Abstract
Background
Cabotegravir is an HIV integrase inhibitor in clinical development with both oral and long-acting (LA) injectable formulations. Cabotegravir is primarily metabolized by uridine 5′-diphospho-glucuronosyltransferase (UGT) 1A1, a known polymorphic enzyme with functional variants that can affect drug metabolism and exposure.
Objectives
To investigate the pharmacogenetic effects of the reduced-function alleles UGT1A1*6, UGT1A1*28 and/or UGT1A1*37 on steady-state pharmacokinetics (PK) and safety of oral cabotegravir (30 mg/day) and intramuscular cabotegravir LA (400 mg every 4 weeks or 600 mg every 8 weeks).
Methods
Plasma cabotegravir PK was assessed in 346 UGT-genotyped participants with and without UGT1A1 functional variants across six studies (four Phase I and two Phase II) of oral cabotegravir, including 215 HIV-infected participants who received oral cabotegravir followed by cabotegravir LA. Changes from baseline in total bilirubin and ALT were assessed in one study (LATTE; NCT01641809).
Results
Statistically significant (P < 0.05) associations were observed between UGT1A1 genotype and plasma cabotegravir PK parameters, with 28%–50% increases following oral cabotegravir [plasma cabotegravir concentration at the end of the dosing interval (Ctau), 1.50-fold; AUCtau, 1.41-fold; and Cmax, 1.28-fold] and 16%–24% increases following cabotegravir LA administration (48 week Ctau, 1.24-fold; AUCtau, 1.16-fold; and Cmax, 1.18-fold) among those with low-versus-normal genetically predicted UGT1A1 activity. A statistically significant (P < 10−5) association between predicted UGT1A1 activity and maximum change in total bilirubin was also observed (2.45-fold asymptomatic increase for low versus normal) without a corresponding change in ALT.
Conclusions
This modest increase in oral and parenteral cabotegravir exposure associated with a reduced function of UGT1A1 is not considered clinically relevant based on accumulated safety data; no dose adjustment is required.
Introduction
Cabotegravir is an HIV-1 integrase strand transfer inhibitor in clinical development, both for HIV-1 treatment, in combination with long-acting (LA) rilpivirine, and as a single agent for prevention of sexually acquired HIV-1 infection. In clinical studies of HIV-1 treatment, cabotegravir was administered as an LA aqueous suspension for intramuscular (IM) injection in the gluteus medius muscle every 4 or 8 weeks following an initial lead-in period of oral dosing. Cabotegravir has low aqueous solubility, resulting in slow, solubility-limited absorption from the IM depot into the systemic circulation following IM administration.1 Cabotegravir LA also exhibits absorption-rate-limited pharmacokinetics (PK), resulting in an extended PK profile that provides sustained plasma concentrations over time and supports infrequent administration. Following a single injection, plasma cabotegravir concentrations can persist for more than a year in some subjects.2,3 Cabotegravir is not readily removed from the IM depot nor dialysable from systemic circulation. Hence, daily oral cabotegravir (immediate-release tablet) is being used as oral lead-in therapy, to assess individual tolerability prior to receipt of LA injections, and is also intended to provide short-term coverage during a lapse in injection dosing.
Cabotegravir is a substrate,4 though not an inhibitor or inducer,5 of uridine 5′-diphospho-glucuronosyltransferase (UGT) and primarily metabolized by UGT1A1 with a minor contribution by UGT1A9.4 The UGT family mediates conjugation of glucuronic acid with exogenous and endogenous substances, such as bilirubin, to increase water solubility for elimination. UGT1A1 is expressed primarily in the liver and gastrointestinal tract6 and is a polymorphic enzyme for which functional variants have been linked to both hereditary disease and altered substrate pharmacology.7–9 Ablation or near-ablation of UGT1A1 activity gives rise to Crigler–Najjar syndrome, a serious and potentially fatal condition characterized by severe hyperbilirubinaemia and kernicterus.10–12 A more common and less serious example of hereditary hyperbilirubinaemia is Gilbert’s syndrome, a generally benign and self-resolving condition characterized by fluctuating levels of unconjugated bilirubin occurring when UGT1A1 function is reduced to approximately 30% of normal.13,14
Genetic polymorphisms reducing UGT enzyme activity may also affect the safety profile of drugs metabolized via this pathway. Several well-characterized, functionally impaired alleles of UGT1A1 linked to Gilbert’s syndrome are implicated in adverse pharmacological and safety outcomes for some drug substrates. Well-studied examples include UGT1A1*6,15 leading to a Gly71Arg amino acid substitution16 found most often in Asian populations,17 and a pair of TA dinucleotide repeat sequence insertion variants in the promotor region (TA7 and TA8) that reduce mRNA expression: UGT1A1*28 (TA7), common in all populations,18 and UGT1A1*37 (TA8),18 which is largely restricted to African populations.19,20UGT1A1*6 and *28 elevate the severity and risk, respectively, of irinotecan-associated neutropenia21,22 and UGT1A1*28 appears to influence the PK and pharmacodynamics of etoposide23 and raloxifene24 as well as influencing the development of hyperbilirubinaemia with UGT1A1-inhibiting drugs such as atazanavir.25 Homozygosity or compound heterozygosity for UGT1A1*6, *28 or *37 has been shown to affect the risk of hyperbilirubinaemia with multiple medicines.25–28
Functional variation in UGT1A9, associated with a minor metabolic pathway for cabotegravir, is also known. Increased UGT1A9 expression due to carriage of UGT1A9*1b, which has a single-base insertion into the promotor region [−118(dT)9>10],29 has been associated with an increased risk of severe diarrhoea on irinotecan-based cancer treatment30 and variability in neonatal clearance of acetaminophen (paracetamol).31 The UGT1A9*1b allele is most common in Asian populations but is also found in Caucasians and African Americans.29,32
Although functional UGT1A1 and/or UGT1A9 variants may potentially impact the PK and safety profile of cabotegravir, there is limited understanding of how metabolic variation affects slow-release LA agents and metabolic profiling is not routinely conducted prior to antiretroviral administration. This is the first pharmacogenomic (PGx) assessment of UGT variation on steady-state cabotegravir exposures following oral immediate-release and LA extended-release administration in healthy and HIV-infected patients participating in Phase I and Phase II studies from the cabotegravir clinical development programme.
Patients and methods
Studies
Pharmacogenetic analyses were conducted retrospectively in participants in ViiV Healthcare-sponsored clinical studies of cabotegravir who provided study-related written informed consent and a separate, optional consent for genetic research. All studies were performed in accordance with the principles originating in the Declaration of Helsinki and with Good Clinical Practice. Protocols and informed consent forms were reviewed and approved by the relevant Institutional Review Boards/Independent Ethics Committees.
Two analyses were performed to assess the influence of UGT allelic variants on steady-state cabotegravir exposure: the impact of UGT1A1 and UGT1A9 on oral cabotegravir PK (Analysis 1), and the impact of UGT1A1 on parenteral cabotegravir LA exposure (Analysis 2). Data sources were four Phase I studies in healthy volunteers and two Phase II studies in HIV-infected patients (Table 1). Full details of three of the Phase I studies and both Phase II studies have been published elsewhere.33–37 Details of the fourth Phase I study are available from the ViiV Healthcare Clinical Study Register (https://www.viiv-studyregister.com/study/19569).
Table 1.
Subjects and studies included in the pooled analysis of oral cabotegravir
| Study | Phase | Purpose of study | No. of subjects with PGx and PK data |
||
|---|---|---|---|---|---|
| C tau | AUCtau | C max | |||
| NCT0314984833 | I | Drug interaction evaluation between rifabutin and oral cabotegravir in healthy volunteers. | 11 | 11 | 11 |
| NCT0146753134 | I | Drug interaction evaluation between oral rilpivirine and oral cabotegravir or dolutegravir in healthy volunteers. | 9 | 9 | 9 |
| NCT0159304635 | I | Safety and pharmacokinetics of cabotegravir LA and rilpivirine LA following an oral lead-in, in healthy volunteers. | 31 | 31 | 31 |
| NCT01754116a | I | Bioavailability of different formulations of LA parenteral cabotegravir, following an oral lead-in, in healthy volunteers. | 9 | 9 | 9 |
| NCT01641809 (LATTE)36 | II | Dose-ranging study of oral cabotegravir plus oral rilpivirine maintenance following induction with oral cabotegravir and nucleoside analogues in treatment-naive HIV-infected patients. The PK analysis was limited to patients who received 30 mg cabotegravir. | 49 | 0 | 0 |
| NCT02120352 (LATTE-2)37 | II | Study of 4 weekly versus 8 weekly maintenance dosing of cabotegravir LA plus rilpivirine LA following induction with oral cabotegravir and nucleoside analogues in treatment-naive HIV-infected patients. | 238 | 0 | 0 |
| Totals | 347 | 60 | 60 | ||
Details available at the ViiV Healthcare Clinical Study Register: https://www.viiv-studyregister.com/study/19569.
The first Phase II study in HIV-infected participants for which clinical data were available (LATTE; NCT01641809)36 administered oral cabotegravir (10, 30 or 60 mg once daily) with two nucleoside analogues for a 24 week induction followed by oral maintenance for 72 weeks with the same cabotegravir dose plus once-daily oral rilpivirine 25 mg. The second Phase II study (LATTE-2; NCT02120352)37 administered daily oral cabotegravir 30 mg plus abacavir/lamivudine for 20 weeks; virological responders were randomized to continue oral treatment, or switched to parenteral cabotegravir LA plus rilpivirine LA on a 4 weekly (400 mg plus 600 mg) or 8 weekly (600 mg plus 900 mg) dosing schedule.
Objectives
Analysis 1 pooled data from all six studies to evaluate the effect of UGT1A1 and UGT1A9 variants on steady-state exposure of once-daily oral cabotegravir 30 mg (with or without nucleoside analogues), the oral lead-in dose used to assess tolerability prior to cabotegravir LA administration in Phase II and Phase III studies. In addition, the effect of UGT1A1 and UGT1A9 variation on maximum on-treatment changes from baseline in ALT and total bilirubin (Tbili) was also assessed in a subset of LATTE participants receiving oral cabotegravir at any dose up to Week 72 in the induction and maintenance phases.
Analysis 2 was conducted later in a subset of Analysis 1 patients from the LATTE-2 study, after additional PK data became available following parenteral dosing during the maintenance phase of LATTE-2. The primary objective of this analysis was to evaluate the effect of UGT1A1 variants on steady-state cabotegravir LA exposure following IM administration with rilpivirine LA every 4 or 8 weeks. A descriptive comparison of the effects of UGT1A1 genotype on cabotegravir LA trough concentrations and oral cabotegravir trough concentrations in the same study was also conducted.
UGT genotyping and activity prediction
UGT1A1 and UGT1A9 genotyping was performed on stored samples from PGx-consented subjects. Germline DNA was extracted from peripheral venous blood by Quest Diagnostics (Valencia, CA, USA or Heston, UK) using the Gentra Puregene Blood Kit on the QIAGEN Autopure LS automated genomic DNA purification platform (QIAGEN, Valencia, CA, USA).
Genotyping was performed by the Bioprocessing Solutions Alliance (BSA) under Brooks Automation Inc. (Piscataway, NJ, USA) using both the Affymetrix Axiom® Biobank Plus GSK custom array and TaqMan® Assay for the UGT1A1*6 allele (rs4148323), GeneScan® for the UGT1A1*28, *36 and *37 alleles (TA5/6/7/8 repeat polymorphism rs8175347) and Sanger sequencing for UGT1A9*1b (rs3832043; Thermo Fisher Scientific, Waltham, MA, USA). All genotype determinations and quality control were performed in accordance with the manufacturers’ protocols. Departure from the Hardy–Weinberg equilibrium was not observed in Caucasians (n = 209) for the genetic variants in the analyses.
Relative enzymatic activity for UGT1A1 and UGT1A9 was estimated semi-quantitatively from allelic carriage. Normal UGT1A1 function was assumed where UGT1A1*6, *28 or *37 were not present. Subjects with a single copy of any of these alleles were classed as having reduced UGT1A1 function; homozygous or compound heterozygous carriage of any two was classed as low functioning. UGT1A1*36 (TA5), a promotor deletion associated with African populations that slightly elevates mRNA transcription,18 was assessed in the same screening assay as the *28 (TA7) and *37 (TA8) promotor variants. Due to the anticipated minor effects of UGT1A1*36, this was considered a WT allele and grouped with the *1 (TA6) WT allele for prediction of UGT1A1 function as described above. UGT1A9-mediated metabolism was considered normal in the absence of UGT1A9*1b, intermediate for heterozygous carriage of *1b and fast for homozygous carriage.
PK and safety assessments
All PGx-consented participants included in the analyses had genetic data for UGT1A1 or UGT1A9 and clinical PK and/or safety data available at the time of analysis. PK parameters included on-treatment steady-state values for plasma cabotegravir concentration at the end of the dosing interval (Ctau), Cmax and the area under the concentration–time curve over the dosing interval (AUCtau).
For oral cabotegravir, all three PK parameters were assessed in Phase I studies. Ctau was assessed in both Phase II studies and was limited to patients receiving cabotegravir 30 mg during the oral induction period. Sampling time varied between the six studies but was not earlier than Day 8 to ensure steady-state conditions were maintained.
For cabotegravir LA, both the 4 weekly and 8 weekly dosing groups in LATTE-2 were included. Mean plasma cabotegravir concentrations were plotted by visit for each UGT1A1 activity stratum. Exposure parameters were assessed at Weeks 32 and 48 of IM dosing and included the individual average cabotegravir trough concentrations (Ctau and pre-dose concentration) across the dosing interval (Cavgi), in addition to Ctau, Cmax and AUCtau. AUCtau for 4 weekly dosing was determined from three samples (pre-dose, 1 week post-dose and Ctau) taken over the Week 24–28 dosing interval for Week 32 AUCtau, or over the Week 40–44 interval for Week 48 AUCtau. For 8 weekly dosing, AUCtau was determined from four PK samples (pre-dose, 1 and 4 weeks post-dose, and Ctau) taken over the Week 24–32 or the Week 40–48 intervals. In addition, final oral trough concentrations (Ctau) in the induction period were also assessed in those who went on to receive cabotegravir LA and compared graphically to cabotegravir LA troughs at Weeks 32 and 48.
Plasma cabotegravir was measured using a validated LC-MS assay with a lower limit of quantification of 10 ng/mL for all studies except LATTE-2 (25 ng/mL). Exposure parameters were calculated using non-compartmental methods in Phoenix WinNonlin 6.3 (Certara Corporation, Princeton, NJ, USA).
Safety endpoints assessed in the LATTE study were maximum on-treatment change from baseline in ALT and Tbili irrespective of cabotegravir dose. Serum ALT and Tbili were measured centrally as previously described4 and converted into multiples of the laboratory upper limit of normal (ULN).
Analyses
Linear regression was used to evaluate both PK and liver chemistry associations with predicted UGT activity. Independent variables were evaluated for inclusion to adjust for non-genetic effects (Table S1, available as Supplementary data at JAC Online) and included in the final model if the P value of association was <0.05. Normal distribution was assessed for each endpoint and those not approximately normal were log-transformed to correct for skewness. Analyses were run for each endpoint and each genetically predicted activity level. The main effect of genotype was modelled with UGT functional classifications as an additive variable (normal: 0; reduced/intermediate: 1; low/fast: 2). P values were reported for each analysis without correction for multiple testing.
Although UGT1A9 genotype data were available for LATTE-2, UGT1A9 associations were not assessed in Analysis 2 as UGT1A9 was not associated with change in Tbili after accounting for predicted UGT1A1 activity (see below).
Results
Pooled analysis of oral cabotegravir
Overall, 347 participants (60 healthy volunteers and 287 HIV-infected patients) who received oral cabotegravir 30 mg once daily in six studies had available PK data and PGx consent, from whom Ctau data were available for all 347 and Cmax and AUCtau for 60 participants each (Table 1). Participants were predominantly male (88%), HIV-infected (83%) and had a mean age of 36 years (range 19–64); 19% were black or African American and 17% were Hispanic or Latino. Table S2 shows baseline characteristics for both analysis populations.
All 347 had UGT1A9 genotype data and were included in the UGT1A9 analyses; one lacked a UGT1A1 genotype and was excluded from the UGT1A1 analyses, resulting in a UGT1A1 PGx-PK dataset of 346 for Ctau and 59 each for Cmax and AUCtau. Of 181 participants overall in LATTE who received oral cabotegravir at any dose, 163 were PGx-consented and had UGT genotype data and were therefore assessed for ALT and Tbili.
Overall, 218/346 (63.0%) participants in the UGT1A1 PGx-PK dataset carried functional polymorphisms in UGT1A1; 209/346 (60.4%) carried one or two copies of UGT1A1*28, 3/346 (0.9%) carried one or two copies of UGT1A1*6 and 12/346 (3.5%) carried one copy of UGT1A1*37. UGT1A1 activity was ‘reduced’ in 162/346 (46.8%) and ‘low’ in 56/346 (16.2%). The distribution of UGT1A1 alleles is shown in Table 2.
Table 2.
Allele distribution and predicted UGT1A1 activity in 346 subjects with UGT1A1 genotype data
| Allelic carriage | Predicted UGT1A1 activity, n (%) |
||
|---|---|---|---|
| normal | reduced | low | |
| *1/*1 (WT) | 119 (34.4) | ||
| *1/*36 | 9 (2.6) | ||
| *1/*28 | 150 (43.4) | ||
| *1/*37 | 6 (1.7) | ||
| *36/*28 | 4 (1.2) | ||
| *1/*6 | 2 (0.6) | ||
| *28/*28 | 49 (14.2) | ||
| *28/*37 | 6 (1.7) | ||
| *6/*6 | 1 (0.3) | ||
| Totals | 128 (37.0) | 162 (46.8) | 56 (16.2) |
In the UGT1A9 PGx-PK dataset, 227 (65.4%) carried UGT1A9*1b; 163 (47.0%) were *1/*1b heterozygotes (‘intermediate’ activity) and 64 (18.4%) were *1b/*1b homozygotes (‘fast’ activity). The remaining 120 (34.6%) were *1/*1 homozygotes with normal predicted activity.
Non-genetic covariates (P < 0.05) in the final linear regression models of oral cabotegravir Ctau, AUCtau and Cmax were study and subject weight and, for Ctau only, subject age. For the maximum on-treatment change from baseline in ALT and Tbili in LATTE, no non-genetic variable reached a P < 0.05 level of association and none were included in the models. Both Ctau and ALT changes were modelled using log-transformed data due to non-normal distribution. Other parameters were modelled without transformation.
Predicted UGT1A1 enzymatic activity was significantly associated with modelled 30 mg oral cabotegravir exposure parameters (P ≤ 0.021) and maximum on-treatment change from baseline in Tbili (P = 7.97 × 10−6); however, there was no significant association with the maximum change in ALT (Table 3). Low UGT1A1 activity increased oral cabotegravir exposure by 28% to 50% relative to normal activity, with the largest increase observed for Ctau. Low UGT1A1 activity also resulted in a 2.45-fold increase in maximum on-treatment change from baseline in Tbili compared with normal activity.
Table 3.
Effect of predicted UGT1A1 activity on steady-state exposure to oral cabotegravir 30 mg/day in pooled analyses of six Phase I and II studies and maximum on-treatment changes in Tbili and ALT in the LATTE study (all patients)
| Endpoint | Total N | Association of genetically predicted UGT1A1 activity with endpoint |
Mean [n] (range) by predicted UGT1A1 activity stratum |
Fold-change in exposure (low versus normal) | |||
|---|---|---|---|---|---|---|---|
| P value | parameter estimate (SE) | normal | reduced | low | |||
| C tau (μg/mL) | 346 | 4.89 × 10–11 | 0.19 (0.03) |
|
|
|
1.50 |
| AUCtau (h·μg/mL) | 59 | 0.0013 | 20.03 (5.89) |
|
|
|
1.41 |
| C max (μg/mL) | 59 | 0.0213 | 0.78 (0.33) |
|
|
|
1.28 |
| Maximum change from baseline (×ULN) | |||||||
| Tbili | 163a | 7.97 × 10–6 | 0.13 (0.03) |
|
|
|
2.45 |
| ALT | 163a | 0.6674 | −0.03 (0.06) |
|
|
|
0.68 |
BL, baseline; SE, standard error.
The analyses of changes in Tbili and ALT include patients who received 10, 30 and 60 mg oral cabotegravir.
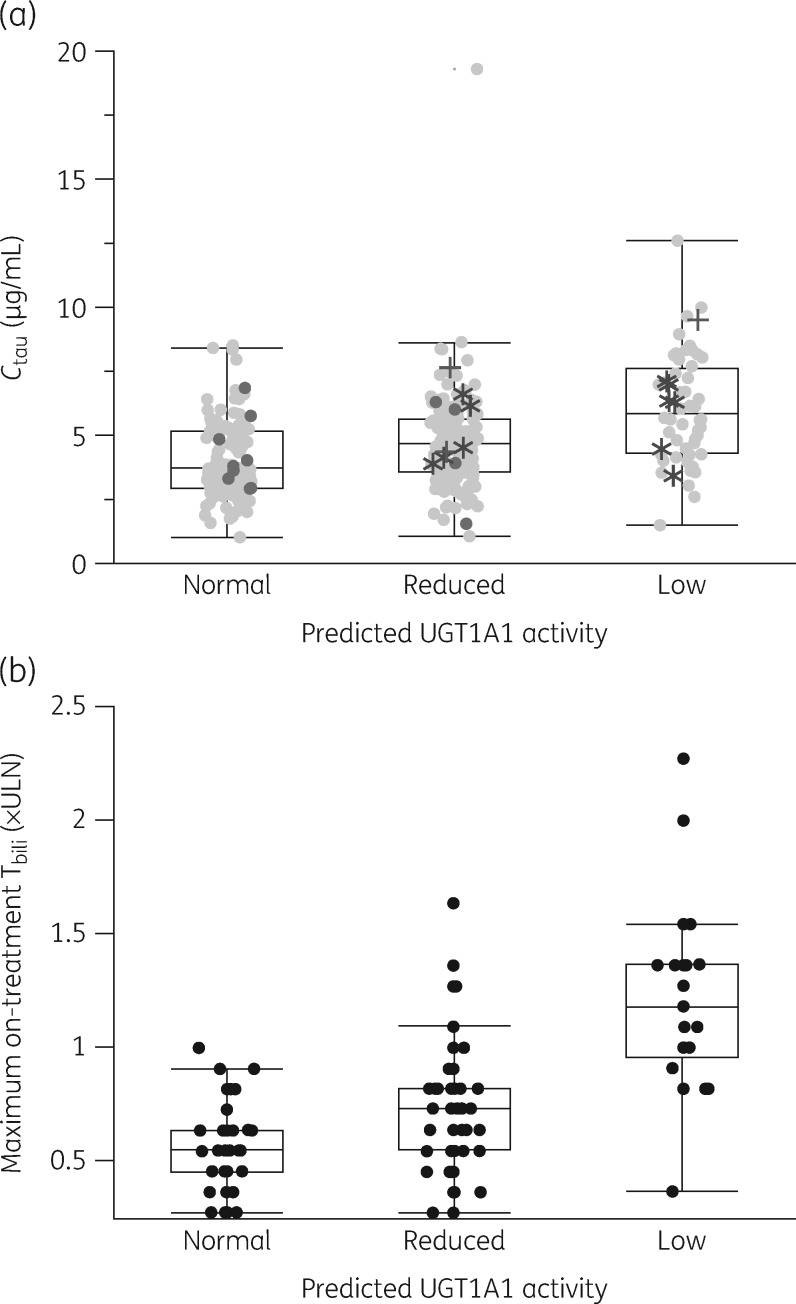
There were too few carriers of UGT1A1*6 and UGT1A1*37 to compare effect size between alleles. However, individual Ctau for UGT1A1*37 and UGT1A1*6 carriers fell within the range of observations for UGT1A1*28 (Figure 1a). PK data for the nine participants heterozygous for *1/*36 were within the range for those homozygous for the *1 WT (Figure 1a).
Figure 1.
Box and whiskers plot showing the effects of predicted UGT1A1 activity on (a) steady-state cabotegravir trough concentrations in pooled data from subjects receiving oral cabotegravir 30 mg/day; and (b) maximum on-treatment Tbili from subjects receiving oral cabotegravir 10, 30 or 60 mg/day in the LATTE study (NCT01641809). Boxes represent the range between the 25th and 75th percentiles, the horizontal lines within the boxes show medians and whiskers show the 5th and 95th percentiles. Panel (a) pale closed circles, UGT1A1*1 homozygotes; dark closed circles, UGT1A1*36 carriers; plus symbols, UGT1A1*6 carriers; asterisks, UGT1A1*37 carriers.
In total, 5/163 participants with Tbili data had on-treatment increases of 1.5× ULN or greater; all had reduced (n = 1) or low (n = 4) predicted UGT1A1 activity (Figure 1b); none experienced a maximum on-treatment ALT elevation ≥3× ULN. The largest increase in Tbili observed was 2.27× ULN in an individual with low predicted UGT1A1 activity, a grade 2 increase of 0.91× ULN over their baseline level of 1.36× ULN.
Predicted enzymatic activity for UGT1A9 was not significantly associated with any exposure measure or ALT change. A P = 0.041 association with change in Tbili initially observed ceased to be significant (P = 0.448) after adjustment of the model for predicted UGT1A1 activity (Table 4), indicating that the initial association may be due to correlated genetic variations in UGT1A1 and UGT1A9 (P = 0.35 for Pearson product-moment correlation coefficient).
Table 4.
Effect of predicted UGT1A9 activity on steady-state exposure to oral cabotegravir 30 mg/day in pooled analyses of six Phase I and II studies, and maximum on-treatment changes in Tbili and ALT in the LATTE study (all patients)
| Endpoint | Total n | Association of genetically predicted UGT1A9 activity with endpoint |
Mean [n] (range) by predicted UGT1A9 activity stratum |
Fold-change in exposure (fast versus normal) | |||
|---|---|---|---|---|---|---|---|
| P value | parameter estimate (SE) | normal | intermediate | fast | |||
| C tau (μg/mL) | 347 | 0.6374 | 0.01 (0.03) |
|
4.72 [162] (1.02–19.30) |
|
0.98 |
| AUCtau (h·μg/mL) | 60 | 0.9108 | −0.77 (6.80) |
|
|
155.58 [8] (103.89–217.46) | 1.04 |
| C max (μg/mL) | 60 | 0.8060 | −0.09 (0.36) |
|
|
|
1.03 |
| Change from BL (×ULN) | |||||||
| Tbili | 163a | 0.0412b | 0.06 (0.03) |
|
|
|
0.69 |
| ALT | 163a | 0.3823 | −0.05 (0.06) |
|
|
|
1.68 |
BL, baseline; SE, standard error.
The analyses of changes in Tbili and ALT include patients who received 10, 30 and 60 mg oral cabotegravir.
No longer significant (P = 0.4481) after accounting for UGT1A1 genetic variation within the model.
Analysis of cabotegravir LA (LATTE-2)
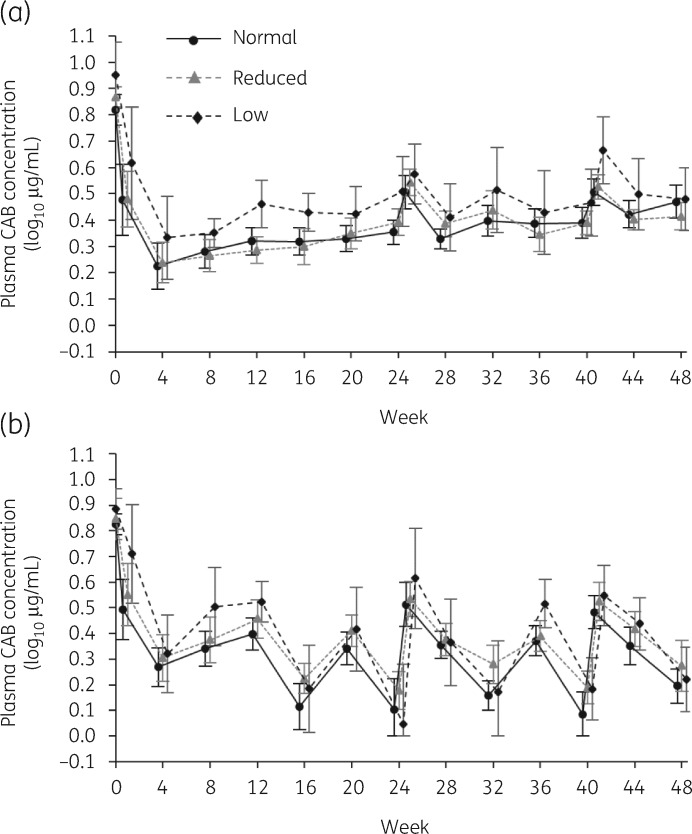
Final oral Ctau data at the end of the induction period were available from 223 PGx-consented participants who subsequently received cabotegravir LA plus rilpivirine LA every 4 or 8 weeks. Of these, cabotegravir LA parameters were available for 215 at Week 32 (4 weekly: 105; 8 weekly: 110) and 213 at Week 48 (4 weekly: 103; 8 weekly: 110). Across the maintenance period, there was little difference between the cabotegravir LA concentration–time profiles of those with normal predicted UGT1A1 activity and those with reduced activity, while consistent but modest elevations in cabotegravir were seen for those with low predicted activity (Figure 2).
Figure 2.
Mean (±95% CI) plasma cabotegravir concentrations by visit in the LATTE-2 study among subjects receiving parenteral cabotegravir LA plus rilpivirine LA maintenance (a) every 4 weeks (400 mg cabotegravir) or (b) every 8 weeks (600 mg cabotegravir) after suppressive treatment on daily oral cabotegravir and nucleoside analogues, according to normal, reduced or low predicted UGT1A1 activity. CAB, cabotegravir.
Non-genetic covariates in the final linear regression models of cabotegravir LA exposure included subject weight and dosing regimen for Ctau, AUCtau and Cavgi, and subject weight alone for Cmax. All parameter distributions were approximately normal and no log-transformation was performed.
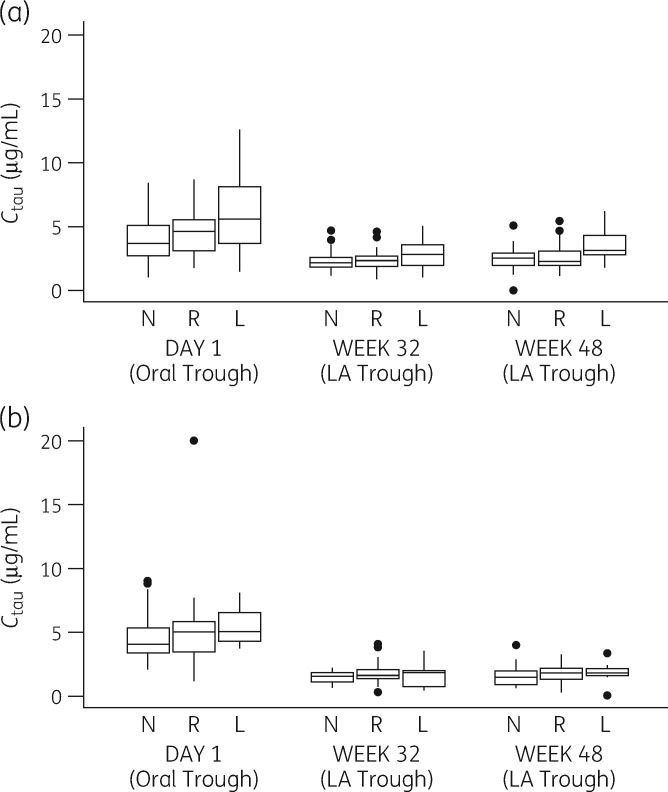
Predicted UGT1A1 activity was significantly associated with all cabotegravir LA exposure parameters at Weeks 32 (Table S3) and 48 (Table 5), with a 16%–24% increase in the value of each for low-versus-normal activity at Week 48. These changes in Cmax, AUCtau and Ctau were numerically smaller than those for oral cabotegravir (Table 3) in the pooled analysis (21%–56% smaller fold-change for low-versus-normal predicted UGT1A1 activity at Week 48). Longitudinal intra-study data from LATTE-2 also show a numerically smaller effect of low-versus-normal predicted UGT1A1 activity on cabotegravir trough concentrations for parenteral dosing compared with the last oral lead-in trough observed in the same treatment group, particularly on the 4 weekly schedule (Figure 3). In the 4 weekly group the low-versus-normal fold-change between mean cabotegravir trough concentrations at Week 48 of LA dosing was 1.42 (3.56 versus 2.51 μg/mL) compared with 1.57 for the last oral trough on Day 1 (6.11 versus 3.88 μg/mL); in the 8 weekly arm, the Week 48 fold-change was 1.15 (1.66 versus 1.44 μg/mL) compared with 1.19 on Day 1 (5.10 versus 4.28 μg/mL).
Table 5.
Effect of predicted UGT1A1 activity on exposure to parenteral cabotegravir LA (400 mg every 4 weeks or 600 mg every 8 weeks) at Week 48 of the cabotegravir LA + rilpivirine LA maintenance phase of LATTE-2 (NCT02120352)
| Endpoint | Total n | Association of genetically predicted UGT1A1 activity with endpoint |
Mean [n] (range) by predicted UGT1A1 activity stratum |
Fold-change in exposure (low vs normal) | |||
|---|---|---|---|---|---|---|---|
| P value | parameter estimate (SE) | normal | reduced | low | |||
| C tau (μg/mL) | 159 | 0.0037 | 0.26 (0.10) |
|
|
|
1.24 |
| AUCtau (h·μg/mL) | 212 | 0.0162 | 250.10 (116.08) |
|
|
|
1.16 |
| C max (μg/mL) | 212 | 0.0047 | 0.41 (0.16) |
|
|
|
1.18 |
| C avgi (μg/mL) | 204 | 0.0094 | 0.19 (0.08) |
|
|
|
1.16 |
SE, standard error.
Figure 3.
Trough cabotegravir concentrations for oral versus IM administration according to predicted UGT1A1 activity in subjects from LATTE-2 (NCT02120352) for (a) IM administration every 4 weeks and (b) IM administration every 8 weeks. L, low predicted UGT1A1 activity; N, normal predicted UGT1A1 activity; R, reduced predicted UGT1A1 activity. On Day 1, oral cabotegravir trough was assessed 20–28 h after the last oral cabotegravir 30 mg dose taken at home. Boxes represent the range between the 25th and 75th percentiles, the horizontal lines within the boxes show medians and whiskers show the 5th and 95th percentiles.
Discussion
Homozygous or heterozygous carriage of UGT1A1*6, *28 or *37 was associated with modest elevations in steady-state cabotegravir exposure for both oral and IM administration. These increases appeared higher for oral dosing in cross-sectional pooled data from six studies (28%–50%) than for IM administration in the LATTE-2 study (16%–24% across all parameters). Longitudinal data from LATTE-2 also suggested a similar or numerically lower effect of low predicted UGT1A1 activity on cabotegravir LA troughs compared with oral dosing in the same patients. Although the caveat applies that no statistical testing was performed, these data are consistent with a supposition that sustained drug delivery from the IM depot may partially mitigate the impact of reduced metabolic clearance compared with the more rapid peak–trough cycling associated with oral administration. These results are of relevance given that there is little information about the influence of metabolic variation on exposure to LA compounds and that metabolic enzyme genotyping is not routinely conducted in clinical practice. Not unexpectedly, UGT1A9*1b-associated functional elevations in the minor UGT1A9 pathway had no statistically relevant independent effect on oral systemic exposure and so were not evaluated for IM dosing.
Based on accumulated safety data for cabotegravir across its development programme, these modest elevations in exposure parameters of 28%–50% (oral) and 16%–24% (parenteral) are not anticipated to be clinically relevant. Of note, elevations in oral AUCtau (41%) and Cmax (28%) associated with low predicted UGT1A1 activity are comparable to data for oral dolutegravir 50 mg/day, a structural analogue of cabotegravir also predominantly metabolized by UGT1A1, though with a notable (∼30%) contribution by cytochrome P450 3A4.38 Using the same allele and activity definitions as herein, a 46% increase in dolutegravir AUCtau and a 32% increase in Cmax was associated with low UGT1A1 activity.39 As with cabotegravir, accumulated safety data for dolutegravir did not indicate any clinical significance for these increases.
On-treatment Tbili increases were associated with reduced or low UGT1A1 function among 163 participants receiving oral cabotegravir, including a small number (n = 5) with moderate (grade 2) elevations above 1.5× ULN. These elevations were clinically asymptomatic, resolved spontaneously and were not accompanied by ALT elevations suggestive of drug-induced liver injury. The elevations are of interest because cabotegravir does not functionally affect UGT1A1 expression or activity7 and on-treatment hyperbilirubinaemia associated with reduced UGT1A1 function is most commonly seen with UGT1A1 inhibitors such as atazanavir,25 tranilast27 or pazopanib.26,28 However, an increased risk of hyperbilirubinaemia associated with UGT1A1*6, *28 and/or *37 was previously observed during treatment with sunitinib,26 a tyrosine kinase inhibitor neither inhibitory towards, nor significantly metabolized by, UGT1A1.40,41
It has been suggested that this hyperbilirubinaemia with sunitinib may have represented a benign manifestation of Gilbert’s syndrome26,42 and it is likely that the grade 2 Tbili elevations in the current analyses also reflected, at least in part, an underlying Gilbert’s condition. However, unlike sunitinib, cabotegravir is a substrate for UGT1A1 and on-treatment bilirubin changes across the entire group with low predicted UGT1A1 function were significantly larger than in the group with normal activity. Thus a general effect, such as substrate competition between cabotegravir and bilirubin for weakly active UGT1A1, may also have contributed to the five asymptomatic grade 2 bilirubin elevations.
Study limitations include the retrospective nature of this PGx evaluation that used data from only a subset of participating trial subjects with optional PGx consent. The analyses were conducted using data from multiple clinical trials, none of which was designed for specific PGx assessments (or sample size considerations). However, the pooled sample size was large compared with most published PGx-PK analyses and represents a racially diverse population. While the representation of some UGT1A1 alleles of interest was limited, consistent with their population frequency, the analysis population included 56 individuals with low predicted UGT1A1 enzyme activity and a further 162 with reduced predicted activity for a robust analysis.
In conclusion, functional reduction of UGT1A1 metabolic capacity associated with carriage of the Gilbert’s syndrome-associated alleles UGT1A1*6, UGT1A1*28 and/or UGT1A1*37 modestly increased exposure to cabotegravir administered either orally or as an LA injection. These increases are not anticipated to be clinically relevant and do not require dose or administration schedule adjustments.
Supplementary Material
Acknowledgements
These data have previously been presented in part at the 17th International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy, Washington, DC, USA, 8–10 June 2016 (Poster P_36); the American Society of Human Genetics Annual Meeting 2016, Vancouver, Canada, 18–22 October 2016 (Poster 1299F); and IDWeek 2018; San Francisco, CA, USA, 3–7 October 2018 (Poster 552).
Funding
This work was supported by ViiV Healthcare. Editorial assistance was provided by Nick Fitch, PhD, of Articulate Science (London, UK) with funding from ViiV Healthcare.
Transparency declarations
P.P., D.M., K.S., and W.R.S. are employees of ViiV Healthcare and stockholders of GlaxoSmithKline. S.F., K.K.B. and K.S.K. are employees and stockholders at GlaxoSmithKline. A.R.H. is a former GlaxoSmithKline employee and a stockholder. Y.L. is a former Parexel employee. L.P., Z.X. and Y.L. have none to declare.
Author contributions
All authors made substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work. All authors were involved in drafting the manuscript and/or revising it critically for important intellectual content, approved the final version for submission and agree to be accountable for all aspects of the manuscript.
Supplementary data
Tables S1 to S3 are available as Supplementary data at JAC Online.
References
- 1. Bari H. A prolonged release parenteral drug delivery system: an overview. Int J Pharm Sci Rev Res 2010; 3: 1–11. [Google Scholar]
- 2. Trezza C, Ford SL, Spreen W. et al. Formulation and pharmacology of long-acting cabotegravir. Curr Opin HIV AIDS 2015; 10: 239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Markowitz M, Frank I, Grant RM. et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV 2017; 4: e331–40. [DOI] [PubMed] [Google Scholar]
- 4. Bowers GD, Culp A, Reese MJ. et al. Disposition and metabolism of cabotegravir: a comparison of biotransformation and excretion between different species and routes of administration in humans. Xenobiotica 2016; 46: 147–62. [DOI] [PubMed] [Google Scholar]
- 5. Reese MJ, Bowers GD, Humphreys JE. et al. Drug interaction profile of the HIV integrase inhibitor cabotegravir: assessment from in vitro studies and a clinical investigation with midazolam. Xenobiotica 2016; 46: 445–56. [DOI] [PubMed] [Google Scholar]
- 6. Court MH, Zhang X, Ding X. et al. Quantitative distribution of mRNAs encoding the 19 human UDP-glucuronosyltransferase enzymes in 26 adult and 3 fetal tissues. Xenobiotica 2012; 42: 266–77. [DOI] [PubMed] [Google Scholar]
- 7. Barbarino JM, Haidar CE, Klein TE. et al. PharmGKB summary: very important pharmacogene information for UGT1A1. Pharmacogenet Genomics 2014; 24: 177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujita K, Sparreboom A.. Pharmacogenetics of irinotecan disposition and toxicity: a review. Curr Clin Pharmacol 2010; 5: 209–17. [DOI] [PubMed] [Google Scholar]
- 9. Stingl JC, Bartels H, Viviani R. et al. Relevance of UDP-glucuronosyltransferase polymorphisms for drug dosing: a quantitative systematic review. Pharmacol Ther 2014; 141: 92–116. [DOI] [PubMed] [Google Scholar]
- 10. Strassburg CP. Pharmacogenetics of Gilbert’s syndrome. Pharmacogenomics 2008; 9: 703–15. [DOI] [PubMed] [Google Scholar]
- 11. Sneitz N, Bakker CT, de Knegt RJ. et al. Crigler-Najjar syndrome in The Netherlands: identification of four novel UGT1A1 alleles, genotype-phenotype correlation, and functional analysis of 10 missense mutants. Hum Mutat 2010; 31: 52–9. [DOI] [PubMed] [Google Scholar]
- 12. Seppen J, Bosma PJ, Goldhoorn BG. et al. Discrimination between Crigler-Najjar type I and II by expression of mutant bilirubin uridine diphosphate-glucuronosyltransferase. J Clin Invest 1994; 94: 2385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kadakol A, Ghosh SS, Sappal BS. et al. Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: correlation of genotype to phenotype. Hum Mutat 2000; 16: 297–306. [DOI] [PubMed] [Google Scholar]
- 14. Bosma PJ, Chowdhury JR, Bakker C. et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med 1995; 333: 1171–5. [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto K, Sato H, Fujiyama Y. et al. Contribution of two missense mutations (G71R and Y486D) of the bilirubin UDP glycosyltransferase (UGT1A1) gene to phenotypes of Gilbert’s syndrome and Crigler-Najjar syndrome type II. Biochim Biophys Acta 1998; 1406: 267–73. [DOI] [PubMed] [Google Scholar]
- 16. Boyd MA, Srasuebkul P, Ruxrungtham K. et al. Relationship between hyperbilirubinaemia and UDP-glucuronosyltransferase 1A1 (UGT1A1) polymorphism in adult HIV-infected Thai patients treated with indinavir. Pharmacogenet Genomics 2006; 16: 321–9. [DOI] [PubMed] [Google Scholar]
- 17. Akaba K, Kimura T, Sasaki A. et al. Neonatal hyperbilirubinemia and mutation of the bilirubin uridine diphosphate-glucuronosyltransferase gene: a common missense mutation among Japanese, Koreans and Chinese. Biochem Mol Biol Int 1998; 46: 21–6. [DOI] [PubMed] [Google Scholar]
- 18. Beutler E, Gelbart T, Demina A.. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc Natl Acad Sci USA 1998; 95: 8170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall D, Ybazeta G, Destro-Bisol G. et al. Variability at the uridine diphosphate glucuronosyltransferase 1A1 promoter in human populations and primates. Pharmacogenetics 1999; 9: 591–9. [PubMed] [Google Scholar]
- 20. Haverfield EV, McKenzie CA, Forrester T. et al. UGT1A1 variation and gallstone formation in sickle cell disease. Blood 2005; 105: 968–72. [DOI] [PubMed] [Google Scholar]
- 21. Hu ZY, Yu Q, Pei Q. et al. Dose-dependent association between UGT1A1*28 genotype and irinotecan-induced neutropenia: low doses also increase risk. Clin Cancer Res 2010; 16: 3832–42. [DOI] [PubMed] [Google Scholar]
- 22. Onoue M, Terada T, Kobayashi M. et al. UGT1A1*6 polymorphism is most predictive of severe neutropenia induced by irinotecan in Japanese cancer patients. Int J Clin Oncol 2009; 14: 136–42. [DOI] [PubMed] [Google Scholar]
- 23. Kishi S, Yang W, Boureau B. et al. Effects of prednisone and genetic polymorphisms on etoposide disposition in children with acute lymphoblastic leukemia. Blood 2004; 103: 67–72. [DOI] [PubMed] [Google Scholar]
- 24. Trontelj J, Marc J, Zavratnik A. et al. Effects of UGT1A1*28 polymorphism on raloxifene pharmacokinetics and pharmacodynamics. Br J Clin Pharmacol 2009; 67: 437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lankisch TO, Moebius U, Wehmeier M. et al. Gilbert’s disease and atazanavir: from phenotype to UDP-glucuronosyltransferase haplotype. Hepatology 2006; 44: 1324–32. [DOI] [PubMed] [Google Scholar]
- 26. Motzer RJ, Johnson T, Choueiri TK. et al. Hyperbilirubinemia in pazopanib- or sunitinib-treated patients in COMPARZ is associated with UGT1A1 polymorphisms. Ann Oncol 2013; 24: 2927–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Danoff TM, Campbell DA, McCarthy LC. et al. A Gilbert’s syndrome UGT1A1 variant confers susceptibility to tranilast-induced hyperbilirubinemia. Pharmacogenomics J 2004; 4: 49–53. [DOI] [PubMed] [Google Scholar]
- 28. Xu CF, Reck BH, Xue Z. et al. Pazopanib-induced hyperbilirubinemia is associated with Gilbert’s syndrome UGT1A1 polymorphism. Br J Cancer 2010; 102: 1371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamanaka H, Nakajima M, Katoh M. et al. A novel polymorphism in the promoter region of human UGT1A9 gene (UGT1A9*22) and its effects on the transcriptional activity. Pharmacogenetics 2004; 14: 329–32. [DOI] [PubMed] [Google Scholar]
- 30. Cui C, Shu C, Cao D. et al. UGT1A1*6, UGT1A7*3 and UGT1A9*1b polymorphisms are predictive markers for severe toxicity in patients with metastatic gastrointestinal cancer treated with irinotecan-based regimens. Oncol Lett 2016; 12: 4231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Linakis MW, Cook SF, Kumar SS. et al. Polymorphic expression of UGT1A9 is associated with variable acetaminophen glucuronidation in neonates: a population pharmacokinetic and pharmacogenetic study. Clin Pharmacokinet 2018; 57: 1325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang J, Cai L, Huang H. et al. Genetic variations and haplotype diversity of the UGT1 gene cluster in the Chinese population. PLoS One 2012; 7: e33988.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ford SL, Lou Y, Lewis N. et al. Effect of rifabutin on the pharmacokinetics of oral cabotegravir in healthy subjects. Antivir Ther 2019; 24: 301–8. [DOI] [PubMed] [Google Scholar]
- 34. Ford SL, Gould E, Chen S. et al. Lack of pharmacokinetic interaction between rilpivirine and integrase inhibitors dolutegravir and GSK1265744. Antimicrob Agents Chemother 2013; 57: 5472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spreen W, Williams P, Margolis D. et al. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr 2014; 67: 487–92. [DOI] [PubMed] [Google Scholar]
- 36. Margolis DA, Brinson CC, Smith GHR. et al. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis 2015; 15: 1145–55. [DOI] [PubMed] [Google Scholar]
- 37. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ. et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017; 390: 1499–510. [DOI] [PubMed] [Google Scholar]
- 38. Reese MJ, Savina PM, Generaux GT. et al. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos 2013; 41: 353–61. [DOI] [PubMed] [Google Scholar]
- 39. Chen S, St Jean P, Borland J. et al. Evaluation of the effect of UGT1A1 polymorphisms on dolutegravir pharmacokinetics. Pharmacogenomics 2014; 15: 9–16. [DOI] [PubMed] [Google Scholar]
- 40. Speed B, Bu HZ, Pool WF. et al. Pharmacokinetics, distribution, and metabolism of [14C]sunitinib in rats, monkeys, and humans. Drug Metab Dispos 2012; 40: 539–55. [DOI] [PubMed] [Google Scholar]
- 41. Fujita K, Sugiyama M, Akiyama Y. et al. The small-molecule tyrosine kinase inhibitor nilotinib is a potent noncompetitive inhibitor of the SN-38 glucuronidation by human UGT1A1. Cancer Chemother Pharmacol 2011; 67: 237–41. [DOI] [PubMed] [Google Scholar]
- 42. Spraggs CF, Xu CF, Hunt CM.. Genetic characterization to improve interpretation and clinical management of hepatotoxicity caused by tyrosine kinase inhibitors. Pharmacogenomics 2013; 14: 541–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.