Abstract
Background
Alemtuzumab is a treatment for highly active multiple sclerosis (MS). Immunosuppression is considered a risk factor for SARS-CoV-2 infection and there is still lack of evidence to guide MS practice.
Methods/results
We describe the clinical and immunological evolution of two MS patients under alemtuzumab treatment who were affected by COVID-19, one of them only one week after receiving her last dose, and both recovered without sequelae.
Conclusion
In selected patients (young, without comorbidities, and with high activity), MS itself could be more dangerous than COVID-19, so we should consider continuing MS treatment as previously planned, including alemtuzumab.
Keywords: Multiple sclerosis, SARS-CoV-2, COVID-19, Alemtuzumab, Immunosuppression
1. Introduction
The new pandemic of COVID-19 is turning our world upside down. Our main concern is people with multiple sclerosis (pwMS) on higher efficacy disease modifying treatments (DMT) because infection risk is increased in them (Willis and Robertson, 2020). Depending on the mechanism of action, they have different risk profiles for infections, including SARS-CoV-2 virus. First communications from registries (Sormani, 2020) are reassuring as pwMS have similar prognosis and risk factors for severe COVID-19 disease to that of the general population: older age and comorbidities, plus other more specific to MS: progressive phenotype, higher disability, and longer disease duration.
It is important to create evidence to guide our MS management. For that, we aim to share our experience about COVID-19 in MS patients treated with alemtuzumab, one of them in the first week after dosing.
1.1. Case 1
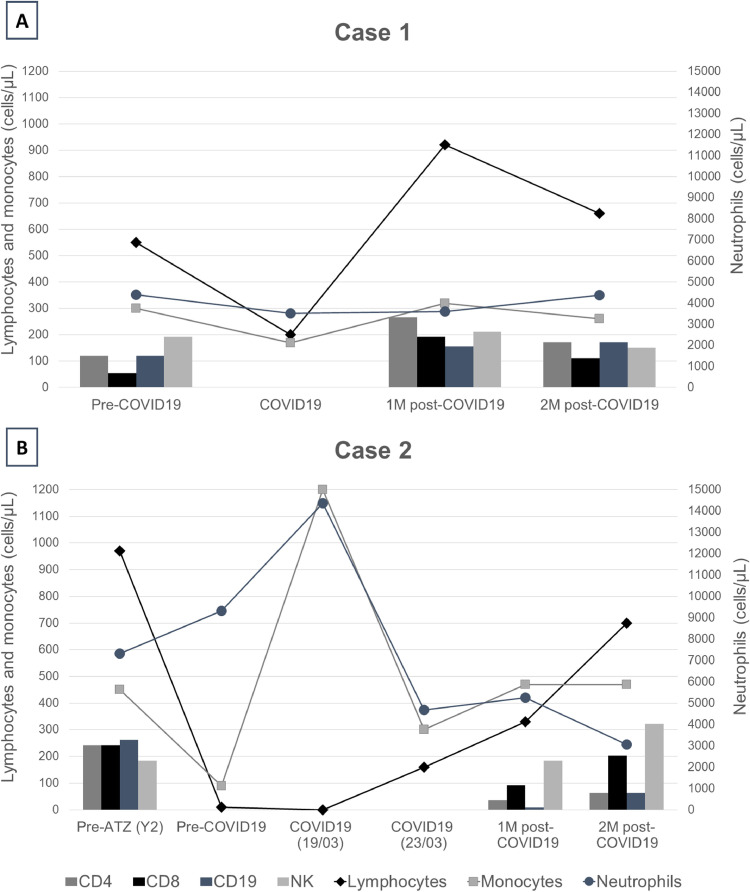
43-year-old male, without comorbidities, and diagnosis of relapsing remitting multiple sclerosis (RRMS) (see Table 1 for further MS and COVID-19 characteristics). He was free of disease activity and EDSS 0, but he maintained a persistent lymphopenia (550 cells/µL) after 11 months from the last alemtuzumab infusion. Five days after developing low-grade fever, cough and myalgias, the patient tested positive for SARS-Cov-2 on reverse transcription-polymerase chain reaction (PCR). First, Paracetamol and home self-isolation was recommended. However, one week later he was admitted to emergency department (ED) as he felt shortness of breath. Chest X ray and blood gas analysis were normal. Total lymphocyte count (TLC) dropped to 200 cells/µL (cells counts and profile is detailed in Fig. 1 a). He was discharged home with 10 days of hydroxychloroquine, lopinavir/ritonavir, and Amoxicillin treatment. Five weeks after the beginning of the symptoms, he was fully recovered, and he returned to work. Then, he had positive IgG test and negative PCR for SARS-CoV-2.
Table 1.
Additional information regarding baseline, multiple sclerosis and COVID-19 characteristics of our two patients.
| Case 1 | Case 2 | |
|---|---|---|
| Baseline | ||
| Age (years)/sex | 43/male | 30/female |
| Comorbidities | None | Ex-smoker for 2 years |
| Multiple sclerosis | ||
| Type | RRMS | RRMS |
| Current EDSS | 0 | 2.5 |
| Disease duration (years) | 14 | 2.5 |
| Last MRI (lesion load) | Low | High |
| Alemtuzumab dosing | ||
| Year 1 | April 2018 | March 2019 |
| Year 2 | April 2019 | March 2020 |
| Previous DMT | Fingolimod Interferon-beta |
— |
| COVID-19 | ||
| Onset | 10-MAR-2020 | 18-MAR-2020 |
| Diagnostic method | ||
| Chest X-ray | Normal | Bilateral pneumonia |
| PCR SARS-CoV-2 (nasopharyngeal swab) | Positive (15-MAR-2020) Negative (06-APR-2020) |
Positive (19-MAR-2020) Negative (25-MAY-2020) |
| Serum IgG SARS-Cov-2 | Positive (07-MAY-2020) | Positive (27-MAY-2020) |
| Maximum lymphopenia (cells/µL) | 200 | 0 |
| Severe disease | No | No |
| Hospitalization (days) | No | Yes (4) |
| Treatment | Lopinavir/ritonavir Hydroxychloroquine Amoxicillin |
Hydroxychloroquine Ceftriaxone/cefditoren |
| Treatment duration | 10 days | 9 days |
| Follow-up | Recovered | Recovered |
Fig. 1.
Temporal evolution of immune cells populations in our two MS patients who developed COVID-19 under alemtuzumab treatment across disease stages. A) Case 1: prior to infection, during COVID-19 and the next 2 months. B) Case 2: prior to alemtuzumab infusion, prior to infection, during COVID-19, and the next 2 months. Total count of lymphocytes (normal values: 1000–4000 cells/µL), neutrophils (normal values:1800–7500 cells/µL) and monocytes (normal values: 130–900 cells/µL) are represented in lines. Lymphocyte profile (CD4, CD8, CD19 and NK cells) are showed in bars, when available. Y2=year 2, 1M=1 month, 2M=2 months.
1.2. Case 2
30-year-old female, no comorbidities (Table 1). She was diagnosed with RRMS after a first disabling relapse and her baseline MRI showed a high T2 lesion load with several gadolinium enhancing ones. One year after alemtuzumab initiation, she was relapse-free and stable in her EDSS of 2.5, but MS was still active in her brain MRI. She received her second-year dose plus pulses of IV methylprednisolone 1 g daily for 3 days. One week later, on March 19th, she consulted in the ED with high fever and cough. Chest X-ray was normal and within her analysis stood out a TLC of 0 cells/µL and a positive PCR for SARS-CoV-2. She did not have respiratory failure and was discharged home on hydroxychloroquine. Acyclovir and trimethoprim-sulfamethoxazole was maintained as prophylaxis. On March 23rd, she was readmitted to the ED due to dyspnea and persistent fever. A new chest-X-ray showed bilateral infiltrates in the lungs and lymphocyte count had risen to 160 cells/µL (Fig. 1b). Then, she was hospitalized for observation and antibiotics were added to prevent bacterial superinfection. Luckily, she did not need supplementary oxygen, and she was discharged 3 days later with significant improvement. One month later, she was also fully recuperated at home. At her last follow-up, her PCR was negative and she tested positive for IgG anti-SARS-Cov2.
2. Discussion
Alemtuzumab, an anti-CD52 monoclonal antibody, is one of the most potent immunosuppressive drugs used in MS, leading to a rapid, profound and prolonged impact in circulatory T and B cells (Baker et al., 2017). An increased risk of infections has been described, being highest during the first month after each infusion, and decreasing over time. This spectrum includes viral infections, mostly by herpes virus (Wray et al., 2019). Therefore, alemtuzumab carries a theoretical high risk of developing COVID-19 if the infection occurs before immune reconstitution and the general recommendation is postponing infusions until the pandemic is controlled (Brownlee et al., 2020; Costa-Frossard França et al., 2020; Giovannoni et al., 2020). Interestingly, only a minority of patients develop severe infections. This means that some immunocompetence is maintained, probably because the remaining lymphocytes are functional, the depletion in lymphoid organs is scarce and the innate immune response is mostly preserved, since macrophages, NK cells and neutrophils have a low CD52 expression (Turner et al., 2013; Wray et al., 2019).
Both of our patients were young, without comorbidities and with low MS-related disability. Although one was admitted to hospital, it was a precaution due to her significant immunosuppression state. Both recovered without sequelae, stopped shedding coronavirus with PCR-negative nasopharyngeal swabs, and developed IgG antibodies against SARS-CoV-2, even with extremely low lymphocyte counts. Similar experiences, with uneventful recoveries despite lymphopenia, have been described in four people with MS who suffer a mild COVID-19 disease one week (Carandini et al., 2020), two months (Guevara et al., 2020) and one year (Matías-Guiu et al., 2020) after their second course of Alemtuzumab.
In our first patient, we observed a significant increase in TCD8+, and TCD4+ to a lesser extent, after the infection, without changes in the rest of the populations. Our second patient showed a significant increase in monocytes and neutrophils during the acute phase of the infection, which might have played a key role in the absence of lymphocytes. Even in the first month after alemtuzumab, she was able to produce a sufficient adaptative response, including antibody production against SARS-CoV-2 and elevation of CD8+ cells.
In times of uncertainty, we should carefully individualize treatment decisions to successfully manage MS. In some pwMS, especially young and otherwise healthy with highly active MS, the risk of a disabling relapse or disease progression might be higher than the risk of severe complications due to SARS-CoV-2 infection (Brownlee et al., 2020). On the other hand, given that innate immunity seems to be essential in the control of the virus (Baker et al., 2020), it is possible that the risk of COVID-19 in our patients is lower than initially expected, since DMT in MS barely affect it. Moreover, a certain degree of immunosuppression might be protective because the severe acute respiratory syndrome (SARS) is related to a dysregulated immune response (Giovannoni et al., 2020). Therefore, in selected patients we should consider continuing DMTs as planned, including alemtuzumab, if safe conditions are ensured: outside of the peak of the outbreak, availability of clean spaces in the hospital for the infusions and a proper self-isolation at home until immune reconstitution.
Consent for publication
Informed consent was obtained from both patients for the publication of this manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Eva Fernández-Díaz: Conceptualization, Data curation, Writing - original draft, Supervision. Julia Gracia-Gil: Conceptualization, Data curation, Writing - review & editing. Jose Gregorio García-García: Conceptualization, Data curation, Writing - review & editing. María Palao: Writing - review & editing. Carlos M Romero-Sánchez: Writing - review & editing. Tomás Segura: Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declared the following potential conflicts of interest: EFD and JGG have received research support, compensation for participating on advisory boards, lecture fees and/or travel support from: Almirall, Bayer, Genzyme-Sanofi, Novartis, Roche and Teva declare no potential conflicts of interest. JGGG has received research support, compensation for participating on advisory boards, lecture fees and/or travel support from: AbbVie, Bayer, Novartis, Angellini and Allergan. MP and CMRS have received support to attend congresses and conferences from Merck. TS has received compensation for participating in scientific advisory boards of Amgen Inc and Boehringer Ingelheim, and funding for travel or speaker honoraria of Bayer Pharmaceuticals and Daiichi-Sankyo. None of these companies are involved in the choice of content, writing or decision to publish this manuscript.
References
- Baker D., Amor S., Kang A.S., Schmierer K., Giovannoni G. The underpinning biology relating to multiple sclerosis disease modifying treatments during the COVID-19 pandemic. Mult. Scler. Relat. Disord. 2020 doi: 10.1016/j.msard.2020.102174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Herrod S.S., Alvarez-Gonzalez C., Giovannoni G., Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol. 2017;74:961–969. doi: 10.1001/jamaneurol.2017.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee W., Bourdette D., Broadley S., Killestein J., Ciccarelli O. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology. 2020 doi: 10.1212/WNL.0000000000009507. doi: 10.1212/WNL.0000000000009507. [DOI] [PubMed] [Google Scholar]
- Carandini T., Pietroboni A.M., Sacchi L., De Riz M.A., Pozzato M., Arighi A., Fumagalli G.G., Martinelli Boneschi F., Galimberti D., Scarpini E. Alemtuzumab in multiple sclerosis during the COVID-19 pandemic: a mild uncomplicated infection despite intense immunosuppression. Mult. Scler. 2020 doi: 10.1177/1352458520926459. 1352458520926459. [DOI] [PubMed] [Google Scholar]
- Costa-Frossard França L., Moreno Torres I., Meca Lallana V., García Domínguez J.M., en representación del grupo de est, en representación del grupo de est Documento EMCAM (Esclerosis Múltiple Comunidad de Madrid) para manejo de pacientes con Esclerosis Múltiple durante la pandemia SARS-CoV2. Rev. Neurol. 2020;70:1. doi: 10.33588/rn.7009.2020155. [DOI] [PubMed] [Google Scholar]
- Giovannoni G., Hawkes C., Lechner-Scott J., Levy M., Waubant E., Gold J. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult. Scler. Relat. Disord. 2020;39 doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara C., Villa E., Cifuentes M., Naves R., de Grazia J. Mild COVID-19 infection in a patient with multiple sclerosis and severe depletion of T-lymphocyte subsets due to alemtuzumab. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matías-Guiu J., Montero-Escribano P., Pytel V., Porta-Etessam J., Matias-Guiu J.A. Potential COVID-19 infection in patients with severe multiple sclerosis treated with alemtuzumab. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P. An Italian programme for COVID-19 infection. Lancet Neurol. 2020;4422:30147. doi: 10.1007/s15010-020-01420-9.Chinese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M.J., LaMorte M.J., Chretien N., Havari E., Roberts B.L., Kaplan J.M., Siders W.M. Immune status following alemtuzumab treatment in human CD52 transgenic mice. J. Neuroimmunol. 2013;261:29–36. doi: 10.1016/j.jneuroim.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Willis M.D., Robertson N.P. Multiple sclerosis and the risk of infection: considerations in the threat of the novel coronavirus, COVID-19/SARS-CoV-2. J. Neurol. 2020:2–4. doi: 10.1007/s00415-020-09822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S., Havrdova E., Snydman D.R., Arnold D.L., Cohen J.A., Coles A.J., Hartung H.P., Selmaj K.W., Weiner H.L., Daizadeh N., Margolin D.H., Chirieac M.C., Compston D.A.S. Infection risk with alemtuzumab decreases over time: pooled analysis of 6-year data from the CAMMS223, CARE-MS I, and CARE-MS II studies and the CAMMS03409 extension study. Mult. Scler. J. 2019;25:1605–1617. doi: 10.1177/1352458518796675. [DOI] [PMC free article] [PubMed] [Google Scholar]