Abstract
Background/Aims
Limited data are available regarding psychosocial distress at the time of diagnosis of ulcerative colitis (UC). We investigated the psychosocial burden and factors related to poor health-related quality of life (HRQL) among patients newly diagnosed with moderate-to-severe UC who were affiliated with the nationwide prospective cohort study.
Methods
Within the first 4 weeks of UC diagnosis, all patients were assessed using the Hospital Anxiety and Depression Scale (HADS), Work Productivity and Activity Impairment questionnaire, Inflammatory Bowel Disease Questionnaire (IBDQ), and 12-Item Short Form (SF-12) health survey. A multiple linear regression model was used to identify factors associated with HRQL.
Results
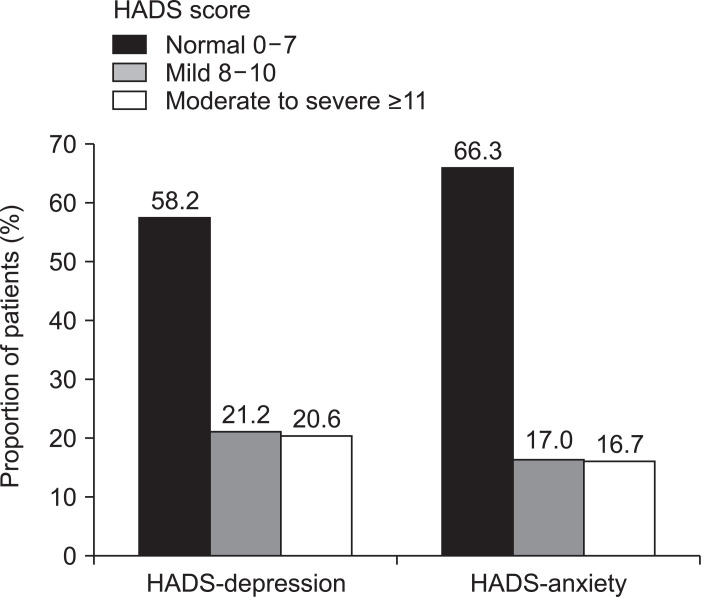
Between August 2014 and February 2017, 355 patients completed questionnaires. Significant mood disorders requiring psychological interventions, defined by a HADS score ≥11, were identified in 16.7% (anxiety) and 20.6% (depression) of patients. Patients with severe disease were more likely to have presenteeism, loss of work productivity, and activity loss than those with moderate disease (all p<0.05). Significant mood disorders had the strongest negative relationship with total IBDQ score, which indicates disease-specific HRQL (β coefficient: –22.1 for depression and –40.0 for anxiety, p<0.001). The scores of all SF-12 dimensions, which indicate general HRQL, were remarkably decreased in the study population compared indirectly with previously reported scores in the general population. The Mayo score, C-reactive protein level, and white blood cell count showed significant negative associations with the IBDQ score (p<0.05).
Conclusions
Psychosocial screening and timely interventions should be incorporated into the initial care of patients newly diagnosed with UC.
Keywords: Colitis, ulcerative; Patient reported outcome measures; Quality of life; Anxiety; Depression
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disorder with alternating periods of relapse and remission that affects the health-related quality of life (HRQL) of affected patients, including physical, mental, and social well-being.1,2 Therefore, HRQL and other patient-reported outcomes (PROs) are now accepted as important endpoints in clinical trials and as treatment targets in real practice for UC and other inflammatory bowel diseases (IBDs).3,4 Previous studies have reported that HRQL in UC is adversely affected by several factors, including patient’s demographics (low socioeconomic status or female sex), disease characteristics (high disease activity and frequent relapses), daily symptoms and impaired activities.1,5-9 In addition, it has been suggested that comorbid psychological disorders such as anxiety and depression have a negative impact on HRQL in UC.10,11 However, several unanswered questions remain regarding psychosocial issues in UC.
First, there is a lack of information on the extent of the psychosocial burden in newly-diagnosed patients with UC. Given diagnostic delay and disease activity, patients with UC often have serious psychosocial problems at the time of diagnosis.12,13 However, limited data are available regarding the unmet psychosocial needs of newly-diagnosed patients with UC.14 Moreover, it is uncertain what comprises the major determinants of HRQL in UC. Finally, available data on the PROs of non-Caucasian patients with UC are also scarce.15,16 The incidence and prevalence of UC in Asian countries have been rapidly increasing;17-19 despite this, psychosocial assessments are overlooked in practice, and only a few studies have addressed these issues.7,20,21
In this background, we aimed to comprehensively quantify the burden of psychosocial distress and to identify significant predictors affecting HRQL among newly-diagnosed patients with UC. All patients were affiliated with the MOSAIK (moderate-to-severe ulcerative colitis in Korea) cohort study. It is a nationwide, prospective inception cohort study, the aims of which is to reveal the natural course of disease in patients with newly-diagnosed active (moderate-to-severe) UC. We prospectively collected comprehensive PRO data via a patient survey and clinical data at regular intervals.
Materials and methods
1. Study population and design
This is an ongoing multicenter, prospective, hospital-based, observational cohort study of newly-diagnosed patients with moderate-to-severe UC recruited from 30 academic teaching hospitals in Korea.22 Inclusion criteria were as follows: aged ≥7 years at the time of enrollment; newly-diagnosed with moderate-to-severe UC at tertiary referral hospital within 4 weeks prior to enrollment. If a patient was to be included after a primary or secondary hospital diagnosis, they had to have received a diagnosis in a tertiary referral hospital within 8 weeks from a primary or secondary hospital; willingness to participate in the study by providing the informed consent. Patients were excluded, if they met at least one of the following criteria: participants in an interventional clinical trial with systemic corticosteroid, biologics (including, but not limited to, infliximab, golimumab, adalimumab, vedolizumab and etc.) or other drugs (including, but not limited to, sulfasalazine, mesalamine, azathioprine, tofacitinib and etc.) for UC; having experienced colectomy such as subtotal colectomy with ileorectostomy or colectomy with ileoanal pouch, Koch pouch, or ileostomy for UC; a current diagnosis of indeterminate colitis, or current diagnosis or history of Crohn’s disease.
Diagnosis was based on symptoms consistent with UC lasting for more than 4 weeks, according to international criteria.23 To establish a diagnosis, at least three of the following four criteria were met: a history of diarrhea, blood or pus in stools, macroscopic appearance on endoscopy, with persistent mucosal inflammation affecting the rectum in continuity with part or all of the colon, microscopic features on biopsy consistent with UC, and no suspicion of Crohn’s disease or indeterminate colitis. Infectious and other acute or chronic non-IBD conditions were excluded from the study.
The disease severity of moderate-to-severe UC was defined according to the Mayo scale; active UC was defined as a full Mayo score of 6–12 points (6–10 points for moderate; ≥11 for severe) and a Mayo endoscopic subscore of ≥2 points on colonoscopy or flexible sigmoidoscopy.24,25 Comprehensive clinical data were prospectively collected from all participants as follows: demographics, comorbidities, body mass index, perinatal history, vaccination history, surgical history, familial history of IBD in first-degree relatives, extraintestinal manifestations, disease-related symptoms, and laboratory tests.
2. Patient-reported outcome measurement tools
All patients were requested to complete four self-questionnaires within the first 4 weeks of diagnosis, including the Hospital Anxiety and Depression Scale (HADS) to assess emotional health, Work Productivity and Activity Impairment (WPAI) questionnaire to assess work disability in UC, and Inflammatory Bowel Disease Questionnaire (IBDQ) and 12-Item Short Form (SF-12) health survey to assess disease-specific and generic HRQL.
3. Assessment of psychological distress
Anxiety and depression symptoms were assessed using the two types of validated Korean version of HADS, each called HADS-Anxiety (HADS-A) and HADS-Depression (HADS-D).26,27 They are 14-item self-reported scale designed to measure depression and anxiety symptoms in patients with a physical illness. Each item on the questionnaire is given a score of 0-3, with higher scores representing a higher level of anxiety or depression. Severity is classified as follows: score of 0–7 (normal), 8-10 (mild), 11–14 (moderate), and ≥15 (severe). A score ≥11 (each of HADS-A≥11, HADS-D≥11) is highly suggestive of a significant mood disorder and indicates a low threshold for pursuing psychological intervention.28
4. Assessment of work disability
Work productivity was assessed using questionnaire for UC (WPAI:UC, Korean-Korea v2.0) which consists of six questions that focus on the previous 7 days. These six questions are combined into four items: hours of work missed due to the disease (absenteeism), degree of reduced productivity while working (presenteeism), regular daily activities (activity impairment) and overall work impairment. The scores range from 0% to 100% with a higher score indicating greater activity impairment and less productivity.29
5. Assessment of HRQL
IBDQ is a widely used disease-specific questionnaire that measures the HRQL in patients with IBD.30 The validated Korean version of IBDQ consists of 32-items that explores four dimensions: bowel symptoms (10 items), systemic symptoms (5 items), emotional function (12 items) and social function (5 items).31 The total score on this questionnaire ranges from 32 to 224 with higher scores indicating a better quality of life. 31,32
SF-12 is a simplified version of the SF-36 and is one of the most popular self-reported multifunctional generic measures of HRQL.33 It is composed of 12 items that assess eight health concepts including (1) physical functioning, (2) role-physical, (3) bodily pain, (4) general health, (5) vitality, (6) social functioning, (7) role-emotional, and (8) mental health. Scores of the eight scales were calculated according to the manual of scoring into the 0 to 100 range.34 A higher SF-12 scores indicate better quality of life. The scores of all SF-12 items in the study cohort were indirectly compared with those of data from the general Korean population in a previous published paper.35
6. Outcome measurements and statistical analysis
The primary outcome of our study was to estimate the burden of psychosocial distress at the time of disease diagnosis in the study cohort. Descriptive statistics for continuous variables are presented as means with standard deviation (SD), and dichotomous variables are presented as frequencies with percentages in parentheses. The PRO measures were compared using the Mann-Whitney U-test or t-test, where appropriate by disease severity on baseline. To identify factors independently associated with quality of life, we used multiple linear regression analysis. The selection of variables included in the multiple linear regression model was based on results of the univariate analyses (p≤0.1) of the relevant variables. All analyses were performed using R software, version 3.4.3 (R foundation for Statistical Computing, Vienna, Austria) and SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA). p-values less than 0.05 were considered statistically significant.
7. Ethical considerations
The study was conducted according to the Declaration of Helsinki and was approved by the institutional review boards of all participating hospitals. Written informed consent was obtained from all participants. The study was registered at www.clinicaltrials.gov (ClinicalTrials.gov identifier: NCT02229344).
Results
1. Demographics and clinical characteristics of the study population
Between August 2014 and February 2017, 368 patients were considered eligible for and invited to participate in this study. Of them, 355 patients who met eligibility criteria were enrolled and completed the questionnaires. The baseline demographics and clinical characteristics are described in Table 1. The mean age at the time of diagnosis was 37.6 years, and the peak age of diagnosis was in the third decade of life (29.6%). The proportion of male patients was 59.2%, with a male to female ratio of 1.4:1. Sixteen patients (4.8%) had a family history of IBD among their first-degree relatives. Approximately half (48.6%) of patients were employed at the time of data collection.
Table 1.
Demographics and Disease Characteristics of the Study Cohort
| Characteristics | No. | Category | Value |
|---|---|---|---|
| Age (yr) at diagnosis | 355 | 37.6±15.2 | |
| Sex | 355 | Male/female | 210 (59.2)/145 (40.8) |
| Body mass index (kg/m2) | 344 | 22.4±3.2 | |
| Smoking | 342 | Never or former | 312 (91.2) |
| Current | 30 (8.8) | ||
| Alcohol | 345 | Never or past | 191 (55.3) |
| Current | 154(44.6) | ||
| Currently employed | 348 | Yes/no | 169 (48.6)/179 (51.4) |
| Family income* | 309 | Low (<200) | 83 (26.9) |
| Middle (≥200 and <400) | 139 (45.0) | ||
| High (≥400) | 87 (28.2) | ||
| Family history of IBD | 334 | UC/CD/others | 12 (3.6)/1 (0.3)/3 (0.9) |
| Symptoms duration† | 354 | 15.9±36.1 | |
| <4 wk | 142 (40.1) | ||
| ≥4 wk | 212 (59.9) | ||
| Total Mayo score | 355 | 8.1±1.6 | |
| Disease severity‡ | 355 | Moderate/severe | 330 (93.0)/25 (7.0) |
| Endoscopic severity§ | 355 | Moderate/severe | 255 (71.8)/100 (28.2) |
| Disease extentII | 349 | Proctitis | 35 (10.0) |
| Left-sided colitis | 162 (46.4) | ||
| Extensive colitis | 152 (43.6) | ||
| ESR, mm/hr | 304 | 20.8±19.3 | |
| C-reactive protein, mg/dL | 324 | 2.6±7.3 | |
| White cell count, 103/μL | 346 | 12.7±60.2 | |
| Hemoglobin, g/dL | 347 | 12.8±2.0 | |
| Albumin, g/dL | 304 | 4.0±0.6 |
Data are presented as the mean±SD or number (%).
IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; ESR, erythrocyte sedimentation rate.
10,000 Korean Won; †Time between the first development of disease-related symptoms and UC diagnosis; ‡Moderate: total Mayo score 6–10; severe: total Mayo score ≥11; §Moderate: Mayo endoscopic subscore=2; severe: Mayo endoscopic subscore=3; IIMontreal classification.
The mean duration from symptom onset to diagnosis was 15.9 (SD, 36.1) weeks; 59.9% of patients firstly began to notice UC-related symptoms more than 4 weeks before the diagnosis. The mean Mayo score was 8.1 (1.6), and 28.2% of patients had severe disease on endoscopy. The disease extent was left-sided in 46.4%, extensive colitis in 43.6%, and proctitis in 10.0% of patients. The mean levels of serum C-reactive protein and hemoglobin were 2.6 mg/dL and 12.8 g/dL, respectively.
2. Psychological comorbidities, work productivity and health-related quality of life
The prevalence of comorbid psychiatric illnesses, defined as a HADS score ≥8, among the patients was 33.7% for anxiety and 41.8% for depression (Fig. 1). Of these, significant mood disorders requiring psychological interventions, defined by a HADS score ≥11, were identified in 16.7% (anxiety) and 20.6% (depression) of patients. There was no significant difference in the mean HADS score according to disease severity (Table 2).
Fig. 1.
Distribution of the study population according to the Hospital Anxiety and Depression Scale (HADS).
Table 2.
Mean Values of Each Patient-Reported Outcome Measure
| PROs | Category | No. | Overall (n=355) | Disease severity | p-value | |
|---|---|---|---|---|---|---|
| Moderate (n=330)* | Severe (n=25)† | |||||
| HADS | Depression | 349 | 6.6±4.5 | 6.5±4.4 | 8.9±5.2 | 0.610 |
| Anxiety | 348 | 6.2±4.4 | 6.2±4.4 | 6.6±4.5 | 0.638 | |
| WPAI:UC | Absenteeism‡ | 149 | 29.1±36.9 | 27.3±35.8 | 54.1±43.5 | 0.102 |
| Presenteeism§ | 143 | 37.8±31.4 | 36.8±30.3 | 62.2±39.6 | 0.037 | |
| Overall work impairmentII | 130 | 45.5±32.7 | 44.1±31.9 | 70.7±37.8 | 0.048 | |
| Social activity impairment¶ | 344 | 46.4±31.2 | 45.2±31.0 | 61.2±31.0 | 0.014 | |
| IBDQ | Bowel system | 344 | 41.7±13.0 | 42.0±13.1 | 36.1±10.9 | 0.027 |
| Emotional health | 347 | 51.7±14.9 | 52.0±14.9 | 46.8±13.6 | 0.097 | |
| Systemic symptoms | 347 | 20.5±6.8 | 20.7±6.8 | 17.0±6.0 | 0.009 | |
| Social function | 309 | 23.2±7.7 | 22.8±7.9 | 18.0±6.8 | 0.003 | |
| Total score | 305 | 137.3±38.8 | 137.5±39.0 | 117.9±33.2 | 0.002 | |
Data are presented as the mean±SD.
PRO, patient-reported outcome; HADS, Hospital Anxiety and Depression Scale; WPAI, Work Productivity and Activity Impairment; UC, ulcerative colitis; IBDQ, Inflammatory Bowel Disease Questionnaire.
Total Mayo score 6–10 points; †Total Mayo score ≥11; ‡Absenteeism: percentage of work time missed=(Q2/[Q2+Q4])×100; §Presenteeism: percentage of impairment while working=(Q5/10)×100; IIPercentage of overall work impairment=(Q2/(Q2+Q4)+[(1–(Q2/(Q2+Q4)))×(Q5/10)])×100; ¶Percentage of social activity impairment=(Q6/10)×100; Q, questionnaire item.
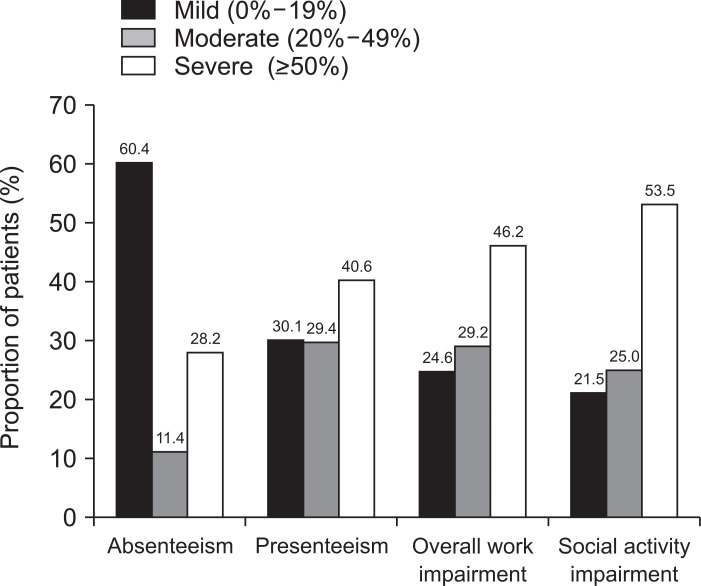
Among all respondents of the WPAI:UC, severe disabilities (defined as a WPAI:UC score ≥50%) were reflected as absenteeism (28.2%), presenteeism (40.6%), work productivity (46.2%), and social activity (53.5%), respectively (Fig. 2). Patients with severe disease were more likely to have presenteeism, overall work impairment, and social activity impairment, when compared to those with moderate disease (62.2% vs 36.8%; 70.7% vs 44.1%; 61.2% vs 45.2%, all p<0.05).
Fig. 2.
Assessment of work productivity and activity impairment using the Work Productivity and Activity Impairment-Ulcerative Colitis (WPAI-UC) questionnaire. Absenteeism: percentage of work time missed; presenteeism: percentage of impairment while working; percentage of overall work impairment=(Q2/(Q2+Q4)+[(1–(Q2/(Q2+Q4)))×(Q5/10)])×100; percentage of social activity impairment= (Q6/10)×100. Q, questionnaire item.
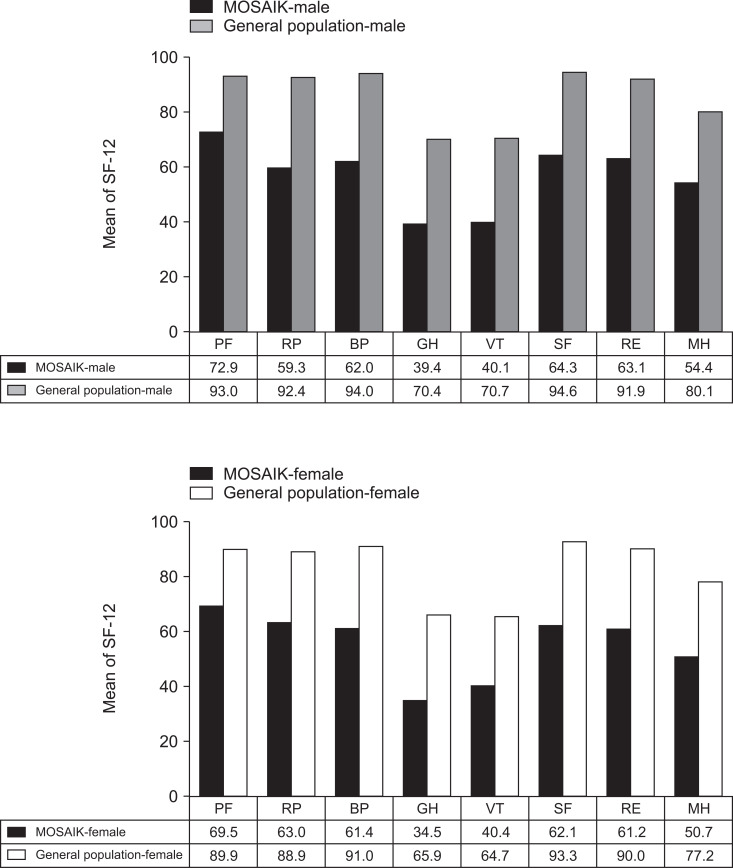
Fig. 3 shows the mean SF-12 component scores in the study cohort in men and women; our data were indirectly compared with those obtained from the general Korean population, which revealed that our study cohort tended to have a worse HRQL than that of the general population.35 With respect to disease-specific HRQL, patients with severe disease activity reported significantly a lower total IBDQ score, compared to those with moderate disease activity (117.9 vs 137.5, p<0.05).
Fig. 3.
12-Item Short Form (SF-12) results in the study cohort in comparison with those previously published for the general Korean population.33
PF, physical functioning; RP, role-physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role-emotional; MH, mental health; MOSAIK, moderate-to-severe ulcerative colitis in Korea.
3. Factors associated with HRQL
In our multiple regression model, significant mood disorders were found to be major determinants of HRQL. The presence of both significant depression and anxiety showed the strongest negative relationships with the total IBDQ score (β coefficient=–22.1 for depression and –40.0 for anxiety, all p<0.001) (Table 3) and almost all of the domains of the SF-12 (Supplementary Table 1). The total IBDQ score also had significant negative relationships with the total Mayo score, serum C-reactive protein (CRP) and white blood cell count (β=–4.1, β=–0.5, and β=–0.1, respectively; all p<0.05), but positive relationship with the hemoglobin level (β=2.3, p=0.02).
Table 3.
Multiple Linear Regression Analysis to Identify Factors Associated with Inflammatory Bowel Disease-Specific Health-Related Quality of Life
| Characteristics | Total IBDQ score | |
|---|---|---|
| β | p-value | |
| HADS-D≥11 | –22.1 | <0.001 |
| HADS-A≥11 | –40.0 | <0.001 |
| Hemoglobin, g/dL | 2.3 | 0.022 |
| CRP, mg/dL | –0.5 | 0.038 |
| WBC, 103/μL | –0.1 | 0.003 |
| Disease severity* | –4.1 | 0.002 |
IBDQ, Inflammatory Bowel Disease Questionnaire; HADS-D, Hospital Anxiety and Depression Scale-Depression; HADS-A, Hospital Anxiety and Depression Scale-Anxiety; CRP, C-reactive protein; WBC, white blood cell.
Total Mayo score. Reference variables: HADS-D/A (<11), disease severity (moderate).
Discussion
This large-scale nationwide prospective cohort study in Korea clearly show that the burden of psychosocial distress is very high among newly-diagnosed patients with UC. The prevalence of psychological comorbidities at the given time was as high as 33.7% for anxiety and 41.8% for depression. Approximately 50% of patients reported severe loss of work productivity (46.2%) and social activity (53.5%). Higher disease activity at the time of diagnosis was more likely to result in loss of work/social activity and low disease-specific HRQL. Our study also highlights that the presence of either significant anxiety or depression (either HADS-A or HADS-D≥11) had strong negative impact on both disease-specific and generic HRQL. These data strongly indicate a need for point-of-care screening and timely interventions for psychosocial distress among newly-diagnosed patients with UC, particularly moderate-to-severe UC.
Generally, patients with IBDs including UC more frequently have greater psychiatric distress, work disability and lower HRQL, compared with general population.1,2,6,10,36,37 Although the rates of psychiatric distress are generally higher in patients with active IBD than inactive IBD,36 there have been no studies conducting a comprehensive psychosocial evaluation in a subset of patients with “newly-diagnosed moderate to severe” UC. The prevalence rates of psychiatric comorbidities in the present study are comparable with those in a recent French nationwide study involving 1,185 patients with IBD (including 462 with UC), although there were considerable differences in the study populations (HADS-D ≥8, 41.8% in our study vs 48.5% in the UC subgroup of French cohort; HADS-A ≥8, 33.7% in our study vs 28.1% in the UC subgroup of French cohort).37 Given the disease activity (moderate-to-severe) of our study cohort, the reported rates of psychological comorbidities in this study seem reasonable. Of interest, approximately 20.0% of patients had clinically significant mood disorders requiring psychological interventions, suggesting the high unmet need of newly-diagnosed patients with moderate-to-severe UC. Psychological comorbidity is a factor contributing to non-adherence to medication and thus leading to adverse outcomes in UC.38,39 In this regard, our results also suggest that psychological screening and timely interventions should be integrated into the initial management of UC. Unfortunately, only a minority of “at risk” patients with IBD are believed to receive proper psychological interventions. In a Dutch study, over 60% of IBD patients with psychological comorbidity symptoms did not receive adequate care.40 In the National Audit report in the United Kingdom, only 12.0% of adult IBD services have a clinical resource for psychological services via a defined referral process.41 Therefore, integrated multidisciplinary care models may be helpful to deliver timely psychological services to patients with IBD.42,43
We provided objective indicators of work disability during the early course of UC, specifically within the first 4 weeks of diagnosis with active UC. We found that work disability was a common and serious problem in this population. The prevalence of severe disability was as high as 28.2% (absenteeism), 40.6% (presenteeism), 46.2% (work productivity), and 53.5% (social activity). Patients with severe disease were more likely to have a high level of work disability in terms of presenteeism and impairment of overall work and social activity. The prevalence of work productivity loss and social activity impairment in our cohort (severe ≥50%: 46.2% and 53.5%, respectively) are considerably higher than those in the UC subgroup of French cohort (18.1% and 33.1%), suggesting that work disability in patients with newly-diagnosed UC is a serious problem requiring social support.37 Recently, the impact on daily activities (disability) was suggested as a key factor contributing to overall UC severity,44 suggesting that the risk stratification by disability grade for an individual patient could guide personalized management plans for UC. Future prospective studies are expected to address this issue.
As expected, the HQRL among the MOSAIK cohort was severely impaired at baseline. The scores of all SF-12 dimensions, reflecting generic HRQL, were remarkably lower, compared with previously reported scores in the Korean general population (Fig. 3). The mean total IBDQ score in our study was much lower than those in other population-based IBD cohorts: 137.3 versus 183.0 in the Inflammatory Bowel Disease in South-East Norway population cohort;45 versus 198.3 in the Spanish study.11 Our results seem reasonable, given that disease activity is a determining factor of the HRQL in UC. Importantly, we confirmed that the presence of either significant anxiety or depression was a major contributing factor for both disease-specific and generic HRQL in our cohort. Comorbid psychological disorders had very strong negative associations with the total IBDQ score (Table 3) and almost all SF-12 domains (Supplementary Table 1). These results strongly suggest that complex biopsychosocial care should be provided as early as possible, particularly in patients with both psychological disorders and poor HRQL.46 To accomplish this goal, it is imperative to implement an adequate screening process in routine practice.47,48
We also found that the IBDQ score had significant negative correlations with the Mayo score, CRP level, and white blood cell count, but a positive association with the hemoglobin level (all p<0.05). These data suggest that common laboratory markers and clinical activity indices (i.e., Mayo score) provide useful predictors for disease-specific HRQL in patients with UC. We expected a longer duration between onset of symptom to diagnosis (i.e., diagnostic delay) would be one of the significant associated factors of low HRQL. The mean duration between symptom onset and diagnosis was approximately 4 months in this study, which was comparable with the previous findings from a Caucasian population.49 Unexpectedly, the delayed diagnosis was not associated with either HRQLs, disease-specific or general measurement tools, according to the multiple linear regression analysis.
Our study had several unique strengths. First, the study population was affiliated with a well-designed nationwide ongoing prospective cohort (MOSAIK), from a country where the incidence and prevalence of UC have rapidly increased in recent years.17-19 The data consistency has been managed tightly under strict diagnostic criteria, quality controls, and data cleaning. Additionally, as stated above, the PROs are now accepted as one of the major end points of clinical trials.3 The MOSAIK cohort includes only newly-diagnosed patients with moderate-to-severe UC, who are potential candidates for future major clinical trials. Our detailed PRO results at the time of diagnosis are meaningful for future clinical trials as well as routine practice. However, our study also had several weaknesses. The study population was comprised only of patients with moderate-to-severe activity from academic referral hospitals, after excluding patients with mild activity or those who were stationed at private clinics/community hospitals. In addition, we currently do not have longitudinal follow-up data of the PROs; it is being collected and will be presented later.
In conclusion, the present study clearly demonstrated that the burden of psychosocial distress was very high among newly-diagnosed patients with active UC, and that significant mood disorders were the major determinants of disease-specific HRQL. Complex biopsychosocial care, including point-of-care psychological screening and interventions, should be integrated into the initial management of newly-diagnosed patients with moderate-to-severe UC.
Supplemental Materials
ACKNOWLEDGMENTS
The authors acknowledge and would like to thank the following members of the moderate-to-severe ulcerative colitis in Korea (MOSAIK) study group for participating in the study: Yoon Jae Kim, Gachon University Gil Medical Center, Incheon; Tae Oh Kim, Inje University College of Medicine, Busan; Geom Seog Seo, Wonkwang University School of Medicine, Iksan; Ja Seol Koo, Korea University College of Medicine, Seoul; Joo Sung Kim, Seoul National University College of Medicine, Seoul; Byung Ik Jang, Yeungnam University College of Medicine, Daegu; Jeong Eun Shin, Dankook University College of Medicine, Cheonan; Ji Won Kim, Seoul National University Boramae Hospital, Seoul; Young Su Park, Seoul National University Bundang Hospital, Seongnam; YoungJa Lee, Medical Affairs, Janssen Korea, Seoul, Korea. This work was supported by Janssen Korea.
Footnotes
CONFLICTS OF INTEREST
This work was supported by Janssen Korea. No other potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study design: C.K.L., H.J.K. Statistical analysis: J.R.M., C.K.L., H.K., B.S., Y.K. Writing of the draft manuscript: J.R.M., C.K.L. Data collection: C.K.L., S.N.H., J.P.I., B.D.Y., J.M.C., S.A.J., K.M.L., D.I.P., Y.T.J., Y.S.P., J.H.C., H.J.K. Critical revision of the manuscript: J.R.M., C.K.L., S.N.H., J.P.I., B.D.Y., J.M.C., S.A.J., K.M.L., D.I.P., Y.T.J., Y.S.P., J.H.C., H.K., B.S., Y.K., H.J.K. All authors approved the final submission.
REFERENCES
- 1.Irvine EJ. Quality of life of patients with ulcerative colitis: past, present, and future. Inflamm Bowel Dis. 2008;14:554–565. doi: 10.1002/ibd.20301. [DOI] [PubMed] [Google Scholar]
- 2.Yarlas A, Rubin DT, Panés J, et al. Burden of ulcerative colitis on functioning and well-being: a systematic literature review of the SF-36® health survey. J Crohns Colitis. 2018;12:600–609. doi: 10.1093/ecco-jcc/jjy024. [DOI] [PubMed] [Google Scholar]
- 3.Williet N, Sandborn WJ, Peyrin-Biroulet L. Patient-reported outcomes as primary end points in clinical trials of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:1246–1256. doi: 10.1016/j.cgh.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Feagan BG, Patel H, Colombel JF, et al. Effects of vedolizumab on health-related quality of life in patients with ulcerative colitis: results from the randomised GEMINI 1 trial. Aliment Pharmacol Ther. 2017;45:264–275. doi: 10.1111/apt.13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han SW, McColl E, Barton JR, James P, Steen IN, Welfare MR. Predictors of quality of life in ulcerative colitis: the importance of symptoms and illness representations. Inflamm Bowel Dis. 2005;11:24–34. doi: 10.1097/00054725-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Hoivik ML, Moum B, Solberg IC, et al. Health-related quality of life in patients with ulcerative colitis after a 10-year disease course: results from the IBSEN study. Inflamm Bowel Dis. 2012;18:1540–1549. doi: 10.1002/ibd.21863. [DOI] [PubMed] [Google Scholar]
- 7.Zheng K, Zhang S, Wang C, Zhao W, Shen H. Health-related quality of life in Chinese patients with mild and moderately active ulcerative colitis. PLoS One. 2015;10:e0124211. doi: 10.1371/journal.pone.0124211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson PR, Vaizey C, Black CM, et al. Relationship between disease severity and quality of life and assessment of health care utilization and cost for ulcerative colitis in Australia: a cross-sectional, observational study. J Crohns Colitis. 2014;8:598–606. doi: 10.1016/j.crohns.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: results of the European Federation of Crohn's and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis. 2007;1:10–20. doi: 10.1016/j.crohns.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Walker JR, Ediger JP, Graff LA, et al. The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol. 2008;103:1989–1997. doi: 10.1111/j.1572-0241.2008.01980.x. [DOI] [PubMed] [Google Scholar]
- 11.Iglesias-Rey M, Barreiro-de Acosta M, Caamaño-Isorna F, et al. Psychological factors are associated with changes in the health-related quality of life in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:92–102. doi: 10.1097/01.MIB.0000436955.78220.bc. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein CN. Psychological stress and depression: risk factors for IBD? Dig Dis. 2016;34:58–63. doi: 10.1159/000442929. [DOI] [PubMed] [Google Scholar]
- 13.Viganò CA, Beltrami MM, Bosi MF, Zanello R, Valtorta M, Maconi G. Alexithymia and psychopathology in patients suffering from inflammatory bowel disease: arising differences and correlations to tailoring therapeutic strategies. Front Psychiatry. 2018;9:324. doi: 10.3389/fpsyt.2018.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen BL, Zoëga H, Shah SA, et al. Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Aliment Pharmacol Ther. 2014;39:811–822. doi: 10.1111/apt.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon JY, Shin JE, Park SH, Park DI, Cha JM. Disability due to inflammatory bowel disease is correlated with drug compliance, disease activity, and quality of life. Gut Liver. 2017;11:370–376. doi: 10.5009/gnl16422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YS, Jung SA, Lee KM, et al. Impact of inflammatory bowel disease on daily life: an online survey by the Korean Association for the Study of Intestinal Diseases. Intest Res. 2017;15:338–344. doi: 10.5217/ir.2017.15.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng SC. Epidemiology of inflammatory bowel disease: focus on Asia. Best Pract Res Clin Gastroenterol. 2014;28:363–372. doi: 10.1016/j.bpg.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Hann HJ, Hong SN, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. 2015;21:623–630. doi: 10.1097/MIB.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 19.Kim JW, Lee CK, Rhee SY, Oh CH, Shim JJ, Kim HJ. Trends in health-care costs and utilization for inflammatory bowel disease from 2010 to 2014 in Korea: a nationwide population-based study. J Gastroenterol Hepatol. 2018;33:847–854. doi: 10.1111/jgh.14027. [DOI] [PubMed] [Google Scholar]
- 20.Kim ES, Cho KB, Park KS, et al. Predictive factors of impaired quality of life in Korean patients with inactive inflammatory bowel disease: association with functional gastrointestinal disorders and mood disorders. J Clin Gastroenterol. 2013;47:e38–e44. doi: 10.1097/MCG.0b013e318266fff5. [DOI] [PubMed] [Google Scholar]
- 21.Ueno F, Nakayama Y, Hagiwara E, Kurimoto S, Hibi T. Impact of inflammatory bowel disease on Japanese patients' quality of life: results of a patient questionnaire survey. J Gastroenterol. 2017;52:555–567. doi: 10.1007/s00535-016-1241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CK, Lee KM, Park DI, et al. A new opportunity for innovative inflammatory bowel disease research: the moderate-to-severe ulcerative colitis in Korea (MOSAIK) cohort study. Intest Res. 2019;17:1–5. doi: 10.5217/ir.2019.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study) Scand J Gastroenterol. 2009;44:431–440. doi: 10.1080/00365520802600961. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 25.D'Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 26.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 27.Oh SM, Min KJ, Park DB. A Study on the standardization of the hospital anxiety and depression scale for Koreans: a comparison of normal, depressed and anxious groups. J Korean Neuropsychiatr Assoc. 1999;38:289–296. [Google Scholar]
- 28.Bernstein CN, Zhang L, Lix LM, et al. The validity and reliability of screening measures for depression and anxiety disorders in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:1867–1875. doi: 10.1093/ibd/izy068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 30.Pallis AG, Mouzas IA, Vlachonikolis IG. The inflammatory bowel disease questionnaire: a review of its national validation studies. Inflamm Bowel Dis. 2004;10:261–269. doi: 10.1097/00054725-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804–810. doi: 10.1016/S0016-5085(89)80080-0. [DOI] [PubMed] [Google Scholar]
- 32.Irvine EJ, Feagan B, Rochon J, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn's Relapse Prevention Trial Study Group. Gastroenterology. 1994;106:287–296. doi: 10.1016/0016-5085(94)90585-1. [DOI] [PubMed] [Google Scholar]
- 33.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Maruish ME, DeRosa MA. A guide to the integration of certified Short Form survey scoring and data quality evaluation capabilities. Quality Metric Incorporated; Lincoln: 2009. [Google Scholar]
- 35.Kim SH, Jo MW, Ahn J, Ock M, Shin S, Park J. Assessment of psychometric properties of the Korean SF-12 v2 in the general population. BMC Public Health. 2014;14:1086. doi: 10.1186/1471-2458-14-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikocka-Walus A, Knowles SR, Keefer L, Graff L. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:752–762. doi: 10.1097/MIB.0000000000000620. [DOI] [PubMed] [Google Scholar]
- 37.Williet N, Sarter H, Gower-Rousseau C, et al. Patient-reported outcomes in a French nationwide survey of inflammatory bowel disease patients. J Crohns Colitis. 2017;11:165–174. doi: 10.1093/ecco-jcc/jjw145. [DOI] [PubMed] [Google Scholar]
- 38.Goodhand JR, Kamperidis N, Sirwan B, et al. Factors associated with thiopurine non-adherence in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:1097–1108. doi: 10.1111/apt.12476. [DOI] [PubMed] [Google Scholar]
- 39.Calloway A, Dalal R, Beaulieu DB, et al. Depressive symptoms predict anti-tumor necrosis factor therapy noncompliance in patients with inflammatory bowel disease. Dig Dis Sci. 2017;62:3563–3567. doi: 10.1007/s10620-017-4800-y. [DOI] [PubMed] [Google Scholar]
- 40.Bennebroek Evertsz' F, Bockting CL, Stokkers PC, Hinnen C, Sanderman R, Sprangers MA. The effectiveness of cognitive behavioral therapy on the quality of life of patients with inflammatory bowel disease: multi-center design and study protocol (KL!C- study) BMC Psychiatry. 2012;12:227. doi: 10.1186/1471-244X-12-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Regueiro MD, McAnallen SE, Greer JB, Perkins SE, Ramalingam S, Szigethy E. The inflammatory bowel disease specialty medical home: a new model of patient-centered care. Inflamm Bowel Dis. 2016;22:1971–1980. doi: 10.1097/MIB.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 42.Arnott I, Glynn M. National audit of inflammatory bowel disease (IBD) service provision: UK IBD audit [Internet] Royal College of Physicians; London: c2014. [cited 2019 Jan 11]. Available from: www.rcplondon.ac.uk. [Google Scholar]
- 43.Lee CK, Melmed GY. Multidisciplinary team-based approaches to IBD management: how might "one-stop shopping" work for complex IBD care? Am J Gastroenterol. 2017;112:825–827. doi: 10.1038/ajg.2017.124. [DOI] [PubMed] [Google Scholar]
- 44.Siegel CA, Whitman CB, Spiegel BMR, et al. Development of an index to define overall disease severity in IBD. Gut. 2018;67:244–254. doi: 10.1136/gutjnl-2016-312648. [DOI] [PubMed] [Google Scholar]
- 45.Huppertz-Hauss G, Lie Høivik M, Jelsness-Jørgensen LP, et al. Health-related quality of life in patients with inflammatory bowel disease 20 years after diagnosis: results from the IBSEN Study. Inflamm Bowel Dis. 2016;22:1679–1687. doi: 10.1097/MIB.0000000000000806. [DOI] [PubMed] [Google Scholar]
- 46.Lee CK, Melmed GY, Mann A, et al. A multidisciplinary approach to biopsychosocial care for adults with inflammatory bowel disease: a pilot study. Inflamm Bowel Dis. 2018;24:2550–2554. doi: 10.1093/ibd/izy215. [DOI] [PubMed] [Google Scholar]
- 47.Szigethy EM, Allen JI, Reiss M, et al. White paper AGA: the impact of mental and psychosocial factors on the care of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2017;15:986–997. doi: 10.1016/j.cgh.2017.02.037. [DOI] [PubMed] [Google Scholar]
- 48.Keefer L, Palsson OS, Pandolfino JE. Best practice update: incorporating psychogastroenterology into management of digestive disorders. Gastroenterology. 2018;154:1249–1257. doi: 10.1053/j.gastro.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 49.Vavricka SR, Spigaglia SM, Rogler G, et al. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:496–505. doi: 10.1002/ibd.21719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.