Abstract
Background/Aims
The utility of serum pepsinogen (sPG) I and the sPGI/II ratio as biomarkers for screening individuals with gastric cancer (GC) has not been established in Korea. The aim of this study was to define the role of sPG, especially sPGII, in GC screening.
Methods
This study enrolled 2,940 subjects, including patients with GC (n=1,124) or gastric dysplasia (n=353) and controls (n=1,463). Tests to determine sPG levels and Helicobacter pylori (HP) infection status were performed. Area under the curve and receiver operating characteristic curve were calculated to identify the optimal cutoff values for sPG. The usefulness of sPG levels for the detection of GC and gastric dysplasia was validated by multivariate logistic regression.
Results
The sPGI/II ratio was associated with the risk of gastric dysplasia and advanced-stage intestinal-type GC (IGC). In contrast, sPGII was associated with the risk of early-stage diffuse-type GC (DGC). Significantly higher risk was indicated by an sPGI/II ratio <3 for gastric dysplasia and advanced-stage IGC and by sPGII levels ≥20 µg/L for early-stage DGC. Positive HP status showed a stronger association with DGC than with IGC. When sPGII level and HP status were combined, the prevalence of DGC was higher in the ≥20 µg/L sPGII and HP-positive group. Age younger than 40 years was strongly related to early-stage DGC, especially in females (odds ratio, 21.00; p=0.006).
Conclusions
sPGII ≥20 ng/mL and positive HP status suggest a risk of early-stage DGC, particularly in young adult females in South Korea.
Keywords: Pepsinogen, Stomach neoplasms, Diffuse type, Helicobacter pylori
INTRODUCTION
The global trend in the prevalence of gastric cancer (GC) has decreased substantially over the last 40 years, but it is still the fifth most frequently diagnosed malignancy and the third leading cause of cancer death worldwide. Above all, incidence rates are markedly elevated in Eastern Asia including South Korea, whereas the rates in North America and Northern Europe are generally low.1 Since considerable numbers are diagnosed at advanced stages due to nonspecific symptoms, screening strategies to detect GC earlier and at a more curable stage have emerged as an important issue. The Korean National Cancer Screening Program for GC using mainly upper endoscopy was initiated in the late twentieth century and has led to a significant reduction in cancer-related mortality.2
As an alternative way, noninvasive mass screening method using serum pepsinogen (sPG) has become popular in Japan. sPGI and sPGII are produced in different parts of the gastric mucosa.3 That is, sPGI is only secreted by gastric chief and mucous neck cells in the fundic glands of the corpus, whereas sPGII is secreted by not only fundic glands but also pyloric glands of the antrum and duodenal mucosa. Previous studies confirmed that low sPGI (<70 µg/L) and a low sPGI/II ratio (<3) are indicators of advanced atrophic gastritis, which is associated with a higher risk of GC.3,4 The theoretical background is that the production of sPGI is reduced in atrophic mucosa and that sPGII increases when the gastric mucosa is inflamed due to Helicobacter pylori (HP) infection. Thus, the sPGI/II ratio is decreased further in association with low sPGI and an increase in PGII in advanced atrophic gastritis.5 However, although these criteria are applicable to intestinal type GC (IGC), which follows Correa’s cascade, questions remain as to whether these criteria can be applied to cases of diffuse type GC (DGC), which has different mechanisms of carcinogenesis.6 However, our team reported that high-risk operative link on gastric atrophy (OLGA)/operative link on gastric intestinal metaplasia (OLGIM) stages are important prediction markers for GC not only for the IGC but also for DGC.7 In South Korea, DGC comprises a relatively high proportion of total GC (42.1%), which is quite different from Japanese studies.8 Thus if there is a useful biomarker for DGC, it will be very useful in South Korea. So far, several previous studies have suggested an association between a high titer of sPGII and DGC.9-11 However, there has been no study about the relationship between sPGII and GC in South Korea.
Therefore, it is necessary to redefine the role of sPG in GC development, especially regarding its utility as a biomarker for DGC to stratify patients who should undergo endoscopy. This will contribute to a further reduction in the GC-related mortality rate in East Asia including China and South Korea. Based on this background, the aim of this study was to identify high-risk patients with GC, including those with DGC, by analyzing sPGI and sPGII.
MATERIALS AND METHODS
1. Study population
From February 2006 to March 2017, 2,940 subjects between the ages of 25 and 80 years who visited Seoul National University Bundang Hospital were prospectively enrolled. Of these, 1,124 patients were diagnosed with GC, and 353 were diagnosed with gastric dysplasia by histological analysis. In all, 1,463 subjects who had no history of previous gastrointestinal surgery or any other malignancy, were enrolled as healthy controls. The pathology records were reviewed in detail for the GC patients who underwent surgery or endoscopic submucosal dissection. GC was classified according to the Lauren classification, and early GC was defined as invasive when it invaded no more deeply than the submucosa, irrespective of lymph node metastasis (T1, any N).12 Thirty GC cases were difficult to classify as either the intestinal type or the diffuse type. All control subjects with dyspepsia symptoms underwent upper endoscopy to exclude other localized gastric disease including gastric mucosa-associated lymphoid tissue lymphoma, gastrointestinal stromal tumor, carcinoid tumor, malignant lymphoma and esophageal cancer. In addition, HP tests were verified. Subjects with benign diseases such as fundic gland hyperplasia, gastric hyperplastic polyp, mild gastritis, reflux esophagitis, or nonerosive reflux disease were assigned to the control group. An experienced interviewer assisted the patients in completing the questionnaires, and blood samples were obtained on the same day of the endoscopy. The study protocol was approved by the Institutional Review Board of the Seoul National University Bundang Hospital (IRB number: B-1610-368-106) and registered at Clinical trials.gov (NCT03380052). Written informed consent was obtained from all subjects following the ethical principles of the Declaration of Helsinki.
2. Endoscopic testing for HP infection and histology
Ten biopsy specimens were obtained from the antrum and the corpus during upper endoscopy. Using these samples, three diagnostic methods were performed to confirm HP infection. Of the 10, four specimens, which were fixed in 10% neutral-buffered formalin and paraffin-embedded, were stained with modified Giemsa, hematoxylin and eosin to evaluate the presence of HP and the stage of OLGA and OLGIM. The other four specimens were cultured for HP at 37°C under microaerobic conditions for 3 to 5 days. The last two specimens were subjected to rapid urease testing (Campylobacter-like organism test, CLO test).13-15
3. Serologic testing for pepsinogen and HP antibody
Fasting blood samples obtained from subjects were immediately placed into a centrifugal separator and stored at –70°C. Serum levels of sPGI and sPGII were measured using a latex-enhanced turbidimetric immunoassay (L-TIA; HBi Corp, Seoul, Korea, imported from Shima Laboratories, Tokyo, Japan). Immunoglobulin G antibody against HP was tested using an enzyme-linked immunosorbent assay (Genedia HP ELISA; Green Cross Medical Science Corp, Yongin, Korea), which has a 97.9% sensitivity and a 92.0% specificity in a Korean population.16
4. Identifying the history of HP infection
Positivity for one test (histology, culture, or CLO test) was defined as definite current HP infection. In addition, the anti-HP antibody test was used for qualitative estimation, especially when three HP tests were negative and all subjects were assessed for HP eradication history. Positivity for the HP serology test and/or an HP eradication history indicated a past HP infection. Overall, both current and past HP infection statuses were considered HP status-positive.
5. Statistical analyses
The Student t-test and one-way analysis of variance were used to compare the baseline characteristics of each group. Area under the curve (AUC) and receiver operating characteristic curve were calculated to find the optimal cutoff values of sPG for the detection of GC and gastric dysplasia compared with controls. AUC of 0.7 or more, sensitivity and specificity of 70% or more were regarded as significant. Then, sPGI, sPGII and the sPGI/II ratio were divided into two categories. Using multivariate logistic regression, odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). If the p-value was 0.05 or lower, the result was regarded as significant.
RESULTS
1. Baseline characteristics
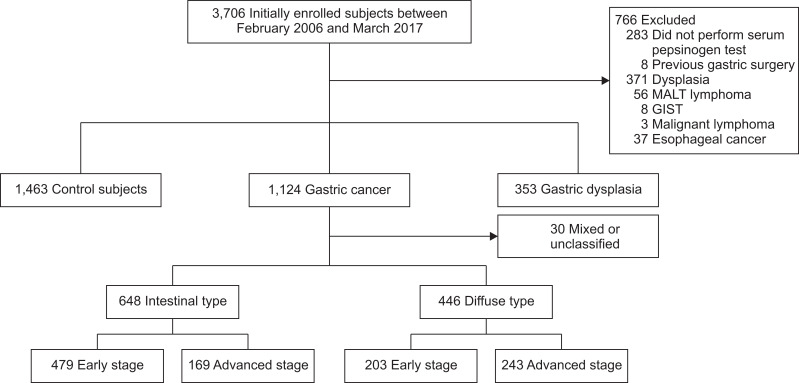
Table 1 shows the baseline characteristics of patients with gastric dysplasia (n=353), GC (n=1,124) and those of the control subjects (n=1,463). The mean age of the control group was 53.4±13.0 years, which was significantly younger than those of the GC (59.75±11.6 years) and gastric dysplasia (62.63±9.4 years) groups. The pooled one-way analysis of variance revealed significant differences in sex, smoking/alcohol history, salty/spicy diet, family history, HP status, sPGI, sPGII, the sPGI/II ratio, OLGA stage, and OLGIM stage among the three groups (Table 1). When GC was histologically categorized as intestinal type or diffuse type, the GC in 30 patients was difficult to classify as either the intestinal type or the diffuse type (Fig. 1). Thus, the remaining 1,094 patients were categorized into the IGC and DGC groups, while 648 were categorized into the IGC group (59.2%) and 446 were classified into the DGC group (40.8%). The differences in sex, age, smoking/alcohol history, salty diet, sPGI and sPGII, OLGA stage, and OLGIM stage were seen among the GC subgroups (Table 1). Subjects with missing data for each variable are presented in Supplementary Table 1.
Table 1.
Baseline Characteristics of the Control Subjects and Those with Gastric Dysplasia and GC
| Characteristic | Total (n=2,940) | Control subjects (n=1,463) | Gastric dysplasia (n=353) | GC (n=1,124) | p-value | Intestinal GC (n=648) | Diffuse GC (n=446) | p-value |
|---|---|---|---|---|---|---|---|---|
| Sex | <0.001* | <0.001* | ||||||
| Female | 1,235 (42.0) | 762 (52.1) | 116 (32.9) | 357 (31.8) | 149 (23.0) | 198 (44.4) | ||
| Male | 1,705 (58.0) | 701 (47.9) | 237 (67.1) | 767 (68.2) | 499 (77.0) | 248 (55.6) | ||
| Age, mean, yr | 56.95±12.6 | 53.4±13.0 | 62.63±9.4 | 59.75±11.6 | <0.001* | 62.96±9.5 | 55.1±12.8 | <0.001* |
| <40 | 304 (10.3) | 240 (16.4) | 5 (1.4) | 59 (5.2) | 6 (0.9) | 52 (11.7) | ||
| 40–59 | 1,258 (42.8) | 695 (47.5) | 119 (33.7) | 444 (39.5) | 207 (31.9) | 222 (49.8) | ||
| ≥60 | 1,378 (46.9) | 528 (36.1) | 229 (64.9) | 621 (55.2) | 435 (67.1) | 172 (38.6) | ||
| Smoking | <0.001* | <0.001* | ||||||
| Never | 1,411 (48.0) | 860 (59.2) | 139 (39.7) | 412 (36.9) | 205 (31.8) | 196 (44.1) | ||
| Ever | 1,510 (51.4) | 593 (40.8) | 211 (60.3) | 706 (63.1) | 439 (68.2) | 248 (55.9) | ||
| Alcohol | 0.002* | 0.010* | ||||||
| Never | 964 (32.8) | 523 (36.0) | 112 (31.9) | 329 (29.5) | 171 (26.6) | 150 (33.9) | ||
| Ever | 1,952 (66.4) | 928 (64.0) | 239 (68.1) | 785 (70.5) | 471 (73.4) | 293 (66.1) | ||
| Salty diet | <0.001* | 0.025* | ||||||
| Not/moderate | 2,047 (69.6) | 1,065 (76.4) | 247 (72.6) | 735 (68.2) | 408 (65.6) | 308 (72.1) | ||
| Strong | 765 (26.0) | 329 (23.6) | 93 (27.4) | 343 (31.8) | 214 (34.4) | 119 (27.9) | ||
| Spicy diet | 0.012* | 0.492 | ||||||
| Not/moderate | 2,028 (69.0) | 1,033 (74.9) | 250 (74.2) | 745 (69.6) | 435 (70.4) | 290 (68.4) | ||
| Strong | 760 (25.9) | 347 (25.1) | 87 (25.8) | 326 (30.4) | 183 (29.6) | 134 (31.6) | ||
| Family history of GC | 0.001* | 0.132 | ||||||
| Negative | 2,205 (75.0) | 1,049 (72.5) | 273 (77.6) | 883 (78.9) | 501 (77.4) | 359 (81.2) | ||
| Positive | 711 (24.2) | 397 (27.5) | 79 (22.4) | 236 (21.1) | 146 (22.6) | 83 (18.8) | ||
| H. pylori status | <0.001* | 0.076 | ||||||
| Negative | 580 (19.7) | 375 (25.9) | 42 (12.0) | 163 (14.5) | 103 (15.9) | 54 (12.1) | ||
| Positive | 2,338 (79.5) | 1,072 (74.1) | 307 (88.0) | 959 (85.5) | 543 (84.1) | 392 (87.9) | ||
| Pepsinogen | ||||||||
| sPGI, µg/L | 62.38±49.47 | 66.13±52.27 | 48.84±42.16 | 61.74±47.08 | <0.001* | 54.36±40.37 | 71.52±53.82 | <0.001* |
| sPGII, µg/L | 21.59±22.98 | 21.23±23.03 | 17.45±13.75 | 23.35±24.97 | <0.001* | 20.82±21.22 | 26.47±27.78 | <0.001* |
| sPGI/II ratio | 3.66±2.73 | 4.17±3.24 | 2.93±1.63 | 3.21±2.05 | <0.001* | 3.15±2.25 | 3.30±1.76 | 0.242 |
| Atrophy† | <0.001* | <0.001* | ||||||
| OLGA 0 | 663 (22.6) | 458 (53.0) | 35 (18.8) | 170 (25.9) | 70 (18.2) | 92 (36.9) | ||
| OLGA I | 517 (17.6) | 266 (30.8) | 46 (24.7) | 205 (31.2) | 120 (31.3) | 78 (31.3) | ||
| OLGA II | 320 (10.9) | 98 (11.3) | 49 (26.3) | 173 (26.3) | 123 (32.0) | 43 (17.3) | ||
| OLGA III | 147 (5.0) | 30 (3.5) | 35 (18.0) | 82 (12.5) | 52 (13.5) | 28 (11.2) | ||
| OLGA IV | 60 (2.0) | 12 (1.4) | 21 (11.3) | 27 (4.1) | 19 (4.9) | 8 (3.2) | ||
| Metaplasia† | <0.001* | <0.001* | ||||||
| OLGIM 0 | 1,197 (40.7) | 865 (63.3) | 49 (14.1) | 283 (25.7) | 101 (15.8) | 173 (40.0) | ||
| OLGIM I | 600 (20.4) | 251 (18.4) | 84 (24.1) | 265 (24.1) | 149 (23.4) | 107 (24.8) | ||
| OLGIM II | 561 (19.1) | 174 (12.7) | 95 (27.3) | 292 (26.5) | 199 (31.2) | 85 (19.7) | ||
| OLGIM III | 322 (11.0) | 59 (4.3) | 72 (20.7) | 191 (17.4) | 131 (20.5) | 57 (13.2) | ||
| OLGIM IV | 135 (4.6) | 18 (1.3) | 48 (13.8) | 69 (6.3) | 58 (9.1) | 10 (2.3) | ||
Data are presented as number (%) or median±SD. Subjects with missing data were shown in Supplementary Table 1.
GC, gastric cancer; H. pylori, Helicobacter pylori; sPG, serum pepsinogen; OLGA, operative link on gastric atrophy; OLGIM, operative link on gastric intestinal metaplasia.
Asterisk indicates statistical significance; †Subjects after excluding non-applicable specimens in the atrophy and intestinal metaplasia due to problems such as improper fixation, inaccurate orientation, and dense inflammation.
Fig. 1.
Study algorithm for the enrollment of the gastric cancer, dysplasia and control groups.
MALT, mucosa-associated lymphoid tissue; GIST, gastrointestinal stromal tumor.
2. Comparison of serologic and histologic features with respect to GC stage
No statistically significant difference was observed between the early- and advanced-stage GC groups with respect to HP status and sPGs (Table 2). When GC was divided according to histological type, HP status was still not different. However, sPGII was higher (p=0.001) and the sPGI/II ratio was lower (p=0.005) in advanced-stage IGC compared with early-stage IGC. In cases of DGC, those with early-stage disease had higher sPGI (p=0.003) and sPGII than those with advanced-stage (p=0.003). In terms of histologic features, advanced-stage IGC showed further progression of atrophic gastritis and intestinal metaplasia than early-stage IGC (Table 2).
Table 2.
Comparison of Serologic and Histologic Features with Respect to GC Stage
| Variable | Total | Intestinal type GC | Diffuse type GC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EGC (n=697) | AGC (n=424) | p-value | EGC (n=479) | AGC (n=169) | p-value | EGC (n=203) | AGC (n=243) | p-value | |
| H. pylori status | 0.572 | 0.797 | 0.297 | ||||||
| Negative | 98 (14.1) | 65 (15.3) | 75 (15.7) | 28 (16.6) | 21 (10.3) | 33 (13.6) | |||
| Positive | 597 (85.9) | 359 (84.7) | 402 (84.3) | 141 (83.4) | 182 (89.7) | 210 (86.4) | |||
| Pepsinogen | |||||||||
| sPGI, µg/L | 61.79±43.53 | 61.75±52.55 | 0.990 | 53.83±38.75 | 55.87±44.73 | 0.571 | 79.71±48.43 | 64.72±57.13 | 0.003* |
| sPGII, µg/L | 22.71±34.35 | 24.48±27.47 | 0.251 | 19.14±17.78 | 25.58±28.36 | 0.001* | 30.76±31.72 | 22.91±23.49 | 0.003* |
| sPGI/II ratio | 3.30±2.19 | 3.07±2.19 | 0.069 | 3.29±2.40 | 2.73±1.68 | 0.005* | 3.31±1.66 | 3.29±1.83 | 0.896 |
| OLGA† | <0.001* | <0.001* | <0.001* | ||||||
| Low risk | 355 (85.3) | 190 (79.8) | 242 (84.9) | 71 (71.7) | 102 (85.0) | 111 (86.0) | |||
| High risk | 61 (14.7) | 48 (20.2) | 43 (15.1) | 28 (28.3) | 18 (15.0) | 18 (14.0) | |||
| OLGIM† | <0.001* | <0.001* | <0.001* | ||||||
| Low risk | 533 (77.4) | 304 (74.5) | 348 (73.3) | 101 (62.0) | 172 (86.4) | 193 (82.8) | |||
| High risk | 156 (22.6) | 104 (25.5) | 127 (26.7) | 62 (38.0) | 27 (13.6) | 40 (17.2) | |||
Data are presented as number (%) or median±SD.
GC, gastric cancer; EGC, early gastric cancer; AGC, advanced gastric cancer; H. pylori, Helicobacter pylori; sPG, serum pepsinogen; OLGA, operative link on gastric atrophy; OLGIM, operative link on gastric intestinal metaplasia.
Asterisk indicates statistical significance; †Subjects after excluding non-applicable specimens in the atrophy and intestinal metaplasia groups due to problems such as improper fixation, inaccurate orientation, and dense inflammation.
3. The effect of HP infection on sPG and OLGA/OLGIM stage
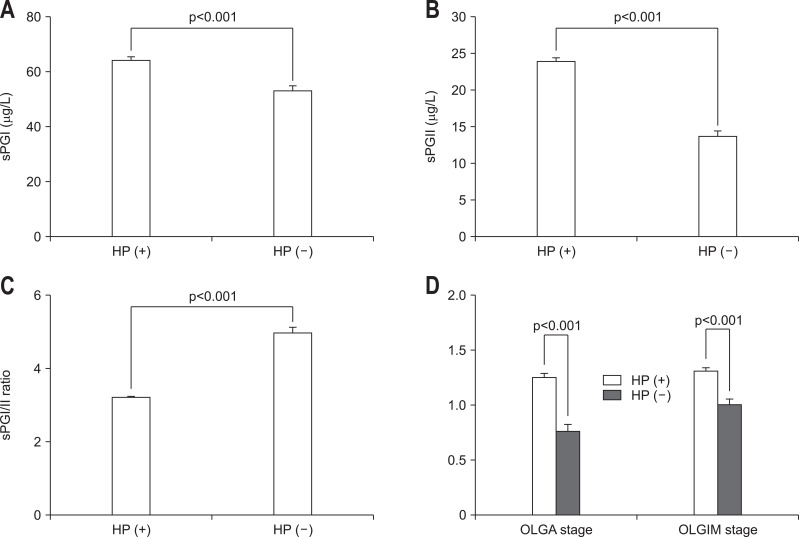
Considerable differences were found in sPGs and were dependent on HP status (Fig. 2). HP status-positive patients had higher sPGI (Fig. 2A), sPGII (Fig. 2B), and a lower sPGI/II ratio (Fig. 2C) (all p<0.001). OLGA/OLGIM stage, an indicator of atrophic gastritis and intestinal metaplasia, was higher in HP status-positive patients (p<0.001) (Fig. 2D).
Fig. 2.
Comparison of serum pepsinogen (sPG) levels and histologic features with respect to Helicobacter pylori (HP) status. HP-positive patients had higher sPGI (A) and sPGII levels (B) and a lower sPGI/II ratio (C) (all p<0.001). OLGA/OLGIM stage, an indicator of atrophic gastritis and intestinal metaplasia, was higher in HP-positive patients (D). Data are presented as the number (%) or median±standard error.
OLGA, operative link on gastritis atrophy; OLGIM, operative link on gastric intestinal metaplasia.
4. Correlation among sPGs, HP status and gastric dysplasia or GC
Table 3 summarizes the risk of gastric dysplasia and GC according to sPGs and HP status. The ORs of the sPGI/II ratio <3 group were 2.77 for gastric dysplasia and 2.25 for GC. Among the GC subtypes, the OR for IGC was higher than for DGC when the sPGI/II ratio was less than 3 (OR, 2.50; p<0.001), especially in advanced-stage disease (OR, 3.40; p<0.001). In contrast, sPGII ≥20 µg/L was higher in DGC (OR, 1.78; p<0.001), and especially in early-stage disease (OR, 3.12; p<0.001) but was not higher in IGC. sPGI <70 µg/L showed similar results in gastric dysplasia (OR, 2.06; p<0.001) and IGC (OR, 1.68; p<0.001) compared to sPGI/II ratio, but overall odd ratios were low. In addition, sPGI was not associated with total GC. Instead, high sPGI was rather more associated with DGC. When the implication of HP status was examined, positive HP status was related to an increased risk for both gastric dysplasia (OR, 2.56; p<0.001) and GC (OR, 2.06; p<0.001). Among GC types, a significantly higher risk for DGC than for IGC, was found when HP status was positive (OR, 2.54; p<0.001).
Table 3.
Correlation among sPGs, HP Status, Gastric Dysplasia, and GC
| Variable | Gastric dysplasia | GC | Intestinal GC | Diffuse GC | ||||
|---|---|---|---|---|---|---|---|---|
| Total | EGC | AGC | Total | EGC | AGC | |||
| sPGI, µg/L | ||||||||
| ≥70 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| <70 | 2.06 (<0.001)* | 1.00 (0.996) | 1.68 (<0.001)* | 1.68 (<0.001)* | 1.84 (0.003)* | 0.68 (0.001)* | 0.48 (<0.001)* | 0.94 (0.680) |
| sPGII, µg/L | ||||||||
| <20 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| ≥20 | 0.72 (0.009)* | 1.32 (<0.001)* | 1.04 (0.722) | 0.97 (0.775) | 1.24 (0.189) | 1.78 (<0.001)* | 3.12 (<0.001)* | 1.12 (0.426) |
| sPGI/II ratio | ||||||||
| ≥3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| <3 | 2.77 (<0.001)* | 2.25 (<0.001)* | 2.50 (<0.001)* | 2.25 (<0.001)* | 3.40 (<0.001)* | 1.93 (<0.001)* | 1.96 (<0.001)* | 1.91 (<0.001)* |
| HP status | ||||||||
| (–) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| (+) | 2.56 (<0.001)* | 2.06 (<0.001)* | 1.84 (<0.001)* | 1.88 (<0.001)* | 1.76 (0.009)* | 2.54 (<0.001)* | 3.03 (<0.001)* | 2.23 (<0.001)* |
Data are presented as odds ratio (p-value). Logistic model adjusted for sex and age.
sPG, serum pepsinogen; HP, Helicobacter pylori; GC, gastric cancer; EGC, early gastric cancer; AGC, advanced gastric cancer.
Asterisk indicates statistical significance.
5. The detective power of sPGII for the diagnosis of GC
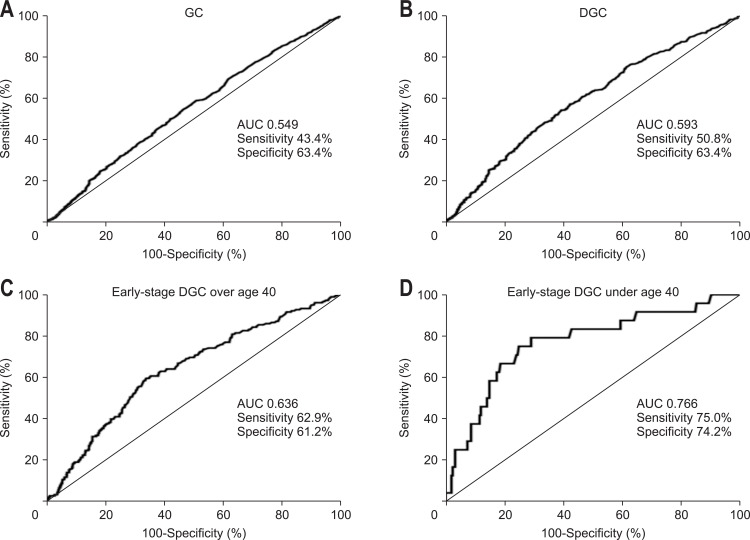
We have calculated the AUC to check whether high sPGII is useful to detect early-stage DGC or not. sPGII did not show significant AUC with sensitivity and specificity for total GC (Fig. 3A) and DGC (Fig 3B). However, when divided into early and advanced cancer, AUC increased in early-stage DGC. Receiver operating characteristic curve revealed that the optimal sPGII cutoff value was 20 µg/L (AUC of 0.636) for the diagnosis of early-stage DGC, with 62.9% sensitivity and 61.2% specificity. When the patients were categorized with age 40, sPGII showed significantly higher diagnostic power for the patients with early-stage DGC under age 40 (Fig. 3D) than those with age equal or greater than 40 (AUC of 0.766, 75.0% sensitivity, 74.2% specificity) (Fig. 3C).
Fig. 3.
Receiver operating characteristic curve and corresponding AUC of sPGII for the diagnosis of gastric cancer (GC). The AUC for sPGII did not show significant sensitivity and specificity for total GC (A) and DGC (B). However, when the patients with early DGC were grouped with age 40 years as the cutoff, sPGII showed significantly higher diagnostic power for patients with early-stage DGC under 40 years of age (AUC 0.766, 75.0% sensitivity, 74.2% specificity) (D) than those 40 years or older (C).
AUC, area under the curve; sPG, serum pepsinogen; DGC, diffuse gastric cancer.
6. Correlation between sPGII and HP status for the risk of DGC
To find the most powerful model for the prediction of DGC by summing up the results above, a risk stratification analysis was performed. Considering the low-risk group as sPGII <20 µg/L with negative HP status, the intermediate-risk (either sPGII ≥20 µg/L or positive HP status) and high-risk groups (both sPGII ≥20 µg/L and positive HP status) were defined according to the combination of HP status and a PGII level ≥20 µg/L (Table 4). In high-risk subjects, the OR of DGC was 3.44 (p<0.001) compared with low-risk subjects. Early-stage DGC (OR, 5.20; p<0.001) showed a higher association with the high-risk group than advanced-stage DGC (OR, 1.92; p=0.013). When we analyzed the subgroups by age and sex, the OR of early-stage DGC for 40 years of age and younger in the high-risk group was 12.76 (p=0.001) and was found to be highest in female under age 40 years (OR, 21.00; p=0.006) (Table 4).
Table 4.
Diffuse-Type GC Risk Stratification by Combining sPGII and HP Status
| Variable | Risk | HP/sPGII | Diffuse GC | Early DGC | Advanced DGC |
|---|---|---|---|---|---|
| Total | Low | – / – | 1 | 1 | 1 |
| Intermediate | + / – | 2.26 (<0.001) | 1.82 (0.040) | 2.53 (<0.001) | |
| – / + | 1.57 (0.193) | 1.55 (0.411) | 1.59 (0.292) | ||
| High | + / + | 3.44 (<0.001) | 5.20 (<0.001) | 1.92 (0.013) | |
| Age ≥40 yr | Low | – / – | 1 | 1 | 1 |
| Intermediate | + / – | 2.21 (<0.001) | 1.73 (0.079) | 2.32 (<0.001) | |
| – / + | 1.55 (0.219) | 1.49 (0.460) | 1.46 (0.391) | ||
| High | + / + | 2.88 (<0.001) | 4.32 (<0.001) | 1.83 (0.021) | |
| Age <40 yr | Low | – / – | 1 | 1 | 1 |
| Intermediate | + / – | 1.63 (0.353) | 1.63 (0.581) | 1.63 (0.442) | |
| – / + | 0.00 (0.999) | 0.00 (0.999) | 0.00 (0.999) | ||
| High | + / + | 8.04 (<0.001) | 12.76 (0.001) | 5.67 (0.003) | |
| Male <40 yr | Low | – / – | 1 | 1 | 1 |
| Intermediate | + / – | 3.38 (0.121) | 3.12 (0.300) | 3.64 (0.235) | |
| – / + | 0.00 (0.999) | 0.00 (0.999) | 0.00 (0.999) | ||
| High | + / + | 5.00 (0.133) | 5.00 (0.272) | 5.00 (0.282) | |
| Female <40 yr | Low | – / – | 1 | 1 | 1 |
| Intermediate | + / – | 4.24 (0.013) | 6.46 (0.084) | 3.50 (0.063) | |
| – / + | 0.00 (0.999) | 0.00 (0.999) | 0.00 (0.999) | ||
| High | + / + | 7.50 (0.003)* | 21.00 (0.006)* | 3.00 (0.542) |
Data are presented as odds ratio (p-value). Low risk, (HP –/PG –); intermediate risk, (HP +/PG –) or (HP –/PG +); high risk, (HP +/PG +). Individuals with PG II of ≥20 µg/L were classified as PG (+). Logistic model adjusted for sex and age.
GC, gastric cancer; sPG, serum pepsinogen; HP, Helicobacter pylori; DGC, diffuse gastric cancer.
Asterisk indicates statistical significance.
DISCUSSION
The levels of sPGI and the sPGI/II ratio are low in cases of atrophic gastritis and are effective biomarkers for GC screening in Japan. However, the role of sPGs in the application of Japan’s standard cutoff values for GC screening in South Korea is debatable. The specificity of sPGI was low, which demonstrates a prediction ability below expectations in our previous study.8 As no attractive biomarker for GC has been established thus far, we decided to investigate the role of sPGs in the development of GC, with a focus on the obscure role of sPGII, which has not been frequently reported. We found that sPGII ≥20 µg/L was associated with the development of DGC, particularly early-stage DGC of young age in this large cohort. The risk of early-stage DGC was significantly increased with sPGII ≥20 µg/L (OR, 3.12) and HP-positive status (OR, 3.03), and when these two conditions were present together, the OR became 12.76. The sPGII level has been known to be associated with the histological changes that reflect the degree of inflammation caused by HP infection in the gastric mucosa. That is, sPGII was higher in HP-associated non-atrophic gastritis and was lower in atrophic gastritis, and HP eradication could reverse the serum level of sPGII.17-20 A previous study also revealed that the level of PGII expression decreased significantly with the degree of malignancy of the gastric mucosa and that the positive rate of PGII expression was regulated by HP infection.21 In this study, DGC patients had a high sPGII level and tended to have more mucosal inflammation than control subjects. These results are consistent with the previous hypothesis that HP-induced active inflammation directly induces DGC without progression through Correa’s cascade.22-25
As is well known, HP induces chronic inflammation in the gastric mucosa, which leads to the sequence of chronic active gastritis, atrophy, intestinal metaplasia, dysplasia and GC. This cascade is considered to be a major process of gastric carcinogenesis, especially IGC.23,26,27 However, 20% to 30% of all GCs in Western countries develop from non-atrophic mucosa, and the background gastric mucosa did not appear to exhibit extensive atrophy in 20% to 40% of GC cases in a Japanese study.23,28-30 Of those GCs with non-atrophic mucosa, a considerable proportion are the DGC histopathological type, which has higher malignancy, greater metastatic potential and a poorer prognosis than IGC. In the present study, the early gastric cancer (EGC) percentage of DGC was 45.5%, which is quite lower than that of IGC at 73.9%. The possible mechanisms of DGC development are that HP-induced inflammation is believed to generate several genetic alterations in the gastric mucosa.23,31 For instance, HP infection induces CpG island methylation and E-cadherin gene inactivation by DNA methylation.32-35 Another explanation is that the cytotoxins induced by HP produce carcinogens such as oxygen free radicals and superoxide, which can trigger mutations in the pepsinogen gene; these mutations can then affect the balance among cell proliferation, differentiation and apoptosis, and as a consequence, lead to DGC.36-38
Previously, a few studies in Japan have reported the relationship between sPGII and GC.9-11 Kikuchi et al.9 reported high sensitivity (83.3% for total GC and 85.0% for EGC) and high specificity (76.9% for hospital control subjects and 75.0% for screening control subjects) for sPGII >14.8 µg/L in a young population. In a study by Yanaoka et al.,10 the risk of DGC increased with sPGII >30 µg/L (hazard ratio, 3.81), which was followed by the group ≤30 and >10 µg/L, and then the group ≤10 µg/L. Another case-control study revealed a significantly higher risk for early-stage DGC in patients with a sPGII level >30 µg/L (OR, 4.1 in men; OR, 7.4 in women); that study yielded results similar to those of our study. However, the number of subjects in the study was quite small (42 subjects with early-stage DGC, 511 age-matched control subjects) and they did not include gastric dysplasia, IGC and advanced-stage DGC in analysis compared with the present study.11
In our study, the high level of sPGII did not show any significant correlation with advanced-stage DGC in contrast to EGC, which needs explanation. A previous study found that the suppression of T cell activation by myeloid-derived suppressor cells was highly correlated with a more advanced-stage of GC and that they contributed to immune dysfunction.39 Weakened inflammatory reactions against HP infection due to immune suppression may result in a decreased level of sPGII. Especially, this tendency looks like to be stronger in the diffuse-type, developed directly by HP-induced inflammation in comparison to the intestinal-type. However, in IGC, sPGII was higher in advanced-stage than early-stage in contrast to diffuse type. In a study by Stemmermann and Nomura,40 sPGII was more likely to be expressed with moderate or extensive intestinal metaplasia than with minimal or no intestinal metaplasia. Cancers that expressed sPGII were found to be of higher stage than those that did not. They assumed that cancers originating from intestinalized glands may subsequently revert to a gastric phenotype.40 Thus we hypothesized that high sPGII in advanced-stage IGC might be derived from postinduction reversion to a gastric phenotype from intestinalized cells. However, in early stage of intestinal type this phenomenon did not frequently occur that sPGII was not so high. The significance of young age and female obtained in our study is very meaningful for the use of sPGII as a biomarker of DGC. The risk of DGC for those under age 40 was higher than those who were older than 40 and female showed higher proportion of DGC than male, which is consistent with the previously known epidemiologic features of DGC.41,42 However, the incidence of GC is very low in those below age 40 years, and the Korean National Cancer Screening Program for GC provides a complimentary endoscopy for those above age 40. Thus, most patients with DGC below age 40 could not be detected at the early stage because no symptoms are present in early-stage disease. Proper evaluation of the risk for early-stage DGC using noninvasive markers in young Koreans would make it possible to perform a careful endoscopic follow-up before DGC development.
sPGI <70 µg/L was insufficient, which is in agreement with what we reported in 2008.8 That is, the odd ratio for GC in comparison to controls was not significant as a biomarker for GC screening in South Korea (OR, 1.00). When limited to IGC, the odd ratio was 1.68 (1.68 in EGC, 1.84 in advanced-stage gastric cancer), but it was still insufficient compared with the findings in Japanese studies.43-47 This result was presumed to be due to not only one reason but to various factors, such as differences in the proportions of IGC and DGC, HP infection rate, eradication rate and testing equipment.43,48-50 An sPGI/II ratio cutoff of <3 was found to have an odd ratio of 2.77 for gastric dysplasia and 2.25 for GC. When GC was classified according to histology and stage, the sPGI/II ratio was more valuable for IGC than for DGC, was more valuable for advanced-stage disease than for early-stage disease, and showed the best performance for advanced-stage IGC (OR, 3.40). These results are thought to be related to different grades of atrophic gastritis and intestinal metaplasia according to histologic type and stage. Our results showed that the OLGA and OLGIM stages were actually higher in IGC than in DGC and in advanced-stage gastric cancer than in EGC. Thus, it appears reasonable that the sPGI/II ratio is an appropriate marker for atrophic gastritis and is closely related to IGC.
Our study also confirmed that HP status itself is an independent risk factor for DGC and it was riskier than IGC. In addition, high-risk OLGA stage as well as high-risk OLGIM stage was associated with not only for IGC but also DGC.7 The present study also showed that high-risk OLGA and OLGIM have taken up a significant portion of DGC (14.4% and 15.5%). Thus, in addition to the direct mechanism of inflammation described above, atrophy and intestinal metaplasia might cause development of DGC in some part.
Our study has several advantages. First, this is the first study to reveal the relationship between sPGII and DGC in South Korea. Previous retrospective Korean study analyzed the serum trefoil factor 3 and sPGI/II ratio in association with DGC, but did not conducted about sPGII.51 Second, nearly 3,000 subjects including those with gastric dysplasia and GC as well as controls were enrolled, which is a higher number compared with the number of subjects in our previous study and other small studies. Third, the grades of atrophic gastritis and intestinal metaplasia were calculated by histologic stage (OLGA/OLGIM) rather than by visual classification. Finally, since the GC stages were classified by histology and HP status, the detailed role of sPGs was evaluated for predicting the risk of GC. There were also limitations in this study. Single center-based case‐control study may lead to selection bias, therefore, the results of this study may not be able to represent the entire South Korean people. Also, a history of using proton pump inhibitors was not investigated in detail in this study which may influence the level of sPGs. However, this study included relatively large population and the history of proton pump inhibitors is not so frequent that the bias may not affect the results much.
In conclusion, our results suggest that a high sPGII level (≥20 µg/L) and positive HP status are useful to detect early-stage DGC in those under age 40, especially in young women. The combination of these two factors in a young population will predict the occurrence of early-stage DGC and will enable a more detailed and intensive endoscopic follow-up.
Supplemental Materials
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Research Foundation (NRF) of Korea to the Global Core Research Center (GCRC) funded by the Korean government (MSIP) (grant number: 2011-0030001).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: N.K. Data acquisition: S.M.B., N.K., Y.J.K., H.S.L., H.Y.K. Data analysis and interpretation: S.M.B., N.K. Drafting of the manuscript; critical revision of the manuscript for important intellectual content: S.M.B., N.K. Statistical analysis: S.M.B., J.L. Administrative, technical, or material support; study supervision: H.Y., C.M.S., Y.S.P., D.H.L.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Jun JK, Choi KS, Lee HY, et al. Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology. 2017;152:1319–1328. doi: 10.1053/j.gastro.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Samloff IM. Pepsinogens, pepsins, and pepsin inhibitors. Gastroenterology. 1971;60:586–604. doi: 10.1016/S0016-5085(71)80065-3. [DOI] [PubMed] [Google Scholar]
- 4.Kodoi A, Yoshihara M, Sumii K, Haruma K, Kajiyama G. Serum pepsinogen in screening for gastric cancer. J Gastroenterol. 1995;30:452–460. doi: 10.1007/BF02347560. [DOI] [PubMed] [Google Scholar]
- 5.Samloff IM, Varis K, Ihamaki T, Siurala M, Rotter JI. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology: a study in relatives of patients with pernicious anemia. Gastroenterology. 1982;83:204–209. doi: 10.1016/0016-5085(82)90176-7. [DOI] [PubMed] [Google Scholar]
- 6.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 7.Yun CY, Kim N, Lee J, et al. Usefulness of OLGA and OLGIM system not only for intestinal type but also for diffuse type of gastric cancer, and no interaction among the gastric cancer risk factors. Helicobacter. 2018;23:e12542. doi: 10.1111/hel.12542. [DOI] [PubMed] [Google Scholar]
- 8.Kang JM, Kim N, Yoo JY, et al. The role of serum pepsinogen and gastrin test for the detection of gastric cancer in Korea. Helicobacter. 2008;13:146–156. doi: 10.1111/j.1523-5378.2008.00592.x. [DOI] [PubMed] [Google Scholar]
- 9.Kikuchi S, Wada O, Miki K, et al. Serum pepsinogen as a new marker for gastric carcinoma among young adults. Research Group on Prevention of Gastric Carcinoma among Young Adults. Cancer. 1994;73:2695–2702. doi: 10.1002/1097-0142(19940601)73:11<2695::AID-CNCR2820731108>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 10.Yanaoka K, Oka M, Yoshimura N, et al. Risk of gastric cancer in asymptomatic, middle-aged Japanese subjects based on serum pepsinogen and Helicobacter pylori antibody levels. Int J Cancer. 2008;123:917–926. doi: 10.1002/ijc.23571. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Yoshihara M, Takata S, et al. Serum screening for detection of high-risk group for early-stage diffuse type gastric cancer in Japanese. J Gastroenterol Hepatol. 2012;27:598–602. doi: 10.1111/j.1440-1746.2011.06893.x. [DOI] [PubMed] [Google Scholar]
- 12.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 13.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Loffeld RJ, Stobberingh E, Flendrig JA, Arends JW. Helicobacter pylori in gastric biopsy specimens: comparison of culture, modified Giemsa stain, and immunohistochemistry. A retrospective study. J Pathol. 1991;165:69–73. doi: 10.1002/path.1711650111. [DOI] [PubMed] [Google Scholar]
- 15.Chey WD, Wong BC Practice Parameters Committee of the American College of Gastroenterology, author. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Kim N, Kim MS, et al. Factors affecting first-line triple therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci. 2014;59:1235–1243. doi: 10.1007/s10620-014-3093-7. [DOI] [PubMed] [Google Scholar]
- 17.Plebani M, Basso D, Cassaro M, et al. Helicobacter pylori serology in patients with chronic gastritis. Am J Gastroenterol. 1996;91:954–958. [PubMed] [Google Scholar]
- 18.Mårdh E, Mårdh S, Mårdh B, Borch K. Diagnosis of gastritis by means of a combination of serological analyses. Clin Chim Acta. 2002;320:17–27. doi: 10.1016/S0009-8981(02)00040-2. [DOI] [PubMed] [Google Scholar]
- 19.Pilotto A, Di Mario F, Franceschi M, et al. Cure of Helicobacter pylori infection in the elderly: effects of eradication on gastritis and serological markers. Aliment Pharmacol Ther. 1996;10:1021–1027. doi: 10.1046/j.1365-2036.1996.88260000.x. [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Miki K, Ichinose M, et al. Changes in evaluation of the pepsinogen test result following Helicobacter pylori eradication therapy in Japan. Inflammopharmacology. 2007;15:31–35. doi: 10.1007/s10787-006-0009-y. [DOI] [PubMed] [Google Scholar]
- 21.Ning PF, Liu HJ, Yuan Y. Dynamic expression of pepsinogen C in gastric cancer, precancerous lesions and Helicobacter pylori associated gastric diseases. World J Gastroenterol. 2005;11:2545–2548. doi: 10.3748/wjg.v11.i17.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sipponen P, Kosunen TU, Valle J, Riihelä M, Seppälä K. Helicobacter pylori infection and chronic gastritis in gastric cancer. J Clin Pathol. 1992;45:319–323. doi: 10.1136/jcp.45.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nardone G, Rocco A, Malfertheiner P. Review article: Helicobacter pylori and molecular events in precancerous gastric lesions. Aliment Pharmacol Ther. 2004;20:261–270. doi: 10.1111/j.1365-2036.2004.02075.x. [DOI] [PubMed] [Google Scholar]
- 24.Correa P. Precursors of gastric and esophageal cancer. Cancer. 1982;50:2554–2565. [PubMed] [Google Scholar]
- 25.Laurén P. Histogenesis of intestinal and diffuse types of gastric carcinoma. Scand J Gastroenterol Suppl. 1991;180:160–164. doi: 10.3109/00365529109093194. [DOI] [PubMed] [Google Scholar]
- 26.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 27.Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–672. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Vauhkonen M, Vauhkonen H, Sipponen P. Pathology and molecular biology of gastric cancer. Best Pract Res Clin Gastroenterol. 2006;20:651–674. doi: 10.1016/j.bpg.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Yanaoka K, Oka M, Mukoubayashi C, et al. Cancer high-risk subjects identified by serum pepsinogen tests: outcomes after 10-year follow-up in asymptomatic middle-aged males. Cancer Epidemiol Biomarkers Prev. 2008;17:838–845. doi: 10.1158/1055-9965.EPI-07-2762. [DOI] [PubMed] [Google Scholar]
- 30.Ohata H, Oka M, Yanaoka K, et al. Gastric cancer screening of a high-risk population in Japan using serum pepsinogen and barium digital radiography. Cancer Sci. 2005;96:713–720. doi: 10.1111/j.1349-7006.2005.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Correa P. Does Helicobacter pylori cause gastric cancer via oxidative stress? Biol Chem. 2006;387:361–364. doi: 10.1515/BC.2006.048. [DOI] [PubMed] [Google Scholar]
- 32.Kang GH, Lee S, Kim JS, Jung HY. Profile of aberrant CpG island methylation along the multistep pathway of gastric carcinogenesis. Lab Invest. 2003;83:635–641. doi: 10.1097/01.LAB.0000067481.08984.3F. [DOI] [PubMed] [Google Scholar]
- 33.Chan AO, Lam SK, Wong BC, et al. Promoter methylation of E-cadherin gene in gastric mucosa associated with Helicobacter pylori infection and in gastric cancer. Gut. 2003;52:502–506. doi: 10.1136/gut.52.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maekita T, Nakazawa K, Mihara M, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989–995. doi: 10.1158/1078-0432.CCR-05-2096. [DOI] [PubMed] [Google Scholar]
- 35.Tamura G, Yin J, Wang S, et al. E-cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst. 2000;92:569–573. doi: 10.1093/jnci/92.7.569. [DOI] [PubMed] [Google Scholar]
- 36.Kuwahara Y, Kono S, Eguchi H, Hamada H, Shinchi K, Imanishi K. Relationship between serologically diagnosed chronic atrophic gastritis, Helicobacter pylori, and environmental factors in Japanese men. Scand J Gastroenterol. 2000;35:476–481. doi: 10.1080/003655200750023723. [DOI] [PubMed] [Google Scholar]
- 37.Backert S, Schwarz T, Miehlke S, et al. Functional analysis of the cag pathogenicity island in Helicobacter pylori isolates from patients with gastritis, peptic ulcer, and gastric cancer. Infect Immun. 2004;72:1043–1056. doi: 10.1128/IAI.72.2.1043-1056.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuniyasu H, Kitadai Y, Mieno H, Yasui W. Helicobactor pylori infection is closely associated with telomere reduction in gastric mucosa. Oncology. 2003;65:275–282. doi: 10.1159/000074481. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol. 2013;190:794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- 40.Stemmermann GN, Nomura AM. The relation of pepsinogen group II (PGII) expression to intestinal metaplasia and gastric cancer. Histopathology. 2006;49:45–51. doi: 10.1111/j.1365-2559.2006.02446.x. [DOI] [PubMed] [Google Scholar]
- 41.Bedikian AY, Khankhanian N, Heilbrun LK, Bodey GP, Stroehlein JR, Valdivieso M. Gastric carcinoma in young adults. South Med J. 1979;72:654–656. doi: 10.1097/00007611-197906000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Isobe T, Hashimoto K, Kizaki J, et al. Characteristics and prognosis of gastric cancer in young patients. Oncol Rep. 2013;30:43–49. doi: 10.3892/or.2013.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshihara M, Sumii K, Haruma K, et al. Correlation of ratio of serum pepsinogen I and II with prevalence of gastric cancer and adenoma in Japanese subjects. Am J Gastroenterol. 1998;93:1090–1096. doi: 10.1111/j.1572-0241.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- 44.Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9:245–253. doi: 10.1007/s10120-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 45.Kitahara F, Kobayashi K, Sato T, Kojima Y, Araki T, Fujino MA. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut. 1999;44:693–697. doi: 10.1136/gut.44.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinis-Ribeiro M, Yamaki G, Miki K, Costa-Pereira A, Matsukawa M, Kurihara M. Meta-analysis on the validity of pepsinogen test for gastric carcinoma, dysplasia or chronic atrophic gastritis screening. J Med Screen. 2004;11:141–147. doi: 10.1258/0969141041732184. [DOI] [PubMed] [Google Scholar]
- 47.Hattori Y, Tashiro H, Kawamoto T, Kodama Y. Sensitivity and specificity of mass screening for gastric cancer using the measurment of serum pepsinogens. Jpn J Cancer Res. 1995;86:1210–1215. doi: 10.1111/j.1349-7006.1995.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oishi Y, Kiyohara Y, Kubo M, et al. The serum pepsinogen test as a predictor of gastric cancer: the Hisayama study. Am J Epidemiol. 2006;163:629–637. doi: 10.1093/aje/kwj088. [DOI] [PubMed] [Google Scholar]
- 49.Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138–143. doi: 10.1002/ijc.11680. [DOI] [PubMed] [Google Scholar]
- 50.Watabe H, Mitsushima T, Yamaji Y, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764–768. doi: 10.1136/gut.2004.055400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JY, Park KS, Lee HG, et al. Comparison of serum trefoil factor 3 with the pepsinogen test for the screening of diffuse-type gastric cancer. Clin Exp Med. 2017;17:403–410. doi: 10.1007/s10238-016-0426-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.