirus Vector) (CTII-nCoV) | CanSino Biologics Inc. Insitute of Biotechnology, Academy of Military Medical Sciences, PLA of China |
Phase II (508) |
On-going | NCT04341389 |
| 15. | Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS (COVID-19) Nanoparticle Vaccine With/Without Matrix-M Adjuvant | Novavax | Phase I (131) |
On-going | NCT04368988 |
| 16. | Safety and Immunogenicity Study of Inactivated Vaccine for Prevention of SARS-CoV-2 Infection (COVID-19) | Sinovac Research and Development Co., Ltd. | Phase I (72) Phase II (350) |
On-going | NCT04383574 |
8. Future perspectives and conclusion
Despite enormous worldwide endeavors to restrict COVID-19 infection caused by SARS-CoV-2, the virus's spread has achieved an epidemic stage. There have been numerous warnings to glean from the worldwide response to the SARS-COV-2 threat. The nonexistence of a trustworthy, reliable, early alert and response system, failure to mount containment actions, a paucity of community commitment for self-isolation, and overdependence on quarantining measures have uncovered the clefts in the competence of health systems worldwide. The COVID-19 outbreak has clearly shown the anemic preparation against evolving and re-emerging dangerous pathogens across the world. The source of the outbreak, the intermediate host, an effective treatment regimen, tools for early diagnosis in asymptomatic patients, and tools to predict the emergence of novel pathogens all remain elusive. It is now well established that SARS-CoV-2 enters cells by attaching to specific ACE-2 receptors, which are widely expressed in the respiratory tract, brain, liver, bile duct, lower GIT, and kidney. Thus, all these organs remain on the verge of damage by COVID-19. Keeping in view the global threat to public health instigated by SARS-CoV-2, an efficient prevention system as well as medication for COVID-19 pneumonia is instantly required. Even though the development of COVIS-19 therapeutics and vaccines is in its childhood stage, but still researchers across the globe have met with some substantial progress starting from elucidating out structure, full genomic sequencing of SARS-CoV-2, pathogenesis of COVID-19 and up to the commencement of clinical trials with COVID-19 drugs and vaccines.
Nevertheless, the development of medication for SARS-CoV-2 is still a prime dilemma for humans, and there is currently no clinically approved drug to combat COVID-19 except remdesivir (recently approved by FDA for emergency use in and only severely ill hospitalized patients). Health care professionals are making use of the repurposed drugs (drugs with limited proof for containing COVID-19) to prevent the spread of SARS-CoV-2. Therefore, in addition to clinical trials of new drugs and testing repurposed drugs, an exceptional strategy is urgently required for the development of new vaccines for COVID-19. Knowledge gained from SARS-CoV and MERS-CoV outbreak implies that SARS-CoV-2 research in the future should emphasize mainly on the creation and development of animal models that summarize and replicate the various clinical facets of COVID-19 and act as contributing factor of development of safe and efficacious drugs/vaccines.
Acknowledgments
Acknowledgment
The authors would like to thank all the research groups worldwide working on COVID-19 for their significant contributions during this outbreak.
Funding
No funding received for this work.
References
- 1.Schlipköter U., Flahault A. Communicable diseases: achievements and challenges for public health. Public Health Rev. 2010;32:90. doi: 10.1007/BF03391594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarner J. Three emerging coronaviruses in two decades: the story of SARS, MERS, and now COVID-19. Am. J. Clin. Pathol. 2020;153:420–421. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paital B., Das K., Parida S.K. Inter nation social lockdown versus medical care against COVID-19, a mild environmental insight with special reference to India. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassan S.A., Sheikh F.N., Jamal S., Ezeh J.K., Akhtar A. Coronavirus (COVID-19): a review of clinical features, diagnosis, and treatment. Cureus. 2020;12 doi: 10.7759/cureus.7355. https://doi [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Bi Y. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang A., Zhao W., Xu Z., Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res. Clin. Pract. 2020;162 doi: 10.1016/j.diabres.2020.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Zhao Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Gennaro, F., Pizzol, D., Marotta, C., Antunes, M., Racalbuto, V., Veronese, N., & Smith, L. (2020). Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int. J. Environ. Res. Public Health 17, E2690. https://2690. doi.org/ 10.3390/ijerph17082690. [DOI] [PMC free article] [PubMed]
- 11.Zhang J., Xie B., Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav. Immun. 2020;87:59–73. doi: 10.1016/j.bbi.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaimes J.A., Whittaker G.R. Feline coronavirus: insights into viral pathogenesis based on the spike protein structure and function. Virology. 2018;517:108–121. doi: 10.1016/j.virol.2017.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Müller M.A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gheblawi, M., Wang, K., Viveiros, A., Nguyen, Q., Zhong, J. C., Turner, A. J., ... & Oudit, G. Y. (2020). Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ. Res.. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed]
- 17.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020 doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infectious Diseases of Poverty. 2020;9:1–7. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020 doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singhal, T. (2020). A review of coronavirus disease-2019 (COVID-19). The Indian Journal of Pediatrics 87, 281–286. http://doi.org/ 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed]
- 23.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020 doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie M., Chen Q. Insight into 2019 novel coronavirus—an updated intrim review and lessons from SARS-CoV and MERS-CoV. Int. J. Infect. Dis. 2020;94:119–124. doi: 10.1016/j.ijid.2020.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han Q., Lin Q., Jin S., You L. Coronavirus 2019-nCoV: a brief perspective from the front line. J. Inf. Secur. 2020;80:373–377. doi: 10.1016/j.jinf.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sungsuwan S., Jongkaewwattana A., Jaru-Ampornpan P. Nucleocapsid proteins from other swine enteric coronaviruses differentially modulate PEDV replication. Virology. 2020;540:45–56. doi: 10.1016/j.virol.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hackbart M., Deng X., Baker S.C. Coronavirus endoribonuclease targets viral polyuridine sequences to evade activating host sensors. Proc. Natl. Acad. Sci. 2020;117(14):8094–8103. doi: 10.1073/pnas.1921485117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan J., Guo J., Fan C., Juan J., Yu X., Li J., Lei D. Coronavirus disease 2019 (COVID-19) in pregnant women: a report based on 116 cases. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siordia Jr, J. A. (2020). Epidemiology and clinical features of COVID-19: a review of current literature. J. Clin. Virol. 127, 104357. doi: 10.1016/j.jcv.2020.104357. [DOI] [PMC free article] [PubMed]
- 33.Lu G., Wang Q., Gao G.F. Bat-to-human: spike features determining ‘host jump’of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015;23:468–478. doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izaguirre G. The proteolytic regulation of virus cell entry by furin and other proprotein convertases. Viruses. 2019;11:837. doi: 10.3390/v11090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moulard M., Decroly E. Maturation of HIV envelope glycoprotein precursors by cellular endoproteases. Biochimica et Biophysica Acta (BBA)-Reviews on Biomembranes. 2000;1469:121–132. doi: 10.1016/S0304-4157(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 36.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. (e00127-e00120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Chen H.D. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu C.M., Wong R.S., Wu E.B., Kong S.L., Wong J., Yip G.W., Soo Y.O., Chiu M.L., Chan Y.S., Hui D., Lee N., Wu A., Leung C.B., Sung J.J. Cardiovascular complications of severe acute respiratory syndrome. Postgrad. Med. J. 2006;82:140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong T.Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.South A.M., Tomlinson L., Edmonston D., Hiremath S., Sparks M.A. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic. Nat. Rev. Nephrol. 2020 doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Phys. Heart Circ. Phys. 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo J., Huang Z., Lin L., Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K.S., Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Military Medical Research. 2020;7:1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu R., Wang L., Kuo H.C., Shannar A., Peter R., Chou P.J., Li S., Hudlikar R., Liu X., Liu Z., Poiani G.J. An update on current therapeutic drugs treating COVID-19. Current Pharmacology Reports. 2020;6:56–70. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Miao X. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA neurology. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiang P., Xu X.M., Gao L.L., Wang H.Z., Xiong H.F., Li R.H. First case of 2019 novel coronavirus disease with encephalitis. ChinaXiv. 2020;202003 [Google Scholar]
- 51.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li, K., Wohlford-Lenane, C., Perlman, S., Zhao, J., Jewell, A. K., Reznikov, L. R., ... & McCray Jr, P. B. (2016). Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J. Infect. Dis. 213, 712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed]
- 53.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hofmann H., Pöhlmann S. Cellular entry of the SARS coronavirus. Trends Microbiol. 2004;12:466–472. doi: 10.1016/j.tim.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Wang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabaan A.A., Al-Ahmed S.H., Sah R., Tiwari R., Yatoo M.I., Patel S.K., Pathak M., Malik Y.S., Dhama K., Singh K.P., Bonilla-Aldana D.K., Haque S., Rodriguez-Morales A.J. SARS-CoV-2/COVID-19 and advances in developing potential therapeutics and vaccines to counter this emerging pandemic virus – a review. Preprints. 2020 doi: 10.20944/preprints202004.0075.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drosten, C., Günther, S., Preiser, W., Van Der Werf, S., Brodt, H. R., Becker, S., ... & Berger, A. (2003). Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348, 1967- 1976. doi:0.1056/NEJMoa030747. [DOI] [PubMed]
- 58.Peiris J.S.M., Lai S.T., Poon L.L.M., Guan Y., Yam L.Y.C., Lim W., Cheng V.C.C. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duan Z.P., Chen Y., Zhang J., Zhao J., Lang Z.W., Meng F.K., Bao X.L. Clinical characteristics and mechanism of liver injury in patients with severe acute respiratory syndrome. Chin. J. Hepatol. 2003;11:493–496. [PubMed] [Google Scholar]
- 60.Lu, Y. C., Yin, C. B., Tang, X. P., Yang, Z., Lei, C. L., Chen, W. S., ... & Jia, W. D. (2004). Clinical characteristics and mechanism of liver function injury in 250 patients with severe acute respiratorv syndrome. China Journal of Modern Medicine 14, 121–123.
- 61.Jiang, T. J., Zhao, M., Zhou, Z. P., Zhou, X. Z., Jiang, S. C., Zhao, J. M., ... & Yan, H. Y. (2004). Clinical features of liver injury in patients with severe acute respiratory syndrome. China Journal of Modern Medicine, 2004, 46.
- 62.Farcas G.A., Poutanen S.M., Mazzulli T., Willey B.M., Butany J., Asa S.L., Kain K.C. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J. Infect. Dis. 2005;191:193–197. doi: 10.1086/426870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chau T.N., Lee K.C., Yao H., Tsang T.Y., Chow T.C., Yeung Y.C., Lai C.L. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan Y.J., Fielding B.C., Goh P.Y., Shen S., Tan T.H., Lim S.G., Hong W. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. J. Virol. 2004;78:14043–14047. doi: 10.1128/JVI.78.24.14043-14047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guan, W. J., Ni, Z. Y., Hu, Y., Liang, W. H., Ou, C. Q., He, J. X., ... & Du, B. (2020). Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv. doi: 10.1056/NEJMoa2002032. [DOI]
- 66.Chai, X., Hu, L., Zhang, Y., Han, W., Lu, Z., Ke, A., ... & Cai, J. (2020). Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. doi: 10.1101/2020.02.03.931766. [DOI]
- 67.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Xiong L. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L., Guo X. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12:244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Tai Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheung K.S., Hung I.F., Chan P.P., Lung K.C., Tso E., Liu R., Ng Y.Y., Chu M.Y., Chung T.W., Tam A.R., Yip C.C. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petrie J.R., Guzik T.J., Touyz R.M. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can. J. Cardiol. 2018;34:575–584. doi: 10.1016/j.cjca.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schoen, K., Horvat, N., Guerreiro, N. F., de Castro, I., & de Giassi, K. S. (2019). Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect. Dis. 19, 1–8. doi: 10.1186/s12879-019-4592-0. [DOI] [PMC free article] [PubMed]
- 73.Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y., Qin C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y., Xu X. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 75.Zhou J., Tan J. Diabetes patients with COVID-19 need better care. Metabolism. 2020 doi: 10.1016/j,metabol.2020.154216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hussain A., Bhowmik B., do Vale Moreira N.C. COVID-19 and diabetes: knowledge in progress. Diabetes Res. Clin. Pract. 2020;162 doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.International Diabetes Federation, 2020. https://diabetesvoice.org/en/news/covid-19-and-diabetes/.
- 78.Jamieson D.J., Theiler R.N., Rasmussen S.A. Emerging infections and pregnancy. Emerg. Infect. Dis. 2006;12:1638. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luo Y., Yin K. Management of pregnant women infected with COVID-19. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Archives of pathology & laboratory medicine. 2020 doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 81.Gajbhiye R., Modi D., Mahale S. Pregnancy outcomes, newborn complications and maternal-fetal transmission of SARS-CoV-2 in women with COVID-19: a systematic review. medRxiv. 2020 doi: 10.1101/2020.04.11.20062356. [DOI] [Google Scholar]
- 82.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., Tong S. Epidemiology of COVID-19 among children in China. Pediatrics. 2020 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 83.Cristiani L., Mancino E., Matera L., Nenna R., Pierangeli A., Scagnolari C., Midulla F. Will children reveal their secret? The coronavirus dilemma. Eur. Respir. J. 2020 doi: 10.1183/13993003.00749-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ioannidis J.P., Axfors C., Contopoulos-Ioannidis D.G. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. medRxiv. 2020 doi: 10.1101/2020.04.05.20054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. Jama. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 86.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment.
- 87.https://www.europeanpharmaceuticalreview.com/news/121787/glenmark-approved-to-supply-favipiravir-as-covid-19-treatment-in-india/.
- 88.https://www.ashp.org/-/media/assets/pharmacy-practice/resourcecenters/Coronavirus/docs/ASHP-COVID-19-Evidence-Table.ashx?.
- 89.Keith P., Day M., Perkins L., Moyer L., Hewitt K., Wells A. A novel treatment approach to the novel coronavirus: an argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit. Care. 2020;24:128. doi: 10.1186/s13054-020-2836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rojas M., Rodriguez Y., Monsalve D.M., et al. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.https://[34]drug-ind-or-deviceexemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma.
- 92.Kim Y.C., Dema B., Reyes-Sandoval A. COVID-19 vaccines: breaking record times to first-in-human trials. npj Vaccines. 2020;5:34. doi: 10.1038/s41541-020-0188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu Y.H., Li J.Y., Wang C., Zhang L.M., Qiao H. The ACE 2 G8790A polymorphism: involvement in type 2 diabetes mellitus combined with cerebral stroke. J. Clin. Lab. Anal. 2017;31 doi: 10.1002/jcla.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ding Y., He L., Zhang Q., et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]