Abstract
Background
High-grade serous ovarian carcinoma (HGSOC) is characterized by TP53 mutations, DNA repair defects, and genomic instability. We hypothesized that prexasertib, a cell cycle checkpoint kinase 1 and 2 inhibitor, would be active in BRCA wild-type HGSOC.
Methods
In this open label, single centre, two-stage proof-of-concept phase 2 study, women aged 18 years or older with measurable, recurrent high-grade serous or high-grade endometrioid ovarian carcinoma were enrolled. All patients must have had either a negative family history of hereditary breast and ovarian cancer or known BRCA wild-type for BRCA wild-type cohort. Other key eligibility criteria were an Eastern Cooperative Oncology Group performance status of 0 or 1 or 2, and adequate haematological, renal, and hepatic function. Patients received intravenous prexasertib 105mg/m2 once every 2 weeks until disease progression, unacceptable toxicity or patient withdrawal of consent. The primary endpoint was investigator-assessed tumour response per protocol based on Response Evaluation Criteria in Solid Tumors, version 1·1 in evaluable patients. The final analysis of this cohort is reported here. This ongoing trial is registered with ClinicalTrials.gov (NCT02203513) and enrolling the patients of BRCA mutation cohort.
Findings
Between January 2015 and November 2016, 28 women (median age 64-year-old [IQR 58–69·5], with median 5 prior systemic therapies [IQR 2·5–5]) were enrolled and received at least one dose of prexasertib. Eight of 24 evaluable patients had a partial response (PR; 33%, 95% CI: 16–55) and 50% had a GCIG CA125 response. The RR in the intention-to-treat population was 29% (8/28, 95% CI: 13–49). The common (>10%) grade 3 or 4 treatment-emergent adverse events were neutropenia (26 [93%] patients), thrombocytopenia (seven [25%] patients), and anaemia (three [11%] patients). Grade 4 neutropenia occurred in 22 (79%) patients after the first dose and was transient ≤ 7 days (median 6 days [IQR 4–8]) without growth factor support; the incidence of febrile neutropenia was 7% (2/28).
Interpretation
We demonstrate clinical activity of prexasertib in BRCA wild-type HGSOC, especially patients with platinum-resistant or refractory ovarian cancer. These results warrant further development for this unmet patient need.
Funding
Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, USA.
Keywords: Prexasertib, cell cycle checkpoint kinase inhibitor, high-grade serous ovarian cancer, BRCA wild-type, CCNE1 amplification and/or overexpression
INTRODUCTION
High-grade serous ovarian carcinoma (HGSOC) is the most lethal gynecologic malignancy in the United States.1 The majority of patients with HGSOC experience relapse at some point in time despite responses to initial cytoreductive surgery and platinum-based chemotherapy, then ultimately develop platinum resistance.2 The prognosis for these patients remains poor and novel therapeutic strategies are needed.2
HGSOC is characterized by a high frequency of TP53 mutations, which disrupt control over the G1/S checkpoint, leaving cells reliant on cell cycle checkpoint-mediated G2/M arrest for DNA repair.3 Cell cycle checkpoint kinases 1 and 2 (CHK1 and CHK2) are crucial components of DNA damage response pathways and are activated by ATR and ATM in response to DNA replication stress or DNA damage.4 CHK1 phosphorylates and inhibits its substrates, phosphatases CDC25C and CDC25A, leading to arrest at the G2/M checkpoint.5,6 Cell cycle arrest is required to allow repair of DNA damage and to address stalled replication forks; collapse into double stranded DNA breaks occurs in the absence of stabilization of stalled replication forks.7 Therefore, CHK1 and CHK2 are reasonable targets to drive tumor cell death in HGSOC.8
Prexasertib mesylate monohydrate (prexasertib; LY2606368) is a selective ATP competitive small molecule inhibitor of CHK1 and CHK2.9 It blocks autophosphorylation and subsequent activation of the CHK proteins, which regulate the activity of CDC25 phosphatases and cyclin-dependent kinases.9 Single agent prexasertib treatment induced DNA damage and apoptosis in preclinical studies10, and potential anticancer activity was observed in patients with solid tumors in phase 1 clinical trial.11 We conducted a proof-of-concept phase 2 single arm study of prexasertib in two cohorts to separately examine the role of replication stress and DNA homologous recombination (HR) repair dysfunction in the background of deleterious germline BRCA mutation-associated HGSOC and in BRCA wild-type HGSOC. In this study, we report the activity of prexasertib monotherapy in recurrent HGSOC patients without germline BRCA mutation.
METHODS
Study design and participants
This study was designed as a signal-seeking study with three independent cohorts, triple negative breast cancer, germline BRCA-mutated and BRCA wild-type ovarian cancer. This report describes the BRCA wild-type ovarian cancer cohort. Eligible patients were age ≥ 18 years and had recurrent sporadic high-grade serous or high-grade endometrioid ovarian carcinoma, either absence of deleterious germline BRCA mutation upon testing or a negative family history of hereditary breast ovarian cancer syndrome (appendix p 5). Other histologic types of ovarian cancer were not eligible. Patients must have had measurable disease by Response Evaluation Criteria In Solid Tumors (RECIST) v1·1 and disease amenable to safe percutaneous biopsy (appendix p 1). There were no restrictions on the number of prior treatment regimens. Other inclusion criteria included radiological progression after one or more lines of therapy, an Eastern Cooperative Oncology Group performance status 0–2, and adequate organ and marrow function, defined as hemoglobin ≥ 100 g/L, absolute neutrophil count ≥ 1·5 ×109 per L, platelet count ≥ 100 ×109 per L, total bilirubin ≤ 1·5 × the upper limit of normal (ULN), alanine aminotransferase and aspartate aminotransferase ≤ 3 × ULN, and serum creatinine ≤ 1·5 × ULN or measured glomerular filtration rate ≥ 45 mL/min per 1·73 m2 (appendix p 5). Study exclusion criteria included concurrent anticancer therapy or any investigational anticancer therapy ≤ 4 weeks before first doses of prexasertib, prior prexasertib or other cell cycle checkpoint kinase inhibitors and central nervous system metastases within 1 year of enrollment (appendix p 1). All patients provided written informed consent before enrollment. The trial was approved by the Institutional Review Board of the Center for Cancer Research, National Cancer Institute, USA.
Procedures
Eligible patients received intravenous prexasertib monotherapy at 105 mg/m2 every two weeks in 4-week cycles. Blood counts were repeated on cycle 1 day 8 to check absolute neutrophil count nadir. 11 Laboratory assessments (including haematology, fasting serum chemistry, and urinalysis) and electrocardiogram were done within 24 hours before each study drug administration during cycle 1 then every 4-week cycle. Clinical response was assessed by the investigator every two cycles by computed tomography (CT) imaging using RECISTv1·1 criteria. Serum CA125 response was investigated every cycle as a post-hoc exploratory end point and was defined as a 50% reduction during treatment with confirmation after 4 weeks according to GCIG criteria.12 Patients were evaluated for toxicity per Common Terminology Criteria for Adverse Events version (CTCAE) v4·0. Events of temporary (≤ 7 days) neutropenia without fever (grade 3 or 4) did not require dose reduction or discontinuation of treatment. Grade 3 or 4 thrombocytopenia > 7 days or any thrombocytopenia requiring platelet transfusion for bleeding resulted in dose reduction to 80 mg/m2 every two weeks. Study treatment was discontinued for progression of disease, intercurrent illness, adverse events not recovering to ≤ grade 1 within a 3-week period, or patient withdrawal of consent (appendix p 1).
For correlative studies, we collected pretreatment fresh frozen core biopsies and paired blood samples (at baseline and on cycle 1 day 15; appendix p 1). Mutations in DNA repair genes were identified by targeted sequencing of tumor DNA using BROCA-HR (appendix p 1).13 CNV analysis for CCNE1 was performed using three TaqMan® minor groove binder (MGB) probes (appendix p 1).14,15 CNV analysis for CCND1 was performed with three MGB probes (appendix p 1).15 RNA sequencing was performed using a HiSeq3000 sequencing system (Illumina, San Diego, CA, USA) at the Center for Cancer Research sequencing facility, National Cancer Institute (appendix p 2). RNA-Seq data on normal ovary tissues were obtained from the Genotype-Tissue Expression project (GTEx project, NIH) on 08/29/2017 (appendix p 2). Immunohistochemistry was used to examine expression of CCNE1 (anti-CCNE1, 1:100 dilution; Abcam, Cambridge, MA, USA) using standard procedures (appendix p 2). Epithelial cell adhesion molecule (EpCAM)-positive CTCs were detected using magnetic pre-enrichment and multiparameter flow cytometry (appendix p 2) as described.16
Outcomes
The primary objective was response rate (RR) by RECISTv1·1 per protocol in the evaluable patients who had undergone CT imaging at baseline and at least one protocol-specified follow-up timepoint. RR for the intention-to-treat population was also reported. Secondary objectives included safety and toxicity evaluation, graded according to the CTCAEv4·0, and progression-free survival (PFS; defined as time from on-study date until the first documented disease progression according to RECIST or death resulting from any cause). Prespecified exploratory objectives were to investigate potential predictive biomarkers (appendix p 1).
Statistical analysis
The study was conducted using Simon’s optimal two-stage phase 2 design to rule out a 5% RR in favor of a 25% RR, with α=0·10 and β=0·10 (http://cancer.unc.edu/biostatistics/program/ivanova/SimonsTwoStageDesign.aspx, last accessed on October 24, 2017). These parameters were chosen for this single arm, signal-seeking study in order to minimize the number of women exposed to a potentially inactive agent and to target a sufficiently high RR to support moving into a definitive trial should this trial be positive. The null hypothesis of 5% was selected to accommodate the inclusion of heavily pretreated patients based on previous study findings, e.g. the GOG126-series. A response in 1 of the first 9 patients sufficed to move to the second stage of accrual, adding another 15 patients. The regimen would be considered sufficiently interesting if ≥ 3/24 patients had a complete response or partial response (PR). The probability of early termination was 63·0% under the null hypothesis. PFS was estimated using the Kaplan-Meier method beginning at the on-study date and continuing until progression or death without progression; patients who have not progressed had their follow-up censored at July 1, 2017 for this evaluation. Safety evaluation was based on all enrolled patients. Patients considered non-evaluable had either no post-baseline CT scan or discontinued after less than 8 weeks without documented progression. All statistical tests for correlative studies analysis utilized a two-sided significance level 0·05. This ongoing trial is registered with ClinicalTrials.gov (NCT02203513).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, or reporting of this trial. JML, ECK, JN, JT and ES had full access to the raw data. The corresponding author (JML) had final responsibility for the decision to submit for publication.
RESULTS
Patient characteristics
Between January 20, 2015 and November 2, 2016, 28 women were enrolled and received at least one dose of prexasertib (Figure 1); four patients were receiving treatment at data cutoff (July 1, 2017), at >16·5 months continuous treatment. Table 1 shows baseline patient characteristics. The majority of patient (22/28 [79%]) had platinum-resistant or -refractory ovarian cancer.
Figure 1.
Consort diagram.
Table 1.
Patient characteristics (N=28)
| Age in years, median (IQR) | 64 (48–69·5) |
|
| |
| ECOG Performance Status, N (%) | |
| 0 | 5 (18%) |
| 1 | 22 (79%) |
| 2 | 1 (3%) |
|
| |
| Platinum-sensitive recurrence, N (%) | 6 (21%) |
| Platinum-resistant recurrence (primary/secondary)*, N (%) | 21 (75%; 2/19) |
| Platinum-refractory disease, N (%) | 1 (4%) |
|
| |
| Median number of prior systemic therapy regimens (IQR) | 5 (2·5–5) |
| Median number of prior cytotoxic chemotherapeutic agents (IQR) | 3 (2–4·5) |
|
| |
| Prior cytotoxic chemotherapy, N (%) | 28 (100%) |
| Prior radiotherapy, N (%) | 2 (7%) |
| Prior PARP inhibitor(s), N (%) | 9 (32%)** |
| Prior bevacizumab, N (%) | 13 (46%) |
| Prior immune checkpoint inhibitor or vaccine, N (%) | 7 (25%) |
|
| |
| Baseline CA125 | |
| Normal (1·9–16·3 units/mL), N (%) | 1 (4%) |
| Abnormal (> 16·3 units/mL), N (%) | 27 (96%) |
All but one patient had results on germline BRCA mutation evaluation by commercial testing prior to enrollment. The single patient had negative family history of hereditary breast and ovarian cancer syndrome and her germline BRCA mutation testing by BROCA-HR later was negative.
Patients were categorized as primary platinum-resistant disease (progression < 6 months after completing first-line platinum therapy) or secondary platinum-resistant (progression ≥ 6 months after first-line platinum therapy but progressed < 6 months after second or last platinum-based therapy).
Five were treated with olaparib on one of several NCI olaparib combination trials. Four patients received PARP inhibitors (olaparib, veliparib or rucaparib) in other clinical trial settings.
Abbreviations: ECOG=Eastern Cooperative Oncology Group.
Clinical outcomes
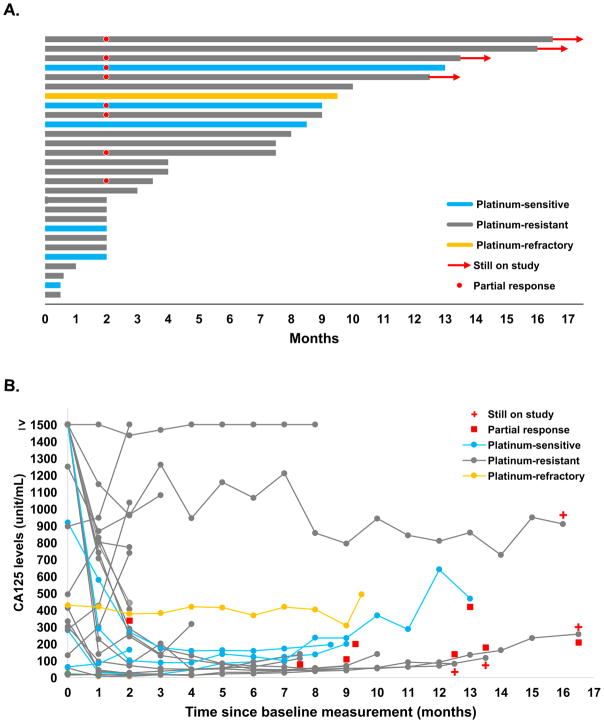
Change from baseline in tumor size and duration on study are shown in Figures 2 and 3. Four of 28 patients were not evaluable for RECIST response. They did not undergo the first restaging scans and were removed from study due to intercurrent illness after the first dose of treatment (one patient), and patient’s withdrawal of consent after 1 or 2 doses due to travel inconvenience (three patients). For all 24 evaluable patients, the median treatment duration was 7·4 months (IQR: 2·1–9·7 months). Eight of 24 evaluable patients attained a PR (33%, 95% CI: 16–55) with a median duration on treatment of 11.4 months (IQR: 8·5 months to undefined; treatment still ongoing at 12·5, 13·5 and 16·5 months), all identified at first tumor reassessment. The RR of the 28 intention-to-treat population was 29% (8/28, 95% CI: 13–49). Eleven of 19 (58%) patients with platinum-resistant or -refractory disease had either PR (6/19 [32%, 95% CI: 13–57]) with a median duration on treatment not reached (IQR: 7·5 months to undefined; treatment still ongoing at 12·5, 13·5 and 16·5 months) or disease stabilization lasting at least 6 months (SD; 5/19 [26%, 95% CI: 9–51]) with a median duration on treatment of 9·5 months (IQR: 8·5–9·8 months). Median potential follow-up was 16·7 months (IQR: 13–26·3 months). The median PFS was 7·4 months (95% CI: 2·1–9·4 months; IQR 2.1 −9.4 months) supplementary Figure S1). For PFS events, 19 had a progression event and one had death on study due to tumour progression. Eleven of 12 patients with a GCIG CA125 response also demonstrated a PR (eight patients) or SD > 6 months (three patients; Figure 2B).
Figure 2. Clinical benefit.
(A) Duration on treatment of 28 enrolled patients. Blue: platinum-sensitive; grey: platinum-resistant; yellow: platinum-refractory disease. The red dot indicates PR and red arrows indicate ongoing treatment at data lock.
(B) Baseline and serial CA125 measurements from 24 evaluable patients. Blue: platinum-sensitive; grey: platinum-resistant; yellow: platinum-refractory disease. Patients with PR by RECIST v1·1 criteria are marked as a red square. Red cross indicates those receiving drug at data lock.
Figure 3. Integrated treatment outcome and mutations in DNA repair genes and CCNE1 amplification or overexpression in pretreatment tumours.
Top: 24 patients with baseline and subsequent imaging reassessment are shown. Best RECIST response is graphed for each patient. Blue: platinum-sensitive; grey: platinum- resistant; yellow: platinum-refractory disease. Red cross indicates those receiving drug at data lock.
Middle: PFS (months), number of prior lines of therapy, and presence of mutations in DNA damage repair genes (black) are listed for each patient.
Bottom: pretreatment CCNE1 copy number variations, mRNA expression, and protein expression are shown for each patient. Tumors are classified by CCNE1 copy number as follows; CCNE1 mean copy number >3: amplification (red) and 2·1–3: copy number gain (yellow). Tumors are considered as CCNE1 mRNA upregulation (pink) if log2 CPM >2 by RNA-Seq. CCNE1 protein expression by IHC is marked as positive (positive or strong positive nuclear staining; black) or negative (grey).
Study ID 48’s core biopsy sample consisted of normal liver tissue with suboptimal quantity of tumor tissue.
Abbreviations: PFS = progression-free survival, CPM = counts per million
Toxicity
All treated patients had at least one any grade treatment-emergent adverse event (TEAE), summarized in Table 2. The most frequently observed toxicity was grade 4 neutropenia (22/28 [79%]); the nadir occurred consistently, approximately 1 week after each dose, and was transient ≤ 7 days (median 6 days [IQR 4–8]). Granulocyte colony-stimulating factor (G-CSF) was administered prophylactically in 79% (22/28) of patients to avoid treatment delays or dose reduction. Two patients (7%) had dose reduction due to recurrent grade 4 neutropenia > 7 days on cycle 4 despite use of filgrastim (one), and recurrent grade 3 anemia on cycle 6 refractory to blood transfusion (one; appendix p 2). Two patients had treatment-related serious adverse events of febrile neutropenia; no deaths or discontinuations occurred as a result of febrile neutropenia. No discontinuation was reported due to TEAEs. The serious adverse events regardless of relationship to study treatment included abdominal pain (four [14%] patients), dehydration (two [7%] patients), urinary tract infection (two [7%] patients), deep vein thrombosis (one [4%] patient), pulmonary embolism (one [4%] patient) and diarrhea associated with E. coli O157 infection (one [4%] patient). One patient experienced seizure-like activity for 45 seconds shortly after optional second research biopsy, approximately 20 hours following prexasertib infusion. Brain MRI showed no anatomical abnormality and no epileptiform activity was observed on electroencephalogram. This event was considered unrelated to the study drug by the investigators, likely associated with conscious sedation, fasting and pain medications during and after biopsy. She tolerated subsequent treatment with prexasertib without events until progression. One death occurred on the study due to tumour progression.
Table 2.
Treatment-related Adverse Events
| Adverse Event | Prexasertib
(N=28) Maximum Grade |
||
|---|---|---|---|
|
| |||
| 1–2 | 3 | 4 | |
|
| |||
| Haematological | |||
|
| |||
| Anaemia* | 23 (82%) | 3 (11%) | - |
| Neutropenia | 1 (4%) | 4 (14%) | 22 (79%)** |
| WBC decreased | 4 (14%) | 14 (50%) | 9 (32%) |
| Platelet count decreased† | 16 (57%) | 4 (14%) | 3 (11%) |
| Febrile neutropenia | - | 2 (7%) | - |
|
| |||
| Non-Haematologic | |||
|
| |||
| Fatigue | 13 (46%) | 2 (7%) | - |
| Fever | 8 (29%) | - | - |
| Allergic reaction | 1 (4%) | - | - |
| Headache | 1 (4%) | - | - |
| Nausea | 18 (64%) | - | - |
| Vomiting | 7 (25%) | 1 (4%) | - |
| Diarrhea | 9 (32%) | 2 (7%) | - |
| Constipation | 3 (11%) | - | - |
| Abdominal pain | 4 (14%) | - | - |
| Anorexia | 4 (14%) | - | - |
| Oral mucositis | 4 (14%) | - | - |
| Dyspepsia | 1 (4%) | - | - |
Data are number of patients (total %). A patient could be counted under more than one preferred term.
9 patients received packed RBC transfusion due to grade 2 anaemia (N=6) and grade 3 anaemia (N=3).
First events of grade 3 and 4 neutropenia were observed from cell counts performed on cycle 1 day 8.
Two patients received platelet transfusion due to bacteremia and grade 3 thrombocytopenia on cycle 1 and due to prolonged grade 4 thrombocytopenia on cycle 1. Patients who were on growth factor support also had transient (< 7 days) grade 3 or 4 thrombocytopenia.
Mutational analysis
Exploratory analysis of the BROCA-HR panel was performed to correlate a potential HR deficiency (HRD) with clinical response to prexasertib (appendix p 3). There was no clear association between clinical response and HRD status (supplementary Table S2).
CCNE1 amplification and/or overexpression
CCNE1 copy number alterations and mRNA expression analyses were performed on pretreatment core biopsy samples from 24 evaluable patients (Figure 3 and supplementary Figure S2A). CCNE1 IHC was performed on the 12 pretreatment tissue samples available (Figure 3). The calculated median log2 CPM value of normal ovarian tissues was −0·035 for CCNE1. 12 of 19 (63%) patients with CCNE1 amplification or CCNE1 mRNA upregulation/protein overexpression had PFS ≥ 6 months on study. Four of 8 PRs (50%) had both CCNE1 amplification or copy number gain and CCNE1 mRNA upregulation. CCNE1 mRNA upregulation without CCNE1 amplification was observed in 58% (14/24) of cases and five patients had both CCNE1 amplification and mRNA upregulation (supplementary Figure S3).
CCND1 amplification
CCND1 copy number alterations analysis was performed on 24 evaluable patients’ baseline tumor samples and there was no association with clinical response (supplementary Figure S2B).
Circulating Tumor Cells
23 of 24 evaluable patients had baseline CTCs and 22 of them had paired CTCs. No associations were observed between baseline and change of CTCs with clinical outcomes (supplementary Figure S4).
DISCUSSION
The findings of this signal-seeking phase 2 study showed that prexasertib monotherapy yielded notable and durable anti-tumour activity in recurrent BRCA wild-type HGSOC patients. We embarked upon a two-step, single arm study targeting a RR of 25% compared to a baseline RR estimated at 5%, recognizing that this population of patients could be heavily pretreated and enriched in women with platinum-resistant disease. The protocol-defined primary objective was met, with 33% of patients achieving a PR. We hypothesized that prexasertib would be active in HGSOC without HR dysfunction; this was in part due to the recognized upregulation/amplification of cyclins E and D.22 Examination of biopsies taken prior to initiation of therapy showed two thirds of women with CCNE1-overexpressing tumours had clinical benefit. These data suggest that prexasertib may be an important new agent for platinum-resistant recurrent ovarian carcinoma and that further studies of CCNE1 expression and treatment outcome are needed.
Platinum resistance is associated with a poor prognosis for women with ovarian carcinoma and almost all patients with recurrent disease ultimately develop platinum resistance.2,17 Although combination chemotherapy with bevacizumab has shown benefit over single-agent chemotherapy, the use of cytotoxic chemotherapy or targeted agent alone has yielded disappointing results, with reported median PFS of 3–4 months and median overall survival of about 12 months in most phase 3 trials.17 In the present study, patients were heavily pretreated, 75% had received ≥ 3 prior regimens. Notably, approximately 60% of patients with platinum-resistant or-refractory disease had clinical benefit from prexasertib, by prolonged SD ≥ 6 months or PR, demonstrating greater than anticipated single agent activity.17 Further examination of this promising agent is warranted.
TP53 mutation is the most well-characterized example of clinical synthetic lethality with cell cycle checkpoint inhibition.18 HGSOC is characterized by a high degree of replication stress that leads to inappropriate replication origin licensing or firing. This results in stalled replication forks and subsequent double stranded DNA breaks.19 CHK1 plays complementary roles in restricting the initiation of replication origins by inhibiting CDK2, which when activated, promotes replication.19 CHK1 inhibitors augment ongoing replication stress by effectively promoting replication origin firing, resulting in more fork stalling and DNA breaks.20 An ongoing challenge is to identify tumours that have reached near-critical levels of replication stress and are likely susceptible to treatment with prexasertib.
CCNE1 is amplified in approximately 20% of primary HGSOCs and associated with chemotherapy resistance.3 Amplified cyclin E is also a known oncogenic driver of unchecked replication, which causes replicative stress and genomic instability.3,21,22 Our post-hoc analysis uncovered most patients with CCNE1 amplification or overexpression had proficient HR. Ovarian tumours with CCNE1 amplification is described to have high levels of HR proficiency as HR may be necessary for the survival of cyclin E-overexpressing cells.21,22 Cyclin E1 is required for activation of CDK2; its overexpression induces DNA damage and replication stress that activates HR repair and may increase sensitivity to CHK1 inhibition.3,23 Our correlative findings should be interpreted with caution as hypothesis-generating given the small number of samples. However, further therapeutic options for cyclin E1-amplified or overexpressed HGSOC is needed.
Earlier clinical trials with CHK1 inhibitors focused on the chemopotentiation potential using combination studies with DNA damaging agents, as no single-agent activity had been observed.20 Although phase 1 trials demonstrated that CHK1 inhibitors could be safely combined with chemotherapy, phase 2 studies failed to meet their primary efficacy endpoints.4 Other cell cycle checkpoint inhibitors, such as those targeting WEE1, ATR, and ATM, are now in clinical investigation with a variety of chemotherapies, and as monotherapies.4 A phase 1 study of AZD1775, WEE-1 inhibitor, monotherapy enrolled 24 patients, nine of whom had BRCA1 or BRCA2 mutation.23 Two of nine BRCA mutated patients (squamous cell cancer of the base of the tongue and HGSOC) attained PR.23 AZD1775 was also tested in combination with chemotherapies in ovarian cancer. AZD1775 and carboplatin demonstrated a median PFS of 5·3 months (2·3–9) in 21 evaluable patients with platinum-refractory or- resistant recurrent, TP53-mutated ovarian carcinoma.24 The selected dose of AZD1775 has been 225 mg twice daily for five total doses per cycle in order to abrogate toxicity seen with continuous dosing, notably neutropenia, thrombocytopenia, and gastrointestinal toxicity.24
There are limited data on safety and anti-tumour activity of cell cycle checkpoint inhibitors.4,20 Our frequency of transient grade 4 neutropenia was similar to the previous phase 1 prexasertib study11 although less frequent dose reduction was required due to prophylactic use of G-CSF. The duration of the neutropenia was brief, and growth factor was able to maintain the biweekly treatment schedule. The degree of dose-responsiveness of clinical activity is unknown. Other CHK inhibitors, AZD7762 (CHK1/CHK2 inhibitor) or MK8776 (CHK1 inhibitor) have been associated with cardiotoxicity, including myocardial infarction and significant QTc changes25,26, which were not observed in our patients, although grade 4 neutropenia was less frequent than prexasertib. The frequency of neutropenia we observed is not unprecedented in this patient population.27 Early studies of paclitaxel in ovarian carcinoma identified neutropenia as the most common dose limiting toxicity.27,28 We demonstrated a higher RR to paclitaxel at a dose of 250 mg/m2, requiring growth factor support due to the frequency of grade 3 and 4 neutropenia.27–29 The doses and schedules used today in ovarian carcinoma were chosen in large part to minimize growth factor supplementation requirements; similar studies may be useful to further understand prexasertib.
Limitations of our study include its small pilot phase 2 trial size, the lack of a comparator arm, and the inability to evaluate 4 patients of the full intention-to-treat population. There were no data on prexasertib in ovarian carcinoma and little data in solid tumours at the time this trial was designed. A pilot study of limited sized cohorts was selected for signal-seeking and preliminary biomarker exploration. Inclusion of a control arm randomization in a subsequent study will allow greater confidence in the RR and PFS estimates for prexasertib. The severity of the neutropenia, 80% grade 4, was countered by its brevity, rare febrile events, and ready response to growth factor support. Similar observations were made during the early development of paclitaxel as dose and schedules were being defined.27,29 There, the marrow toxicity of the standard and dose intense three-weekly schedules has been offset by the lower dose weekly schedule. Further exploration of prexasertib dose and schedule could be considered. Nine of the 28 patients had prior exposure to PARP inhibitor (PARPi) therapy, an unusual frequency for a group of women without BRCA mutation. These patients were referred after participation in an olaparib-containing study within our group30 or other PARPi clinical trials of which many were available and enrolling women without identified BRCA mutation. There are no defined cumulative toxicities of the PARPi class of agents, thus we do not believe that this prior exposure contributed to prexasertib toxicity.
In conclusion, we demonstrated prexasertib monotherapy is tolerable and clinically active in heavily pretreated BRCA wild-type recurrent HGSOC. This activity may be associated with tumour CCNE1 amplification and/or overexpression, requiring prospective validation. An ongoing phase 2 study of prexasertib in BRCA-mutated ovarian carcinoma cohort, accruing now in second stage, will provide insight into the possible clinical synergy of prexasertib in germline BRCA mutation setting. Those results will be reported separately as an independent cohort. The encouraging anti-tumour activity observed in platinum-resistant or -refractory ovarian carcinoma patients warrants further development in a randomized trial that also considers assessment of patient reported outcomes.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We searched PubMed and the databases of the American Society of Clinical Oncology, European Society for Medical Oncology, and the Society of Gynecologic Oncology to identify all studies that included a cell cycle checkpoint kinase inhibitor, focusing on studies that included efficacy and safety as either a primary or secondary endpoint. We searched the studies published between July 1, 2014, and July 1, 2017, that included the search terms “cell cycle checkpoint inhibitor”, “prexasertib”, “LY2606368”, or “PF477736, AZD7762, MK-8776, GDC-0425”, “monotherapy”, “single agent” AND “ovarian cancer”, or “gynecologic cancer”. Our search identified several ongoing studies of single agent of the cell cycle checkpoint kinase inhibitor, most of which are early phase clinical trials without long-term follow-up (> 1 year) for activity and safety data. No studies have reported durable clinical activity and safety of the monotherapy for ovarian cancer patients treated with prexasertib.
Added value of this study
To our knowledge, the present study provides the first prospective phase 2 trial results supporting the cell cycle checkpoint kinases 1 and 2 inhibitor, prexasertib in BRCA wild-type recurrent ovarian carcinoma. Post-hoc analysis in the population of patients with CCNE1 amplified and/or overexpressed tumors suggest potential activity of prexasertib in this population.
Implications of all of the available evidence
Our results support future investigation of prexasertib in diseases with high prevalence of replication stress, particularly in patients with BRCA wild-type recurrent platinum-resistant HGSOC, a clinical unmet need.
Acknowledgments
This research was fully funded by the Intramural Research Program of the NIH, National Cancer Institute (NCI), Center for Cancer Research (CCR; Grant No. ZIA BC011525 [JML]), USA. Prexasertib was supplied to the CCR, NCI under a Cooperative Research and Development Agreement (CRADA) between the CCR/NCI and Eli Lilly. Research funding was provided to CCR by Eli Lilly under a CRADA (JML). Funding was also provided to EMS by Stand Up To Cancer – Ovarian Cancer Research Fund Alliance – National Ovarian Cancer Coalition Dream Team Translational Research Grant (Grant Number: SU2C-AACR-DT16-15). Stand Up to Cancer is a program of the Entertainment Industry Foundation; research grants are administered by the American Association for Cancer Research, a scientific partner of Stand Up To Cancer.
We thank Drs. Bernard W. Parker and Ana Nunes, Nicole Houston RN, Erin Nichols RN, Ms. Mireya Gomez and Mr. Ethan S. Brill for their contributions in clinic. We thank Drs. Bao Tran, Maggie Cam and Xiongfong Chen, for their expertise in generating the RNA sequencing data used in this study.
This article is dedicated to Ms. Jinyoung Choi, who died from breast cancer.
Footnotes
Author contributions
JML and ECK conceived and designed the study. AZ, SL, CMA, LM, IE, JML and ECK enrolled and treated patients and obtained the data. JN, ES, MIH, JT, MJL, MB, MM, DAB, AZ, SL, CMA, LM, SS, JML and ECK contributed to data analysis and interpretation. JML, ECK, DAB, MM, ES, MIH, SS and JN developed the tables and figures. All authors reviewed the report and approved the final version. JML gave final approval of the report.
CONFLICTS OF INTEREST
All authors declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Naumann RW, Coleman RL. Management strategies for recurrent platinum-resistant ovarian cancer. Drugs. 2011;71:1397–412. doi: 10.2165/11591720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. 2015;5:1137–54. doi: 10.1158/2159-8290.CD-15-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin AB, McNeely SC, Beckmann RP. Achieving Precision Death with Cell-Cycle Inhibitors that Target DNA Replication and Repair. Clin Cancer Res. 2017;23:3232–40. doi: 10.1158/1078-0432.CCR-16-0083. [DOI] [PubMed] [Google Scholar]
- 5.Patil M, Pabla N, Dong Z. Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell Mol Life Sci. 2013;70:4009–21. doi: 10.1007/s00018-013-1307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zannini L, Delia D, Buscemi G. CHK2 kinase in the DNA damage response and beyond. J Mol Cell Biol. 2014;6:442–57. doi: 10.1093/jmcb/mju045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–23. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 8.Sakurikar N, Eastman A. Will targeting Chk1 have a role in the future of cancer therapy? J Clin Oncol. 2015;33:1075–7. doi: 10.1200/JCO.2014.60.0767. [DOI] [PubMed] [Google Scholar]
- 9.King C, Diaz HB, McNeely S, et al. LY2606368 Causes Replication Catastrophe and Antitumor Effects through CHK1-Dependent Mechanisms. Mol Cancer Ther. 2015;14:2004–13. doi: 10.1158/1535-7163.MCT-14-1037. [DOI] [PubMed] [Google Scholar]
- 10.Lowery CD, VanWye AB, Dowless M, et al. The Checkpoint Kinase 1 Inhibitor Prexasertib Induces Regression of Preclinical Models of Human Neuroblastoma. Clin Cancer Res. 2017;23:4354–4363. doi: 10.1158/1078-0432.CCR-16-2876. [DOI] [PubMed] [Google Scholar]
- 11.Hong D, Infante J, Janku F, et al. Phase I Study of LY2606368, a Checkpoint Kinase 1 Inhibitor, in Patients With Advanced Cancer. J Clin Oncol. 2016;34:1764–71. doi: 10.1200/JCO.2015.64.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rustin GJ, Vergote I, Eisenhauer E, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1. 1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG) Int J Gynecol Cancer. 2011;21:419–23. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 13.Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;18:75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 14.Pils D, Bachmayr-Heyda A, Auer K, et al. Cyclin E1 (CCNE1) as independent positive prognostic factor in advanced stage serous ovarian cancer patients - a study of the OVCAD consortium. Eur J Cancer. 2014;50:99–110. doi: 10.1016/j.ejca.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Etemadmoghadam D, deFazio A, Beroukhim R, et al. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. Clin Cancer Res. 2009;15:1417–27. doi: 10.1158/1078-0432.CCR-08-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kauffman EC, Lee MJ, Alarcon SV, et al. Lack of Impact of Robotic Assisted Laparoscopic Radical Prostatectomy on Intraoperative Levels of Prostate Cancer Circulating Tumor Cells. J Urol. 2016;195:1136–42. doi: 10.1016/j.juro.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomao F, Marchetti C, Romito A, et al. Overcoming platinum resistance in ovarian cancer treatment: from clinical practice to emerging chemical therapies. Expert Opin Pharmacother. 2017;18:1443–1455. doi: 10.1080/14656566.2017.1328055. [DOI] [PubMed] [Google Scholar]
- 18.Dobbelstein M, Sorensen CS. Exploiting replicative stress to treat cancer. Nat Rev Drug Discov. 2015;14:405–23. doi: 10.1038/nrd4553. [DOI] [PubMed] [Google Scholar]
- 19.Gaillard H, Garcia-Muse T, Aguilera A. Replication stress and cancer. Nat Rev Cancer. 2015;15:276–89. doi: 10.1038/nrc3916. [DOI] [PubMed] [Google Scholar]
- 20.Puigvert JC, Sanjiv K, Helleday T. Targeting DNA repair, DNA metabolism and replication stress as anti-cancer strategies. FEBS J. 2016;283:232–45. doi: 10.1111/febs.13574. [DOI] [PubMed] [Google Scholar]
- 21.Karst AM, Jones PM, Vena N, et al. Cyclin E1 deregulation occurs early in secretory cell transformation to promote formation of fallopian tube-derived high-grade serous ovarian cancers. Cancer Res. 2014;74:1141–52. doi: 10.1158/0008-5472.CAN-13-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patch AM, Christie EL, Etemadmoghadam D, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–94. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 23.Do K, Wilsker D, Ji J, et al. Phase I Study of Single-Agent AZD1775 (MK-1775), a Wee1 Kinase Inhibitor, in Patients With Refractory Solid Tumors. J Clin Oncol. 2015;33:3409–15. doi: 10.1200/JCO.2014.60.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leijen S, van Geel RM, Sonke GS, et al. Phase II Study of WEE1 Inhibitor AZD1775 Plus Carboplatin in Patients With TP53-Mutated Ovarian Cancer Refractory or Resistant to First-Line Therapy Within 3 Months. J Clin Oncol. 2016;34:4354–61. doi: 10.1200/JCO.2016.67.5942. [DOI] [PubMed] [Google Scholar]
- 25.Daud AI, Ashworth MT, Strosberg J, et al. Phase I dose-escalation trial of checkpoint kinase 1 inhibitor MK-8776 as monotherapy and in combination with gemcitabine in patients with advanced solid tumors. J Clin Oncol. 2015;33:1060–6. doi: 10.1200/JCO.2014.57.5027. [DOI] [PubMed] [Google Scholar]
- 26.Sausville E, Lorusso P, Carducci M, et al. Phase I dose-escalation study of AZD7762, a checkpoint kinase inhibitor, in combination with gemcitabine in US patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73:539–49. doi: 10.1007/s00280-014-2380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohn EC, Sarosy G, Bicher A, et al. Dose-intense taxol: high response rate in patients with platinum-resistant recurrent ovarian cancer. J Natl Cancer Inst. 1994;86:18–24. doi: 10.1093/jnci/86.1.18. [DOI] [PubMed] [Google Scholar]
- 28.Link CJ, Jr, Bicher A, Kohn EC, et al. Flexible granulocyte colony-stimulating factor dosing in ovarian cancer patients who receive dose-intense taxol therapy. Blood. 1994;83:1188–92. [PubMed] [Google Scholar]
- 29.Abu-Rustum NR, Aghajanian C, Barakat RR, Fennelly D, Shapiro F, Spriggs D. Salvage weekly paclitaxel in recurrent ovarian cancer. Semin Oncol. 1997;24:S15-62–S15-67. [PubMed] [Google Scholar]
- 30.Lee JM, Peer CJ, Yu M, et al. Sequence-Specific Pharmacokinetic and Pharmacodynamic Phase I/Ib Study of Olaparib Tablets and Carboplatin in Women’s Cancer. Clin Cancer Res. 2017;23:1397–406. doi: 10.1158/1078-0432.CCR-16-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.