Abstract
Background/Aims
Desmoplasia is a prominent feature of pancreatic ductal adenocarcinoma (PDA). Stromal desmoplasia reflects the low cellularity that is characteristic of PDA, and it may play a role in PDA chemoresistance. In this retrospective study, we evaluated the relationship between tumor cellularity in resected PDA specimens and long-term patient outcomes.
Methods
We retrospectively reviewed the data from 175 patients who underwent PDA resection between January 2010 and December 2015 at Seoul National University Bundang Hospital, and analyzed their clinicopathological features and the relationship between tumor cellularity (high vs low based on a cutoff of 30% cellularity) and patient outcomes.
Results
The high-cellularity group had significantly shorter overall survival (OS) (18.7 months vs 26.6 months, p=0.006) and disease-free survival (11.0 months vs 16.9 months, p=0.031) than the low-cellularity group. Multivariate analysis revealed that high tumor cellularity was an independent risk factor for poor OS (hazard ratio, 2.008; 95% confidence interval, 1.361 to 2.962; p<0.001). Adjuvant therapy improved OS in the low-cellularity group (16.3 months vs 41.3 months, p=0.001) but not in the high-cellularity group (15.9 months vs 24.4 months, p=0.107).
Conclusions
Tumor cellularity in PDA specimens may be a prognostic and predictive biomarker that could aid in identifying patients who would benefit from adjuvant therapy for PDA. (Gut Liver 2020;14:521-528)
Keywords: Tumor cellularity; Biomarker; Carcinoma, pancreatic ductal; Prognosis; Chemotherapy, adjuvant
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDA) is the fourth leading cause of cancer-related deaths and has a 5-year survival of 8% in Western countries.1,2 The prognosis for patients with PDA remains dismal despite recent modest improvements that have resulted from the optimized use of systemic chemotherapy.3,4 The only curative treatment for PDA is complete resection, which is only possible in 10% to 20% of patients.2,5,6 Even after potentially curative resection, locoregional recurrences and distant metastases are common, and the 5-year survival rates remain poor.7-11
The histological characteristics of PDA often include abundant desmoplastic stroma with low tumor cellularity.12,13 There is increasing evidence that the stromal desmoplasia of PDA helps impair drug delivery to tumor cells and increases resistance to chemotherapy.14-17 A recent clinical trial has also demonstrated that targeting the stromal compartment in pancreatic cancer may have antitumor effects and enhance the sensitivity to chemotherapy.18 Although few studies have evaluated the clinical implications of tumor cellularity in PDA, this parameter is a significant prognostic indicator in other solid tumors, such as gastrointestinal stromal tumors19 and breast cancers.20 Therefore, this retrospective study aimed to explore whether tumor cellularity could predict prognosis and response to adjuvant therapy among PDA patients.
MATERIALS AND METHODS
1. Patients and clinicopathological variables
The study’s retrospective protocol was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB number: SNUBH B-1804/463–104) and informed consent was waived. Clinicopathological data were retrieved for all 225 patients who underwent surgical resection of PDA between January 2010 and December 2015 at our center. Patients were excluded from the analysis if they received neoadjuvant treatment, did not have histological confirmation of the PDA diagnosis, had a macroscopically positive resection (R2), or died within 30 days after surgery (Fig. 1). Thus, 175 patients who underwent potentially curative resection were included in the present study.
Fig. 1.
Flowchart illustrating inclusion/exclusion criteria of the study.
SNUBH, Seoul National University Bundang Hospital; PDA, pancreatic ductal adenocarcinoma.
2. Assessment of tumor cellularity
Hematoxylin and eosin stained histology slides from all resected cases were retrieved from the archives of the Seoul National University Bundang Hospital, Department of Pathology, and assessed by two pathologists with expertise in pancreaticobiliary pathology (H.K. and S.A.). For all cases, the entire tumor was submitted for histological evaluation, which is the routine practice of the institution. One representative microscopic slide containing the highest cellularity was selected for each case, and after examining the entire slide at scanning power (×10) microscopy, one most representative area containing the highest cellularity was selected for tumor cellularity assessment. In order to reduce intra- and inter-observer variability in estimating tumor cellularity, five random cases were initially evaluated using a quantitative approach (Supplementary Fig. 1). Briefly, ×100 magnification fields were captured on an Olympus BX50 microscope (Tokyo, Japan), and the photomicrograph images were analyzed using ImageJ analysis software version 1.47 (National Institutes of Health, Bethesda, MD, USA; downloaded from imagej.nih.gov/ij). Tumor cellularity was defined as the total area occupied by tumor cells divided by the area of the entire ×100 field, expressed as a percentage. Using the captured images and corresponding morphometric analyses for guidance, the tumor cellularity of the remaining cases was estimated independently by two pathologists, both blinded to the other’s cellularity score, the patient identification and outcome status. The intraclass correlation score and the Pearson correlation coefficient between the two pathologists were 0.650 (95% confidence interval [CI], 0.475 to 0.766) and r=0.789 (p<0.001), respectively. For discrepant cases, a consensus was arrived at using a multiheaded microscope.
3. Adjuvant therapy and outcomes
Adequate adjuvant therapy was defined as receiving ≥4 cycles of chemotherapy with or without radiation. Chemotherapeutic regimens included gemcitabine monotherapy (intravenous gemcitabine at 1,000 mg/m2 over 30 minutes on days 1, 8, and 15, followed by 1-week rest), gemcitabine plus erlotinib (intravenous gemcitabine at 1,000 mg/m2 over 30 minutes on days 1, 8, and 15, plus oral erlotinib at 100 mg daily for 28 days), and 5-fluorouracil plus leucovorin (bolus intravenous leucovorin at 20 mg/m2 followed by bolus intravenous 5-fluorouracil at 425 mg/m2 on days 1–5 every 4 weeks). The outcomes were defined as disease-free survival (DFS; time from surgery to the first instance of recurrence or the date of last follow-up) and overall survival (OS; time from diagnosis to death or the date of last follow-up).
4. Statistical analysis
All statistical analyses were performed using STATA/SE software version 14.0 (STATA Corp., College Station, TX, USA). The chi-square test or Fisher exact test was used to analyze categorical variables. The optimal cutoff value for defining low or high tumor cellularity was determined using receiver operating characteristic curve analysis. Univariate analyses of DFS were performed using the Kaplan-Meier method and the log-rank test. Significant variables from the univariate analyses, and those that met the proportional hazard assumptions were also analyzed in a multivariate Cox proportional hazard model. Differences were considered statistically significant at p-values <0.05.
RESULTS
1. Baseline characteristics
The patient and disease characteristics are summarized in Table 1. The median age was 66.2 years (range, 35.7 to 88.3 years) and 59.4% of patients were men. The majority of the PDAs (68.0%) were located in the head of the pancreas. The median tumor size was 3.0 cm (range, 1.2 to 9.5 cm). Lymph node metastasis was identified in 110 cases (62.9%). The histological classifications were: well differentiated in 18 cases (10.3%), moderately differentiated in 138 cases (78.9%), and poorly differentiated in 19 cases (10.8%). Angiolymphatic invasion was present in 100 cases (57.1%), venous invasion was present in 80 cases (45.7%), and perineural invasion was present in 153 cases (87.4%). Microscopically negative margins (R0 resection) were achieved in 140 patients (80.0%). The median preoperative serum CA19-9 level was 128.0 U/mL (range, 0.1 to 6,500.0 U/mL). Among the 175 cases, adjuvant chemotherapy was provided to 108 patients (61.7%) and adequate adjuvant chemotherapy was identified for 67 patients (38.3%).
Table 1.
Patient Characteristics
| Characteristics | No. (%) |
|---|---|
| Age, yr | |
| <65 | 71 (40.6) |
| ≥65 | 104 (59.4) |
| Sex | |
| Male | 104 (59.4) |
| Female | 71 (40.6) |
| Tumor location | |
| Head | 119 (68.0) |
| Body/tail/others | 56 (32.0) |
| Median tumor size, cm | |
| <3 | 75 (42.9) |
| ≥3 | 100 (57.1) |
| Lymph node involvement | |
| Absent | 65 (37.1) |
| Present | 110 (62.9) |
| Histology (differentiation) | |
| Well | 18 (10.3) |
| Moderate | 138 (78.9) |
| Poor | 19 (10.8) |
| Angiolymphatic invasion | |
| Absent | 75 (42.9) |
| Present | 100 (57.1) |
| Venous invasion | |
| Absent | 95 (54.3) |
| Present | 80 (45.7) |
| Perineural invasion | |
| Absent | 22 (12.6) |
| Present | 153 (87.4) |
| Resection margin | |
| R0 | 140 (80.0) |
| R1 | 35 (20.0) |
| Median preoperative CA19-9, U/mL | |
| <128 | 87 (49.7) |
| ≥128 | 88 (50.3) |
| Adequate adjuvant therapy | |
| Yes | 67 (38.3) |
| No | 108 (61.7) |
R0, no residual tumor; R1, microscopic residual tumor (corresponds to positive resection margins); CA19-9, carbohydrate antigen 19-9.
2. Clinicopathological characteristics of PDA according to tumor cellularity
The median tumor cellularity percentage was 25.9% (range, 10% to 90%). The receiver operating characteristic curve analysis revealed that the optimal cutoff value for cellularity was 30%, which provided an area under the receiver operating characteristic curve of 0.655 (95% CI, 0.572 to 0.738). Thus, patients were classified into a low-cellularity group (<30%, 107 patients) and a high-cellularity group (≥30%, 68 patients). The high-cellularity group had a significantly higher prevalence of poor histological differentiation (p<0.001) (Table 2).
Table 2.
Correlation between Tumor Cellularity and the Clinicopathological Characteristics of Pancreatic Ductal Adenocarcinoma
| Characteristics | Tumor cellularity <30% (n=107) | Tumor cellularity ≥30% (n=68) | p-value |
|---|---|---|---|
| Age, yr | 0.656 | ||
| <65 | 42 (59.1) | 29 (40.9) | |
| ≥65 | 65 (62.5) | 39 (37.5) | |
| Sex | 0.257 | ||
| Male | 60 (57.7) | 44 (42.3) | |
| Female | 47 (66.2) | 24 (33.8) | |
| Tumor location | 0.055 | ||
| Head | 67 (56.3) | 52 (43.7) | |
| Body/tail/others | 40 (71.4) | 16 (28.6) | |
| Median tumor size, cm | 0.371 | ||
| <3 | 43 (57.3) | 32 (42.7) | |
| ≥3 | 64 (64.0) | 36 (36.0) | |
| Lymph node involvement | 0.687 | ||
| Absent | 41 (63.1) | 24 (36.9) | |
| Present | 66 (60.0) | 44 (40.0) | |
| Histology (differentiation) | <0.001 | ||
| Well | 16 (88.9) | 2 (11.1) | |
| Moderate | 86 (62.3) | 52 (37.7) | |
| Poor | 5 (26.3) | 14 (73.7) | |
| Angiolymphatic invasion | 0.227 | ||
| Absent | 42 (56.0) | 33 (44.0) | |
| Present | 65 (65.0) | 35 (35.0) | |
| Venous invasion | 0.735 | ||
| Absent | 57 (60.0) | 38 (40.0) | |
| Present | 50 (62.5) | 30 (37.5) | |
| Perineural invasion | 0.497 | ||
| Absent | 12 (54.5) | 10 (45.5) | |
| Present | 95 (62.1) | 58 (37.9) | |
| Resection margin | 0.816 | ||
| R0 | 85 (60.7) | 55 (39.3) | |
| R1 | 22 (62.9) | 13 (37.1) | |
| Median preoperative CA19-9, U/mL | 0.131 | ||
| <128 | 58 (66.7) | 29 (33.3) | |
| ≥128 | 49 (55.7) | 39 (44.3) | |
| Adequate adjuvant therapy | 0.516 | ||
| Yes | 43 (64.2) | 24 (35.8) | |
| No | 64 (59.3) | 44 (40.7) | |
Data are presented as number (%).
R0, no residual tumor; R1, microscopic residual tumor (corresponds to positive resection margins); CA19-9, carbohydrate antigen 19-9.
3. Survival analysis according to tumor cellularity
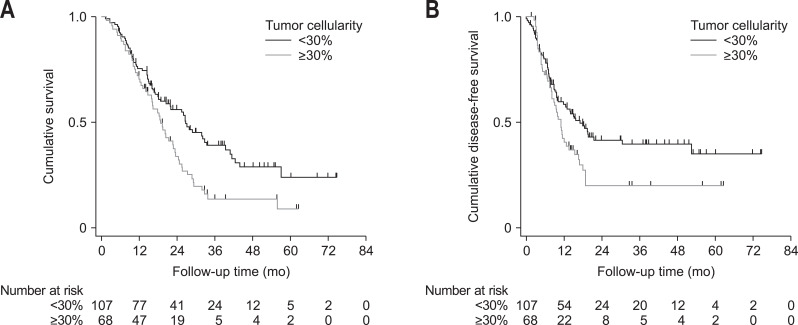
The median OS was 22.6 months (95% CI, 18.1 to 26.5 months), and the high-cellularity group had a significantly shorter median OS than the low-cellularity group (18.7 months [95% CI, 15.8 to 22.7 months] vs 26.6 months [95% CI, 18.5 to 33.3 months]: hazard ratio [HR], 1.674; 95% CI, 1.159 to 2.418; p=0.006) (Fig. 2A). The univariate analyses revealed that shorter median OS was associated with older age (HR, 1.643; 95% CI, 1.127 to 2.396; p=0.01), tumor size >3 cm (HR, 1.758; 95% CI, 1.217 to 2.539; p=0.003), lymph node involvement (HR, 2.160; 95% CI, 1.438 to 3.245; p<0.001), angiolymphatic invasion (HR, 1.747; 95% CI, 1.198 to 2.548; p=0.004), venous invasion (HR, 1.858; 95% CI, 1.287 to 2.684; p=0.001), not adequate adjuvant therapy (HR, 2.207; 95% CI, 1.477 to 3.297; p<0.001), and tumor cellularity ≥30% (HR, 1.674; 95% CI, 1.159 to 2.418; p=0.006) (Table 3). According to the multivariate analysis, independent predictors of poor survival included tumor cellularity ≥30% (HR, 2.008; 95% CI, 1.361 to 2.962; p<0.001), older age (HR, 1.476; 95% CI, 1.004 to 2.170; p=0.048), tumor size >3 cm (HR, 1.723; 95% CI, 1.171 to 2.535; p=0.006), lymph node involvement (HR, 1.716; 95% CI, 1.093 to 2.692; p=0.014), venous invasion (HR, 1.755; 95% CI, 1.196 to 2.574; p=0.004), and not adequate adjuvant therapy (HR, 2.064; 95% CI, 1.371 to 3.109; p=0.001) (Table 3). The median DFS was 12.9 months (95% CI, 9.8 to 16.9 months). Relative to the low-cellularity group, the high-cellularity group had a significantly shorter median DFS (11.0 months [95% CI, 8.0 to 13.5 months] vs 16.9 months [95% CI, 10.0 to 30.3 months]: HR, 1.536; 95% CI, 1.039 to 2.270; p=0.031) (Fig. 2B).
Fig. 2.
Kaplan-Meier estimates for (A) overall survival (HR, 1.674; 95% CI, 1.159–2.418; p=0.006) and (B) disease-free survival (HR, 1.536; 95% CI, 1.039–2.270; p=0.031) for patients stratified by tumor cellularity in of pancreatic ductal adenocarcinoma.
HR, hazard ratio; CI, confidence interval.
Table 3.
Prognostic Factors for Survival Identified by Univariate and Multivariate Analyses
| Characteristic | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age, yr | ||||
| <65 | Reference | Reference | ||
| ≥65 | 1.643 (1.127–2.396) | 0.010 | 1.476 (1.004–2.170) | 0.048 |
| Sex | ||||
| Male | Reference | |||
| Female | 0.741 (0.507–1.081) | 0.120 | ||
| Tumor location | ||||
| Head | Reference | |||
| Body/tail/others | 1.073 (0.727–1.584) | 0.721 | ||
| Median tumor size, cm | ||||
| ≤3 | Reference | Reference | ||
| >3 | 1.758 (1.217–2.539) | 0.003 | 1.723 (1.171–2.535) | 0.006 |
| Lymph node involvement | ||||
| Absent | Reference | Reference | ||
| Present | 2.160 (1.438–3.245) | <0.001 | 1.716 (1.093–2.692) | 0.014 |
| Angiolymphatic invasion | ||||
| Absent | Reference | Reference | ||
| Present | 1.747 (1.198–2.548) | 0.004 | 1.208 (0.792–1.841) | 0.380 |
| Venous invasion | ||||
| Absent | Reference | Reference | ||
| Present | 1.858 (1.287–2.684) | 0.001 | 1.755 (1.196–2.574) | 0.004 |
| Perineural invasion | ||||
| Absent | Reference | |||
| Present | 1.811 (0.993–3.302) | 0.052 | ||
| Resection margin | ||||
| R0 | Reference | |||
| R1 | 1.484 (0.962–2.290) | 0.074 | ||
| Median preoperative CA19-9, U/mL | ||||
| <128 | Reference | |||
| ≥128 | 1.253 (0.864–1.818) | 0.233 | ||
| Adequate adjuvant therapy | ||||
| Yes | Reference | Reference | ||
| No | 2.207 (1.477–3.297) | <0.001 | 2.064 (1.371–3.109) | 0.001 |
| Tumor cellularity, % | ||||
| <30 | Reference | Reference | ||
| ≥30 | 1.674 (1.159–2.418) | 0.006 | 2.008 (1.361–2.962) | <0.001 |
HR, hazard ratio; CI, confidence interval; R0, no residual tumor; R1, microscopic residual tumor (corresponds to positive resection margins); CA19–9, carbohydrate antigen 19–9.
4. Survival benefit of adjuvant therapy according to tumor cellularity
The median OS were 16.0 months in the no adjuvant therapy subgroup and 31.8 months in the adjuvant therapy subgroup (HR, 0.453; 95% CI, 0.303 to 0.676; p<0.001). In the low-cellularity group, patients who received adjuvant therapy had a much longer median OS than those who did not (41.3 months vs 16.3 months: HR, 0.380; 95% CI, 0.218 to 0.659; p=0.001). However, in the high-cellularity group, adjuvant therapy provided only a small, non-significant increase in OS (24.4 months vs 15.9 months: HR, 0.616; 95% CI, 0.342 to 1.110; p=0.107) (Fig. 3).
Fig. 3.
Kaplan-Meier estimates of overall survival by the receipt of adjuvant therapy in the (A) low-cellularity group (HR, 0.380; 95% CI, 0.218–0.659; p=0.001) and (B) high-cellularity group (HR, 0.616; 95% CI, 0.342–1.110; p=0.107).
HR, hazard ratio; CI, confidence interval.
DISCUSSION
Our results indicate that high PDA tumor cellularity was associated with poorer histological differentiation and worse survival and that the benefit of adjuvant therapy on OS was significantly diminished in the high-cellularity group relative to the low-cellularity group. Therefore, tumor cellularity can serve as a potential predictive biomarker for adjuvant therapy in PDA patients.
Although surgery is the only treatment that can potentially provide long-term survival in PDA cases, only a small subset of patients is eligible for surgery at the time of diagnosis because of invasive nature of the disease and the high recurrence rate.21 Therefore, chemotherapy remains an important treatment modality for PDA patients, and it is important to identify biomarkers that can predict the response to chemotherapy and subsequent survival outcomes.
Relative to other epithelial malignancies, the general histological features of PDA are abundant desmoplastic stroma and relatively sparse tumor cellularity, with the stromal compartment often exceeding 80% of the tumor volume.12,22 However, there are limited clinicopathological data regarding the relationship between PDA tumor cellularity and patient outcomes, which prompted us to perform this analysis of tumor cellularity in resected PDA specimens from 175 consecutive patients, as well as the relationship of tumor cellularity with survival and adjuvant chemotherapy response.
Our findings agree with those of Heid et al.,23 who recently demonstrated that high preoperative tumor cellularity was a negative prognostic factor for PDA. In that study, the researchers evaluated tumor cellularity based on the apparent diffusion coefficient parameter from diffusion-weighted magnetic resonance imaging, and suggested that subgroups of PDA with high tumor cellularity could be identified non-invasively.23 Our data provide histological confirmation that PDA tumor cellularity is a strong independent predictor of survival outcomes, and also indicate that adjuvant chemotherapy may provide significant benefits to PDA patients with low cellularity. Thus, including tumor cellularity assessments in pathology reports may help guide the selection of adjuvant treatment regimens. It may also be possible to assess tumor cellularity using small specimens from endoscopic ultrasound-guided fine needle aspiration biopsies, although prospective studies are needed to determine whether this approach could be used to guide the management of advanced stage PDA.
Tumor cellularity is relatively sparse in PDA (median proportion, 25.9% in the present study), and these tumors typically have abundant desmoplastic stroma that accounts for most of the tumor volume. There are limited data regarding the clinicopathological significance of desmoplastic stroma in human malignancies, and research has recently focused on the roles that the tumor stroma play in tumor initiation, progression, and resistance to chemotherapy and radiotherapy.24,25 A study of resected PDAs has also indicated that high stromal content was associated with poor long-term outcomes.26 In contrast, our previous study of a subset of PDA cases revealed that high cancer-associated fibroblast (CAF) counts were associated with significantly better survival outcomes, which supports recent experimental findings that the tumor stroma may have a protective role rather than an enhancing role for any aggressive behavior.27-30 Because the influence of PDA tumor cellularity on patient survival and response to chemotherapy is unclear, the present study focused on the epithelial cell compartment of PDA. However, we did not find significant inverse correlations between the expression of CAF-related markers and tumor cellularity (data not shown), which is unsurprising because the tumor microenvironment is a complex network of many cell types (e.g., fibroblasts, endothelial cells, and inflammatory cells) interacting with the extracellular matrix.24
The mechanism behind the relationships between high tumor cellularity and poor patient outcomes and PDA chemoresistance remains unclear. It is possible that tumors with high cellularity have relatively sparse amounts of CAFs, which could result in accelerated tumor growth, invasiveness, and chemoresistance. For example, Rhim et al.30 reported that reducing the amount of stromal content by genetically deleting sonic hedgehog in a mouse model of PDA resulted in more aggressive behavior, undifferentiated histology, increased angiogenesis, and accelerated cellular proliferation. Furthermore, depletion of stromal myofibroblasts in another mouse model of PDA resulted in enhanced tumor aggressiveness and shorter survival.29 However, in vitro studies have revealed increased invasiveness and migration of PDA cancer cells when they are co-cultured with pancreatic stellate cells, as well as CAFs, protecting the cancer cells from the effects of chemoradiation therapy.31
The present study has two important limitations. First, this retrospective, single-center study focused on surgically resected PDAs, which led to the exclusion of patients with advanced PDA. Thus, validation is needed in further prospective studies of independent cohorts, preferably with the addition of preoperative biopsy findings. Second, patients who received neoadjuvant treatment were excluded to eliminate any effects of preoperative treatment on tumor cellularity. Therefore, a separate study is needed to determine whether cellularity assessments provide clinical value for patients who receive neoadjuvant therapy.
In conclusion, the present study revealed that tumor cellularity significantly and independently predicted OS and DFS after potentially curative resection of PDA. In addition, adjuvant therapy was more effective for patients with low tumor cellularity, which suggests that assessing tumor cellularity may help guide the post-operative management of PDA patients.
Supplemental Materials
REFERENCES
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malvezzi M, Carioli G, Bertuccio P, et al. European cancer mortality predictions for the year 2016 with focus on leukaemias. Ann Oncol. 2016;27:725–731. doi: 10.1093/annonc/mdw022. [DOI] [PubMed] [Google Scholar]
- 6.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayahara M, Nagakawa T, Ueno K, Ohta T, Takeda T, Miyazaki I. An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer. 1993;72:2118–2123. doi: 10.1002/1097-0142(19931001)72:7<2118::AID-CNCR2820720710>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/S1091-255X(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 10.Staley CA, Lee JE, Cleary KR, et al. Preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for adenocarcinoma of the pancreatic head. Am J Surg. 1996;171:118–124. doi: 10.1016/S0002-9610(99)80085-3. [DOI] [PubMed] [Google Scholar]
- 11.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990;211:447–458. doi: 10.1097/00000658-199004000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 13.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 15.Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michl P, Gress TM. Improving drug delivery to pancreatic cancer: breaching the stromal fortress by targeting hyaluronic acid. Gut. 2012;61:1377–1379. doi: 10.1136/gutjnl-2012-302604. [DOI] [PubMed] [Google Scholar]
- 17.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hingorani SR, Harris WP, Hendifar AE, et al. High response rate and PFS with PEGPH20 added to nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients with high-HA tumors: interim results of a randomized phase II study. J Clin Oncol. 2015;33(15 Suppl):4006. doi: 10.1200/jco.2015.33.15_suppl.4006. [DOI] [Google Scholar]
- 19.Martín J, Poveda A, Llombart-Bosch A, et al. Deletions affecting codons 557-558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS) J Clin Oncol. 2005;23:6190–6198. doi: 10.1200/JCO.2005.19.554. [DOI] [PubMed] [Google Scholar]
- 20.Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer: a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/S1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 22.Lunardi S, Muschel RJ, Brunner TB. The stromal compartments in pancreatic cancer: are there any therapeutic targets? Cancer Lett. 2014;343:147–155. doi: 10.1016/j.canlet.2013.09.039. [DOI] [PubMed] [Google Scholar]
- 23.Heid I, Steiger K, Trajkovic-Arsic M, et al. Co-clinical assessment of tumor cellularity in pancreatic cancer. Clin Cancer Res. 2017;23:1461–1470. doi: 10.1158/1078-0432.CCR-15-2432. [DOI] [PubMed] [Google Scholar]
- 24.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 25.Bijlsma MF, van Laarhoven HW. The conflicting roles of tumor stroma in pancreatic cancer and their contribution to the failure of clinical trials: a systematic review and critical appraisal. Cancer Metastasis Rev. 2015;34:97–114. doi: 10.1007/s10555-014-9541-1. [DOI] [PubMed] [Google Scholar]
- 26.Erkan M, Michalski CW, Rieder S, et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1155–1161. doi: 10.1016/j.cgh.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Park H, Lee Y, Lee H, et al. The prognostic significance of cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. Tumour Biol. 2017;39:1010428317718403. doi: 10.1177/1010428317718403. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen MF, Mortensen MB, Detlefsen S. Key players in pancreatic cancer-stroma interaction: cancer-associated fibroblasts, endothelial and inflammatory cells. World J Gastroenterol. 2016;22:2678–2700. doi: 10.3748/wjg.v22.i9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.