Abstract
Objective
This systematic review aimed to synthesize early data on typology and topography of brain abnormalities in adults with COVID-19 in acute/subacute phase.
Methods
We performed systematic literature search via PubMed, Google Scholar and ScienceDirect on articles published between January 1 and July 05, 2020, using the following strategy and key words: ((covid[Title/Abstract]) OR (sars-cov-2[Title/Abstract]) OR (coronavirus[Title/Abstract])) AND (brain[Title/Abstract]). A total of 286 non-duplicate matches were screened for original contributions reporting brain imaging data related to SARS-Cov-2 presentation in adults.
Results
The selection criteria were met by 26 articles (including 21 case reports, and 5 cohort studies). The data analysis in a total of 361 patients revealed that brain abnormalities were noted in 124/361 (34%) reviewed cases. Neurologic symptoms were the primary reason for referral for neuroimaging across the studies. Modalities included CT (-angiogram, -perfusion, -venogram), EEG, MRI (-angiogram, functional), and PET. The most frequently reported brain abnormalities were brain white matter (WM) hyperintensities on MRI 66/124 (53% affected cases) and hypodensities on CT (additional 23% affected cases), followed by microhemorrhages, hemorrhages and infarcts, while other types were found in <5% affected cases. WM abnormalities were most frequently noted in bilateral anterior and posterior cerebral WM (50% affected cases).
Conclusion
About a third of acute/subacute COVID-19 patients referred for neuroimaging show brain abnormalities suggestive of COVID-19-related etiology. The predominant neuroimaging features were diffuse cerebral WM hypodensities / hyperintensities attributable to leukoencephalopathy, leukoaraiosis or rarefield WM.
Keywords: CNS, Neurologic, Leukoencephalopathy, Leukoaraiosis, Microangiopathy, COVID-19, SARS-Cov-2, Infection
1. Introduction
Over 12 million individuals worldwide have tested positive for Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) coronavirus 19 (COVID-19) up to date (Coronavirus disease (COVID-19) Pandemic. Geneva: World Health Organization, 2020). The pandemic has triggered massive quantities of scientific publications reporting data on COVID-19 of clinical- and scientific-relevance. The typical presentation of SARS-CoV-2 involves fever and respiratory symptoms. However, the recognition of neuroinvolvement of COVID-19 is increasing daily since the initial indications in February 2020 (Li et al., 2020). Currently, PubMed database search alone for the keywords “covid”/”sars-cov-2”/”coronavirus” and “neurologic”/”CNS” results in over 120,000 matches. Cohort studies and case reports describe various brain manifestations suggestive of COVID-19 etiology. At the time of “flattening the epidemic curve”, this growing body of research characterizing acute/subacute phase of infection calls for a synthesis.
The aim of this systematic review is to provide a synthesis of early evidence of brain abnormalities in patients with COVID-19 in acute/subacute phase, with the focus on (1) frequency of particular brain abnormality types, and (2) topographical distribution of registered brain abnormalities.
2. Methods
2.1. Search strategy and study selection
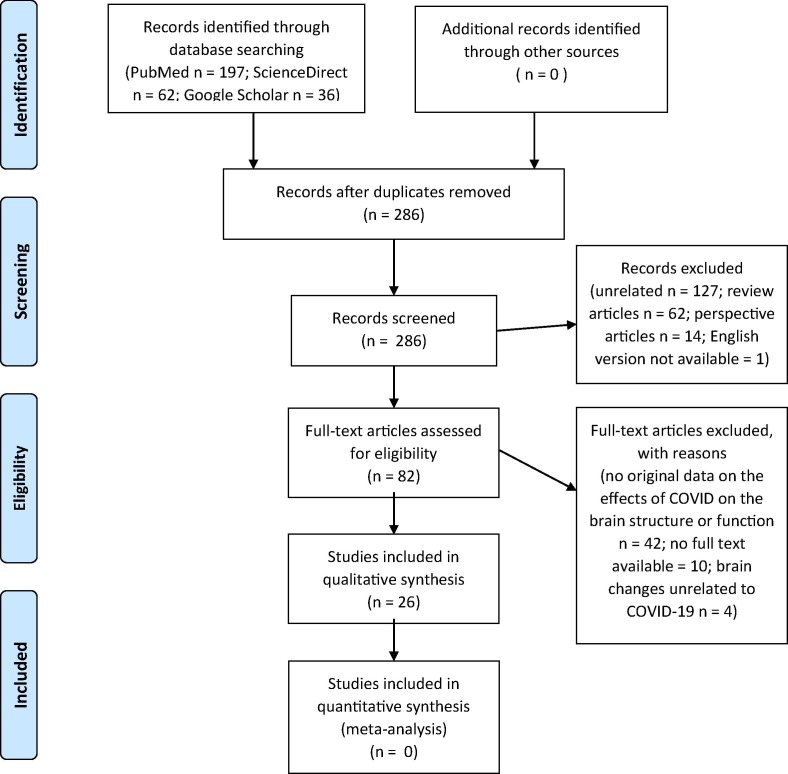
A systematic search of literature was performed in line with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Hutton et al., 2015, Moher et al., 2009) (Fig. 1 ). Search was implemented for PubMed, GoogleScholar, and ScienceDirect databases. The search strategy and keywords was as follows: ((covid[Title/Abstract]) OR (sars-cov-2[Title/Abstract]) OR (coronavirus[Title/Abstract])) AND (brain[Title/Abstract]). Search was limited to articles published between January 01 and July 05, 2020. The review protocol was not previously registered. Initial search was screened for duplicates. Then, two independent authors (ARE and SC) identified potential articles through (1) screening titles and abstracts, and (2) screening full text using inclusion and exclusion criteria (below). Search was finalized on July 06, 2020.
Fig. 1.
PRISMA (2009) flow diagram of the study.
2.2. Inclusion and exclusion criteria
Original contributions, which presented data on brain structural and/or functional abnormalities (or absence of such) suggestive of COVID-19 etiology, were included in the current systematic review. Articles were excluded in case of no original neuroimaging data, no full text, no available English version of the article, or in case of reviews, letters to editor, correspondence, perspective, and opinion not containing original data of interest.
2.3. Data extraction
Data was extracted by two independent authors (ARE and SC) with the use of standardized form where rows contained information about the authors and year of publication, while columns indicated the following: study type (i.e., case report or cohort study), number of patients who completed at least one brain scanning session, age, sex, survival status, pre-existing medical conditions, RNA PCR fluid (CSF) status for SARS-CoV-2, early symptoms of COVID-19 (i.e., before hospital admission), symptoms of COVID-19 at/after hospital admission, symptoms of COVID-19 the day of brain scan (separately for 1st brain imaging and follow-ups), brain imaging interpretation, procedures performed on the brain during that hospital visit/stay, brain imaging modality, brain imaging results (separately for each scanning session in case of follow-ups).
3. Results
3.1. Study selection and characteristics
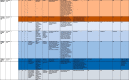
Initial search resulted in a collection of 295 records. Duplicates were removed, leaving 286 original contributions. Screening titles and abstracts excluded unrelated articles (n = 127); review articles (n = 62); perspective articles (n = 14); articles with English version not available (n = 1). The remaining 82 potential articles were entered into full text screening using inclusion and exclusion criteria. This step excluded articles with no original data on the brain structure or function with suggested relevance to COVID-19 (n = 42); no full text available (n = 10). Out of the identified 30 eligible articles, one article was excluded from the synthesis as patient SARS-CoV-2+ status was not confirmed neither in the swab specimen nor real-time polymerase chain reaction in the cerebrospinal fluid (Haddadi et al., 2020). Additional three article were excluded as the relationship between brain abnormalities and COVID-19 infection was noted by the authors as improbable. In detail, the authors attributed reported brain abnormalities rather to other/pre-existing medical conditions, previous pathological situations or interpreted them as potentially coincidental with COVID-19 (Morrasi et al., 2020, Degeneffe et al., 2020, Petrescu et al., 2020). Therefore, we entered a total of 26 articles (including 21 case reports, and 5 cohort studies) into the final synthesis. All 361 participants from 26 studies were patients with confirmed COVID-19 infection (with swab and/or CSF test) (Coronavirus disease (COVID-19) Pandemic. Emergency use ICD codes for COVID-19 disease outbreak. Geneva: World Health Organization, 2020). Brain abnormalities suggestive of COVID-19 etiology were present in 124/361 (34%) reported cases. Available demographic and illness characteristics are shown in Table 1 .
Table 1.
Characteristics of the included studies.
 |
 |
 |
 |
Notes. N/A – non-applicable, M – male, F – female, GCS – Glasgow Coma Score, CT – Computed Tomography, MRI – Magnetic Resonance Imaging, CNS –Cerebrospinal Fluid, CSF – Chemical-physical cerebrospinal fluid, PCR– Polymerase chain reaction, RNA- Ribonucleic acid, RT-PCR – Real-time polymerase chain reaction.
* Authors provided neuroimaging results for 11/27 cases. The inclusion of those 11 cases was based onnoted abnormalities interms of white matter T2 hyperintensities (more than expected for age-related microangiopathy based on visual qualitative assessment) and/or microhemorrhages (defined as ≤ 4 mm in size). Microhemorrhages confined to any areas of acute/subacute infarcts were excluded.
** 242 out of 3661 patients were MRI scanned. The authors reportthe most common clinical indications for brain imaging in their cohort to be: altered mental status (n = 102), syncope/fall (n = 79), or focal neurologic deficit (n = 30).
3.2. Typology of brain abnormalities in COVID-19
The most frequent brain abnormalities were brain WM hyperintensities on MRI and hypodensities on CT, which together accounted for 76% of affected cases (Table 2 ). Hyperintensities in cerebral WM were reported in 66/124 (53% affected cases). Those abnormalities were noted in bilateral medial temporal lobes [Z] (Virhammar et al., 2020), frontal, occipital, parietal [C (Anzalone et al., 2020): 4/21 cases], all of the above plus temporal lobes [D (Asfar et al., 2020); P (Kandemirli et al., 2020): 12/27; W (Radmanesh et al., 2020): 10/11 cases; Q (Kremer et al., 2020): 16/37]. Changes were also registered in insular cortex [P (Kandemirli et al., 2020): 3/27], subinsular regions [Z] (Virhammar et al., 2020), cingulate gyri [P (Kandemirli et al., 2020): 3/27], cerebral peduncle and internal capsule [β] (Zoghi et al., 2020), thalamus [Z (Virhammar et al., 2020); D (Asfar et al., 2020); H (Fischer et al., 2020), midbrain [Z] (Virhammar et al., 2020), pons [D (Asfar et al., 2020); β (Zoghi et al., 2020), parahippocampal gyri and basal ganglia [H] (Fischer et al., 2020), splenium of corpus callosum [L (Hayashi et al., 2020); β (Zoghi et al., 2020), olfactory nerves/bulb [R (Li et al., 2020), W (Petrescu et al., 2020) and gyrus rectus [W] (Petrescu et al., 2020), or described as diffuse [α (Zanin et al., 2020), W (Radmanesh et al., 2020): 10/11 cases; Q (Kremer et al., 2020): 11/37; U (Parsons et al., 2020). Three patients showed lateralized hyperintensities: one case of right prefrontal involvement [K] (le Guennec et al., 2020), one case of right temporal lobe, inferior horn of lateral ventricle and hippocampus [S] (Moriguchi et al., 2020), and one case of left WM, cortical and deep gray matter and midbrain [A] (Abdi et al., 2020). Diffuse leukoencephalopathy was further reported in 4/124 (3%) in bilateral cerebellar hemispheres and middle cerebellar peduncles [W (Radmanesh et al., 2020): 4/11].
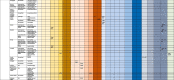
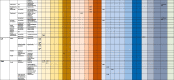
Table 2.
Brain imaging features in patients with COVID-19 in acute/subacute phase.
 |
 |
 |
 |
 |
 |
 |
 |
Notes. “x” indicates the presence of abnormality on brain scan, CT – Computed Tomography; MRI – Magnetic Resonance Imaging, EEG – Electroencephalography, N/A – non-applicable, * acute, surrounded by edema and caused midline shift
** became chronic
*** re-reabsorbing with persistent perilesional brain edema and midline shift
†with associated mass effect and cortical sulcal effacement
†† three focal seizures lasting approximately 30 s each
††† focal status epilepticus
‡consistent with mild microvascular disease but without acute intracranial lesion
‡‡ no evidence of brain edema
‡‡‡ no signs of cerebral vasospasm
**microhemorrhages varied between 5 and 6 to innumerable. Predominantly punctate, smaller than 3-mm in size. no concomitant larger intracranial hemorrhage. One patient with microhemorrhages has a prior brain MRI available (7 days before current hospital admission), which revealed that all hemorrhages were new. 4 in 7 patients had CT 3–7 days before MRI - no punctate microhemorrhages shown.
**No patients with altered mental status as the indication for brain imaging demonstrated acute or subacute infarct or acute intracranial hemorrhage
***the authors did not clearly state if hyperintensities comprised all cases of abnormalities.
¥ White matter microangiopathy was more than expected for age in 26 patients and in additional 108 patients as much as expected for age.
¥¥ posterior frontal and temporo-parieto-occipital symmetric bilateral hypodensity of the subcortical white matter.
¥¥¥ Default Mode Network was studied based on four nodes: the medial prefrontal cortex, the posterior cingulate cortex, and bilateral inferior parietal lobules
$ extensive and isolated WM microhemorrhages
$$ the signal alteration in the cortex completely disappeared
$$$ the olfactory bulbs were thinner and slightly less hyperintense
δ improved brain swelling
Hypodensities were noted in additional 29/124 (23% affected cases), and were primarily registered as diffuse changes in bilateral WM [E (Cariddi et al., 2020); X (Radmanesh et al., 2020): 26/242 cases]. Two case studies described hypodensities in amygdala [F] (Dixon et al., 2020), supratentorial leptomeningeal [N] (Hepburn et al., 2020), left occipital lobe [F] (Dixon et al., 2020) in WM and gray matter).
Other brain abnormalities were reported as follows. Microhemorrhages in WM were noted in 16/124 (13%) with bilateral diffuse presentation [W (Radmanesh et al., 2020): 5/11], in corpus callosum [W (Radmanesh et al., 2020): 4/7; Y (De Stefano et al., 2020), and putamen [F] (Dixon et al., 2020), bilateral juxtacortical WM and internal capsule [Y] (De Stefano et al., 2020); or diffuse [Q (Kremer et al., 2020): 9/37]. Infarct was reported in 13/124 (10%) and involved bilateral anterior [X (Radmanesh et al., 2020): 9/242] and posterior [X (Radmanesh et al., 2020): 4/242] circulation territories. Hemorrhages were noted in 7/124 (6%) and included: bilateral posterior parieto-occipital area (J) (Franceschi et al., 2020) and amygdala [F] (Dixon et al., 2020); as well as left frontal [T] (Muhammad et al., 2020) and occipital areas [E] (Cariddi et al., 2020); right temporal area [E] (Cariddi et al., 2020); temporal plus frontal lobes and Sylvian fissure [B] (Al-olama et al., 2020); and right posterior parieto-occipital area [I] (Franceschi et al., 2020); brain stem and pons [F] (Dixon et al., 2020); and corpus callosum [I] (Franceschi et al., 2020); and intraventricular layering in the occipital horns of lateral ventricles [U] (Parsons et al., 2020). Swelling/edema, restricted diffusion was reported in 4/124 (3%) in bilateral WM with diffuse presentation [F] (Dixon et al., 2020), in posterior parieto-occipital regions [I (Franceschi et al., 2020), J (Franceschi et al., 2020), thalamic nuclei [F] (Dixon et al., 2020), subinsular regions [F] (Dixon et al., 2020), basal ganglia [J] (Franceschi et al., 2020), cingulate gyri [F] (Dixon et al., 2020), cerebellar hemispheres [J] (Franceschi et al., 2020), right frontal lobe [J] (Franceschi et al., 2020), and right temporal lobe [O] (Kadono et al., 2020), as well as brain stem, pons and splenium [F] (Dixon et al., 2020). Seizures were noted in 4/124 (3%) in bilateral fronto-temporal regions [N (Hepburn et al., 2020); α (Zanin et al., 2020)], right frontal [K] (le Guennec et al., 2020) and right centropatieral area [M] (Hepburn et al., 2020). EEG demonstrated wave slowing in 4/124 (3%) patient cases [G (Espinosa et al., 2020), H (Fischer et al., 2020), U (Parsons et al., 2020) Z (Virhammar et al., 2020). CT-angio revealed increased enhancement in 1/124 (1%) patient case bilateral supratentorial leptomeningeal [B] (Al-olama et al., 2020). Ischemia was characterized in another patient case (1/124 (1%) in left frontal lobe [T] (Muhammad et al., 2020). Hematoma was also identified in one case report (1/124 (1%) and located in right subdural and frontal area [B] (Al-olama et al., 2020). Smaller olfactory bulb was noted in one case report 1/124 (1%). One report on spontaneous brain activity revealed no abnormalities in the Default Mode Network [H] (Fischer et al., 2020).
3.3. Topography of brain abnormalities in COVID-19
Diffuse subcortical and deep WM abnormalities were the most prominent. A cumulative of 62/124 (50%) of cases presented brain abnormality in either anterior areas [D (Asfar et al., 2020); N (Hepburn et al., 2020); α (Zanin et al., 2020), X (Radmanesh et al., 2020): 9/242 cases, Z (Virhammar et al., 2020) or posterior regions [I (Franceschi et al., 2020), J (Franceschi et al., 2020), X (Radmanesh et al., 2020): 4/242 cases] or anterior-posterior regions [C (Anzalone et al., 2020): 4/21 cases; E (Cariddi et al., 2020); P (Kandemirli et al., 2020): 4/27 cases; W (Radmanesh et al., 2020): 10/11 cases; Q (Kremer et al., 2020): 20/37]. Of those, several patients additionally presented brain abnormalities which were lateralized [I (Franceschi et al., 2020); E (Cariddi et al., 2020); J (Franceschi et al., 2020), cerebellar (W (Radmanesh et al., 2020): 4/11 cases], located in cortex [C (Anzalone et al., 2020): 4/21 cases], deep brain structures [D (Asfar et al., 2020); P (Kandemirli et al., 2020): 3/27 cases], scattered in juxtacortical WM [Y] (De Stefano et al., 2020), or diffuse [α] (Zanin et al., 2020). Unspecified brain location for brain waves slowing on EEG recording was reported in four cases [G (Espinosa et al., 2020); H (Fischer et al., 2020); U (Parsons et al., 2020); Z (Virhammar et al., 2020).
Anterior brain regions were affected bilaterally in 45/124, i.e., 36% of patients with brain abnormalities. Those primarily involved juxta/subcortical and deep white matter (WM) hyperintensities in medial temporal lobe [Z] (Virhammar et al., 2020), frontal and temporal lobes [W (Radmanesh et al., 2020): 10/11 cases], frontal lobe [P (Kandemirli et al., 2020): 4/27 cases, including 1/27 also in temporal lobe], or temporal lobe (D (Asfar et al., 2020), Q (Kremer et al., 2020): 16/37; R (Li et al., 2020), or gyrus rectus and olfactory bulb (V) (Politi et al., 2020). Seizures were noted with the EEG in fronto-temporal regions for two patients [N (Hepburn et al., 2020); α (Zanin et al., 2020)]. One study reported infarcts in anterior circulation territories [X (Radmanesh et al., 2020): 9/242 cases].
Posterior brain regions presented bilateral abnormalities in 22/124 (18% of patients with brain abnormalities). One patient showed subcortical WM hypodensities reaching from occipito-parieto-temporal reaching toward posterior frontal tracts [E] (Cariddi et al., 2020). Subcortical and deep WM hyperintensities were diffuse [U] (Parsons et al., 2020), included occipital and parietal regions [P (Kandemirli et al., 2020): 4/27 and 3/27 cases respectively], or were accompanied by mild restricted diffusion in subcortical and deep WM in occipital lobe [W (Radmanesh et al., 2020): 10/11 cases, including 7 cases with additional abnormalities in juxtacortical WM]. Two other cases showed focal vasogenic/cytotoxic edema [I (Franceschi et al., 2020), J (Franceschi et al., 2020)] in posterior parieto-occipital regions, while one was further accompanied by restricted diffusion and hemorrhages [J] (Franceschi et al., 2020). Another study reported infarcts in posterior circulation territories [X (Radmanesh et al., 2020): 4/242 cases].
Exclusively right cerebral hemisphere abnormalities were noted in 8/124 (6%) affected cases and were not specific to any one particular location or type of abnormality. Hyperintensities were noted in temporal mesial lobe, inferior horn of lateral ventricle and hippocampus in one patient [S] (Moriguchi et al., 2020)). One case report showed restricted diffusion with associated edema in frontal lobe [J] (Franceschi et al., 2020). Another patient showed subdural and frontal intracerebral hematoma, accompanied by subarachnoid hemorrhage in frontal, temporal regions and Sylvian fissure [B] (Al-olama et al., 2020). Intraventrivular hemorrhage was noted in one case [U] (Parsons et al., 2020). Focal seizures in centroparietal regions were noted in another two case reports [M (Hepburn et al., 2020); K (le Guennec et al., 2020)]. One case report revealed hemorrhage in posterior parieto-occipital region [I] (Franceschi et al., 2020). Another case reported severe brain swelling in the right temporal lobe, which was previously injured by hemorrhagic infarction [O] (Kadono et al., 2020).
Exclusively left cerebral hemisphere abnormalities were reported in 3/124 (2%) affected cases. Those included diffuse hyperintensities in WM, cortical and deep gray matter [A] (Abdi et al., 2020), hypodensity in occipital cortex and WM [F] (Dixon et al., 2020), and aneurysmal hemorrhage with delayed cerebral ischemia in frontal lobe [T] (Muhammad et al., 2020).
Cerebellar abnormalities were evident in 7/124 (6%) affected cases, and involved white matter hypodensity [N] (Hepburn et al., 2020) or diffuse leukoencephalopathy [W (Radmanesh et al., 2020): 4/11 cases], restricted diffusion with associated edema [J] (Franceschi et al., 2020), and increased enhancement on CT-angio [B] (Al-olama et al., 2020).
Deep brain structures were affected in 9/124 (7%) affected cases, out of which 4 comprised insula and cingulate gyri abnormalities [P (Kandemirli et al., 2020): 3/27 cases], and swelling and restricted diffusion with peripheral enhancement [F] (Dixon et al., 2020). The same patient [F] (Dixon et al., 2020) also showed swelling and restricted diffusion with peripheral enhancement in thalamus and putamen, as well as hypodensity/hemorrhage in amygdala [F] (Dixon et al., 2020). Four cases showed internal capsul hyperintensities [β] (Zoghi et al., 2020) or microbleeds [Y] (De Stefano et al., 2020), hyperintensities in thalamic nuclei [D (Asfar et al., 2020); Z (Virhammar et al., 2020)] and subinsula [Z] (Virhammar et al., 2020), or cerebral peduncle [β] (Zoghi et al., 2020). Additionally, restricted diffusion with edema was noted in basal ganglia (no details available) in one patient [J] (Franceschi et al., 2020).
The midline structures of the brain were affected in 12/124 (10%) affected cases and mainly included abnormalities in the corpus callosum, i.e., hyperintensities [L (Hayashi et al., 2020); β (Zoghi et al., 2020)], hemorrhage [I] (Franceschi et al., 2020), microhemorrhages [W (Radmanesh et al., 2020)]: 4/7 cases; Y (De Stefano et al., 2020), and swelling and restricted diffusion [F] (Dixon et al., 2020). Additionally, one of those patients [F] (Dixon et al., 2020) showed signs of swelling and hemorrhage in brain stem and hemorrhage in pons. Hyperintensities were noted in midbrain [A (Abdi et al., 2020); Z (Virhammar et al., 2020)] and pons [D (Asfar et al., 2020); β (Zoghi et al., 2020)].
Only 6/361 patients were scanned with CTP, CT-/MR-angio. In 4 of those 6 cases, the results were not showing arteriovenous malformation or aneurysms or acute vascular occlusion, or were unremarkable. Two patients showed frontal subarachnoid hemorrhage or ischemia, one of them only on the follow-up scan.
In the majority of reviewed cases 237/361 (66%), CT/MRI did not reveal any acute/subacute brain abnormalities that were attributed to COVID-19 as the most probable cause. Those included 17/21 patients [C] (Anzalone et al., 2020), 15/27 [P] (Kandemirli et al., 2020), and 205/242 [X] (Radmanesh et al., 2020). Additionally, one study did not report neuroimaging results for 16/27 patients as they did not show white matter T2 hyperintensities and/or microhemorrhages W (Radmanesh et al., 2020). However, such description does not allow to uniformly determine whether brain scans in those 16 patients were unremarkable.
Finally, three case reports showed brain abnormalities (in the form of cortical hyperintensities) on the initial scan, but a complete resolution of lesions at 1-month follow-up scan [C (Anzalone et al., 2020); K (le Guennec et al., 2020)]; V (Politi et al., 2020). Additionally, one case showed EEG signal abnormalities that were no longer present at around two weeks after Sars-CoV-2 detection [Y] (De Stefano et al., 2020).
4. Discussion
This systematic review provides a synthesis of early evidence on brain abnormalities suggestive of COVID-19 etiology in patients in acute/subacute phase. Collectively, published reports show that out of patients with available brain imaging, 66% patients do not present brain manifestations of presumed COVID-19 etiology. Various brain abnormalities were present in the remaining 34% reviewed cases. Together, this suggests that early neurologic symptoms, which were the reason for referral for brain imaging, may appear earlier than the brain structural changes can be detected with the available technology. Future studies should consider employing myelin imaging or WM tractography based on diffusion-weighted imaging data to provide additional description of more intricate brain WM changes in COVID-19. Alternatively, transient neurologic symptoms may also be related to acute/subacute brain alterations at the level of functional networks. This hypothesis can be examined for example with the use of resting state functional MRI sequences. This methodology may be especially useful considering the respiratory complications in COVID-19.
The primary neuroimaging feature involved WM hyperintensities on or MRI hypodensities on CT, which was observed in 76% of the affected cases. These changes were primarily diffuse in the cerebral WM, however, the provided examples of brain scans for cohort studies [W (Radmanesh et al., 2020), X (Radmanesh et al., 2020)] also reveal the increased density of WM changes in close proximity to the ventricles. As the brain images were not provided for all reported cases, we cannot verify whether the increased periventricular presentation is a common characteristics. At the same time, the involvement of cerebellar, midline- or deep brain structures was reported infrequently. Together, the exhibited topographical pattern of the WM abnormalities allows us to speculate about attributing these changes to leukoencephalopathy, leukoaraiosis (LA) or rarefield WM not restricted to periventricular area. This interpretation is in line with the notion made by the Authors of the original articles [F (Dixon et al., 2020), W (Radmanesh et al., 2020)]. LA is one of the most prominent characteristics of the aging brain, often asymptomatic and only revealed with neuroimaging. However, the analyzed data further suggest that the prevalence of LA is higher in this patient population than expected for age. Other possible interpretations may include encephalitis as suggested in several reports (Anzalone et al., 2020, Asfar et al., 2020, Espinosa et al., 2020, Hayashi et al., 2020, Kremer et al., 2020), acute necrotizing encephalitis (Virhammar et al., 2020), encephalomyelitis (Abdi et al., 2020, Zoghi et al., 2020), demyelination (Zanin et al., 2020, Parsons et al., 2020, Zoghi et al., 2020), or microangiopathy (Fischer et al., 2020). Therefore, we encourage future studies to report more detailed description of the WM changes in order to establish differential characteristics of COVID-19-related vs. age-related changes in WM. One way to address this as well as to enable future meta-analyses, is to report the scores on the Fazekas scale (Fazekas et al., 1987).
The potential neuropathological associations of LA may include hypoxia, hypoperfusion, as well as demyelination or axonal loss, with consequent disconnection syndromes. However, the potential pathogeneses of brain abnormalities in COVID-19 patients remain unclear and are beyond the scope of this systematic review. We restricted the analyses to the synthesis of available evidence regarding types and topography of registered brain abnormalities. Future longitudinal studies are needed to address the mechanisms of brain manifestations, neurologic sequelae in COVID-19, and the directional relationship between neuroinvasive actions of SARS-CoV-2 and respiratory failure.
Other types of brain abnormalities were less frequently observed and included aneurysm, hematoma, hemorrhage and seizure. These brain abnormalities were reported infrequently as compared to LA cases. Thus, it can be hypothesized, that if the presentation of these conditions is related to COVID-19, than perhaps it may be enhanced or accelerated with systemic inflammation rather than directly triggered by the infection. The neuropathological associations of these brain abnormalities should be examined in the future studies.
Importantly, in three patient cases with cortical hyperintensities, there was a resolution of lesions noted on a 30-day follow-up. Comparisons with other reports are limited as only two more research teams presented an extensive follow-up brain scan in one patient [F (Dixon et al., 2020); U (Parsons et al., 2020)]. Also, one of the patients with EEG showed resolution of signal abnormalities at around 2-week mark following Sars-CoV-2+ detection [Y] (De Stefano et al., 2020). The hypothesis on transient character of brain abnormalities should be assessed in future research.
This systematic review has limitations. It is based on the available evidence with the assumption that the original contributions report all evident brain abnormalities and their proposed interpretation of the relationship with COVID-19 is accurate. Neuroimaging findings were excluded from the current review and analysis in cases where the authors reported them to be unrelated to the COVID-19, coincidental, or where the authors provided a different explanation for the findings. For example, one study reported 134/242 patients to show WM hypodensities/hyperintensities, out of which in 108 changes were “as much as expected for age” (Radmanesh et al., 2020). Importantly, as the relationship between brain structure/function and COVID-19 infection is not clear yet, such interpretations may lead to underreporting brain issues in this patient population and the current results should be treated with caution. Furthermore, our literature search only included articles with title and/or abstract containing the word “brain” and at least one of the following “covid”/“sars-cov-2”/“coronavirus”. As this holds a potential of missing original contributions of interest, we checked the results of the following extended search strategies: ((covid[Title/Abstract]) OR (sars-cov-2[Title/Abstract]) OR (coronavirus[Title/Abstract])) AND (brain[Title/Abstract]) OR (CNS[Title/Abstract]), which yielded 106,581 results; and ((covid[Title/Abstract]) OR (sars-cov-2[Title/Abstract]) OR (coronavirus[Title/Abstract])) AND (brain[Title/Abstract]) OR (neurologic[Title/Abstract]), which yielded 83,533 results as of July 06, 2020. However, for the purpose of a timely contribution on early evidence of abnormalities due to COVID-19 only in the brain and not other parts of the CNS, we analyzed the data from the initial, more narrow and precise search. Our future research plans involve a more holistic literature search employing the above extended the search strategies. Another limitation is posed by the reasons for referral to CT/MRI/EEG imaging in the analyzed studies as well as bias related to the case reports, such as the selection of patient cases for presentation. Missing data on neurologic symptoms in original articles did not allow us to analyze the relationships with the revealed brain abnormalities patterns. Due to few published cohort studies, we incorporated case reports into a cumulative synthesis, but we were unable to employ meta-analytic approach. Future systematic reviews should include meta-analysis of larger cohort studies once they become available.
5. Conclusion
We found that brain images in acute/subacute patients with COVID-19 are predominantly characterized by diffuse cerebral WM hyperintensities/hypodensities. The available evidence allows to speculate about the higher prevalence of leukoencephalopathy, leukoaraiosis or rarefield WM in this patient population than expected for age. Large cohort studies reporting details of registered brain abnormalities are needed in order to establish (1) the incidence of brain abnormalities, (2) neurologic sequelae, and (3) pathophysiological associations of neuroinvasion in COVID-19.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2020.07.014.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Coronavirus disease (COVID-19) Pandemic. Geneva: World Health Organization. 2020. https://covid19.who.int/. Accessed July 10, 2020.
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton B., Salanti G., Caldwell D.M., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Morrasi M., Bagatto D., Cobelli M., et al. Stroke in patients with SARS-CoV-2 infection: case series. J. Neuol. 2020 doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdi S., Ghorbani A., Farzad F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J. Neurol. Sci. 2020;416 doi: 10.1016/j.jns.2020.117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-olama M., Rashid A., Garozzo D. COVID-19-associated meningoencephalitis complicated with intracranial hemorrhage: a case report. Aca Neurochirurgica. 2020 doi: 10.1007/s00701-020-04402-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone N., Castellano A., Scotti R., et al. Multifocal laminar cortical brain lesions: a consistent MRI fnding in neuro–COVID–19 patients. J. Neurol. 2020 doi: 10.1007/s00415-020-09966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfar H., Yassin Z., Kalantari S., et al. Evolution and resolution of brain involvement associated with SARS- CoV2 infection: a close Clinical – Paraclinical follow up study of a case. Multip. Sclerosis Related Disorders. 2020;43 doi: 10.1016/j.msard.2020.102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariddi L.P., Damavandi P.T., Carimati F., et al. Reversible encephalopathy syndrome (PRES) in a COVID–19 patient. J. Neurol. 2020 doi: 10.1007/s00415-020-10001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degeneffe A., Bruneau M., Spitaels J., et al. Acute hemorrhage after intracerebral biopsy in COVID-19 patients: report of 3 cases. J. W. Neu. 2020:15235. doi: 10.1016/j.wneu.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L., Varley J., Gontsarova A., et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol. Neuroimmunol. Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa P.S., Rizvi Z., Sharma P., et al. Neurological complications of Coronavirus Disease (COVID-19): encephalopathy, MRI brain and cerebrospinal fluid findings: case 2. Cureus. 2020 doi: 10.7759/cureus.7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D., Threlkeld Z.D., Bodien Y.G., et al. Intact brain network function in an unresponsive patient with COVID-19. Ann. Neurol. 2020 doi: 10.1002/ana.25838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi A.M., Ahmed O., Giliberto L., Castillo M. Hemorrhagic posterior reversible encephalopathy syndrome as a manifestation of COVID-19 infection. AJNR Am. J. Neuroradiol. 2020 doi: 10.3174/ajnr.A6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Guennec L., Devianne J., Jalin L., et al. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia. 2020 doi: 10.1111/epi.16612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadi K., Ghasemian R., Shafizad M. Basal ganglia involvement and altered mental status: a unique neurological manifestation of coronavirus disease 2019. Cureus. 2020 doi: 10.7759/cureus.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Sahasi Y., Baba Y., Okura H. COVID-19-associated mild encephalitis/encephalopathy with a reversible splenial lesion. J. Neurol. Sci. 2020;415 doi: 10.1016/j.jns.2020.116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn M., Mullaguri N., George P., et al. Acute symptomatic seizurres in critically ill patients with COVID-19: is there an association? Neurocrit. Care. 2020 doi: 10.1007/s12028-020-01006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadono Y., Nakamura Y., Ogawa Y., et al. A case of COVID-19 infection presenting with a seizure following severe brain edema. Seizure: Euro J Epilepsy. 2020;80:53–55. doi: 10.1016/j.seizure.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandemirli S., Dogan M., Sarikaya Z.T., et al. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020 doi: 10.1148/radiol.2020201697. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer S., Lersy F., de Seze J., et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Neuroradiol. 2020 doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.W., Syue L.S., Tsai Y.S., et al. Anosmia and olfactory tract neuropathy in a case of COVID-19. J. Microbiol., Immun. Infect. 2020 doi: 10.1016/j.jmii.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. IJID. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad S., Petridisa A., Cornelius A.F., Hanggi D. Severe brain haemorrhage and concominant COVID-19 infection: a neurovascular complication of COVID-19. BBI. 2020 doi: 10.1016/j.bbi.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T., Banks S., Bae C., et al. COVID–19–associated acute disseminated encephalomyelitis (ADEM) J. Neurol. 2020 doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrescu A.M., Tassig D., Bouilleret V. Electroencephalogram (EEG) in COVID-19: a systematic retrospective study. Neurophysiol. Clin. 2020 doi: 10.1016/j.neucli.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi L.S., Salsano E., Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient With Coronavirus Disease 2019 (COVID-19) and anosmia. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2125. [DOI] [PubMed] [Google Scholar]
- Radmanesh A., Derman A., Lui Y.W., et al. COVID-19-associated diffuse leukoencephalopathy and microhemmorhages. Radiology. 2020 doi: 10.1148/radiol.2020202040. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmanesh A., Raz E., Zan E., et al. Brain imaging use and findings in COVID-19: a signle academic center experience in the epicenter of disease in the United States. ANJR Am. J. Neuroradiol. 2020 doi: 10.3174/ajnr.A6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano P., Nencha U., De Stefano L., et al. Focal EEG changes indicating critical illness associated cerebral microbleeds in a Covid-19 patient. J. CNP. 2020:125–129. doi: 10.1016/j.cnp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virhammar J., Kumlien E., Fallmar D., et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020 doi: 10.1212/WNL.0000000000010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin L., Saraceno G., Panciani P.P., et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020 doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghi A., Ramezani M., Roozbeh M., et al. A case of possible atypical demyelinating event of the central nervous system following COVID-19. J. MSARD. 2020 doi: 10.1016/j.msard.2020.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F., Chawluk J.B., Alavi A., et al. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am. J. Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Coronavirus disease (COVID-19) Pandemic. Emergency use ICD codes for COVID-19 disease outbreak. Geneva: World Health Organization. 2020. https://www.who.int/classifications/icd/covid19/en/. Accessed July 10, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.