Abstract
Background
Coronavirus disease 2019 (COVID-19) remains a worldwide pandemic with a high mortality rate among patients requiring mechanical ventilation. The limited data that exist regarding the utility of extracorporeal membrane oxygenation (ECMO) in these critically ill patients show poor overall outcomes. This report describes our institutional practice regarding the application and management of ECMO support for patients with COVID-19 and reports promising early outcomes.
Methods
All critically ill patients with confirmed COVID-19 evaluated for ECMO support from March 10, 2020, to April 24, 2020, were retrospectively reviewed. Patients were evaluated for ECMO support based on a partial pressure of arterial oxygen/fraction of inspired oxygen ratio of less than 150 mm Hg or pH of less than 7.25 with a partial pressure of arterial carbon dioxide exceeding 60 mm Hg with no life-limiting comorbidities. Patients were cannulated at bedside and were managed with protective lung ventilation, early tracheostomy, bronchoscopies, and proning, as clinically indicated.
Results
Among 321 patients intubated for COVID-19, 77 patients (24%) were evaluated for ECMO support, and 27 patients (8.4%) were placed on ECMO. All patients were supported with venovenous ECMO. Current survival is 96.3%, with only 1 death to date in more than 350 days of total ECMO support. Thirteen patients (48.1%) remain on ECMO support, and 13 patients (48.1%) have been successfully decannulated. Seven patients (25.9%) have been discharged from the hospital. Six patients (22.2%) remain in the hospital, of which 4 are on room air. No health care workers who participated in ECMO cannulation developed symptoms of or tested positive for COVID-19.
Conclusions
The early outcomes presented here suggest that the judicious use of ECMO support in severe COVID-19 may be clinically beneficial.
Dr Kon discloses a financial relationship with Medtronic, Inc and Breethe, Inc; Dr Cerfolio with Bovie, Community Health Services, Covidien/Medtronic, C-SATS, Davol/Bard, Ethicon, Google/Verb, Intuitive Surgical, KCI/Acelity Company, Myriad Genetics, Pinnacle, TEGO Corporation, and ROLO-7 Consulting Firm; Dr Montgomery with bioStrategies Group, Inc, CSL Behring, CTI Clinical Trial and Consulting Services, Inc, Hansa, Intas Pharmaceuticals, LIM, PeerView Operative Services America, LLC, Recombinetics, RMEI, eGenesis, Genzyme/Sanofi, Regeneron, Takeda/Shire, and Viela Bio; Dr Moazami with Medtronic, Inc; Dr Galloway with Medtronic, Inc, and Edward Lifesciences.
The Supplemental Tables can be viewed in the online version of this article [https://doi.org/10.1016/j.athoracsur.2020.07.002] on https://www.annalsthoracicsurgery.org.
Coronavirus disease 2019 (COVID-19) results from infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A severe respiratory distress syndrome requiring endotracheal intubation develops in approximately 15% to 20% of hospitalized patients with COVID-19, and the mortality rate in these patients is extremely high (50%-90%).1, 2, 3, 4, 5 The World Health Organization declared COVID-19 a pandemic on March 11, 2020.6 The number of people with this disease has increased exponentially since that time, with more than 2.8 million cases and 190,000 deaths reported worldwide.7
The literature regarding the use of extracorporeal membrane oxygenation (ECMO) support in COVID-19 patients is scarce, and most reports have involved only a small number of patients with poor outcomes. A pooled analysis of 17 patients reported a 94.1% mortality rate,4 and another study of 12 patients reported an early mortality rate of 42%, with 33% of the surviving patients still on ECMO and 25% decannulated but remaining hospitalized.8 On the basis of these data and anecdotal reports from Europe and China,9 there is skepticism among physicians regarding the effectiveness of ECMO use in severe COVID-19.
However, previous reports of the use of ECMO in influenza10 and other typical causes of adult respiratory distress syndrome11 , 12 were more promising. Given the paucity of data on the use of ECMO in COVID-19 and theoretical advantages that it might offer in this population, our institution has continued the judicious use of ECMO support for patients with COVID-19. The hypothesis of this study is that patient and lung recovery with the use of ECMO in severe COVID-19 is achievable and that a nihilistic approach to these patients is unwarranted. This report describes our experience with this approach during the surge phase of COVID-19 in New York City.
Patients and Methods
Patient Population
This is a retrospective analysis of all patients admitted to New York University Langone Health (NYULH) Manhattan campus from March 10, 2020, to April 24, 2020, with COVID-19 and severe respiratory failure requiring mechanical ventilation who were evaluated for ECMO support. COVID-19 was diagnosed by nasal pharyngeal swab for reverse transcriptase- polymerase chain reaction (RT-PCR) assay in all patients. The NYULH Institutional Review Board approved this human subjects study (IRB #: i20-00611), and data were collected from direct medical record review.
Patient Selection
A multidisciplinary team consisting of a cardiothoracic surgeon, critical care physician, and pulmonologist evaluated all patients referred for ECMO. Entry and inclusion criteria were based on the arterial partial pressure of oxygen (Pao 2)–to–fraction of inspired oxygen (Fio 2) ratio (P/F ratio), arterial blood gas, ventilator settings, and patient functional status, comorbidities, and hemodynamic status. ECMO support was only offered to patients with a P/F ratio of less than 150 mm Hg or a pH of less than 7.25 with a partial pressure of arterial carbon dioxide (Paco 2) exceeding 60 mm Hg. Patients undergoing active cardiopulmonary resuscitation were not considered candidates for ECMO support. Additionally, patients with confirmed neurologic injury, known malignancy with poor prognosis, multisystem organ failure, with the exception of acute kidney injury during the current hospitalization, and age older than 65 years were not deemed appropriate ECMO candidates. All patients deemed appropriate for further support underwent venovenous (VV)-ECMO as the initial cannulation strategy, regardless of hemodynamic status or vasopressor requirement.
ECMO Cannulation
All patients placed on VV-ECMO were cannulated in the intensive care unit (ICU) at the bedside. The primary cannulation strategy was through a percutaneous right femoral venous drainage cannula and right internal jugular (IJ) venous return cannula. Only if this access was not attainable was alternate access pursued. All personnel wore full personal protection equipment (PPE) per institutional policies (hair cover, N95 mask, face shield, gown, and 2 layers of gloves) upon entering the room. Two cardiothoracic surgeons with extensive experience in ECMO were present for each cannulation, with 1 cannulating the neck and 1 cannulating the femoral vein concurrently to minimize overall procedure and exposure time. Ultrasound guidance was used for all cannulation access.
Management of ECMO Patients
Ventilation/oxygenation
All patients were managed with pressure control ventilation with a peak inspiratory pressure (PIP) of less than 25 mm Hg, positive end-expiratory pressure (PEEP) of 10 to 14 mm Hg, respiratory rate of 16 breaths/min or less, and Fio 2 of 0.40 or less. Ventilator support was not increased beyond these thresholds for persistent hypoxia, which was tolerated if no evidence of organ injury was present. ECMO circuit flow was titrated to oxygenation needs but did not exceed revolutions per minute thresholds for possible hemolysis. In cases where persistent hypoxia could not be corrected by circuit flow, red blood cell transfusion thresholds were modified to achieve adequate tissue perfusion.13 Conversely, flow was maintained above 3 L/min to avoid oxygenator thrombus formation. Oxygenator Fio 2 was maintained at 1.0 for the entirety of support. Paco 2 management was controlled using the ECMO circuit by varying the sweep gas flow rate for a goal Paco 2 of less than 45 mm Hg, and mechanical ventilation was not altered. When the sweep gas flow rate was less than 0.5 L/min, the gas flow was disconnected for 2 hours, with a repeat arterial blood gas. Patients with a Paco 2 of less than 45 mm Hg and a P/F ratio exceeding 200 on 2 sequential clamp trials greater than 24 hours apart were deemed appropriate for ECMO decannulation. This was performed at bedside in all cases.
Tracheostomy/airway management
With the goal of decreasing sedation requirements and improving pulmonary toilet, early tracheostomy was planned within 3 days of ECMO cannulation. Bronchoalveolar lavage (BAL) was performed on every patient shortly after cannulation to identify potential coinfection that would alter treatment and to clear secretions. Subsequent toilet bronchoscopies were performed as clinically indicated.
Anticoagulation
Therapeutic anticoagulation with an intravenous heparin infusion was initiated on every patient with a goal anti-factor Xa level exceeding 0.15 IU/mL and a partial thromboplastin time of less than 70 seconds based on our previously published data.14 If thrombocytopenia developed, the patient was evaluated for heparin-induced thrombocytopenia (HIT) and transitioned to a therapeutic intravenous bivalirudin infusion with a goal partial thromboplastin time of 40 to 60 seconds. The heparin-coated systems in patients who were HIT-positive were exchanged for nonheparin-coated systems.
Sedation/paralytics
In the early part of the series, patients were sedated to achieve a Richmond agitation-sedation scale score of −4 to prevent dyssynchrony with mechanical ventilation, and many patients required pharmacologic paralysis. As the series progressed, more aggressive paralytic and sedation weans were initiated after tracheostomy placement. After the initial 9 cases, pharmacologic paralysis was terminated upon the initiation of ECMO support for all subsequent patients.
Prone positioning
Although initial patients on ECMO were not routinely placed prone, care strategy evolved to selectively prone patients as clinically indicated based on oxygenation and imaging. Patients were manually proned with a team consisting of an anesthesiologists, a perfusionist, ICU nurses, and a respiratory therapist. No proning beds were used.
COVID-19 Targeted therapy
On the basis of an interim institutional guidance protocol, all patients received azithromycin/hydroxychloroquine therapy for 5 days. No specific COVID-19 therapies were added after cannulation, but they were continued if previously planned (Supplemental Table 1). However, selective use of moderate-dose steroids was added for patients with persistent or uptrending inflammatory clinical markers in the absence of obvious coinfection.
Outcomes
The primary outcome for this study was survival and lung recovery as defined by weaning off ECMO, mechanical ventilation, and supplemental oxygen. Secondary outcomes were freedom from ECMO-associated complications and COVID-19 infection among health care providers from patient transmission.
Results
Patients Evaluated for ECMO
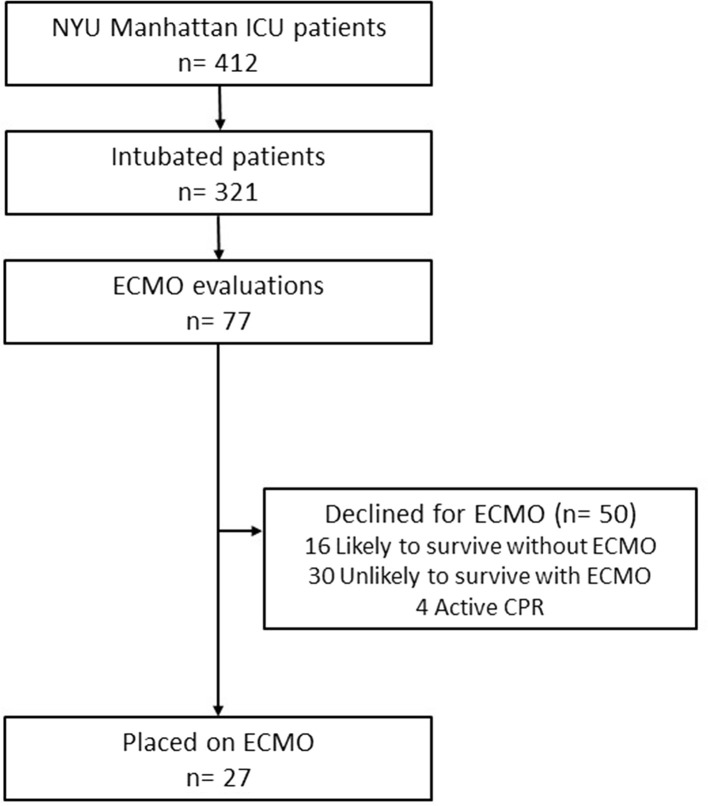
From March 10, 2020, to April 24, 2020, more than 1900 confirmed COVID-19 patients were admitted to NYULH Manhattan campus. During that interval, 412 patients were admitted to the ICU, 321 patients required endotracheal intubation, and 77 patients were evaluated for ECMO. Of these, 27 patients met our patient selection criteria, and 50 patients were deemed not to be appropriate candidates for ECMO support. Four patients were declined because they were undergoing active cardiopulmonary resuscitation (Figure 1 ).
Figure 1.
Flowchart for extracorporeal membranous oxygenation (ECMO) evaluations and outcomes of patients with coronavirus disease 2019 (COVID-19). (CPR, cardiopulmonary resuscitation; ICU, intensive care unit; NYU, New York University.)
Characteristics of Patients Placed on ECMO
The patients were a median age of 40 years (interquartile range [IQR], 30.5-47 years). All patients had a body mass index (BMI) of 25 kg/m2 or higher (median, 32 kg/m2; IQR, 29-37 kg/m2). No patients had a history of stroke or neurologic impairment, and all were ambulatory with normal activities of daily living before their current admission for COVID-19. Eighteen patients (67%) had no major comorbidity other than obesity.
The median PIP was 31 mm Hg (IQR, 28-35 mm Hg), PEEP was 14 mm Hg (IQR, 12-16 mm Hg), and Fio 2 was 0.90 (IQR, 0.75-1.00). The median P/F ratio was 84 mm Hg (IQR, 70-118 mm Hg). The pH in 15 patients (56%) was less than 7.25, with a Paco 2 exceeding 60 mm Hg. All patients were intubated for 7 days or less (median, 2; IQR, 1-4 days) before cannulation. The median Murray score was 3.5 (IQR, 3.4-3.8). At the time of cannulation 11 patients (41%) were hemodynamically unstable and required inotropic agents or vasopressors (Table 1 , Supplemental Table 2).
Table 1.
Characteristics of All Patients Cannulated for Extracorporeal Membrane Oxygenation
| Variable | All ECMO Patients (N = 27) | Recovereda (n = 11) |
|---|---|---|
| Sex | ||
| Male | 23 (85) | 10 (91) |
| Female | 4 (15) | 1 (9) |
| Age, y | 40 (30.5-47) | 37 (28.5-44) |
| Body mass index, kg/m2 | 32 (29-37) | 33 (30.45-37) |
| Comorbidities | ||
| Active malignancy | 0 (0) | 0 (0) |
| Asthma/chronic obstructive pulmonary disease | 2 (7) | 2 (18) |
| Long-term steroid use | 2 (7) | 0 (0) |
| Coronary artery disease | 1 (4) | 0 (0) |
| Diabetes | 4 (15) | 0 (0) |
| End-stage renal disease on dialysis | 0 (0) | 0 (0) |
| HIV/AIDS | 2 (7) | 1 (9) |
| Hypertension | 5 (19) | 1 (9) |
| Known pulmonary embolism or deep venous thrombosis | 3 (11) | 1 (9) |
| Stroke | 0 (0) | 0 (0) |
| Total patients with any comorbidity | 9 (33) | 3 (27) |
| Creatinine level, mg/dL | ||
| On admission | 0.97 (0.82-1.09) | 0.94 (0.83-1.02) |
| At evaluation | 0.83 (0.73-1.56) | 0.83 (0.78-1.06) |
| Acute kidney injury requiring renal replacement | 1 (4) | 0 (0) |
| Ventilator settings before ECMO | ||
| Peak inspiratory pressure, mm Hg | 31 (28-35) | 30 (28-38) |
| Positive end-expiratory pressure, mm Hg | 14 (12-16) | 15 (12-17) |
| Respiratory rate, breaths/min | 25 (22-28) | 24 (21-26) |
| Fio2, % | 90 (75-100) | 90 (75-100) |
| Arterial blood gas values | ||
| pH | 7.28 (7.22-7.39) | 7.28 (7.26-7.41) |
| Pao2, mm Hg | 74 (63-87.5) | 73 (65-85) |
| Paco2, mm Hg | 63 (44-80) | 46 (43.5-68.5) |
| Pao2/Fio2 ratio, mm Hg | 84 (70-118) | 88 (78-114) |
| Murray score | 3.5 (3.4-3.8) | 3.5 (3.5-3.8) |
| Paralytic use | 26 (96) | 10 (91) |
| Proning | 22 (82) | 7 (64) |
| Inhaled nitric oxide use | 7 (26) | 2 (18) |
| Vasopressor/inotrope requirement | 11 (41) | 3 (27) |
| Days on ventilator before cannulation | 2 (1-4) | 3 (1-4) |
Categoric data are presented as n (%) and continuous data as the median (interquartile range).
ECMO, extracorporeal membrane oxygenation; Fio2, fraction of inspired oxygen; Paco2, partial pressure of arterial carbon dioxide; Pao2, partial pressure of arterial oxygen.
Recovered: Alive, discharged, or awaiting discharge; weaned off ECMO support, mechanical ventilation, and supplemental oxygen.
ECMO Cannulation Variables
All patients were placed on VV-ECMO and were cannulated at the bedside in the ICU without fluoroscopic equipment. Ultrasound was used for access of the femoral vein and right IJ vein in all cases. Right IJ and right femoral venous cannulation was achieved in 25 patients (93%). One patient with a history of hemodialysis and kidney transplant had an occluded right IJ vein and required placement of the return cannula in the contralateral femoral vein. In another patient, we were unable to confirm appropriate wire position from the right femoral vein, so the drainage cannula was placed through the left femoral vein (Supplemental Table 3). No vascular injuries or major bleeding complications related to cannulation occurred. Mean total in-room time for cannulation was 21 ± 5 minutes.
Patient Outcomes
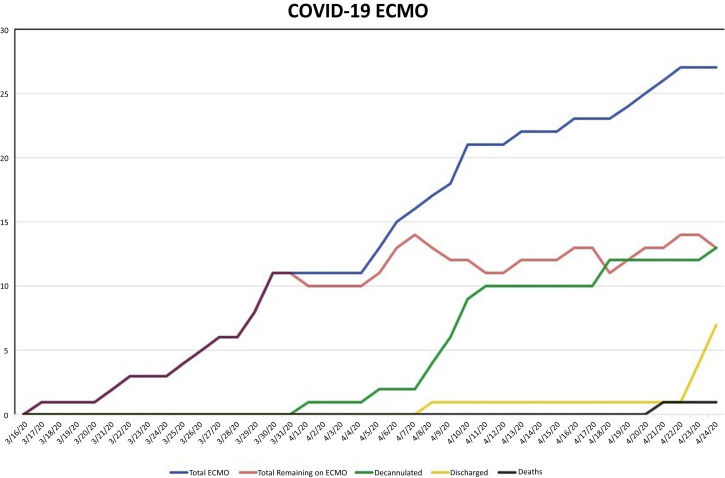
To date, the primary outcome was observed in 11 patients (41%) who have been weaned from ECMO support, mechanical ventilation, and supplemental oxygen. Two additional patients have been weaned and decannulated from ECMO support but remain on mechanical ventilation with modest settings. Seven patients (26%) have been discharged from the hospital (Figure 2 ). Survival after decannulation is 100% to date. Thirteen patients (48%) remain on ECMO support. One patient (4%) died on ECMO support. This patient had shown significant lung recovery and was approaching decannulation, but acutely suffered a pulseless electrical activity arrest from an unknown cause. The median time on ECMO for all patients was 11 days (IQR, 10-14 days), as of April 24, 2020, with a median time of 11 days (IQR 10-14 days) on ECMO for all patients who were decannulated (Table 2 , Supplemental Table 4).
Figure 2.
Longitudinal extracorporeal membrane oxygenation (ECMO) use and outcomes for coronavirus disease 2019 (COVID-19). Graph demonstrates the total number of patients requiring ECMO (blue), the number of patients remaining on ECMO (red), the total number of decannulated patients (green), the total number of discharged patients (yellow), and the total number of deaths (black) illustrated by date.
Table 2.
Outcomes of Patients Supported With Extracorporeal Membrane Oxygenation
| Variable | All ECMO Patients (N = 27) | Recovereda (n = 11) |
|---|---|---|
| Duration of ECMO, d | 11 (10-14) | 11 (10-14) |
| Days from ECMO to tracheostomy | 1 (1-2.75) | 2 (1-4) |
| Duration of mechanical ventilation post-ECMO decannulation, d | N/A | 6 (5-9.5) |
| Hospital length of stay, d | 23 (19.5-29) | 29 (25.5-35.5) |
| Positive bronchoalveolar lavage | 17 (63) | 5 (46) |
| Candida | 8 (30) | 2 (18) |
| Enterobacter | 2 (7) | 1 (9) |
| Escherichia coli | 3 (11) | 0 (0) |
| Klebsiella | 2 (7) | 1 (9) |
| Staphylococcus aureus | ||
| Methicillin sensitive | 1 (4) | 0 (0) |
| Methicillin resistant | 4 (15) | 2 (19) |
| Serratia | 2 (7) | 0 (0) |
| Other | 2 (7) | 0 (0) |
| Complications | ||
| Acute renal failure requiring renal replacement | 2 (7) | 1 (9) |
| Bleeding requiring intervention | 1 (4) | 0 (0) |
| Cannula repositioning | 1 (4) | 0 (0) |
| Myocardial infarction | 0 (0) | 0 (0) |
| New pulmonary embolism | 0 (0) | 0 (0) |
| New deep venous thrombosis | 5 (19) | 4 (36) |
| Pneumothorax requiring treatment | 4 (15) | 0 (0) |
| Stroke | 0 (0) | 0 (0) |
| Deaths | 1 (3.7) | N/A |
Continuous data are presented as the median (interquartile range) and categorical data as n (%).
ECMO, extracorporal membrane oxygenation; N/A, not applicable.
Recovered: Alive, discharged or awaiting discharge; weaned off ECMO support, mechanical ventilation and supplemental oxygen
ECMO-associated complications occurred in 11 patients (41%). Acute renal failure developed in 2 patients (7%), who required a new need for renal replacement therapy. Thrombocytopenia developed in 5 patients (19%), which were positive for HIT by platelet factor 4 antibody. However, only 2 patients had HIT confirmed by a serotonin release assay. All were transitioned to bivalirudin for systemic anticoagulation. One patient, who had a left femoral venous drainage cannula and right IJ venous return cannula, required repositioning of the drainage cannula to the right femoral vein in a hybrid operating room. Pneumothoracies requiring chest tube placement developed in 4 patients (15%) at some point during their ECMO support; none were related to cannulation. These pneumothoracies developed 3 to 24 days after cannulation. Clinically significant bleeding requiring a surgical intervention, which was secondary to tracheostomy site bleeding, developed in only 1 patient (4%). In the 24 patients who presented without a history of deep venous thrombosis or pulmonary embolism, a deep venous thrombosis developed in 5 patients (19%). Five patients (19%) required a circuit exchange, equivalent to 1.4 changes per 100 ∙ ECMO ∙ days. No myocardial infarcts or neurologic complications were noted. No patients required conversion to venoarterial ECMO.
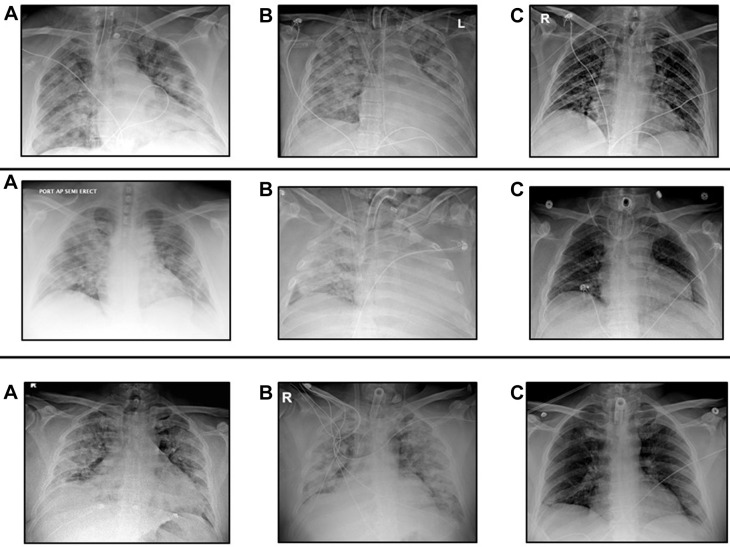
All patients underwent an early tracheostomy, with a median of 1 day (IQR,1-2.75 days) from ECMO initiation to tracheostomy placement. One patient, with a BMI exceeding 50 kg/m2, required an open tracheostomy. One patient had a previous neck operation, and required a tracheostomy revision in the operating room, which represents the bleeding complication noted previously. All patients underwent bronchoscopy with BAL after cannulation. BAL results in 16 patients (59%) were positive BAL, demonstrating superinfection with a bacterial or fungal pneumonia. All patients with a positive BAL were administered broad-spectrum antibiotic coverage and then transitioned to targeted therapy once sensitivities were obtained. Nine patients (33%) were proned during ECMO support. All patients decannulated from ECMO demonstrated near resolution of pulmonary infiltrates that were present at time of cannulation (Figure 3 ).
Figure 3.
Representative chest roentgenogram progression in 3 patients. Serial chest roentgenograms from patients placed on extracorporeal membrane oxygenation (ECMO) support: (A) before ECMO support, (B) while on ECMO support, and (C) predischarge.
Health Care Provider Exposure
The ECMO team included 4 cannulating physicians and 16 perfusionists who participated across the 27 bedside cannulations. To date, no symptoms of fever, general malaise, cough, or shortness of breath have developed in any of these 20 team members, nor have they tested positive for COVID-19. Among these team members, 17 (85%) agreed to testing, and all have been negative by nasal pharyngeal swab for RT-PCR assay.
Comment
While COVID-19 spreads across the globe, health care systems continue to be overwhelmed with critically ill patients who require ICU care and mechanical ventilation. Many of these intubated patients will have progression of the disease that leads to a fatal outcome. In a recent report of 5700 patients hospitalized with COVID-19 from another New York City Health System, the mortality rate of patients who required intubation was 88.1%.5 However, for a select portion of these patients who remain critically ill despite optimal medical therapy, ECMO support appears to have a valuable role in preventing death.
Our experience differs from other published data which suggested that ECMO is of limited value for patients with COVID-19. Although still early in many of these patients’ clinical courses, these initial outcomes are encouraging, with an overall current survival of 96%, with nearly half of the patients already weaned from ECMO support, mechanical ventilation, and supplemental oxygen. Furthermore, a significant number of these patients have been discharged from the hospital.
The reason for the increased early survival observed in this study is likely multifactorial. An established ECMO program was already in place with the infrastructure and expertise to care for patients with complex disease. Our screening process for the use of ECMO involves a team of physicians from multiple subspecialties as well as specific selection criteria similar to those outlined by the Extracorporeal Life Support Organization recommendations for ECMO use in patients with COVID-19.15 Only patients with a previously normal functional status and no preexisting life-threatening medical comorbidities were placed on ECMO. After initiation of ECMO, our specialized multidisciplinary team of cardiothoracic surgeons, intensivists, and pulmonologists managed the care of these patients.
All patients received the same treatments as patients with traditional causes of adult respiratory distress syndrome, and this was the overarching theme of their care. Protective lung ventilation strategies with low inspiratory pressures and low Fio 2 were used for every patient to prevent ventilator-associated lung injury. In addition, patients who would benefit from proning due to continued hypoxemia on full ECMO support were manually proned.
Tracheostomies were performed early in all patients using a novel percutaneous technique that minimized aerosolization,16 with a potentially decreased risk of viral transmission to health care providers. The routine use of tracheostomy allowed for better airway secretion management and ultimate ventilator weaning after ECMO decannulation. Early tracheostomies also facilitated faster weaning of sedation and paralytics, which allowed some patients to participate with physical therapy and sit in a chair while on ECMO.
In addition, toilet bronchoscopies were performed commonly in these patients as clinically indicated. The addition of bronchoscopies and BAL helped diagnose the presence of concurrent infection. The global rate of superimposed bacterial and fungal pneumonia in COVID-19 patients is unknown, because BAL is not routinely performed due to fear of increasing the risk of viral transmission to health care workers. To mitigate this concern, the ventilator was placed on standby to reduce the risk of aerosolization during bronchoscopies. The combination of these unique strategies likely contributed to our promising early outcomes.
Another important finding is that our technique appears to be safe for our health care providers. All providers who participated in the cannulation of ECMO patients remain negative for COVID-19 based on symptoms or testing. This is likely the result of a combination of the full PPE used by all health care providers and an ECMO cannulation strategy designed to minimize the amount of time spent in the room for the providers. All ICU rooms at our institution are also single-bed negative-pressure rooms. Of note, those providers who performed tracheostomies, bronchoscopies, and BAL also all remain COVID-19 negative.16
This report describes a large United States, single-institution experience with ECMO support for COVID-19 patients. The early outcomes are encouraging, with an overall 96% survival, and 41% of patients have demonstrated lung recovery to date. This report describes the patient selection criteria and management strategies that likely contributed to these outcomes.
The major limitation of this study is that it only fully evaluates early outcomes, because 48% of patients remain on ECMO support. However, the patients who remain on ECMO support generally represent a more recent cohort that is following a similar trajectory to those who have already demonstrated lung recovery. Moreover, the length of time on ECMO support for our cohort exceeds the length of time on support for other publications that describe a dramatically higher mortality. Further follow-up will determine the overall 30-day and 90-day mortality for these patients and their ultimate degree of lung recovery and functional status. Although limited by its early outcomes, this report demonstrates the life-saving capability of ECMO support in appropriately selected patients with severe COVID-19.
Acknowledgments
The authors wish to acknowledge Bridget Toy, BSN, RN, Kimberly Sureau, NP, Brigitte Sullivan, MBA, Daniel H. Sterman, MD, and the New York University Langone Health perfusionist team for their contribution to the care of these patients.
Supplementary Data
References
- 1.Intensive Care National Audit & Research Centre ICNARC Report on COVID-19 in Critical Care. April 2020. https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports Available at:
- 2.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUS of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry B.M., Lippi G. Letter to the Editor. Poor survival in extracorporeal membranous oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care. 2020;58:27–28. doi: 10.1016/j.jcrc.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Coronavirus disease 2019 (COVID-19). Situation Report - 51. March 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10 Available at:
- 7.Johns Hopkins University & Medicine Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. April 2020. https://coronavirus.jhu.edu/map.html Available at:
- 8.Zeng Y., Cai Z., Xianyu Y., Yang B.X., Song T., Yan Q. Prognosis when using extracorporeal membrane oxygenation (ECMO) for critically ill COVID-19 patients in China: a retrospective case series. Crit Care. 2020;24:148. doi: 10.1186/s13054-020-2840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs J.P., Stammers A.H., Louis J., St., et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in COVID-19: experience with 32 patients. ASAIO J. 2020;66:722–730. doi: 10.1097/MAT.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 11.Spinelli E., Bartlett R.H. Relationship between hemoglobin concentration and extracorporeal blood flow as determinants of oxygen delivery during venovenous extracorporeal membrane oxygenation: a mathematical model. ASAIO J. 2014;60:688–693. doi: 10.1097/MAT.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 12.Peek G.J., Mugford M., Tiruvoipati R., et al. Efficacy and economic assessment of conventional ventilator support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicenter randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 13.Combes A., Hajage D., Capellier G., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 14.Arnouk S., Altshuler D., Lewis T.C., et al. Evaluation of anti-Xa and activated partial thromboplastin time monitoring of heparin in adult patients receiving extracorporeal membrane oxygenation support. ASAIO J. 2020;66:300–306. doi: 10.1097/MAT.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 15.Extracorporeal Life Support Organization Extracorporeal Life Support Organization COVID-19 Interim Guidelines: A consensus document from an international group of interdisciplinary ECMO providers. April 2020. https://www.elso.org/Portals/0/Files/pdf/ELSO%20covid%20guidelines%20final.pdf Available at: [DOI] [PMC free article] [PubMed]
- 16.Angel L., Kon Z.N., Chang S.H., et al. Novel percutaneous tracheostomy for critically ill patients with COVID-19. Ann Thorac Surg. 2020;110:1006–1011. doi: 10.1016/j.athoracsur.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.