Abstract
Background
Artemisinin-based combination therapies (ACTs) have significantly contributed to reduce Plasmodium falciparum malaria burden in Vietnam, but their efficacy is challenged by treatment failure of dihydroartemisinin/piperaquine ACT in Southern provinces.
Objectives
To assess the efficacy of dihydroartemisinin/piperaquine for uncomplicated P. falciparum malaria in Gia Lai, Central Vietnam, and determine parasite resistance to artemisinin (ClinicalTrials.gov identifier NCT02604966).
Methods
Sixty patients received either dihydroartemisinin/piperaquine (4 mg/kg/day, 3 days; n = 33) or artesunate monotherapy (4 mg/kg/day, 3 days; n = 27) followed by dihydroartemisinin/piperaquine (AS + DHA/PPQ). Clinical phenotypes were determined during a 42 day follow-up and analysed together with ex vivo susceptibility to antimalarials and molecular markers of drug resistance.
Results
Day 3 positivity rate was significantly higher in the AS + DHA/PPQ arm compared with dihydroartemisinin/piperaquine (70.4% versus 39.4%, P = 0.016). Parasite clearance time was 95.2 h (AS + DHA/PPQ) versus 71.9 h (dihydroartemisinin/piperaquine, P = 0.063) and parasite clearance half-life was 7.4 h (AS + DHA/PPQ) versus 7.0 h (dihydroartemisinin/piperaquine, P = 0.140). Adequate clinical and parasitological response at Day 42 was 100% in both arms. By RT–qPCR, 36% (19/53) patients remained positive until Day 7. No recurrences were detected. kelch13 artemisinin resistance mutations were found in 87% (39/45) of isolates and 50% (20/40) were KEL1/C580Y. The piperaquine resistance marker plasmepsin-2 was duplicated in 10.4% (5/48). Isolates from Day 3-positive patients (n = 18) had higher ex vivo survival rates to artemisinin compounds (P < 0.048) and prevalence of kelch13 mutations (P = 0.005) than Day 3-negative patients (n = 5). The WHO definition of artemisinin resistance was fulfilled in 60% (24/40) of cases.
Conclusions
Although dihydroartemisinin/piperaquine remained effective to treat P. falciparum, the high Day 3 positivity rate and prevalence of KEL1 strains calls for continuous monitoring of dihydroartemisinin/piperaquine efficacy in Central Vietnam.
Introduction
Artemisinin-based combination therapy (ACT) is the currently recommended first-line treatment for Plasmodium falciparum malaria and has been an important contributor to the reduction of the global malaria burden over the past two decades.1 However, the emergence and spread of resistance to ACTs in South-East Asia constitutes a challenge for countries targeting malaria elimination as well as a potential threat to malaria control in high endemicity areas.2 Standard ACT regimens combine the action of a fast-acting artemisinin derivative (such as dihydroartemisinin or artesunate, which reduces parasite load in the first 72 h) with that of a partner drug with a longer half-life (such as piperaquine, mefloquine or lumefantrine) that clears remaining parasites.3 Delayed parasite clearance from the blood after artemisinin treatment is the first indicator of artemisinin resistance and was first reported in Cambodia in 2008.4,5 Although even infections caused by artemisinin-resistant parasites are eventually cleared thanks to combined drug actions within an ACT, the presence of concomitant resistance to partner drugs has already led to high levels of treatment failure. This has been the case for the ACT dihydroartemisinin/piperaquine in Cambodia and Vietnam,6–8 and for artesunate/mefloquine in the Thai–Myanmar border,9 requiring changes in the ACT policy in these areas.5
At the parasitological level, non-synonymous mutations in the P. falciparum gene kelch13 (encoding the K13 propeller domain protein) are associated with a higher parasite survival rate after artemisinin exposure.10–12 Since the identification of this genetic marker, the WHO defines confirmed artemisinin resistance as the presence of ≥5% of patients carrying validated K13 mutations, all of whom remain parasitaemic 72 h after treatment initiation (Day 3) or have a parasite clearance half-life (PC½) ≥5 h.13 Likewise, piperaquine resistance associates with gene duplications in the plasmepsin-2 (pm2) and plasmepsin-3 gene cluster and mutation E415G in exonuclease (exo415).14,15 Genomic epidemiology studies have identified parasite genetic backgrounds (PGBs) prone to the emergence of K13 mutations,16,17 as well as a lineage of MDR P. falciparum, characterized by the presence of both K13-C580Y mutation and pm2 amplification (KEL1/PLA1), that spread within Cambodia and to neighbouring South-East Asian countries such as Vietnam.18–21 In addition, both dihydroartemisinin/piperaquine treatment failure and in vitro piperaquine-resistant phenotypes have been attributed to parasites with emerging mutations in the chloroquine resistance transporter gene (crt).21–24
Vietnam has experienced a sharp reduction in malaria cases in the past two decades, reaching 4813 confirmed cases in 2018 (an ∼90% reduction compared with the year 2000).25 About two-thirds of cases correspond to uncomplicated P. falciparum infections,26 which since 2007 have been treated with dihydroartemisinin/piperaquine, although drug combinations containing dihydroartemisinin/piperaquine plus trimethoprim (named CV8) were already part of national treatment policy in 2003.27 Susceptibility to dihydroartemisinin/piperaquine remained relatively stable until 2010–11. Between 2011 and 2016, parasite clearance time (PCT) and Day 3-positive rates steadily increased in the Southeast (from to 22% to 57% in Binh Phuoc) and Central Highlands provinces (from 0% to 68% in Gia Lai or 30% in Quang Nam).8,28–31 Treatment failure with dihydroartemisinin/piperaquine was first confirmed in 2015 in Binh Phuoc province, where the proportion of recrudescence reached >50% in 2018,8,23,29 and later on in Dak Nong province.5 Most of the recurring infections in Binh Phuoc were found to be K13-pm2 or exo415 double mutants.8,23,29 On the other hand, artemisinin-specific clearance time and treatment failure using artemisinin-based monotherapy have not been assessed in the Central Highlands, which accounted for almost half of all confirmed malaria cases in the country in 2018 (n = 2346).25
In this article, we present results of a therapeutic efficacy study that assessed the efficacy of dihydroartemisinin/piperaquine and determined the level of resistance to the artemisinin-derivative component in Gia Lai province (Central Highlands), including a detailed characterization of P. falciparum ex vivo drug susceptibility and drug resistance genetic profiles.
Patients and methods
Study site
The study was conducted between April 2015 and January 2017 in Chu R’Cam commune, Krong Pa district (Gia Lai, Vietnam). A field laboratory was set up in Chu R’Cam health centre, and patient recruitment was extended to the nearby communes of Ia R’Sai and Ia R’Suom, all of them within 20 km distance from the main district hospital (see Methods S1 for further details; available as Supplementary data at JAC Online). In 2014, Gia Lai province reported 4367 microscopically confirmed malaria cases, of which 2191 occurred in Krong Pa (1051 P. falciparum and 1124 Plasmodium vivax).32,33 Since 2010, the province has been considered an area of ‘suspected partial artemisinin resistance’ by the WHO, after the National Institute of Malariology, Parasitology and Entomology (NIMPE) reported >10% Day 3-positive slides in a trial conducted in Phu Thien district.34
Study design and trial procedures
The study was designed as a randomized open-label 42 day follow-up study to evaluate the clinical and parasitological responses after treatment of uncomplicated P. falciparum infections. Patients presenting with fever (≥37.5° C) and/or history of fever during the previous 48 h were screened for malaria by blood smears from finger pricks. Those with P. falciparum mono-infections >500 parasites/μL were invited to enrol. Haemoglobin was measured using Hb201+ System (Hemocue). Individuals with P. vivax, mixed infections, severe malaria, antimalarial drug hypersensitivity, regular use of medication that may interfere with antimalaria pharmacokinetics, signs of other febrile diseases, chronic medical conditions, ongoing pregnancy or breastfeeding women were excluded. Prior to treatment administration, patients were asked to donate 5 mL of whole blood collected by venepuncture. A 200 μL aliquot was transferred into an EDTA microtainer, 100 μL were mixed with 500 μL of RNAprotect (Qiagen) for subsequent RNA isolation, and the remaining volume was used for drug susceptibility assays and isolate cryopreservation. Parasitaemia was monitored by light microscopy at the health centre every 12 h until 72 h (Day 3), or until parasite clearance was confirmed by two consecutive negative blood films (see Methods S2). Additional finger pricks were performed on Day 7, 14, 28, 35 and 42.
Treatment
Patients were randomly assigned to one of the two treatment arms. Those under dihydroartemisinin/piperaquine received Eurartesim® daily for 3 days (40 mg dihydroartemisinin + 320 mg piperaquine/tablet; Sigma-Tau, Bologna, Italy), using the following weight-based daily dose: 13 to <24 kg, 1 tablet; 24 to <36 kg, 2 tablets; 36 to <75 kg 3 tablets; and 75 to 100 kg, 4 tablets. Artesunate monotherapy consisted of Co-Artesun® (50 mg artesunate/tablet; Guilin Pharmaceutical, Shangai, China) once daily at a target dose of 4 mg/kg/day for 3 days, followed by an additional 3 day course of Eurartesim®. Treatment was given under direct observation. Those vomiting their medication within the first 30 min received another full dose, whereas those vomiting between 30 and 60 min received a half dose.
Laboratory procedures
Susceptibility to artemisinin derivatives artesunate and dihydroartemisinin (Sigma–Aldrich) was measured by ring stage survival assay (RSA) in samples with parasitaemia ≥0.1% at time 0 h (see Methods S3).35,36 Percentage parasite survival was calculated after 72 h of culture as the parasitaemia in the drug-exposed well (6 h exposure) compared with parasitaemia in the unexposed control well. Susceptibility to chloroquine and piperaquine (Sigma–Aldrich) was determined by schizont maturation assays (SMAs; see Methods S3).37,38 Parasites were cultured on plates pre-dosed with seven 2-fold serial dilutions of drugs until the number of schizonts in the control well reached 40% (or until 42 h). Half-maximal inhibitory concentrations (IC50) were calculated using WWARN’s IVART Tool (http://www.wwarn.org/tools-resources/toolkit/analyse/ivart).39
Total parasite density was determined by quantitative reverse transcription–PCR (RT–qPCR) amplification of Pf18S A-type rRNA transcripts (pfA18S) in a LightCycler 480, whereas gametocyte densities were determined in separate reactions for all pfA18S-positive samples, by targeting Pfs25 transcripts, as previously described (see Methods S4).40
Prevalence of drug resistance markers was measured on parasite DNA extracted from Day 0 EDTA samples (see Methods S5). Copy number variations (CNVs) in pm2 and mdr1 (markers of mefloquine resistance)41 were determined by qPCR using the ubiquitin-conjugated enzyme gene as the reference gene and the 3D7 strain as calibrator. Genotyping of parasite barcode (101 SNPs) and SNPs in K13, exo415, crt and the artemisinin resistance parasite genetic background (arps10, ferredoxin, crt, and mdr2) was performed at the Wellcome Sanger Institute (Cambridge, UK) as part of the MalariaGEN SpotMalaria Project, using either capillary sequencing or MS of PCR amplicons in a MassARRAY® System (Agena BioScience; see Methods S5). Samples with sufficient quality were additionally processed for WGS and used to determine KEL1 lineage (based on five mutations in chromosome 13)20 as well as crt mutations associated with piperaquine resistance or dihydroartemisinin/piperaquine treatment failure (T93S, H97Y/L, F145I, I218F, M343L and G353V).21–24
Endpoints and definitions
Primary endpoints were parasite clearance estimates by light microscopy obtained from the loge parasitaemia–time profile using the WWARN Parasite Clearance Estimator (http://www.wwarn.org/tools-resources/toolkit/analyse/parasite-clearance-estimator-pce).42 PC½ was defined as the time in hours needed for the parasite density to decrease by 50% during the log-linear phase of the curve, which in susceptible parasites is <5 h. PCT was defined as the time in hours from the start of treatment to the first of the two consecutive negative blood slides, which in susceptible parasites is defined as <72 h. Treatment outcome was classified as early treatment failure, late clinical failure, late parasitological failure or adequate clinical and parasitological response (ACPR).43 Following WHO criteria, confirmed artemisinin resistance was defined as ≥5% of K13 validated mutations (F446I, N458Y, M476I, Y493H, R539T, I543T, P553L, R561H or C580Y) found in patients positive on Day 3 or in infections with PC½ ≥5 h.5,13
Sample size and statistics
Previous studies in Vietnam had reported Day 3 positivity rates of 27% for artesunate (4 mg/kg/day) and 22% for dihydroartemisinin/piperaquine (2.4 mg/kg/day) in Binh Phuoc province (2011), and 29% for dihydroartemisinin/piperaquine in Quang Nam province (2013).28,31 Assuming an initial Day 3 positivity rate of 30% in each treatment group, with a confidence level of 95% and a precision of 15%, 36 patients were estimated to be needed per treatment arm. Comparisons between both groups were performed using Chi-square, Fisher’s exact or Wilcoxon rank sum tests, as required. PCT in each group was estimated using Kaplan–Meier survival curves and log-rank test. Data analysis was performed in Stata 11.0 (Statacorp) and plotted using Prism8 (GraphPad). P values <0.05 were considered statistically significant.
Ethics
Ethics approval was obtained from ethics committees at the National Institute of Malariology, Parasitology and Entomology (351/QD-VSR), Vietnam’s Ministry of Health (QD2211/QD-BYT), Institute of Tropical Medicine Antwerp (936/14) and Antwerp University Hospital (14/15/182). All procedures followed national and institutional standards and were carried out in accordance with the Declaration of Helsinki. The trial was registered at ClinicalTrials.gov under identifier NCT02604966.
Results
Enrolment, baseline characteristics and follow-up
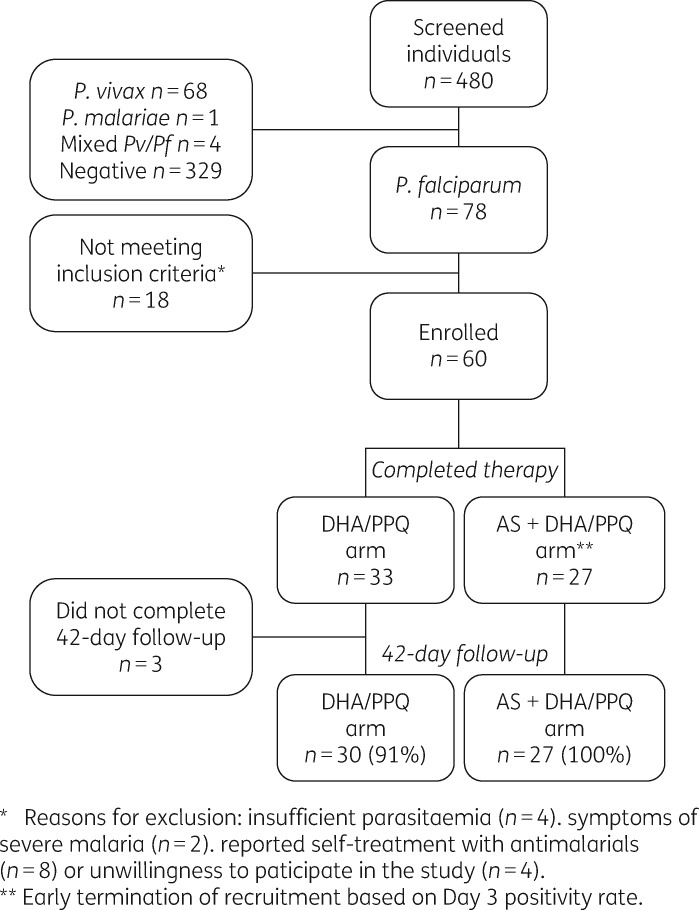
A total of 480 febrile individuals were screened during the study period, of whom 151 (31.5%) had malaria and 78 (16.2%) a P. falciparum mono-infection (Figure 1). Sixty patients [33 dihydroartemisinin/piperaquine and 27 artesunate + dihydroartemisinin/piperaquine (AS + DHA/PPQ)] met the inclusion criteria and were included in primary endpoint analysis.
Figure 1.
Study flow chart. AS, artesunate; DHA, dihydroartemisinin; PPQ, piperaquine.
The two arms did not differ significantly in their demographic characteristics, history of malaria or clinical and parasitological characteristics at enrolment (Table 1). The youngest patient was 8 years old (n = 2). None of the 60 participants reported taking any antimalarial drug in the 14 days prior to the visit. Both treatment regimens were well tolerated; one patient vomited artesunate tablets shortly after intake of the first dose and received another full dose. Fifty-seven patients (94%) completed the 42 day follow-up.
Table 1.
Characteristics of patients at enrolment by treatment arm
| Variable |
n/N (%) or median (IQR) |
P value | |
|---|---|---|---|
| DHA/PPQ (N = 33) | AS + DHA/PPQ (N = 27) | ||
| Age group (years) | |||
| 8–15 | 6/33 (18.2) | 5/27 (18.5) | 1.000 |
| > 15-25 | 9/33 (27.3) | 8/27 (29.6) | |
| > 25-45 | 18/33 (54.5) | 14/27 (51.9) | |
| Weight (kg) | 53 (47–58) | 53 (42–56) | 0.705 |
| Gender (males) | 29/33 (87.9) | 22/27 (81.5) | 0.718 |
| Ethnic group | |||
| JaRai | 28/33 (84.8) | 24/27 (88.9) | 0.719 |
| Kinh | 5/33 (15.2) | 3/27 (11.1) | |
| Had malaria last year | 13/25 (52) | 13/17 (76.5) | 0.195 |
| Fever | |||
| ≥37.5°C | 29/33 (87.9) | 22/27 (81.5) | 0.718 |
| temperature (°C), all | 38.3 (37.7–39.1) | 38.3 (37.5–39.1) | 0.577 |
| temperature (°C), febrile | 38.8 (38.2–39.4) | 38.9 (38.3–39.4) | 0.747 |
| Haemoglobin (Hb) (g/dL) | 13.6 (12.2–14.9) | 13.9 (12.2–15.6) | 0.494 |
| Anaemia (Hb <11 g/dL) | 4/33 (12.1) | 2/27 (7.4) | 0.681 |
| Main clinical symptoms: | |||
| headache | 32/33 (97) | 26/27 (96.3) | 1.000 |
| fatigue | 32/33 (97) | 25/27 (92.6) | 0.583 |
| chills | 24/33 (72.7) | 16/27 (59.3) | 0.288 |
| dizziness | 22/33 (66.7) | 16/27 (59.3) | 0.599 |
| nausea | 7/33 (21.2) | 3/27 (11.1) | 0.488 |
| Parasitological data by microscopy: | |||
| asexual parasitaemia (p/μL)a | 10166 (5311–19 459) | 15832 (8454–29 650) | 0.393 |
| gametocyte prevalence | 2/33 (6.1) | 1 (3.7) | 1.000 |
DHA, dihydroartemisinin; AS, artesunate; PPQ, piperaquine.
Geometric mean (95% CI).
Drug efficacy and study endpoints
More than half of the patients [53% (32/60)] remained positive at Day 3 by light microscopy, and this rate was significantly higher in the AS + DHA/PPQ arm [70.4% (95% CI 61.9%–78.9%)] compared with the dihydroartemisinin/piperaquine arm [39.4% (95% CI 31.1%–47.7%), P = 0.016]. The high Day 3 positivity rate in the AS + DHA/PPQ arm, detected in an evaluation of the preliminary results, prompted the termination of recruitment in this arm, a decision agreed between the study team and the NIMPE/National Malaria Control Program (NMCP).
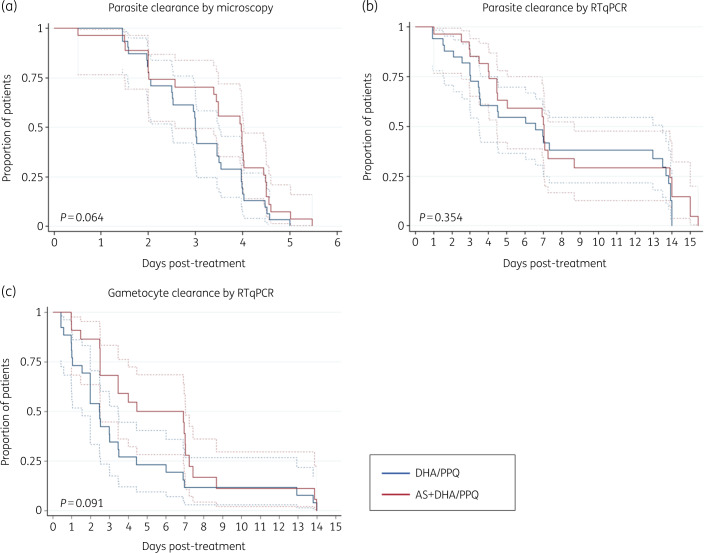
Patients that remained positive at Day 3 in the dihydroartemisinin/piperaquine arm had higher initial parasitaemia than Day 3-negative patients [31 896 parasites/μL (95% CI 13 931–73 027) versus 4835 parasites/μL (2154–10 852), respectively, P = 0.002], but densities did not differ in the AS + DHA/PPQ arm [22 237 parasites/μL (95% CI 11 936–41 426) versus 7066 parasites/μL (1328–37 621), respectively; P = 0.222]. Day 3-positive and negative patients did not differ in age (P = 0.759 for dihydroartemisinin/piperaquine and P = 0.466 for AS + DHA/PPQ) or in the exact drug dose adjusted by body weight (P = 0.246 for dihydroartemisinin and piperaquine and P = 0.130 for artesunate). None of the patients positive at or after Day 3 presented fever. Based on light microscopy, all infections were cleared by Day 6 (Figure 2a) and per-protocol ACPR was 100% in both arms. Overall, the median PCT was 95.2 h (range 12.1–131.5) and the median PC½ was 7.3 h (range 2.0–11.8). In 81% of patients (42/52), the PC½ was longer than 5 h. Both PCT and PC½ medians were longer in patients who first received artesunate monotherapy compared with dihydroartemisinin/piperaquine (Table 2 and Figure 2a), albeit the differences were not statistically significant. Parasitaemia at Day 0 did not correlate with PCT or PC½.
Figure 2.
Kaplan–Meier curves for time to parasite clearance by treatment arm. (a) Asexual parasitaemia by light microscopy (n = 60); (b) asexual parasitaemia by PfA18S RT–qPCR (n = 50); (c) gametocytes by Pfs25 RT–qPCR (n = 50). Time was computed in hours since treatment administration. No measurements were conducted between Day 7 and Day 14 (some patients were sampled on Day 15 for logistical reasons). Dashed lines indicate 95% CI. P values were calculated using log-rank test. DHA/PPQ, dihydroartemisinin/piperaquine; AS, artesunate.
Table 2.
Study endpoints
|
n/N (%) or median (IQR) |
P value | ||
|---|---|---|---|
| DHA/PPQ (N = 33) | AS + DHA/PPQ (N = 27) | ||
| Primary endpoints | |||
| PCT (h) | 71.9 (48.2–95.2) | 95.2 (48.2–107.6) | 0.063 |
| PC½ (h)a | 7.0 (5.7–7.8) | 7.4 (6.6–9.1) | 0.140 |
| Positive at Day 3 | |||
| by light microscopy | 13/33 (39.4) | 19/27 (70.4) | 0.016 |
| by RT–qPCR | 26/33 (78.8) | 14/18 (77.8) | 0.539 |
| Secondary endpoints | |||
| early treatment failureb | 0 (0) | 0 (0) | – |
| late clinical failurec | 0 (0) | 0 (0) | – |
| late parasitological failured | 0 (0) | 0 (0) | – |
| ACPRe | 30/30 (100) | 27/27 (100) | 1.000 |
DHA, dihydroartemisinin; AS, artesunate; PPQ, piperaquine; PCT, parasite clearance time; PC½, parasite clearance half-life; ACPR, adequate clinical and parasitological response.
Time needed for parasitaemia to reduce by half. N (DHA/PPQ) = 27; N (AS + DHA/PPQ) = 25.
Parasitaemia at Day 2 > Day 0 or parasitaemia at Day 3 with fever or parasitaemia at Day 3 ≥25% of parasitaemia on Day 0.
Parasitaemia with fever or signs of severe malaria between Day 4 and Day 42.
Parasitaemia between Day 7 and Day 42.
Absence of parasitaemia by Day 42 without previously meeting any of the criteria for treatment failure.
RT–qPCR analysis of infections revealed a Day 3 positivity rate of 78% (40/51), with no significant differences between treatment arms (Table 2). No recurrences were detected by RT–qPCR during the follow-up. However, several patients remained RT–qPCR positive until Day 7 (Figure 2b). The Day 7 RT–qPCR positivity rate did not differ between treatment arms and was associated with having higher initial parasitaemia [24 937 parasites/μL (95% CI 13 824–44 984) versus 7953 parasites/μL (4145–15 260), P = 0.020].
Gametocytes were present in 76.0% (38/50) of infections at Day 0 by RT–qPCR, most of them submicroscopic [92% (35/38)]. There were no differences between treatment arms in baseline gametocyte carriage (P = 0.735) or gametocyte density (P = 0.963). The median time to clear gametocytes was 60 h (IQR 37–84) for dihydroartemisinin/piperaquine and 72 h (60–137) for AS + DHA/PPQ (P = 0.355). The gametocyte clearance rate was slower during artesunate monotherapy and differed significantly until Day 7 (P = 0.005, log-rank test, Figure 2c).
Ex vivo susceptibility to antimalarial drugs
Twenty-three out of the 57 attempted RSAs (40.3%) had a growth rate >1 in control wells and were included in the analysis. The median parasite survival relative to drug-free control was 38.5% (IQR 10.7%–88.3%) after dihydroartemisinin exposure and 37.2% (15.7%–100.8%) after artesunate exposure. Parasites isolated from patients positive at Day 3 (n = 18) had higher survival rates to ex vivo artemisinin-derivative exposure than those who cleared before Day 3 (n = 5, P < 0.048; Table 3). There was no correlation between ex vivo survival rates to artemisinin and in vivo parasite clearance by PC½ (Figure S1).
Table 3.
Ex vivo drug susceptibility and molecular markers of drug resistance by light microscopy Day 3 positivity
| All |
DHA/PPQ |
AS + DHA/PPQ |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 3-negative |
Day 3-positive |
P | Day 3-negative |
Day 3-positive |
P | Day 3-negativea |
Day 3-positive |
P | |||||||
| Ex vivo drug assays | N | N | N | N | N | N | |||||||||
| DHA survival rateb | 5 | 5.4 (0–10.7) | 18 | 48.5 (22.4–88.4) | 0.048 | 4 | 8.1 (2.7–81.8) | 7 | 44.1 (35.2–90.2) | 0.130 | 0 | – | 11 | 52.9 (19.1–88.4) | – |
| AS survival rateb | 5 | 13.7 (5.4–14.3) | 18 | 43.4 (25.2–101.0) | 0.021 | 4 | 14.0 (9.5–49.2) | 7 | 39.5 (30.1–115.0) | 0.089 | 0 | – | 11 | 47.3 (21.4-101.0) | – |
| PPQ IC50 (nM)c | 12 | 35.9 (24.0–53.7) | 17 | 45.4 (34.8–59.2) | 0.352 | 5 | 24.5 (16.6–36.1) | 7 | 44.5 (36.3–54.7) | 0.007 | 7 | 47.1 (25.1–88.7) | 10 | 46.0 (28.6–74.1) | 0.770 |
| CQ IC50 (nM)c | 12 | 48.2 (37.1–62.4) | 17 | 70.0 (53.8–91.2) | 0.037 | 5 | 47.1 (34.2–64.8) | 7 | 85.0 (61.6–117.1) | 0.028 | 7 | 48.9 (30.5–78.5) | 10 | 61.2 (40.4–92.6) | 0.329 |
| Drug resistance markers | n/N | (%) | n/N | (%) | n/N | (%) | n/N | (%) | n/N | (%) | n/N | (%) | |||
| K13 non-synonymous mutations | 14/20 | (70) | 25/25 | (100) | 0.005 | 11/14 | (79) | 10/10 | (100) | 0.180 | 3/6 | (50) | 15/15 | (100) | 0.015 |
| K13-C580Y | 8/20 | (40) | 20/25 | (80) | 0.012 | 7/14 | (50) | 9/10 | (90) | 0.079 | 1/6 | (17) | 11/15 | (73) | 0.046 |
| KEL1 lineaged | 5/18 | (28) | 15/22 | (68) | 0.011 | 5/13 | (38) | 6/9 | (67) | 0.193 | 0/5 | (0) | 9/13 | (69) | 0.009 |
| PGB of artemisinin resistance | |||||||||||||||
| arps10-(V127M+D128H) | 16/22 | (73) | 26/29 | (90) | 0.150 | 12/16 | (75) | 9/10 | (90) | 0.617 | 4/6 | (67) | 17/19 | (89) | 0.234 |
| fd-D193Y | 11/22 | (50) | 24/29 | (83) | 0.017 | 10/16 | (63) | 10/10 | (100) | 0.052 | 1/6 | (17) | 14/19 | (74) | 0.023 |
| crt-(N326S+I356T) | 8/22 | (36) | 20/29 | (69) | 0.026 | 7/16 | (44) | 8/11 | (73) | 0.239 | 1/6 | (17) | 12/18 | (11) | 0.061 |
| mdr2-T484I | 9/22 | (41) | 4/28 | (14) | 0.051 | 5/16 | (31) | 1/10 | (10) | 0.352 | 4/6 | (67) | 3/18 | (17) | 0.038 |
| exonuclease-E415G | 0/23 | (0) | 0/30 | (0) | – | 0/17 | (0) | 0/11 | (0) | – | 0/6 | (0) | 0/19 | (0) | – |
| pm2 multicopy | 2/22 | (9) | 3/26 | (12) | 0.581 | 2/18 | (11) | 1/11 | (9) | 0.684 | 0/4 | (0) | 2/15 | (13) | 0.614 |
| mdr1 multicopy | 2/21 | (10) | 2/26 | (8) | 0.610 | 1/17 | (6) | 0/11 | (0) | 0.607 | 1/4 | (25) | 2/15 | (13) | 0.530 |
| crt-CVI(E/D)T haplotypes | 17/22 | (77) | 26/27 | (96) | 0.077 | 15/17 | (88) | 11/11 | (100) | 0.505 | 2/5 | (40) | 15/16 | (94) | 0.028 |
DHA, dihydroartemisinin; AS, artesunate; PPQ, piperaquine; CQ, chloroquine; PGB, parasite genetic background.
No successful RSA in Day 3-negative samples in the AS + DHA/PPQ arm.
Median (IQR).
Geometric mean (95% CI).
Presence of at least three of the following SNPs in Chr13: positions 1700345, 1717359, 1718288, 1739315 and 1862741 (Table S1).
Susceptibility to piperaquine and to chloroquine, which is first-line treatment for P. vivax in Vietnam, was determined in 56 SMAs. Twenty-nine (52%) assays reached 40% schizonts in the control well before 42 h and were included in the analysis. Another 14 (25%) reached between 10% and 40% schizonts, and 13 (23%) did not mature. The IC50 geometric means were 41.2 nM (95% CI 33.1–51.2) for piperaquine and 60.0 nM (49.6–72.6) for chloroquine. Parasites isolated from patients that remained positive at Day 3 after dihydroartemisinin/piperaquine exposure had a higher IC50 than those who cleared before Day 3 for both piperaquine and chloroquine (P = 0.007 and P = 0.028, respectively; Table 3, Figure S2). No differences were observed in those under artesunate monotherapy. A total of 13 cultures (23.2%) were successful for both RSA and SMA; in these samples, we did not observe a significant correlation between piperaquine or chloroquine IC50 and survival rates to artemisinin (Spearman’s rho < 0.472, P > 0.103).
Molecular markers of drug resistance
K13 non-synonymous mutations were found in 87% (39/45) of Day 0 samples (Table S1). Out of these, 71.8% (n = 28) corresponded to C580Y, 12.8% to Y511H (n = 5), 7.7% to C469F (n = 3) and 7.7% to P553L (n = 3), all of which had previously been reported in South-East Asia.9,44–47 The KEL1 lineage represented 50% (20/40) of all samples and 83% (20/24) of those with C580Y mutation. Eight different haplotypes of PGB associated with artemisinin resistance were identified. The double V127M + D128H substitution in arps10 was the most common mutation [82% (42/51)]. Most of the parasites with the C580Y mutation [93% (25/27)] were found in a background of triple arps10, fd and mdr2 mutations (MHY**I; Table 4).
Table 4.
Parasite genetic background (PGB) associated with artemisinin resistance and K13 mutations
| K13 non-synonymous mutations, n (%)b |
||||||
|---|---|---|---|---|---|---|
| PGB haplotypea | n | WT | C469F | Y511H | P553L | C580Y |
| VDDNIT | 5 | 2 (40) | 3 (60) | 0 | 0 | 0 |
| MHDNIT | 3 | 3 (100) | 0 | 0 | 0 | 0 |
| VDDSTT | 1 | 1 (100) | 0 | 0 | 0 | 0 |
| VDYSTT | 2 | 0 | 0 | 2 (100) | 0 | 0 |
| MHYSTT | 1 | 0 | 0 | 1 (100) | 0 | 0 |
| MHDNII | 5 | 0 | 0 | 0 | 3 (60) | 2 (40) |
| MHYNII | 8 | 0 | 0 | 0 | 0 | 8 (100) |
| MHYSTI | 17 | 0 | 0 | 0 | 0 | 17 (100) |
Order of SNPs: arps10-V127M and D128H, fd-D193Y, crt-N326S, crt-I356T, mdr2-T484I.
Percentages indicate the proportion of each K13 mutations per haplotype.
Overall, K13 mutants had a significantly longer PC½ [7.0 h (95% CI 6.3–7.8)] than WT parasites [2.9 h (2.2–3.8),P = 0.002; Figure 3]. There was a trend towards longer PC½ in C580Y mutants compared with parasites carrying other non-synonymous mutations [7.6 h (95% CI 7.0–8.6) versus 6.6 h (6.1–7.1), P = 0.072]. Similarly, the rate of K13 mutants was higher among persistent infections at Day 3 [100% (25/25)] compared with Day 3 negatives [70% (14/20); P = 0.005; Table 3]. Unfortunately, no RSAs were successful among K13 WT parasites, limiting further associations with ex vivo phenotypes. Taking the in vivo and molecular data together, 24 out of the 40 individuals with complete data available fulfilled WHO criteria of artemisinin resistance, resulting in a confirmed resistance rate of 60% (and a minimum rate of 40% out of 60 enrolled patients). All these 24 K13-validated mutants had PC½ ≥5 h, whereas 20 were also Day 3 positive.
Figure 3.
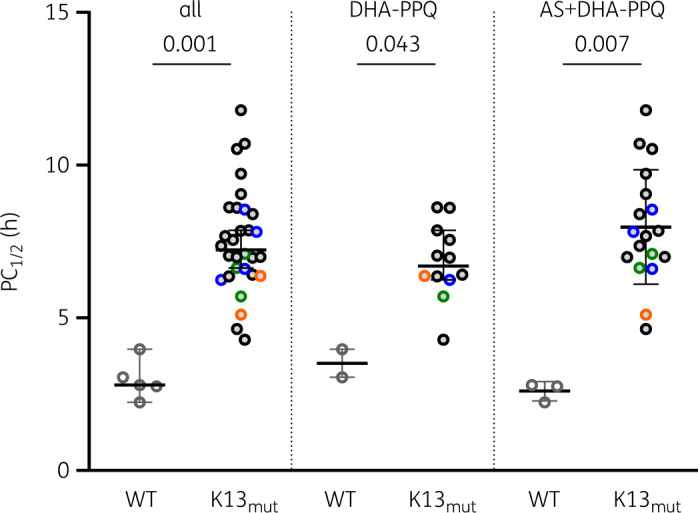
Parasite clearance and K13 non-synonymous mutations. Parasite clearance slope half-life (PC½) for K13 WT parasites and K13 mutants (K13mut; C469F in green, Y511H in blue, P553L in orange, C580Y in black).
There were no parasites with the piperaquine resistance-associated mutation exo415 (n = 56), but 10.4% (5/48) had pm2 duplication [mean copy number 2.3 (95% CI 1.7–3.4); Table S1]. On the other hand, mdr1 gene duplications, indicative of mefloquine resistance and common in areas with a high level of resistance to the ACT artesunate/mefloquine,9 were found in 8.5% (4/47) of the isolates. Double K13 + pm2 mutants were found in three samples [8.6% (3/35)], all of them with single parasite clones [complexity of infection (COI) = 1; Table S1]. Two were from the KEL1 lineage, were positive at Day 3 (after artesunate monotherapy) and had a PC½ of 7 and 7.8 h; the third sample (K13-Y511H) also had a duplication in mdr1 but infection was cleared before Day 3.
Finally, the chloroquine-resistant CVIET/CVIDT haplotype was found in 87.5% (42/48) of isolates and correlated with higher IC50 values [n = 18; 61.8 nM (95% CI 49.1–77.7)] compared with WT CVMNK [n = 4; IC50 33.6 nM (95% CI 20.0–56.3); P = 0.041]. There was no evidence of emerging crt mutations previously associated with piperaquine resistance or treatment failure (n = 40).
Discussion
The results show that more than half of the P. falciparum patients treated with artemisinin-based drugs in Gia Lai province had delayed clearance, were predominantly infected by K13 mutant parasites and had parasites with high survival rates to artemisinin ex vivo. Following the WHO definition, our data confirmed artemisinin resistance in at least 40% of P. falciparum infections. However, the lack of treatment failure and the low prevalence of polymorphisms in piperaquine resistance markers suggest that the dihydroartemisinin/piperaquine formulation remained effective to treat uncomplicated P. falciparum malaria in Gia Lai province in 2017.
Our data provide updated information on dihydroartemisinin/piperaquine efficacy in the Central Highlands and Gia Lai province, the region with the highest malaria burden in the country.25 Results from the reference dihydroartemisinin/piperaquine arm (Day 3 positivity = 39.4%, PCT = 72 h, PC½ = 7 h; n = 33) confirm the increasing trends in delayed clearance observed in Vietnam since 2010, with all indicators being higher than those reported in Gia Lai in 2011 (Day 3 positivity = 0%, PCT = 37 h, PC½ = 2 h; n = 55) or in the neighbouring province of Quang Nam in 2013 (Day 3 positivity = 29%, PCT = 61 h, PC½ = 6.2 h; n = 89).8,31 Although Day 3 positivity rates reported here are lower than those reported for Krong Pa in 2015 (55%–68%), the frequency of C580Y (67%) was highly coincident (67%–76%).8,30 The differences in in vivo efficacy may be explained by the type of drugs used in each trial. Indeed, whereas patients in the present study were treated with Eurartesim® (Sigma-Tau), a drug produced under Good Manufacturing Practices, the dihydroartemisinin/piperaquine drugs commonly used in Vietnam and in other therapeutic efficacy studies (branded as Artecan® or Arterakine®) are not, and concerns regarding potential underdosing have been raised in the past.31,48
Importantly, we were able to assess the efficacy of artemisinin independently from its ACT partner drug by including a monotherapy arm. Results clearly show that Day 3 positivity was higher in individuals under artesunate monotherapy as compared with those under ACT, irrespective of their initial parasitaemia. This rate was considerably higher than those reported in the few previous studies with artesunate monotherapy conducted in Binh Phuoc (2% in 2009 and 27% in 2011–13) and in Ninh Thuan (0% in 2016).12,28,49,50
At the parasitological level, artemisinin resistance was evident in pre-treatment parasite isolates showing both high survival to dihydroartemisinin and artesunate exposure in the RSA and a high prevalence of K13 non-synonymous mutations, with predominance of the KEL1 lineage. Remarkably, although KEL1 prevalence was low in Vietnam in 2013,20 the prevalence we report for Gia Lai (50% in 2017) is similar to that found in Western Cambodia in 2013,20 suggesting a rapid substitution of local strains by those spreading eastwards from the Cambodian province of Ratanakiri. Most of the parasites with C580Y were found in a background of the arps10, fd and mdr2 mutant haplotype (25/27), characteristic of founder populations strongly associated with artemisinin resistance previously identified in Southeast Vietnam.16 Recently, a study by MalariaGEN has shown that some previously rare crt mutations emerged in KEL1 parasites after 2016 in several Vietnamese provinces at frequencies ranging from 7% to 24%.21 However, we did not find evidence of any of these mutations in Gia Lai between 2015 and 2017.
Despite the high levels of artemisinin resistance, no treatment failures were reported, and only those patients with higher initial parasitaemia had detectable parasite genetic material until Day 7. On one hand, good ACPR results may be explained by the contribution of naturally acquired antibodies to parasite clearance. The study population was mostly adult (82% >15 years old), and it has been suggested that antibodies have a greater effect on clearing K13-mutant parasites than WT parasites.51 On the other hand, the low prevalence of piperaquine resistance markers suggests that partner drug efficacy remained unaffected and played a key role in P. falciparum clearance. Although the observation of a higher piperaquine IC50 in parasites from Day 3-positive versus Day 3-negative patients may suggest that there is some degree of piperaquine resistance (dihydroartemisinin/piperaquine arm only), it is possible that IC50 measurement does not reflect true parasite susceptibility to piperaquine. Research published shortly after the start of our clinical trial suggested that a 48 h exposure to 200 nM piperaquine (followed by a further 24 h of culture) better mimics the in vivo piperaquine pharmacological dose.52 Moreover, the similar association found between Day 3 positivity and chloroquine IC50 could also indicate some degree of cross-resistance between chloroquine-resistant strains and piperaquine.53 Finally, we cannot exclude that the lack of treatment failures was also partially explained by a better adherence to treatment under clinical trial conditions compared with real life.
The main limitations of our study were: first, the decline in malaria transmission observed in Gia Lai province during the study period as compared with previous years did not allow us to reach the target of 36 patients in the dihydroartemisinin/piperaquine arm (n = 30); similarly, recruitment in the AS + DHA/PPQ arm was stopped at 27 patients, in this case forced by the high Day 3 positivity rates that were double the initial estimates. Second, the reduced number of successful ex vivo assays together with the lack of culture replicates, which reflect the challenges of these assays in field conditions, limit the interpretations on drug susceptibility phenotypes in stratified analysis.
In conclusion, there was a high level of artemisinin resistance in Gia Lai province in early 2017 but no dihydroartemisinin/piperaquine treatment failure. Despite the good clinical outcomes, the high prevalence of KEL1 and the detection of KEL1 + pm2 duplications suggest there is a high risk that dihydroartemisinin/piperaquine treatment failure will emerges in Central Vietnam if partner drug resistance increases, based on the experience from Southern provinces.23 Vietnam has already opted to change the ACT policy from dihydroartemisinin/piperaquine to artesunate/pyronaridine in Binh Phuoc and Dak Nong provinces, but, until implementation is achieved, these and all remaining provinces will continue to use dihydroartemisinin/piperaquine. Under these circumstances, continuous monitoring of ACT efficacy is essential for adequate patient management and timely ACT switches (if needed) in order to ultimately meet malaria elimination targets. As therapeutic efficacy studies are becoming increasingly difficult to conduct due to low malaria transmission levels in South-East Asia, molecular surveillance tools including artemisinin and partner drug resistance markers as proxy indicators of in vivo resistance are urgently needed as part of NMCP strategies.
Supplementary Material
Acknowledgements
We sincerely thank all individuals that agreed to enrol in the trial. We are especially grateful to the staff from Chu R Cam health centre for their assistance and collaboration, to local and provincial authorities for their support, and to all staff at the NIMPE Department of Malaria Treatment. We would also like to thank Sigma-tau and Guilin Pharmaceutical for providing the study drugs, the WorldWide Antimalarial Resistance Network (WWARN) for clinical trial protocol guidelines, and the staff of Wellcome Sanger Institute (Sample Management, Genotyping, Sequencing and Informatics teams) for their contribution to the analysis of samples.
Funding
This work was supported by the Belgium Development Cooperation (DGD) under the Framework Agreement Program between DGD and ITM (FA3-III Vietnam, 2014–2016). This publication uses data from the MalariaGEN SpotMalaria Project, coordinated by the MalariaGEN Resource Centre supported by Wellcome (Grant ID 098051 and 090770).
Transparency declarations
None to declare.
Author contributions
Conceived and designed the study: E.R.V., N.V.H., N.X.X., A.E. and A.R.U.; performed the experiments: E.R.V., N.V.H., J.H.K., R.M.H., N.T.T.H., N.T.H.N., P.G., N.L.H. and T.T.M.; analysed the data: E.R.V., N.V.H., J.H.K. and A.R.U.; contributed reagents/data collection/materials/analysis tools: R.M.H., N.L.H., N.T.T.D. and A.E.; supervised the study activities: E.R.V., N.V.H., T.T.D., B.Q.P., N.X.X., A.E. and A.R.U; wrote the paper: E.R.V. and A.R.U. All authors read and approved the final version of the manuscript.
Supplementary data
Additional Methods (S1 to S5), Figures S1 and S2 and Table S1are available as Supplementary data at JAC Online.
References
- 1. Bhatt S, Weiss DJ, Cameron E. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015; 526: 207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Menard D, Dondorp A.. Antimalarial drug resistance: a threat to malaria elimination. Cold Spring Harb Perspect Med 2017; 7: a025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. White NJ. Antimalarial drug resistance. J Clin Invest 2004; 113: 1084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noedl H, Se Y, Schaecher K. et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 2008; 359: 2619–20. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Artemisinin resistance and artemisinin-based combination therapy efficacy. Status report. 2018. https://apps.who.int/iris/handle/10665/274362.
- 6. Saunders DL, Vanachayangkul P, Lon C.. Dihydroartemisinin–piperaquine failure in Cambodia. N Engl J Med 2014; 371: 484–5. [DOI] [PubMed] [Google Scholar]
- 7. Amaratunga C, Lim P, Suon S. et al. Dihydroartemisinin–piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis 2016; 16: 357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thanh NV, Thuy-Nhien N, Tuyen NTK. et al. Rapid decline in the susceptibility of Plasmodium falciparum to dihydroartemisinin–piperaquine in the south of Vietnam. Malar J 2017; 16: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Phyo AP, Ashley EA, Anderson TJC. et al. Declining efficacy of artemisinin combination therapy against P. falciparum malaria on the Thai–Myanmar border (2003–2013): the role of parasite genetic factors. Clin Infect Dis 2016; 63: 784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ariey F, Witkowski B, Amaratunga C. et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2013; 505: 50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WWARN K13 Genotype-Phenotype Study Group. Association of mutations in the Plasmodium falciparum Kelch13 gene (Pf3D7_1343700) with parasite clearance rates after artemisinin-based treatments— a WWARN individual patient data meta-analysis. BMC Med 2019; 17: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashley EA, Dhorda M, Fairhurst RM. et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2014; 371: 411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Artemisinin and artemisinin-based combination therapy resistance. Status Report. 2017. https://apps.who.int/iris/handle/10665/255213.
- 14. Witkowski B, Duru V, Khim N. et al. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype–genotype association study. Lancet Infect Dis 2017; 17: 174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amato R, Lim P, Miotto O. et al. Genetic markers associated with dihydroartemisinin–piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype–phenotype association study. Lancet Infect Dis 2017; 17: 164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miotto O, Amato R, Ashley EA. et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet 2015; 47: 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MalariaGEN. Genomic epidemiology of artemisinin resistant malaria. Elife 2016; 5: pii: e08714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Imwong M, Suwannasin K, Kunasol C. et al. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect Dis 2017; 17: 491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imwong M, Hien TT, Thuy-Nhien NT. et al. Spread of a single multidrug resistant malaria parasite lineage (PfPailin) to Vietnam. Lancet Infect Dis 2017; 17: 1022–3. [DOI] [PubMed] [Google Scholar]
- 20. Amato R, Pearson RD, Almagro-Garcia J. et al. Origins of the current outbreak of multidrug-resistant malaria in southeast Asia: a retrospective genetic study. Lancet Infect Dis 2018; 18: 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamilton WL, Amato R, van der Pluijm RW. et al. Evolution and expansion of multidrug-resistant malaria in southeast Asia: a genomic epidemiology study. Lancet Infect Dis 2019; 19: 943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ross LS, Dhingra SK, Mok S. et al. Emerging Southeast Asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat Commun 2018; 9: 3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van der Pluijm RW, Imwong M, Chau NH. et al. Determinants of dihydroartemisinin–piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis 2019; 19: 952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dhingra SK, Small-Saunders JL, Ménard D. et al. Plasmodium falciparum resistance to piperaquine driven by PfCRT. Lancet Infect Dis 2019; 19: 1168–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute of Malariology Entomology and Parasitology. Malaria Report 2018. 2019. [Internal Report].
- 26.World Health Organization. World Malaria Report 2018 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/en/.
- 27. Tran TH, Dolecek C, Pham PM. et al. Dihydroartemisinin–piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomised clinical trial. Lancet 2004; 363: 18–22. [DOI] [PubMed] [Google Scholar]
- 28. Hien TT, Thuy-Nhien NT, Phu NH. et al. In vivo susceptibility of Plasmodium falciparum to artesunate in Binh Phuoc Province, Vietnam. Malar J 2012; 11: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Phuc BQ, Rasmussen C, Duong TT. et al. Treatment failure of dihydroartemisinin/piperaquine for Plasmodium falciparum malaria, Vietnam. Emerg Infect Dis 2017; 23: 715–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pau MC, Pantaleo A, Tsamesidis I. et al. Clinical impact of the two ART resistance markers, K13 gene mutations and DPC3 in Vietnam. PLoS One 2019; 14: e0214667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thriemer K, Hong NV, Rosanas-Urgell A. et al. Delayed parasite clearance after treatment with dihydroartemisinin–piperaquine in Plasmodium falciparum malaria patients in central Vietnam. Antimicrob Agents Chemother 2014; 58: 7049–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institute of Malariology Entomology and Parasitology. Malaria Report 2014. 2015. [Internal Report].
- 33. Goldlust SM, Thuan PD, Giang DDH. et al. The decline of malaria in Vietnam, 1991–2014. Malar J 2018; 17: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Status report on artemisinin resistance. 2014. http://www10.who.int/malaria/publications/atoz/update-artemisinin-resistance-sep2014/en/.
- 35.WWARN Procedures. Ring stage Survival Assays (RSA) to evaluate the in vitro and ex vivo susceptibility of Plasmodium falciparum to artemisinins. 2013. https://www.wwarn.org/sites/default/files/attachments/procedures/INV10-Standard-Operating-Procedure-Ring-Stage-Survival-Assays-v1.2.pdf.
- 36. Witkowski B, Amaratunga C, Khim N. et al. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis 2013; 13: 1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Basco LK, Field application of in vitro assays sensitivity of human malaria parasites antimalarial drugs. 2007. https://apps.who.int/iris/handle/10665/43610.
- 38. Marfurt J, Chalfein F, Prayoga P. et al. Comparative ex vivo activity of novel endoperoxides in multidrug-resistant Plasmodium falciparum and P. vivax. Antimicrob Agents Chemother 2012; 56: 5258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Woodrow CJ, Dahlström S, Cooksey R. et al. High-throughput analysis of antimalarial susceptibility data by the WorldWide Antimalarial Resistance Network (WWARN) in vitro analysis and reporting tool. Antimicrob Agents Chemother 2013; 57: 3121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wampfler R, Mwingira F, Javati S. et al. Strategies for detection of Plasmodium species gametocytes. PLoS One 2013; 8: e76316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Price R, Uhlemann A, Brockman A.. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 2004; 364: 438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Flegg JA, Guerin PJ, White NJ. et al. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J 2011; 10: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. Methods for surveillance of antimalarial drug efficacy. 2009. https://www.who.int/malaria/publications/atoz/9789241597531/en/.
- 44. Nyunt MH, Soe MT, Myint HW. et al. Clinical and molecular surveillance of artemisinin resistant falciparum malaria in Myanmar (2009–2013). Malar J 2017; 16: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Win AA, Imwong M, Kyaw MP. et al. K13 mutations and pfmdr1 copy number variation in Plasmodium falciparum malaria in Myanmar. Malar J 2016; 15: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thuy-Nhien N, Tuyen NK, Tong NT. et al. K13 propeller mutations in Plasmodium falciparum populations in regions of malaria endemicity in Vietnam from 2009 to 2016. Antimicrob Agents Chemother 2017; 61: e01578–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ménard D, Khim N, Beghain J. et al. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med 2016; 374: 2453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ringwald P, Dondorp AM.. Severe malaria not responsive to artemisinin derivatives in man returning from Angola to Vietnam. Emerg Infect Dis 2015; 21: 1264–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thanh NV, Toan TQ, Cowman AF. et al. Monitoring for Plasmodium falciparum drug resistance to artemisinin and artesunate in Binh Phuoc Province, Vietnam: 1998–2009. Malar J 2010; 9: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Phong NC, Chavchich M, Quang HH. et al. Susceptibility of Plasmodium falciparum to artemisinins and Plasmodium vivax to chloroquine in Phuoc Chien Commune, Ninh Thuan Province, south-central Vietnam. Malar J 2019; 18: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ataide R, Ashley EA, Powell R. et al. Host immunity to Plasmodium falciparum and the assessment of emerging artemisinin resistance in a multinational cohort. Proc Natl Acad Sci USA 2017; 114: 3515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Duru V, Khim N, Leang R. et al. Plasmodium falciparum dihydroartemisinin–piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: retrospective and prospective investigations. BMC Med 2015; 13: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Basco LK, Ringwald P.. In vitro activities of piperaquine and other 4-aminoquinolines against clinical isolates of Plasmodium falciparum in Cameroon. Antimicrob Agents Chemother 2003; 47: 1391–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.