Abstract
Background
Initiation of combination antiretroviral therapy (cART) soon after HIV-1 infection limits the establishment of viral reservoirs. Thus, early treated individuals are preferred candidates to evaluate novel viral remission strategies. However, their cART-dependent HIV-1 DNA decay dynamics are still poorly defined. This can hamper the design and interpretation of results from clinical trials intended to further reduce viral reservoirs.
Objectives
To clarify the duration of cART needed for the HIV-1 reservoir to be stabilized in early treated individuals.
Methods
We characterized the longitudinal decline of total HIV-1 DNA levels by droplet digital PCR in 21 individuals initiating cART within 6 months after estimated HIV-1 acquisition. Measurements were taken at cART initiation, after 6 months and annually until Year 4. Correlations between virological and clinical parameters were statistically analysed. Statistical modelling was performed applying a mixed-effects model.
Results
Total HIV-1 DNA experienced a median overall decrease of 1.43 log10 units (IQR = 1.17–1.69) throughout the 4 years of follow-up. Baseline levels for total HIV-1 DNA, viral load, absolute CD4+ T cell count and CD4+/CD8+ ratio correlate with final HIV-1 DNA measurements (R2 = 0.68, P < 0.001; R2 = 0.54, P = 0.012; R2 = −0.47, P = 0.031; and R2 = −0.59, P = 0.0046, respectively). Statistical modelling shows that after 2 years on cART the viral reservoir had reached a set point.
Conclusions
A waiting period of 2 years on cART should be considered when designing interventions aiming to impact latent HIV-1 reservoir levels and viral rebound kinetics after cART discontinuation, in order to facilitate interpretation of results and enhance the chance of viral control.
Introduction
HIV-1 infection is characterized by the establishment of a persistent viral reservoir of latently infected cells. Combination ART (cART) suppresses HIV-1 replication and prevents disease progression but it cannot eliminate the latent reservoir, so infection remains chronic. However, early initiation of cART has been proposed as a way to restrict the seeding of the viral reservoir, as individuals who initiate cART during the acute/early phase of infection show a reduction of viral reservoir size compared with those who started cART in the chronic stage.1,2 The smaller reservoir and the potentially preserved immune competence make early treated patients the preferred candidates to test novel clinical interventions aiming to achieve drug-free remission of HIV-1 infection.
The frequency of HIV-1-infected cells, measured as total HIV-1 DNA copies/106 cells, rapidly increases with the course of infection and exponentially decreases upon cART initiation as the predominant, short-lived, productively infected cells rapidly disappear and new infections are blocked.3,4 Afterwards, the persistence of a population of long-lived latently infected cells is evidenced by an HIV-1 DNA plateau.5 The size of the reservoir at this set point differs between individuals but remains fairly constant over time. Previous studies have shown that the HIV-1 reservoir in chronically treated individuals experiences a sustained but slow decline on long-term cART, with a median half-life of 12 years.6 Once this phase has been reached, the decay of HIV-1 DNA is not significant if measured within short time periods, as assessed in most clinical trials.
Of note, the evaluation of changes in total HIV-1 DNA levels is the most widely used endpoint in clinical trials aiming to impact the viral reservoir, based on the robustness of this measurement and its strong correlation with time to viral rebound after cART interruption.7,8 However, the size and the stability of the HIV-1 reservoir are rarely inclusion/exclusion criteria for clinical trials. Therefore, patients enrolled during the first years after cART initiation might still be undergoing the initial cART-mediated HIV-1 DNA decay. This fact may become a confounding factor, leading to misinterpretation of the results if trials are not sufficiently powered, even if controlled with a placebo arm. One option to increase the chances of differentiating an effect of the intervention might be to recruit individuals only once they have all reached a stable set point in the size of their viral reservoir. This may also be desirable in clinical trials that include a treatment-interruption phase, as viral control after treatment interruption is more likely if the viral reservoir is smaller.9 Although different studies describe the decay kinetics of HIV-1 DNA following early treatment initiation,1,2,4,10,11 none has specifically evaluated the time on cART needed to reach the stabilization of the reservoir in early treated individuals and no assumption can be inferred from previous data, given the varying results. To solve this gap in knowledge, we have performed a longitudinal study of the HIV-1 DNA decay after early cART initiation and used statistical modelling to specifically determine the timespan required to reach reservoir stability.
Materials and methods
Ethics
All patients provided written informed consent before enrolment into the cohort. The study was approved by the institutional ethics review board of Germans Trias i Pujol University Hospital (Early_cART Cohort, PI-14-072), in accordance with the Declaration of Helsinki and national standards.
Study design and participants
We included 21 individuals from a longitudinal, prospective cohort of patients with confirmed recent HIV-1 infection who started cART within 6 months of estimated HIV-1 acquisition. This cohort was established in the Germans Trias i Pujol University Hospital (Badalona, Spain) and recruitment began in 2013. By December 2017, we had selected all the individuals from this cohort that fulfilled our inclusion criteria. All sample processing and analysis took place during 2018.
Criteria for documented acute/early infection include plasma HIV-1 RNA with antibody (enzyme immunoassay) negativity, Gag p24 antigen assay positivity, an indeterminate western immunoblot signal or the absence of a p31 band in a positive western blot. Days since estimated HIV-1 acquisition and Fiebig staging at treatment initiation are defined according to the time since known exposure, acute antiretroviral syndrome and/or results of clinical laboratory tests at the time of HIV-1 diagnosis.12 For cases in which exposure or acute retroviral syndrome were unclear or absent, the estimated date of infection of subjects with an HIV-1 positive antibody test following an earlier negative antibody test was calculated as the middle timepoint between these two tests. Viral load determinations were carried out before treatment initiation and after treatment initiation, at Weeks 4, 12, 24, 36 and 48 (during the first year) and every 6 months thereafter. The inclusion criteria for selecting the 21 individuals for the current study were: (i) being effectively suppressed (HIV-1 RNA <50 copies/mL, allowing non-consecutive blips <200 copies/mL) for ≥4 years; (ii) lacking documented periods of non-adherence to cART; (iii) receiving no immunotherapies; and (iv) having stored samples available at 0, 0.5, 1, 2, 3 and 4 years after cART initiation.
Quantification of HIV-1 DNA
CD4+ T cells were purified from cryopreserved PBMCs using the ‘CD4+ T Cell Isolation Kit, human’ (Miltenyi Biotec). Lysed extracts were used for HIV-1 DNA quantification by droplet digital PCR (ddPCR, Bio-Rad), as previously described.13 Briefly, primer/probe sets annealing at the 5′ LTR, Gag or integrase conserved regions of the HIV-1 genome were used, depending on the individual, to circumvent underestimation due to viral sequence variability, and the RPP30 cellular gene measurement was used for data normalization. All quantifications were performed in duplicate and the average limit of detection of HIV-1 DNA was 3 copies/106 CD4+ T cells.
Statistical analysis and data modelling
Association between HIV-1 DNA values and other clinical and virological variables was assessed by Spearman’s rank correlation coefficient using the GraphPad Prism v5 software.
HIV-1 DNA dynamics were modelled by fitting a previously described asymptotic one-exponential decay model based on: (i) the limited sample size, which did not allow for the use of more complex models; and (ii) previously reported observational data showing HIV-1 DNA stabilization beyond 4 years after ART initiation.5,14 The equation of the model is as follows:
where vDNA stands for the natural logarithm of total HIV-1 DNA; Asym stands for HIV-1 DNA level at the set point; R0 stands for HIV-1 DNA level at t = 0 years; r stands for the decay rate; and t stands for the time since cART initiation. To infer the timepoint from which no further HIV-1 DNA decay would be detected in eventual clinical trials, we determined when the difference between the estimated model and the reservoir set point would become non-significant. To establish this threshold, we used the equation:
where d is the value of the effect size boundary estimated by power analysis for a Student’s t-test and σ is the population standard deviation of HIV-1 DNA when the set point has been reached (σ = 0.55, as estimated from our data). Effect size boundary was estimated as the value below which the probability to reject the false null hypothesis of equal means (difference is assumed) was less than 0.2 considering a sample size of n = 50 and a one-sided t-test. The timepoint (x) at which the global dynamics model crossed the threshold is then given by the equation:
For data analysis and visualization, we used R v3.4 and GraphPad Prism v7.
Results and discussion
In the present study, we aimed to model the HIV-1 DNA dynamics in early treated patients, to infer the timepoint from which further significant decay of the viral reservoir is no longer detectable. This parameter is critical for the design of clinical trials evaluating new therapeutic strategies aiming to impact the HIV-1 reservoir size. It may provide guidance for the interpretation of such studies and maximize the effect of viral control after cART interruption.
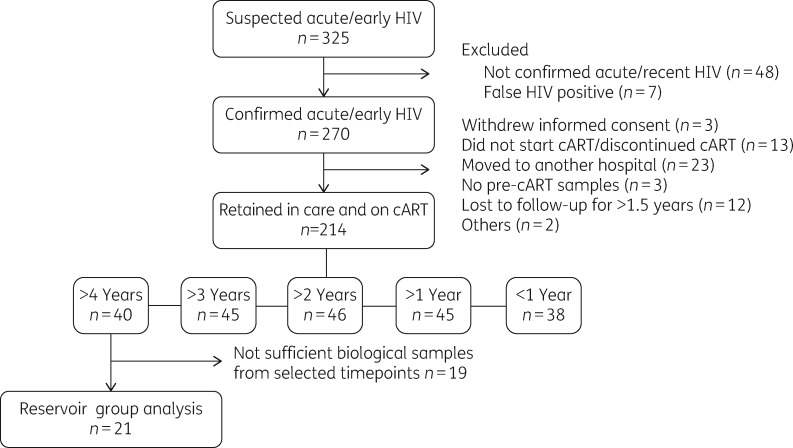
For that purpose, we characterized the evolution of total HIV-1 DNA levels in 21 early treated patients, from a cohort of 214 subjects who initiated cART during confirmed acute/early HIV infection (Figure 1). Demographics and clinical parameters of this subgroup were not significantly different from those of the rest of members of the Early_cART cohort (Table 1). All 21 participants had started cART within a median of 65 days (IQR = 47–75) since estimated HIV-1 acquisition. One participant started cART at Fiebig stage I, 18 at Fiebig stage V and 2 at Fiebig stage VI. All individuals were MSM, with a median age of 32 years (IQR = 27–36) at diagnosis and a median plasma viral load of 5.2 HIV-1 RNA log10 copies/mL (IQR = 4.2–5.7) at cART initiation. A majority (86%, 18 out of 21) of first-line cART regimens were based on integrase inhibitors (raltegravir in all cases). All patients were effectively suppressed during the follow-up. Longitudinal measurement of proviral HIV-1 DNA was performed in CD4+ T cells isolated from cryopreserved PBMCs at 0, 0.5, 1, 2, 3 and 4 years after cART initiation.
Figure 1.
Disposition of patients in the Early_cART cohort and subjects included in the present study.
Table 1.
Demographic and baseline characteristics of the patients included in the study (reservoir group, n = 21) and the whole Early_cART cohort (total cohort, n = 214), prior to cART initiation
| Reservoir group (n = 21) | Total cohort (n = 214) | |
|---|---|---|
| Age (years), median (IQR) | 32 (27–36) | 33 (27–39) |
| Gender; male, n (%) | 21 (100) | 212 (99) |
| Group risk; MSM, n (%) | 21 (100) | 203 (95) |
| Pre-cART log10 HIV-1 RNA (copies/mL), median (IQR) | 5.2 (4.2–5.7) | 5.0 (4.4–5.7) |
| Pre-cART CD4+ T cell count (cells/mm3), median (IQR) | 418 (304–500) | 466 (331–607) |
| Pre-cART CD4+/CD8+ ratio, median (IQR) | 0.48 (0.29–0.75) | 0.53 (0.38–0.85) |
| Time from HIV acquisition to cART (days), median (IQR) | 65 (47–75) | 67 (40–92) |
| Time from cART initiation to first VL <50 copies/mL (days), median (IQR) | 91 (32–131) | 69 (28–126) |
| Fiebig stage at cART initiation, n (%) | ||
| I–IV | 1 (4.8) | 27 (12.6) |
| V | 18 (85.7) | 114 (53.3) |
| VI | 2 (9.5) | 73 (34.1) |
| First-line cART regimen, n (%) | ||
| three drugs, INSTI based | 18 (85.7) | 200 (93.4) |
| three drugs, PI based | 2 (9.5) | 10 (4.7) |
| others | 1 (4.8) | 4 (1.9) |
VL, viral load; INSTI, integrase strand transfer inhibitor.
The absence of significant differences between the reservoir group and the rest of individuals belonging to the Early_cART cohort (n = 193) was assessed by performing Fisher’s test for ‘gender’ and ‘group risk’, χ2 test for ‘Fiebig stage at cART initiation’ and ‘first line cART regimen’ and a Mann–Whitney U test for the rest of parameters.
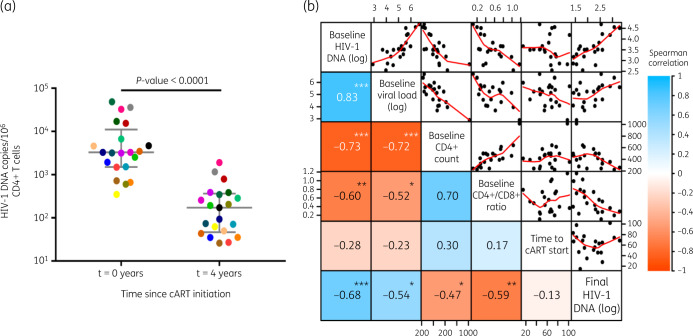
Before initiating cART, total HIV-1 DNA in peripheral blood, which reflected the number of infected cells during the period of untreated viral replication, showed a median level of 3263 copies/106 CD4+ T cells (IQR = 1499–11 072) (Figure 2a). At the end of the study, after 4 years of suppressive cART, the remaining proviral DNA showed a massively reduced median level of 172 HIV-1 DNA copies/106 CD4+ T cells (IQR = 47–366, P < 0.0001) (Figure 2a). In most patients, viral DNA levels had undergone a 10- to 100-fold overall decrease (median = 1.43 log10 units; IQR = 1.17–1.69).
Figure 2.
HIV-1 DNA in peripheral CD4+ T cells from early treated patients during cART and correlation with other viral and clinical parameters. (a) Change in HIV-1 DNA (median and IQR) in peripheral CD4+ T cells before cART initiation and after 4 years of cART. Wilcoxon test P value is shown. Each coloured dot represents an individual patient (n = 21). (b) Heat map showing the association between different clinical parameters before cART initiation (baseline) and at Year 4 (final). In the left-hand side of the matrix, the Spearman’s rank correlation coefficient (rho) is shown inside the boxes and represented as a colour code. The P value for significant correlations is also shown inside the boxes (***P < 0.001, **P < 0.01, *P < 0.05). In the right-hand side of the matrix, correlations are shown in diagrams with points representing single patients and red lines representing the trend. Axes are located on the sides of the matrix. HIV-1 DNA and RNA levels are represented as log10 copies/106 CD4+ T cells or copies/mL of blood, respectively. CD4+ T cell count is represented as cells/mm3. Time to cART start from HIV-1 estimated date of acquisition is represented as days.
We also evaluated the association between the HIV-1 reservoir size and different clinical and virological parameters (Figure 2b). As expected, baseline levels for total HIV-1 DNA and viral load, prior to cART initiation, significantly and positively correlated with each other (P < 0.001) and with HIV-1 DNA levels at the end of the study (P < 0.001 and P = 0.012, respectively). Also, absolute CD4+ T cell count and CD4+/CD8+ ratio at baseline were inversely correlated with final HIV-1 DNA measurements (P = 0.031 and P = 0.0046, respectively). However, we found no correlation between final HIV-1 DNA levels and the timespan between estimated HIV-1 infection and cART initiation (P = 0.561), probably due to low inter-individual variability (IQR = 47–75 days) of this parameter in the study group.
As expected, individual dynamics of all patients showed a quick decrease in viral DNA levels during the first months of cART (Figure 3). This sharp drop was later restrained and finally HIV-1 DNA levels were relatively stabilized. The different rates of decay corresponded to the initial rapid loss of the major compartment of short-lived productively infected CD4+ T cells and the subsequent slower clearance of latently infected CD4+ T cell subpopulations with longer half-lives.15 Similar results were recorded in former studies, both in early treated and chronically treated patients, although the size of the long-lived reservoir was found to be smaller in early treated patients.1,16 The early initiation of cART, when HIV-1 DNA levels were still relatively low, probably explains the HIV-1 DNA levels on ART also being lower. The detection of a very strong correlation between HIV-1 DNA levels at cART initiation and subsequent measurements in our study would be in line with this notion.
Figure 3.
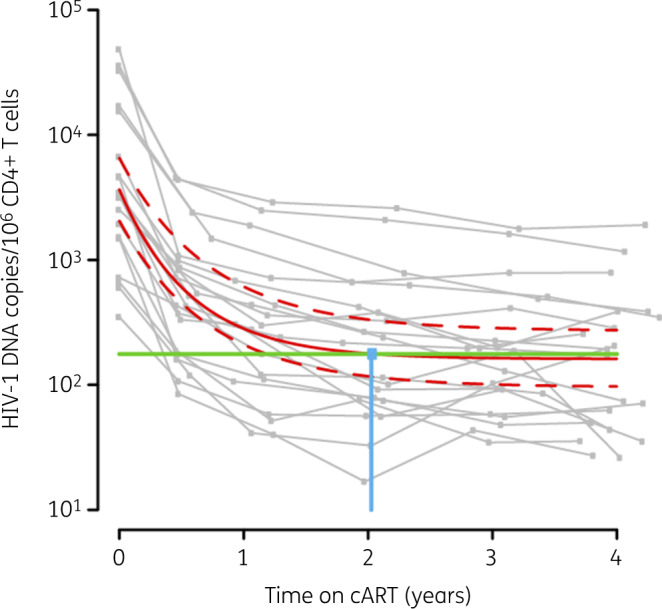
Statistical model for HIV-1 DNA dynamics during cART in early treated patients. Global exponential decay kinetics, adjusted with a linear mixed-effects model, are represented by the red line, flanked by 95% CI (red dashed lines) and shown superimposed over the individual dynamics in grey. Significant difference from the final HIV-1 DNA level determined by the model is represented by the threshold (80%; green line). The intersection point of the model and the threshold is indicated by the blue dot and line.
Our main objective was to determine how much time is needed, upon early cART initiation, to reach these stable HIV-1 DNA levels. To answer this question, we first adjusted our data from the 21 individual dynamics to the global model that best described their behaviour using a statistical mixed-effects model (Figure 3). In the resulting dynamics, HIV-1 DNA exponentially decayed towards the reservoir set point. The defined model was used to provide an in silico estimation of the timepoint from which no further significant HIV-1 DNA decay would be detected in eventual clinical trials. Thus, we based our study on statistical power estimations considering a conservative scenario with a hypothetical sample size of n = 50 and a one-sided test. According to our estimation, 2 years of early cART would ensure that the stable reservoir set point has been achieved.
Previous reports showed discrepancies in the decay kinetics of the HIV-1 reservoir in early treated individuals,1,2,4,10,11 probably due to differences in the study design and analytical approaches. For this reason, our longitudinal study was specifically designed to enable a statistical estimation of the time needed on cART to achieve stable HIV-1 DNA levels. Our results indicate that in early treated individuals the viral reservoir reaches a set point 2 years after cART initiation. Results from previous studies suggested a slower stabilization of the HIV-1 DNA set point after ART initiation in acute and/or early treated individuals, in the range of 3–7 years;4,11 however, total HIV-1 DNA measurement methodology and timepoints included in the analysis, as well as design of data analysis, differed from the present one and none included statistical modelling of longitudinal data. Additionally, population size and characteristics, including diversity of HIV subtypes, may influence such differences.
Our study also has some potential limitations. Firstly, we analysed a relatively small number of individuals (n = 21), although they were representative of the total cohort (Table 1). Secondly, most participants were receiving integrase inhibitor-based regimens, while the cART regimen may differ in other clinical settings. However, we do not anticipate differential effects on the long-term decay dynamics of the viral reservoir due to the use of different antiretroviral combinations, provided they are fully suppressive cART regimens.6,17,18 Also, timing of cART initiation is a crucial variable defining HIV-1 DNA decay dynamics. As the vast majority of our participants started cART at Fiebig stage V (Table 1), this might raise the question of whether a similar result would be obtained with a different set of individuals including patients treated since earlier phases. In fact, previous studies show a significantly sharper initial decay of total HIV-1 DNA among acute-treated individuals starting cART within the first month after infection—comprising Fiebig stages I to IV—when compared with those early treated patients starting cART later.16 Interestingly, the difference in slopes between the groups becomes non-significant after 8 months of treatment. This suggests that the time at which HIV-1 DNA levels reach the set point would be similar in all early treated patients, regardless of their Fiebig staging. Even in the case of chronically treated individuals, the time needed to achieve HIV-1 DNA stabilization might be similar; despite a plateau being observed after 4 years of cART, no further analysis had been performed in timepoints between Years 1 and 4 to investigate the exact point of stabilization.5 Future studies directly addressing the comparison of time to reservoir stabilization between early and chronically treated individuals would be of interest.
In this study the longitudinal dynamics of total HIV-1 DNA levels have been investigated, but not other valuable measures of HIV-1 persistence such as cell-associated HIV-1 RNA, intact proviruses or replication-competent reservoir.19,20 Despite the real added value of these alternative approaches, as proviral DNA overestimates the true size of the reservoir due to the high proportion of defective proviruses, this was the only measurement that allowed us to compare viral dynamics in early treated individuals with other patient cohorts described in previous studies. Forthcoming studies comparing data from total HIV-1 DNA measurements, quantitative viral outgrowth assay (QVOA) and the recently developed intact proviral DNA assay (IPDA) might provide key information on the stability of the true viral reservoir in early versus chronically treated individuals.21,22
In conclusion, early cART clinical trials aiming to mobilize the viral reservoir, especially those including a treatment-interruption phase, might benefit from taking into consideration that 2 years on suppressive cART are needed to stabilize the reservoir. After this time, the impact of therapeutic interventions on the viral reservoir can be more precisely measured. In addition, since viral reservoir size has been related to the likelihood of viral rebound once treatment is stopped, introducing a waiting period of 2 years to allow the reservoir to reach lower levels will also enhance the chance that viral control is achieved after treatment interruption.
Acknowledgements
Preliminary results from the present work were presented at the ‘XVII Jornada de Virologia’ (Virology Day) organized by the Societat Catalana de Biologia (Catalan Society of Biology), 30 October 2018, Barcelona, Spain (Abstract #P8). They were also presented at the ‘X Congreso Nacional GeSIDA’ (National Congress of HIV/AIDS Research Group) organized by GeSIDA and SEIMC (Spanish Society of Infectious Diseases and Clinical Microbiology), 6–9 November 2018, Madrid, Spain (Abstract PO-41).
We thank R. Ayén, L. Gómez and E. Grau for sample processing.
Funding
The Early_cART cohort is funded in part by the HIVACAT Catalan research programme for an HIV vaccine. This work was supported in part by research agreement with AELIX Therapeutics S.L. (Barcelona). A.B.-G. is supported by the Spanish Ministry of Education, Culture and Sport (FPU17/04766), B.M. is supported by ‘Instituto de Salud Carlos III’ (JR 13/00024) and S.M.-L. is supported by ‘Agència de Gestió d’Ajuts Universitaris i de Recerca’ (2013FI_B00275).
Funding sources had no involvement in the design, development, analysis, interpretation and publication of results from the present study.
Transparency declarations
B.M. is a consultant for AELIX Therapeutics S.L., outside the submitted work. C.B. is founder, CSO and shareholder of AELIX Therapeutics S.L. All other authors: none to declare.
Author contributions
All authors made substantial contributions to the conception and design of the work or to the acquisition, analysis and interpretation of the data. M.C.P., V.U., B.M., C.B. and J.M.-P. contributed to the study conception and design. L.B., P.C. and B.M. contributed to patients’ recruitment and clinical management. A.B.-G., M.C.P. and S.M.-L. performed the experimental work. V.U. performed the statistical analysis. A.B.-G., M.C.P., V.U., J.M.-P., B.M. and C.B. contributed to data analysis and interpretation, and drafted the manuscript including figures and tables; all authors revised it critically for important intellectual content and approved the final version submitted for publication.
References
- 1. Hocqueloux L, Avettand-Fènoël V, Jacquot S. et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother 2013; 68: 1169–78. [DOI] [PubMed] [Google Scholar]
- 2. Jain V, Hartogensis W, Bacchetti P. et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 2013; 208: 1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ananworanich J, Schuetz A, Vandergeeten C. et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012; 7: e33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ananworanich J, Chomont N, Eller LA. et al. HIV DNA set point is rapidly established in acute HIV infection and dramatically reduced by early ART. EBioMedicine 2016; 11: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Besson GJ, Lalama CM, Bosch RJ. et al. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin Infect Dis 2014; 59: 1312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Golob JL, Stern J, Holte S. et al. HIV DNA levels and decay in a cohort of 111 long-term virally suppressed patients. AIDS 2018; 32: 2113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hurst J, Hoffmann M, Pace M. et al. Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nat Commun 2015; 6: 8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leth S, Schleimann MH, Nissen SK. et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV 2016; 3: e463–72. [DOI] [PubMed] [Google Scholar]
- 9. Williams JP, Hurst J, Stöhr W. et al. HIV-1 DNA predicts disease progression and post-treatment virological control. Elife 2014; 3: e03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koelsch KK, Boesecke C, McBride K. et al. Impact of treatment with raltegravir during primary or chronic HIV infection on RNA decay characteristics and the HIV viral reservoir. AIDS 2011; 25: 2069–78. [DOI] [PubMed] [Google Scholar]
- 11. Buzon MJ, Martin-Gayo E, Pereyra F. et al. Long-term antiretroviral treatment initiated at primary HIV-1 infection affects the size, composition, and decay kinetics of the reservoir of HIV-1-infected CD4 T cells. J Virol 2014; 88: 10056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fiebig EW, Wright DJ, Rawal BD. et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003; 17: 1871–9. [DOI] [PubMed] [Google Scholar]
- 13. Morón-López S, Puertas MC, Gálvez C. et al. Sensitive quantification of the HIV-1 reservoir in gut-associated lymphoid tissue. PLoS One 2017; 12: e0175899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu H, Ding AA.. Population HIV-1 dynamics in vivo: applicable models and inferential tools for virological data from AIDS clinical trials. Biometrics 1999; 55: 410–8. [DOI] [PubMed] [Google Scholar]
- 15. Palmer S, Josefsson L, Coffin JM.. HIV reservoirs and the possibility of a cure for HIV infection. J Intern Med 2011; 270: 550–60. [DOI] [PubMed] [Google Scholar]
- 16. Laanani M, Ghosn J, Essat A. et al. Impact of the timing of initiation of antiretroviral therapy during primary HIV-1 infection on the decay of cell-associated HIV-DNA. Clin Infect Dis 2015; 60: 1715–21. [DOI] [PubMed] [Google Scholar]
- 17. Markowitz M, Evering TH, Garmon D. et al. A randomized open-label study of 3- versus 5-drug combination antiretroviral therapy in newly HIV-1-infected individuals. J Acquir Immune Defic Syndr 2014; 66: 140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ostrowski M, Benko E, Yue FY. et al. Intensifying antiretroviral therapy with raltegravir and maraviroc during early human immunodeficiency virus (HIV) infection does not accelerate HIV reservoir reduction. Open Forum Infect Dis 2015; 2: ofv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bruner KM, Hosmane NN, Siliciano RF.. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol 2015; 23: 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baxter AE, O’Doherty U, Kaufmann DE.. Beyond the replication-competent HIV reservoir: transcription and translation-competent reservoirs. Retrovirology 2018; 15: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bruner KM, Murray AJ, Pollack RA. et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med 2016; 22: 1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bruner KM, Wang Z, Simonetti FR. et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019; 566: 120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]