Abstract
Background
Pneumonia with severe respiratory failure represents the principal cause of death in COVID-19, where hyper-inflammation plays an important role in lung damage. An effective treatment aiming at reducing the inflammation without preventing virus clearance is thus urgently needed. Tocilizumab, an anti-soluble IL-6 receptor monoclonal antibody, has been proposed for treatment of patients with COVID-19.
Methods
A retrospective cohort study at the Montichiari Hospital, Brescia, Italy, was conducted. We included consecutive patients with COVID-19 related pneumonia at the early stage of respiratory failure, all treated with a standard protocol (hydroxychloroquine 400 mg daily, lopinavir 800 mg plus ritonavir 200 mg per day). We compared survival rate and clinical status in a cohort of patients who received additional treatment with tocilizumab once (either 400 mg intravenous or 324 mg subcutaneous) with a retrospective cohort of patients who did not receive tocilizumab (referred to as the standard treatment group). All outcomes were assessed at the end of the follow-up, that correspond to death or complete recovery and discharge from the hospital.
Findings
158 patients were included, 90 of which received tocilizumab. 34 out of 68 (50%) patients in the standard treatment group and 7 out of 90 (7.7%) in the tocilizumab group died. Tocilizumab significantly improved survival compared to standard care (multivariate HR: 0.057; 95% C.I = 0.017- 0.187, p < 0.001). No differences between the two administration routes of tocilizumab were observed. No tocilizumab-related infections and/or side effects were observed.
Interpretation
Early treatment with tocilizumab could be helpful to prevent excessive hyper-inflammation and death in COVID-19 related pneumonia. Low dose administration of tocilizumab is not associated with adverse events.
Funding
none
Research in context.
Evidence before this study
Since the coronavirus disease 2019 (COVID-19) outbreak, evidence has emerged that patients may develop interstitial pneumonia with severe respiratory failure. This represents the principal cause of death in COVID-19 related infection, where hyper-inflammation plays an important role in lung damage. Tocilizumab, an anti-soluble IL-6 receptor monoclonal antibody, has been proposed for treatment of patients with COVID-19. However, data so far are limited, both in the term of efficacy and safety and only small or uncontrolled studies using high dose of tocilizumab have been published on patients in severe clinical conditions and the safety profile is still unknown.
Added value of this study
The current retrospective study describes the beneficial clinical effect of early IL-6 blockade with low dose tocilizumab in patients with COVID-19 and respiratory failure. Furthermore, both the intravenous and subcutaneous administration routes demonstrated to be equally effective and safe.
Implications of all the available evidence
Our study suggests that early administration of low dose tocilizumab, before the appearance of a respiratory distress requiring assisted ventilation, modulates excessive hyper-inflammation and reduces mortality caused by COVID-19. This result, together with the excellent safety profile and the ease of administration, suggests that the early administration of low dose of this agent deserves consideration in controlled trials for the treatment of COVID-19.
Alt-text: Unlabelled box
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has severely affected northern Italy and especially the province of Brescia. The disease has been characterized by a very high mortality rate being interstitial pneumonia with respiratory failure the main cause of death for COVID-19 [1].
Previous studies have shown that immunological hyper-activation, referred to as “cytokine storm” [2], can be a contributory cause of interstitial damage in the lungs of COVID-19 patients, leading to a more severe clinical course. During this state of immunological hyper-activation, peripheral CD4 and CD8 T cells counts were substantially reduced while their status was hyper-activated. Furthermore, an increased concentration of highly pro-inflammatory CCR6+ T helper 17 (Th17) in CD4 T cells and a high concentration of cytotoxic granules in CD8 lymphocytes in peripheral blood was found in dead patients infected with SARS-CoV-2 [3]. Importantly, as shown by previous literature, increased circulating levels of pro-inflammatory cytokines (e.g. interleukin –IL- 1B, IL-6, IL-12) and chemokines (CXCL10 and CCL2) are associated with pulmonary inflammation and extensive lung involvement in SARS patients [4,5]. In this scenario, Acute Respiratory Distress Syndrome (ARDS) is the ultimate result of cytokine storm [3].
Within the pro-inflammatory cytokines, Interleukin 6 (IL-6) plays a key role in the pathogenesis of the COVID-19 related cytokine storm [2]. IL-6 seems to be also responsible for the activation of T helper 17 (Th17) cells in the dendritic cell-T cell interaction [6]. In COVID-19 affected patients, a high Th17 cells activation could result from a virus-driven increased production of IL-6 by the immune system [2]. Several studies showed that the serum levels of IL-6 are increased in COVID-19 patients and that its circulating levels are positively related to disease severity [7], [8], [9]. Indeed, levels of IL-6 have been found to be directly associated with severe lung damage [10,11], and a recent meta-analysis of six studies investigating IL-6 concentration in COVID-19 demonstrated 2.9 fold higher levels in patients with complicated COVID-19 compared with patients with non-complicated disease [12]. An excessive and dysregulated production of IL-6 is thus considered a potential negative prognostic factor for survival during COVID-19, being higher levels of IL-6 related to a higher mortality rate [10]. For this reason, high serum IL-6 levels were suggested to be a reliable biomarker of COVID-19 progression [13], [14], [15]. The role of hyper-inflammation in COVID-19 is so important that guidelines for the diagnosis and treatment of SARS-CoV-2 infected pneumonia recommended cytokine monitoring to reduce mortality [14,15].
Tocilizumab, a humanized anti-soluble IL-6 receptor monoclonal antibody, has been proposed to be useful in the treatment of COVID-19 disease. By blocking the IL-6 receptor interaction, tocilizumab inhibits the IL-6 mediated signal transduction, reduces the availability of IL-6 and regulated immunological activity [16], [17], [18]. For this reason, some authors recommended its use in critically ill COVID-19 patients with significant elevated IL-6 [7]. However, the usefulness of tocilizumab is still controversial. While some studies describing the clinical and radiological improvement of patients with severe clinical conditions due to COVID-19 related pneumonia treated with high dose of tocilizumab reported an increased survival [19], other studies failed to observe beneficial effect of tocilizumab in severe patients [20,21]. Furthermore, it should be noted that IL-6 inhibition has potential hazards of inducing infectious diseases [22].
Thus, the role of inhibiting the link between IL-6 and the soluble receptor on COVID-19 evolution remains to be fully evaluated [23]. Interestingly, a recent paper suggested that treatment with tocilizumab could be useful not only to target symptoms but also to modulate the disease itself in its early phase [24], preventing excessive inflammation and lung damage [7]. In order to test this hypothesis, in the current paper, we describe 158 patients with COVID-19, ninety of whom were treated early in the disease course with single - low dose of tocilizumab. Our a priori hypothesis was twofold. First, mortality rate was expected to be lower in patients early treated with tocilizumab compared to controls, who were treated with standard therapy only. Second, a low dose of tocilizumab would is not expected to be associated with de novo infections.
2. Methods
2.1. Patients
We conducted a retrospective cohort study at the Montichiari Hospital, a tertiary health-care Centre in Brescia, Italy, which was designated as a COVID-19 hub by Italian health authorities. Patients consecutively admitted to Montichiari Hospital were retrospectively included in the study if they met the following inclusion criteria: 1) confirmed COVID-19 infection as determined by a positive reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assay of a specimen collected on a nasopharyngeal swab; 2) bilateral pulmonary interstitial opacities on chest imaging that were not fully explained by congestive heart failure or other forms of volume overload; 3) a respiratory failure showing at least one of the following conditions: respiratory rate ≥ 30 breaths/min; peripheral capillary oxygen saturation (SpO2) ≤ 93% while breathing ambient air or ratio of the partial pressure of oxygen in arterial blood to the fractional concentration of oxygen in inspired air (PaO2/FiO2) ≤ 300 mmHg. In line with our rationale of including only patients in the early phase of infection, the following exclusion criteria were applied: 1) presence of a critical respiratory syndrome that requires mechanical or invasive ventilation at hospital admission; 2) presence of severe clinical conditions as revealed by transaminase 5 times the upper limit of the normal value; neutrophils <500 mmc; platelets <50.000 mmc [17].
This retrospective study has been conducted in accordance with the declaration of Helsinki and its later amendments and was approved by the Ethical Committee of the Spedali Civili of Brescia.
2.2. Study design
Due to the emergency situation in the province of Brescia, northern Italy, it was impossible to carry out a properly randomized controlled trial. Patients admitted to the hospital between February 26th and March 13th underwent a standard therapy (hydroxychloroquine 400 mg daily, lopinavir 800 mg daily plus ritonavir 200 mg per day) [25,26] as for standard protocol administered at our institution at the time (hereafter defined to as “standard treatment group”). Patients admitted after March 13th patients received off-label a single low dose administration of tocilizumab in addition to standard therapy (hereafter defined to as “tocilizumab group”). Inclusion of patients in the standard treatment or in the tocilizumab group (400 mg intravenously -i.v.- or 324 mg subcutaneous -s.c.-) was determined by the availability of the drug at the moment, as in a previous study [23]. All patients receiving standard therapy only retrospectively full-filled eligibility criteria for tocilizumab treatment. All patients gave written informed consent for off-label use of tocilizumab. Administration of tocilizumab occurred upon worsening of the respiratory functions, as described in the inclusion criteria, in accordance with the aim of the study to treat patients early in the course of the respiratory distress. This usually occurred the day of hospital admission or the day after.
During hospitalization, patients in both groups were assisted with non-invasive (i.e. low flow nasal cannula; high flow mask; Continuous Positive Airway Pressure –CPAP-) or invasive (i.e. mechanical ventilation) oxygen therapy, according to their needs. Patients were followed-up until the end of the clinical observation, defined as death or complete recovery and discharge from the hospital with SpO2>94% while breathing in ambient air.
2.3. Data extraction
The clinical record of each patient was retrospectively analyzed and, for each patient, the following information were extracted and recorded in a dedicated database: patients anonymized ID; age at admission, gender; inclusion criteria; comorbidities; date of first flu symptom; date of admission to the hospital; date of tocilizumab administration (if pertinent); tocilizumab administration route (if pertinent); serum procalcitonin (both at the hospital admission and at the time of discharge) to evaluate subclinical bacterial infections; de novo infections and de novo respiratory infections; the need for daily respiratory support (low flow cannula, high flow mask, CPAP; SpO2; Peep; FiO2); daily body temperature; daily C Reactive Protein (CRP); complete laboratory test results; date of discharge or death; data of admission and of discharge from Intensive Care Unit, where mechanical ventilation has been administered (if pertinent).
2.4. Statistical analyses
Data were analyzed with SPSS version 24.0 (Chicago, IL, USA). We report categorical variables as number (%) and continuous variables as mean (standard deviation) or median (range) depending on whether the data are normally distributed or not. Statistical significance was assessed by means of chi-squared for dichotomous variables, or by means of the two independent sample t-test or the Mann-Whitney U test for continuous variable depending on whether the data are normally distributed or not. For longitudinal analysis, data were analyzed using paired sample t test or Wilcoxon test depending on whether the data are normally distributed or not. Regarding laboratory results, if one or more laboratory test resulted to statistically differ between tocilizumab and standard treatment group with a potential clinical relevance, we were interested in understanding the effect of tocilizumab administration route and posology on these laboratory test results. Thus, a repeated measures ANOVA was performed on laboratory results with potential clinical relevance using group (324 mg vs 400 mg) as independent variable and Time (two levels: pre-therapy; 5 days post therapy follow up) as dependent variable.
The primary endpoint was the survival rate in patients treated with tocilizumab in addition to standard therapy (tocilizumab group), and only with standard therapy (standard treatment group). The survival rate was assessed by Kaplan–Meier (KM) plot using group (tocilizumab vs controls) as between factor; death as event and time to death/discharge as time variable. Data were censored at the end of the observation, that corresponds to discharge from the hospital for patients with a complete recovery as the event (i.e. death) was not observed. Hazard Ratio (HR) with 95% confidence intervals (CI) were calculated by means of the Cox proportional-hazard model, adjusting for the following baseline variables: age, gender, diabetes, hypertension or heart diseases; serum CRP and respiratory support needed at hospital admission (both this variables included in the multivariate model to correct for disease severity ad admission); time elapsed from symptoms onset to hospital admission (hereafter referred as to “time to hospitalization”, included in the multivariate model to correct for treatment delay).
2.5. Role of the funding source
No funding was received for this study. All the authors had full access to the raw data and to patient's clinical records.
3. Results
The results are described in accordance with the STROBE guidelines [27].
One hundred and fifty eight patients were included in the current study: sixty-eight patients received standard care, while 90 patients were treated with tocilizumab in addition to standard care (43 (47.7%) received 400 mg i.v. once, whereas 47 (52.3%) received 324 mg s.c. once, according to the availability of the drug). Baseline demographic and clinical characteristics, including laboratory test results, of the groups of patients in standard care or treated with tocilizumab are reported in Table 1.
Table 1.
Demographic and clinical characteristics of the two groups of patients. Number denotes mean (standard deviation) (a); raw number (percentages) (b); median [interquartile range] (c). Statistical significance was evaluated using two independent sample t-test (a); chi square (b); Mann-Whitney U test (c). P value reports the associated p value (statistical significance p<0.05). CRP = C-Reactive Protein; bpm = beats per minute.
| Normative values | Controls (n=68) | Tocilizumab (n=90) | Significance | P value | |
|---|---|---|---|---|---|
| Age (years)a | – | 71 (14.6) | 62.9 (12.5) | -3.706 | <0.001 |
| Ethnicity (Caucasian)b | – | 65 (95.5%) | 85 (94.4%) | 0.105 | 0.745 |
| Gender (males)b | – | 49 (72%) | 64 (71.1%) | 0.017 | 0.896 |
| Diabetes (yes) b | – | 21 (30.9%) | 14 (15.5%) | 5.276 | 0.022 |
| Hypertension (yes) b | – | 36 (42.9%) | 41 (45.5%) | 0.846 | 0.358 |
| Heart disease(yes) b | – | 22 (32.3%) | 11 (12.2%) | 9.500 | 0.002 |
| Time to hospitalization (days)a | – | 6 (3.1) | 9.1 (8.1) | 3.001 | <0.001 |
| Clinical characteristics at hospital admission | |||||
| Heart rate (bpm) a | – | 90.0 (16.0) | 91.0 (16.0) | 0.390 | 0.697 |
| Systolic blood pressure (mm/Hg) a | – | 127.0 (22.0) | 130.0 (23.0) | 0.918 | 0.360 |
| Diastolic blood pressure (mm/Hg) a | – | 73.0 (11.0) | 74.0 (10.9) | 0.285 | 0.776 |
| Temperature (°C)c | <37 | 37.5 [36–39.5] | 37.5 [36-40] | 0.460 | |
| Laboratory Results at hospital admission | |||||
| CRP (mg/L)a | <0.5 | 83.8 (64.1) | 121 (77.5) | 3.212 | 0.002 |
| Procalcitonin (ng/L)c | <0.1 | 0.14 [<0.1–3.2] | 0.25 [<0.1-7.5] | 0.980 | |
| White blood cells (x10³ per μL) a | 4.00-10.80 | 6.6 (3.9) | 7.0 (3.7) | 0.635 | 0.527 |
| Lymphocytes (x10³ per μL) a | 0.90-4.00 | 1.1 (0.6) | 9.9 (0.8) | -0.491 | 0.624 |
| Neutrophils (x10³ per μL) a | 1.50-8.00 | 4.7 (3.0) | 5.6 (3.5) | 1.444 | 0.151 |
| Platelets (x10³ per μL) a | 130-400 | 188 (89) | 224 (103) | 2.252 | 0.026 |
| Glycemia (mg/dl)b | 76-115 | 118 [87–476] | 114 [76-314] | 0.061 | |
| Urea (mg/dl) b | 17-49 | 38 [21–154] | 35 [17-143] | 0.140 | |
| Creatinine (mg/dl) a | 0.60-1.00 | 1.1 (0.5) | 1.0 (0.3) | -2.369 | 0.016 |
| Sodium (mmol/L) a | 136-145 | 135.9 (3.6) | 136.6 (5.3) | 0.790 | 0.431 |
| Potassium (mmoL/L) a | 3.4-4.5 | 3.9 (0.5) | 3.8 (0.4) | -1.471 | 0.143 |
| Chlorine (mmol/L) a | 89-107 | 97.3 (4.1) | 97.4 (4.2) | 0.227 | 0.821 |
| Bilirubin (mg/dL) a | <1.20 | 0.5 (0.2) | 0.6 (0.2) | 0.982 | 0.328 |
| Aspartate Transaminase -AST (U/L) a | 18-34 | 52.7 (37.8) | 56.7 (38.2) | 0.623 | 0.534 |
| Alanine amionotrasferase -ALT (U/L) a | 10-35 | 35.4 (31.1) | 46.9 (35.5) | 2.015 | 0.046 |
| Gamma glutamyl trasferase- gGT (U/L) b | 6-42 | 34 [14–555] | 45.5 [11-360] | 0.053 | |
| Alkaline phosphatase -ALP (U/L) a | 44-107 | 70.7 (51.1) | 63.0 (34.3) | -1.001 | 0.319 |
| Lactate dehydrogenase- LDH (U/L) a | 135-225 | 326.0 (146.7) | 371.3 (131.1) | 1.192 | 0.237 |
| Creatine kinase- CK (U/L) b | 26-192 | 102.5 [18–843] | 113 [21–918] | 0.048 | |
| Prothrombine time -Pt (sec) b | 9.4-12.5 | 13.1 [10.9–18.4] | 13.5 [10.8–22.2] | 0.145 | |
| Activated partial thromboplastine time- aPTT (sec) b | 24-38 | 32.2 (5.1) | 33.1 (5.2) | -1.033 | 0.303 |
| International normalized ratio -INR c | 0.9-1.2 | 1.2 [0.9–4.6] | 1.2 [1.0–2.0] | 0.165 | |
3.1. Effect of early administration of tocilizumab on mortality rate
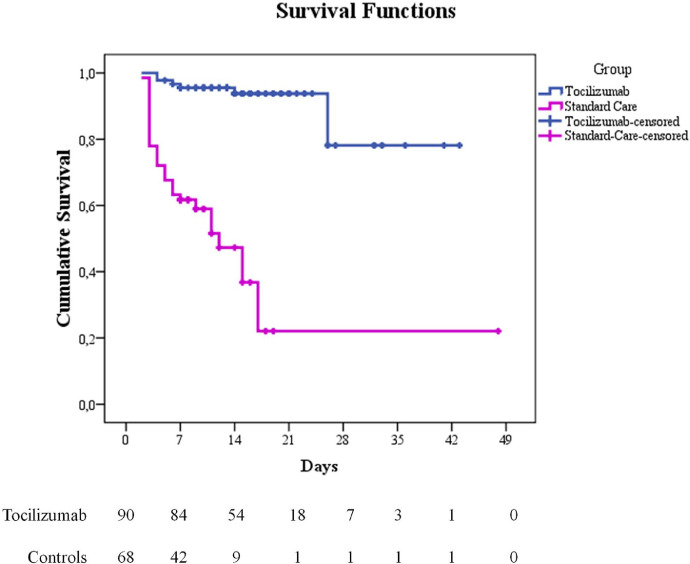
Seven deaths were observed in the group of patients treated with tocilizumab (7 out of 90 patients, 7.7%, mean age=74) while 34 deaths occurred in the control group (34 out of 68 patients, 50%, mean age=78). The Cox proportional hazard model (adjusted for age, gender, diabetes, hypertension, heart disease; CRP; respiratory support needed at hospital admission; time to hospitalization) showed a significantly greater survival rate of tocilizumab patients as compared to controls (multivariate H.R. for death: 0.057; 95% C.I = 0.017- 0.187, p < 0.001, Fig. 1). The data revealed that the risk of death increases by 6% for each year of age, making older age a risk factor for death in COVID-19. In addition, having diabetes or heart disease increases the risk of death 3.2 or 3 times, respectively. Results are reported in Table 2.
Fig. 1.
Kaplan-Meier survival curve for tocilizumab (blue line) and control (violet line) group. Analysis run using Group (tocilizumab vs controls) as factor; death as event and time to death/discharge as time variable. Multivariate Hazard Ratio (H.R. for death: 0.057; 95% C.I = 0.017- 0.187, p < 0.001) is adjusted for baseline characteristics.
Table 2.
Results from the Cox Proportional Hazard model. GROUP = main variable (tocilizumab vs. controls); HR = Hazard Ratio; CI = Confidence Intervals; CRP = C-Reactive Protein.
| 95% C.I. for HR |
||||
|---|---|---|---|---|
| Variable | HR | Lower | Upper | p value |
| GROUP | 0.057 | 0.017 | 0.187 | <0.001 |
| Age | 1.069 | 1.026 | 1.114 | 0.001 |
| Gender | 1.727 | 0.797 | 3.743 | 0.166 |
| Diabetes | 3.272 | 1.477 | 7.245 | 0.003 |
| Hypertension | 1.634 | 0.710 | 3.758 | 0.248 |
| Heart disease | 3.001 | 1.422 | 6.332 | 0.004 |
| CRP at admission | 1.006 | 1.000 | 1.011 | 0.044 |
| Time to hospitalization | 1.001 | 0.913 | 1.098 | 0.978 |
| Respiratory support | 1.728 | 1.141 | 2.619 | 0.010 |
3.2. Clinical longitudinal follow up in the two groups
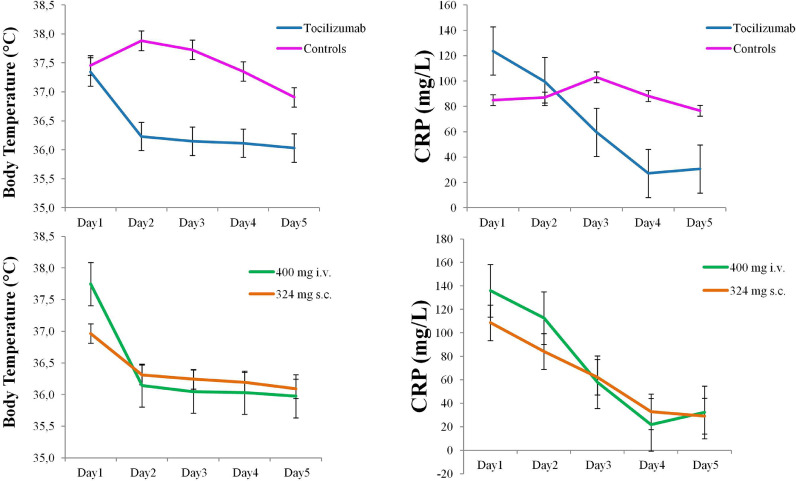
Considering the pharmacodynamic of tocilizumab [28], an immediate effect on inflammatory indices (CRP and body temperature) was expected. Patients were then closely monitored for the first five days after the beginning of therapeutic interventions in both groups. Fig. 2 (upper panel) showed the drastic reduction of fever and CRP in patients treated with tocilizumab but not in controls. As CRP was not expected to fall within the normal range in few days, CRP was now measured further.
Fig. 2.
Longitudinal data. Graphs show the longitudinal evolution of the body temperature and of the CRP (C Reactive Protein) after the hospital admission in the two groups of patients. The first five days after admission to the hospital were reported, where Day 1 refers to the beginning of therapeutic intervention in both groups. The upper panel shows the longitudinal body temperature and CRP data in tocilizumab and control groups, whereas the lower panel shows the longitudinal body temperature and CRP data in the tocilizumab group divided in the two administration routes. Error bars denotes standard error of the mean.
As reported in the methods, all patients were provided with respiratory support according to their needs. Respiratory support needed at hospital admission is reported in Table 3. During the observation, six patients in the standard therapy group (8.82%) and 13 patients in the tocilizumab group (14.4%) needed mechanical ventilation.
Table 3.
Respiratory data and Follow up data of the two groups. Numbers denotes row numbers (percentages) or median [interquartile range]. SpO2 = peripheral capillary oxygen saturation; FiO2 = Fractional concentration of oxygen in inspired air; CPAP = Continuous Positive Airway Pressure; Peep = positive end expiratory pressure.
| Controls (n=68) | Tocilizumab (n=90) | |
|---|---|---|
| Respiratory support needed at hospital admision | ||
| 1) No respiratory support | 20 (29.4%) | 18 (20%) |
| SpO2 (%) | 95 [88–98] | 93 [88–97] |
| 2) Low flow cannula | 13 (19.1%) | 8 (8.8%) |
| SpO2 (%) | 95 [92–97] | 94 [92–98] |
| FiO2 (%) | 28 [24–31] | 31 [24–31] |
| 3) High flow mask | 24 (35.3%) | 42 (46.6%) |
| SpO2 (%) | 95 [91–98] | 94 [88–100] |
| FiO2 (%) | 60 [35–100] | 60 [40-100] |
| 4) CPAP | 11 (16.2%) | 22 (24.4%) |
| SpO2 (%) | 94 [88–98] | 95 [89–99] |
| FiO2 (%) | 50 [40-60] | 55 [40–100] |
| Peep (cm H2O) | 13 [10–12.5] | 13 [10–20] |
| Longitudinal follow up | ||
| Patients needing mechanical ventilation | 6 (8.82%) | 13 (14.4%) |
| Respiratory System Infections | 4 (5.9%) | 6 (6.6%) |
| Etiological agent for respiratory system infections | Streptococcus Epidermidis (n=1) Clamydia Pneumoniae (n=1) Mycoplasma Pneumoniae (n=1) Staphilococcus Hominis (n=1) |
Mycoplasma Pneumoniae (n=2) Clamidya Pneumoniae (n=2) Staphilococcus Hominis (n=1) Cytomegalovirus (n=1) |
| Pulmunary Embolism | Not known | 12 (13.3%) |
| Total deaths | 34 (50%) | 7 (7.7%) |
During the longitudinal follow up, no mechanical ventilation associated pneumonia were observed in our cohort. Four patients within the standard treatment group (5.9%) and six patients within the tocilizumab group (6.6%) manifested de novo respiratory system infections. Details on the etiological agents are reported in Table 3. Twelve patients in tocilizumab group (13.3%) manifested pulmonary embolism, three of whom died. As during the first phase of the outbreak the association between COVID-19 and pulmonary embolism [29] was not known and postmortem examinations were not done at our site, the prevalence of pulmonary embolism in the standard therapy group is not known. Both respiratory system infections and pulmonary embolism are known to be associated with COVID-19 [30], [31], [32], [33], [34], [35], thus the role of tocilizumab in their manifestation could be ruled out. For this reason, these data will not be further discussed. No infections or additional safety concerns related to tocilizumab emerged.
3.3. Effect of tocilizumab on laboratory test results
Furthermore, the short term effect of single low dose of tocilizumab administration was evaluated. Laboratory test results were compared before tocilizumab administration and 5 days after tocilizumab administration in 81 patients (n=7 dead patients were excluded; n=2 patients had a follow up shorter than 5 days and laboratory test results are not available). As shown in Table 4, few statistical differences emerged between baseline and follow up, however only few of them are clinically relevant [36]. A decrement in heart rate (from 90.5 at baseline to 73 bpm at follow up), in body temperature (from 37.5 to 36°C) and in CRP (from 108 to 22 mg/L) are indicative of improving clinical conditions. An increment in alanine amionotrasferase (ALT) from 48 to 91 U/L has been observed. This is very commonly observed as a result of tocilizumab and is clearly indicated in the patient information leaflet (PIL) [37].
Table 4.
Longitudinal clinical and laboratory results of patients treated with tocilizumab. Data are reported at baseline (before tocilizumab administration) and at 5 days follow up. Number denotes mean (standard deviation) (a); median [interquartile range] (b). Statistical significance was evaluated using paired sample t-test (a); Wilcoxon test (b). P value reports the associated p value (statistical significance p<0.05). Asterisk (*) denotes clinical significance.
| Normative values | Pre-tocilizumab treatment (n=81) | 5 days post-tocilizumab treatment (n=81) | Significance | P value | |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Heart rate (bpm) a | - | 90 (15.0) | 73 (13.0) | 8.427 | <0.001* |
| Systolic blood pressure (mm/Hg) a | - | 129 (22.) | 131 (19.0) | -0.458 | 0.648 |
| Diastolic blood pressure (mm/Hg) a | - | 73 (11.0) | 76 (10.0) | -1.954 | 0.056 |
| Temperature (°C)b | <37 | 37.5 [36-40] | 36.0 [36-37.5] | -5.690 | <0.001* |
| Laboratory Results | |||||
| CRP (mg/L)a | <0.5 | 108.4 (74.3) | 22.2 (36.6) | 9.950 | <0.001* |
| Procalcitonin (ng/L)b | <0.1 | 0.25 [<0.1–7.5] | 0.1 [<0.01-0.56] | -2.293 | 0.022 |
| White blood cells (x10³ per μL) a | 4.00-10.80 | 6.9 (3.9) | 7.4 (3.1) | -0.790 | 0.432 |
| Lymphocytes (x10³ per μL) a | 0.90-4.00 | 0.9 (0.4) | 1.1 (0.5) | -2.828 | 0.006 |
| Neutrophils (x10³ per μL) a | 1.50-8.00 | 5.5 (3.7) | 6.2 (5.6) | -0.678 | 0.500 |
| Platelets (x10³ per μL) a | 130-400 | 225 (106) | 337 (282) | -3.436 | 0.001 |
| Glycemia (mg/dl)b | 76-115 | 115 [76-314] | 100.5 [64-323] | -0.909 | 0.363 |
| Urea (mg/dl) b | 17-49 | 35 [16-143] | 35 [16-88] | -0.675 | 0. 500 |
| Creatinine (mg/dl) a | 0.60-1.00 | 0.9 (0.2) | 0.8 (0.2) | 7.108 | <0.001 |
| Sodium (mmol/L) a | 136-145 | 136.5 (5.1) | 140.1 (2.9) | -4.707 | <0.001 |
| Potassium (mmoL/L) a | 3.4-4.5 | 3.8 (0.5) | 4.0 (0.5) | -3.454 | 0.001 |
| Chlorine (mmol/L) a | 89-107 | 97.5 (3.9) | 101.0 (9.1) | -2.970 | 0.004 |
| Bilirubin (mg/dL) a | <1.20 | 0.5 (0.3) | 0.5 (0.2) | 1.622 | 0.115 |
| Aspartate Transaminase -AST (U/L) a | 18-34 | 58.6 (42.4) | 58.9 (42.3) | -0.057 | 0.995 |
| Alanine amionotrasferase -ALT (U/L) a | 10-35 | 48.6 (39.6) | 91.2 (110.3) | -3.322 | 0.002* |
| Gamma glutamyl trasferase- gGT (U/L) b | 6-42 | 45 [11-360] | 70 [8-609] | -1.734 | 0.083 |
| Alkaline phosphatase -ALP (U/L) a | 44-107 | 65.1 (37.3) | 67.0 (29.0) | -0.373 | 0.711 |
| Lactate dehydrogenase- LDH (U/L) a | 135-225 | 358.6 (138.5) | 324.7 (118.5) | 0.415 | 0.681 |
| Creatine kinase- CK (U/L) b | 26-192 | 112 [11–878] | 44 [7-1218] | -3.176 | 0.001 |
| Prothrombine time -Pt (sec) b | 9.4-12.5 | 13.4 [10.8-22.2] | 12.9 [11-15.8] | -4.119 | <0.001 |
| Activated partial thromboplastine time- aPTT (sec) b | 24-38 | 32.7 (4.0) | 29.6 (5.4) | 4.351 | <0.001 |
| International normalized ratio -INR c | 0.9-1.2 | 1.2 [1.0-2.0] | 1.2 [1.0-1.4] | 0.504 | 0.674 |
3.4. Effect of tocilizumab administration route
We then considered only patients treated with tocilizumab (n=90) and we explored whether the two different administration routes (400 mg i.v. or 324 mg s.c.) had an impact on patients outcome. Baseline demographic and clinical characteristics of the two groups of patients are reported in Table 5. Out of the seven deaths observed in patients treated with tocilizumab, one occurred in the 400 mg i.v. group (age=64) and 6 in the 324 mg s.c. group (mean age=76). The Cox proportional hazard model (adjusted for age, gender, diabetes, hypertension, heart disease; CRP, respiratory support needed at hospital admission; time to hospitalization) revealed that survival rate did not statistically differ between the two administration routes (multivariate H.R. for death: 5.234; 95% C.I = 0.241- 113.633, p = 0.292). The two groups did not differ in the time to discharge (mean time to discharge: 18 days -95% C.I. [15.3-20.7]- in the 400 mg i.v. group and 14.2 days -95% C.I. [12.1-16.3]- in the 324 mg s.c. group; baseline characteristics adjusted Cox proportional hazard model: multivariate H.R.: 1.556; 95% C.I = 0.940- 2.479, p = 0.086). Furthermore, the quick decrement of body temperature and CPR after tocilizumab administration is similar between the two administration routes, as shown in the lower panel of Fig. 2. Finally, as an increment in the ALT emerged as a potential safety concern, we were interested in understanding the potential impact on administration route and posology on ALT increase. The repeated measures ANOVA revealed a non significant critical interaction Group x Time (F=2.18; p=0.14). Despite this, the Newman Keuls post hoc test revealed that, while the ALT results did not change significantly in the 324 s.c. mg group (from 50.1±40.9 to 74.0±46.3 U/L, p=0.15), ALT significantly increase in patients in the 400 i.v. mg group (from 44.4±28.3 to 103.1±141.3, p=0.004).
Table 5.
Demographic and clinical characteristics of the two groups of patients. Number denotes mean (standard deviation) (a); raw number (percentages) (b); median [interquartile range] (c). Statistical significance was evaluated using two independent sample t-test (a); chi square (b); Mann–Whitney U test (c). CRP = C-Reactive Protein.
| 324 mg s.c. (n=47) | 400 mg i.v. (n=43) | Significance | P value | |
|---|---|---|---|---|
| Age (years)a | 66.8 (11.3) | 58.7 (12.6) | 3.182 | 0.002 |
| Gender (males)b | 34 (72.3%) | 30 (69.7%) | 0.072 | 0.788 |
| Diabetes (yes) b | 7 (14.9%) | 7 (16.2%) | 0.003 | 0.856 |
| Hypertension (yes) b | 24 (51%) | 17 (39.5%) | 1.203 | 0.273 |
| Heart disease(yes) b | 6 (12.7%) | 5 (11.6%) | 0.027 | 0.869 |
| Time to hospitalization (days)a | 10 (5.7) | 8.1 (10.1) | 1.068 | 0.289 |
| CRP at admission (mg/L)a | 109 (73.5) | 134 (80.5) | -1.550 | 0.125 |
| Temperature at admission (°C)c | 36 [36-39] | 38 [36-40] | 0.003 | |
| Procalcitonin (ng/L)c | 0.19 [<0.1-5.2] | 0.17 [<0.1-7.5] | 0.822 |
4. Discussion
The current study describes the positive impact of a single low dose of tocilizumab in addition to standard therapy in a relatively early phase of SARS-CoV-2 disease in a cohort of 90 patients compared to 68 patients treated with standard therapy only. All patients had laboratory confirmed infection and were followed-up until the discharge from the hospital or death. First and most important, this study found that the risk of death for patients treated with tocilizumab is 94% lower than the one of patients treated with standard therapy only. Early treatment with a single low dose of tocilizumab is thus effective. Second, this efficacy profile is not related to the administration route, as the two groups (400 i.v. vs 324 s.c.) show the same survival rate. Third, the effect of tocilizumab on inflammatory indices is very quick and does not depend on administration route. Finally, our study also revealed that low dose of tocilizumab administration is safe, as no tocilizumab related infections or safety concerns have been observed. Among COVID-19 patients, about 25% present severe complications including acute respiratory distress syndrome (ARDS) with a rapid worsening of clinical conditions leading to the need of mechanical or invasive ventilation to support respiratory functions [5]. Although the viral invasion and the direct cytopathic effect are critical for a worsening of the clinical course, there is evidence that a deranged immune response can be implicated in ARDS [3]. A significant increase of white blood cells, in particular of neutrophils, has been observed in patients with a severe disease, while a significant reduction of both CD8+ and CD4+ T lymphocytes cells was detected [38]. Among of immunologic biomarkers, the highest levels of IL-6 and serum ferritin were observed in severe subacute form of respiratory disease and in non-survivors COVID-19 patients [9]; in the same way, inflammatory cytokines as IL-2 and IFN-γ show an high serum levels in patient with severe course of SARS and MERS [5,39]. As cytokine storm has been proposed to have a pivotal role in organ injury in COVID-19, tocilizumab has been suggested as a possible treatment in SARS-CoV-2 infection. Indeed, anti-IL6 therapeutic strategy has been shown to be effective in cytokine response syndrome (CRS) [40]. A recommended protocol for tocilizumab used in COVID-19 [41], requires a first dose of 4-8 mg/kg and an additional infusion after 12 hours for patients with worsening or poor clinical response. This protocol, however, lead to inconsistent results. Previous literature indeed report small or absent [20,21] effect of tocilizumab in a very small cohort of patients, making the results statistically unreliable. Additional studies provide data of single arm of patients only (i.e. the control group was not available) [42], [43], [44], [45], [46], making the real efficacy of tocilizumab obscure. In the absence of specific treatment for COVID-19, the main objective of clinicians should be to promote virus clearance allowing immune system to over-ride viral infection, whereas preventing organ damage due to excessive inflammation. In this regard, early tocilizumab administration was expected to be useful to modulate the cytokine storm and prevent the consolidation of lung damage, while the low dose was expected to be useful to reduce hyper inflammation without abolishing the immune response to the virus [24]. In the current study, a single low dose of tocilizumab (400 mg e.v. or 324 mg s.c., regardless of the patients’ weight) has been administered at the early stage of the respiratory failure. Our study shows a higher survival rate in the treated group compared to the patients who were treated with standard therapy only with a 94% treatment impact on the risk of death, fully supporting the efficacy of administration of low dose of tocilizumab early in the disease course.
In accordance with previous data [47], the current study also supports that older age is a risk factor for death and that the probability to die increases by 6% for each year of age. Several reports also suggested that concomitant chronic illness may have an impact on mortality rate [1,48]. The current results confirmed diabetes and cardiopathy as risk factors for worse outcome Indeed, patients with diabetes have a 3.2 fold higher risk of death compared to patients without diabetes, and patients with cardiopathy have a 3 fold higher risk of death compared to patients without cardiopathy [49,50]. In our cohort hypertension is not associated with an increased risk of death but this could depend on the sample size.
The favorable outcome in the tocilizumab cohort is independent from administration routes, as the survival rates and the respiratory recovery are similar between patients receiving 400 mg i.v. and patients receiving 324 mg s.c. The higher number of deaths observed in the latter (6 deaths vs 1) is easily justified by the older average age in the subcutaneously treated group compared to the intravenous one. The equivalence between the two administration routes is further supported by the the similar hospitalization time and by the equally rapid pharmacological effect, as evidenced by the quick decrease in CRP and body temperature analysis in both groups, in accordance with previous studies [51]. These data confirm the pharmacodynamic equivalence studies that analyzed the impact of tocilizumab administration route and confirm what has already been found in the rheumatological field [28,52]. Despite the similar effect of the two administration routes on tocilizumab efficacy, the current data also provide preliminary support that the potential hepatotoxicity might be dose dependent, being the observed increment in ALT higher in patients receiving 400 mg compared to patients receiving 324 mg. This elevation in ALT is known and expected during tocilizumab therapy, is not associated with clinically relevant increases in direct bilirubin and is not associated with clinical evidence of hepatic insufficiency. Importantly, the PIL clearly indicate that this elevation did not result in permanent or clinically evident hepatic injury in clinical trials [37].
Critically, the treatment with a low dose of tocilizumab was safe. During the follow-up period we did not observe any adverse drug reactions; in particular, secondary infections or intestinal perforations, both threatening complications of the administration of tocilizumab [53,54], did not occur. Serum level of procalcitonin remained stable and within the lower limits (<0.5 ng/mL), denoting the absence of bacterial infections in our patients. These complications were instead observed using higher doses of tocilizumab in patients with COVID-19 [42].
This study presented some limitations. The most important one refers to the lack of randomization resulting in not matched groups. Indeed, patients treated with standard care were older and with higher prevalence of comorbidities compared to patients treated with tocilizumab. Contrarily, patients treated with tocilizumab were admitted to the hospital later during the disease course, as supported by the longer time elapsed from symptoms onset to hospitalization. Despite we are confident that the multivariate approach applied in the current paper (Cox proportional hazard model) removed the potential biasing effect of these unmatched variables on the primary results, the current data need to be further supported. A second important limitation is that an additional control group, including patients treated with tocilizumab during the late stage of respiratory failure is missing. This unfortunately prevents us for claiming that early administration of tocilizumab is more effective than late administration. However, it is worth noting that previous literature provides discordant and thus inconclusive findings on tocilizumab effectiveness in severe COVID-19 [20,21], thus suggesting that its late administration, when lung damage already happened, might be less effective in reducing mortality. Finally, the patient's inclusion strategy applied does not allow to definitely rule out the potential impact of unmeasured and unconscious confounding factors on the results, as for instance the acquired clinical experience of managing the disease. It is here important to underline that the patients included timely received the needed respiratory support and the standard therapy as for protocol at our site. Furthermore, none of the included patients received anticoagulant therapy modulating the thromboembolic risk [55]. We are thus confident that no relevant confounding factors explaining such an important decrement in mortality rate were present.
Despite the number of patients included is quite limited, to our knowledge this represents the biggest study comparing patients receiving tocilizumab with a control group receiving standard therapy only. However, in line with our rationale, only patients in the early stage of respiratory failure were enrolled. The current results cannot thus be generalizable to the wider population of COVID-19 patients, as for instance patients in critical conditions, where tocilizumab beneficial effect seems to be more limited.
In conclusion, the results described in the current paper are clinically relevant since they demonstrate a reduction of the mortality rate of 94% in patients receiving a single low dose of tocilizumab early in the course of the disease. The effect of additional variables on the results observed has reasonably been ruled out and the safety profile of this drug is excellent. Further multicenter and randomized trials are needed to confirm the efficacy and safety of early administration of low dose of tocilizumab in larger populations.
Declaration of Competing Interest
All authors declare no competing interests.
Acknowledgments
Acknowledgments
The authors are grateful to the Montichiari COVID-19 Study Group, that includes: Aloisi Gaetano, Amolini Claudia, Angelini Osvaldo, Armellini Andrea, Bernardi Silvia, Bianchi Alessandra, Bianco Gianluca, Boari Gianluca, Bonetti Silvia, Bonzi Bianca, Bosisio Marco, Botteri Emanuele, Braglia Orlandini Federico, Bregoli Laura, Capistrano Mariano, Cappello Giovanni, Caprioli Michela, Castagna Ilaria, Cervellini Patrizio, Colombi Marta, Chiarini Giulia, Croatto Enrico, D'Avanzo Luigi, Desenzani Paolo, Domeneghini Elio, Faustini Cristina, Ferrari Toninelli Giulia, Gallico Agnese, Grassini Chiara, Guarinoni Vittoria, Lopotinschi Diane, Leali Daria, Lucente Daniela, Malerba Paolo, Manzoni Francesca, Mascadri Cristina, Massucco Alberto, Megaro Alberico, Montalto Jacopo, Morandi Riccardo, Nardin Matteo, Nicosia Franco, Pasini Giancarlo, Saottini Michele, Spitti Carla, Torrisi Sebastiano, Trombetta Luca, Turini Daniele, Tusi Claudia, Villa Simona, Viola Sara, Viola Claudio, Volpini Andrea, Zanotti Eros. The authors are grateful to Dr. Elena Bortolato for statistical support.
Funding
This research received no funds.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100459.
Appendix. Supplementary materials
References
- 1.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. 2020/02/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coperchini F, Chiovato L, Croce L. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.05.003. 2020/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z, Shi L, Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. 2020/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. 2017/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. 2020/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. 2010/06/29. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Wu Z, Li JW. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. 2020/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Liu HG, Liu W. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:203–208. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. 2020/03/14. [DOI] [PubMed] [Google Scholar]
- 9.McGonagle D, Sharif K, O'Regan A. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. 2020/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25948. 2020/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.04.009. 2020/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coomes EA and Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis. 101101/20200330200480582020. [DOI] [PMC free article] [PubMed]
- 13.Ulhaq ZS, Soraya GV. Interleukin-6 as a potential biomarker of COVID-19 progression. Med Mal Infect. 2020;50:382–383. doi: 10.1016/j.medmal.2020.04.002. 2020/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun X, Wang T, Cai D. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.04.002. 2020/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin YH, Cai L, Cheng ZS. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. 2020/02/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alzghari SK, Acuna VS. Supportive treatment with tocilizumab for COVID-19: a systematic review. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104380. 2020/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18:164. doi: 10.1186/s12967-020-02339-3. 2020/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Li L, Shen A. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Investig. 2020 doi: 10.1007/s40261-020-00917-3. 2020/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alattar R, Ibrahim TBH, Shaar SH. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol. 2020 doi: 10.1002/jmv.25964. 2020/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rimland C, Morgan C, Bell G, et al. Clinical characteristics and early outcomes in patients with COVID-19 treated with 2 tocilizumab at a United States academic center. 101101/20200513201004042020.
- 21.Campochiaro C, Della-Torre E, Cavalli G. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. 2020/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Zhong Y, Pan L. Treat 2019 novel coronavirus (COVID-19) with IL-6 inhibitor: are we already that far. Drug Discov Ther. 2020;14:100–102. doi: 10.5582/ddt.2020.03006. 2020/05/08. [DOI] [PubMed] [Google Scholar]
- 23.Remy KE, Bracknridge SC, Francois B. Immunotherapies for COVID-19: lessons learned from sepsis. Lancet Resp Med. 2020 doi: 10.1016/S2213-2600(20)30217-4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transpl. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. 2020/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu CM, Cheng VC, Hung IF. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. 2004/02/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincent MJ, Bergeron E, Benjannet S. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. 2005/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. 2007/12/08. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Georgy A, Rowell L. Pharmacokinetics and pharmacodynamics of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, following single-dose administration by subcutaneous and intravenous routes to healthy subjects. Int J Clin Pharmacol Ther. 2013;51:443–455. doi: 10.5414/CP201819. 2013/04/04. [DOI] [PubMed] [Google Scholar]
- 29.Wichmann D, Sperhake JP, Lutgehetmann M. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann Intern Med. 2020 doi: 10.7326/M20-2003. 2020/05/07. [DOI] [PubMed] [Google Scholar]
- 30.Bompard F, Monnier H, Saab I. Pulmonary embolism in patients with Covid-19 pneumonia. Eur Respir J. 2020 doi: 10.1183/13993003.01365-2020. 2020/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poyiadji N, Cormier P, Patel PY. Acute pulmonary embolism and COVID-19. Radiology. 2020 doi: 10.1148/radiol.2020201955. 2020/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bikdeli B, Madhavan MV, Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. 2020/04/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klok FA, Kruip M, van der Meer NJM. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. 2020/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan BE, Lim KGE, Chong VCL. COVID-19 and mycoplasma pneumoniae coinfection. Am J Hematol. 2020;95:723–724. doi: 10.1002/ajh.25785. 2020/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai CC, Wang CY, Hsueh PR. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.05.013. 2020/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranganathan P, Aggarwal R, Pramesh CS. Common pitfalls in statistical analysis: odds versus risk. Perspect Clin Res. 2015;6:222–224. doi: 10.4103/2229-3485.167092. 2015/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khanna D, Denton CP, Lin CJF. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate) Ann Rheum Dis. 2018;77:212–220. doi: 10.1136/annrheumdis-2017-211682. 2017/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0198. 2020/03/03. [DOI] [PubMed] [Google Scholar]
- 39.Wong CK, Lam CW, Wu AK. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. 2004/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta P, McAuley DF, Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. 2020/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trial C.https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001110-38. Clinical trials for eudract_number:2020-001110-38.
- 42.Toniati P, Piva S, Cattalini M. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102568. 2020/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X, Han M, Li T. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. 2020/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klopfenstein T, Zayet S, Lohse A. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. 2020 doi: 10.1016/j.medmal.2020.05.001. 2020/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo P, Liu Y, Qiu L. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. 2020/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sciascia S, Apra F, Baffa A. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020 2020/05/03. [PubMed] [Google Scholar]
- 47.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. 2020/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. 2020/02/25. [DOI] [PubMed] [Google Scholar]
- 49.Cook TM. The importance of hypertension as a risk factor for severe illness and mortality in COVID-19. Anaesthesia. 2020 doi: 10.1111/anae.15103. 2020/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. 2020/03/29. [DOI] [PubMed] [Google Scholar]
- 51.Nishimoto N, Terao K, Mima T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–3964. doi: 10.1182/blood-2008-05-155846. 2008/09/12. [DOI] [PubMed] [Google Scholar]
- 52.Lauper K, Mongin D, Iannone F. Comparative effectiveness of subcutaneous tocilizumab versus intravenous tocilizumab in a pan-European collaboration of registries. RMD Open. 2018;4 doi: 10.1136/rmdopen-2018-000809. 2018/11/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogata A, Kato Y, Higa S. IL-6 inhibitor for the treatment of rheumatoid arthritis: a comprehensive review. Mod Rheumatol. 2019;29:258–267. doi: 10.1080/14397595.2018.1546357. 2018/11/15. [DOI] [PubMed] [Google Scholar]
- 54.Vikse J, Henry BM. Tocilizumab in COVID-19: beware of risk of intestinal perforation. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.106009. 2020/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kollias A, Kyriakoulis KG, Dimakakos E. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189:846–847. doi: 10.1111/bjh.16727. 2020/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.