Abstract
Headache is a common source of pain in children after traumatic brain injury (TBI); however, relatively little is known about nonheadache pain in this pediatric population. The present review seeks to map the extant literature to determine the prevalence, characteristics, and impact of nonheadache pain in children post-TBI of all severities. We found that of 109 studies published on pain in children after a TBI, 95 (87%) were focused exclusively on headache pain and only 14 (13%) reported on nonheadache pain or overall pain, with half (n = 7) in the form of case studies. Overall, the level of evidence was low, with only three level 1 high-quality prospective studies. In one study, over half (57.1%) of adolescents who experienced persistent pain post-TBI reported pain in multiple body sites (e.g., back, lower limb, and neck).1 For each additional noncephalic pain site, the risk for developing chronic migraine is also increased. Nevertheless, pain in body regions other than the head is often not assessed systematically in pediatric TBI research. Findings of the current review suggest that pain assessment in children post-TBI needs improvement, given that pain is linked to worse recovery, poorer quality of life, and can be long-lasting. More rigorous examination of nonheadache pain and its role in impeding recovery in children post-TBI is imperative and has the potential to improve the care and management of children with TBI. We conclude with recommendations for pain assessment, discuss gaps in the literature, and highlight directions for future research.
Keywords: : pain assessment, pediatrics, traumatic brain injury
Introduction
Headache is a common symptom after pediatric traumatic brain injury (TBI).1,2 A systematic review concluded that chronic headache pain is a common complication after pediatric TBI, even for minor injuries.3 This raises concerns given that children with pain are likely to grow up to become adults with chronic pain4 and to develop associated mental health problems,5 resulting in enormous economic,6 psychological,7 and social costs8 for individuals and society. Indeed, post-injury headache is an important area of research. However, headache is not the only type of pain that may be present after a TBI. Any biological tissue that contains nociceptive fibers is a potential source of pain in persons with TBI.9 Noncephalic pain is commonly comorbid in people with headache or migraine.2 Recent research found that after controlling for demographics, individuals were 15% more likely to have persistent chronic migraine for each additional noncephalic pain site (e.g., face, neck or shoulders, and back).2 Nevertheless, to the best of our knowledge, no published reviews have examined the prevalence and impact of nonheadache pain in children post-TBI.
A systematic review of pain in adult TBI reported an alarmingly high chronic pain prevalence of 57.8%, and discussed other nonheadache pain syndromes as potential sequelae, such as complex regional pain syndrome, heterotopic ossification, peripheral neuropathic pain, and neuromuscular spasticity.10 Few studies have investigated mechanisms of pain after a TBI. The pathogenic etiologies of chronic pain post-TBI are thought to include musculoskeletal, vascular, neurogenic, visceral, and iatrogenic mechanisms.11 However, these findings from adult studies may or may not generalize to children, given that numerous important differences characterize TBI in youth compared to adulthood. For instance, children show an increased propensity for apoptosis (programmed/regulated cell death), different age-dependent parameters for cerebral blood flow and metabolism, and an increased likelihood of early post-traumatic seizures.12 Given the dissimilarities noted in pathophysiology, pain experiences post-TBI may differ across development.
The purpose of the current review was to map the nonheadache pain literature in the context of pediatric TBI based on several factors: study design, types of pain investigated, and methods used for pain assessment. Our goal was to elucidate what is known about the types of nonheadache pain that are present in children post-TBI. This is an important goal because both TBI and chronic pain are linked to poor outcomes across nearly every domain of functioning, from cognitive, social, and physical, to economic and familial.7 Therefore, pain may further complicate and exacerbate poor outcomes post-TBI in these vulnerable youths.
Methods
Study inclusion and exclusion criteria
Published studies were included that fulfilled three main criteria: 1) The study was specific to the pediatric population (between the ages 0 and 18 years, or participants of those ages were analyzed separately if older participants were included); 2) participants had a history of TBI; and 3) the study provided quantitative and/or qualitative information about pain (nonheadache pain or overall pain) whether from child or parent report. We included all study designs, including descriptive case studies, case series, cohort studies, case-control studies, and clinical trials. Pain could be measured using any method. Articles were excluded if they did not specifically examine pain in children after a TBI or only reported on headache pain. Studies were also excluded if they were not written in English. No restriction was imposed on sex, race, geographical residence, or source of population (e.g., community, hospital, registry, or health administrative data).
The primary outcomes of interest were pain characteristics reported in children at any time point before or after TBI. Preferably, the diagnosis of TBI was based on internationally recognized criteria, such as the World Health Organization Collaborating Center Task Force on Mild Traumatic Brain Injury,13 although studies using other methods (e.g., self-report) or local clinical judgment were also included.
Search strategy
The search for relevant publications included studies up to October 2016. The search strategy was developed using MeSH terms in MEDLINE (OvidSP), as well as relevant keywords. The terms “traumatic brain injury” or “concussion” or “brain injury” were exploded and searched in the title and/or abstract. The term ”pain” was exploded, and “child” or other synonymous terms (e.g., adolescent, infant, and pediatrics) were searched in the title and/or abstract. In consultation with a librarian, the search strategy was adapted for use in other databases (EMBASE, PsycINFO, CINAHL, SportDiscus, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews). The full search strategy can be viewed in the Appendix.
Study selection
We selected studies in three stages (title screen, abstract screen, and full text screen) completed by two independent reviewers (V.K. and M.V.) based on the inclusion/exclusion criteria described above. Any disagreement was resolved between the two reviewers. Further, the citation lists of all included articles were scanned for any articles that may have been missed. The two reviewers (V.K. and M.V.) extracted data from included studies using a standardized data collection form. The level of the evidence was assessed using the “Levels of Evidence for Primary Research” for prognostic studies.14
Results
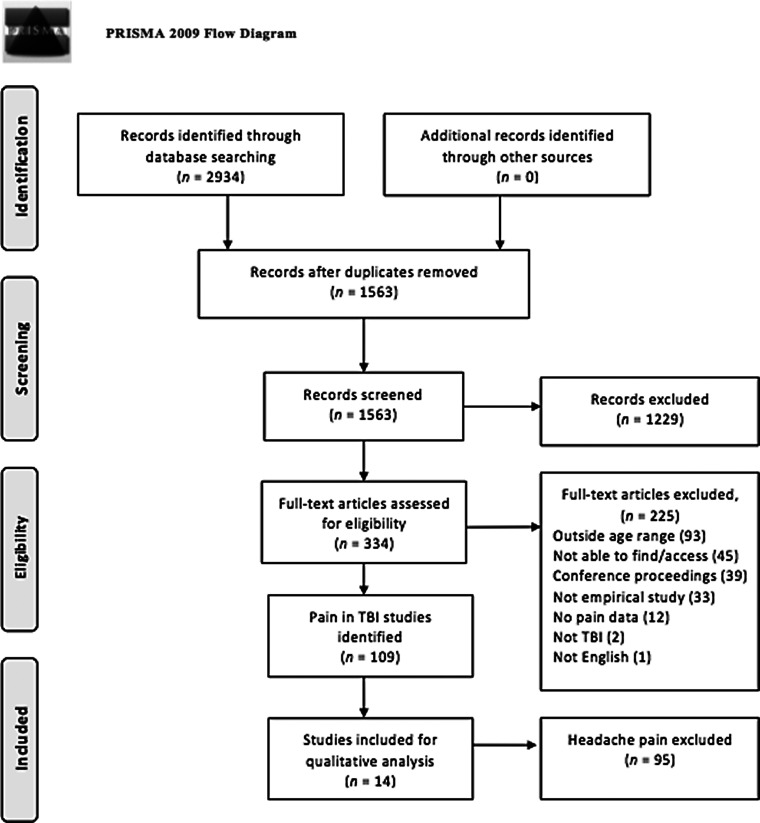
The search strategy yielded 1563 papers. The number was reduced to 450 and then 334 following the title screen and abstract screen, respectively. Full text reviews of the 334 studies further reduced the number to 109 (Fig. 1). Of the 109 studies published from the year 1806 to 2016 examining pain in children post-TBI, only 14 (13%) reported on nonheadache pain or overall pain (pain anywhere in the body and not specifying the location of this pain). Almost half of these 14 studies (n = 6; 43%) were descriptive case studies. The details of each study can be found in Table 1.
FIG. 1.
Flow diagram of studies identified for possible inclusion. Source: from Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097. TBI, traumatic brain injury.
Table 1.
Results of Published Studies (n = 14) Evaluating Pain in Children After Traumatic Brain Injury, Sorted by Method of Pain Assessment
| Author, year, location | Study design | Case/ control (n) | Case severity | Source of cases | Source of controls | Age of cases (years) | Sex of cases (M/F) | Time post-injury | Pain assessment method | Findings | Level of evidencea |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain by body region (n = 1) | |||||||||||
| Tham and colleagues, 2013, USA | Propsective cohort |
144/NA | Mild to severe TBI | 10 Washington study hospitals and 1 hospital in Pennsylvania | NA | M = 15.7; SD = ±1.2 |
100/44 | 3, 12, 24, 36 months | 4 questions about pain in the past week (child report): 1) Numerical rating scale, 0–10 2) Pain “other than a headache that bothered you” 3) If yes, identify where (8 options) 4) Numerical rating scale of the pain intensity in the other identified area(s) |
Pain prevalence did not decline over the period from 3 months to 36 months after TBI. In the persistent pain subgroup, 57.1% endorsed pain involving more than 1 anatomic region. Adolescents with persistent pain had significantly higher levels of depressive symptoms, PTSD symptomatology, and poorer HRQOL compared to the infrequent pain subgroup (p < 0.0001). |
1 |
| Overall pain rating (n = 3) | |||||||||||
| Batailler and colleagues, 2014, France | Prospective cohort |
127/1283 Selected out of 1283 persons in the database, but not analyzed as a control |
Mild or moderate TBI (M-AIS) attributed to a road accident |
Pediatric ESPARR cohort | Rhone registry |
n = 31 (age 0–5); n = 87 (age 6–11); n = 99 (age 12–16) |
82/45 | 6 months and 1 year | Child Health Questionnaire (parents form)-50: bodily pain | Significant correlation between body pain and quality of life (r = 0.482; p < 0.001) 77.5% of children who had not fully recovered by 1 year post-injury had body pain; 10.8% had body pain in the children who did recovery completely. |
1 |
| Brown, Kenardy and Dow, 2014, Australia | Prospective cohort | 195/NA | Mild to severe TBI | 3 Australian hospitals | NA | M = 10.78; SD = ±2.49 (range, 6–15 years) |
137/59 | 3, 6, 18 months | Child Health Questionnaire (parent form): bodily pain | CHQ mean pain score (standardized) was 77.23 at 3 months, 80.01 at 6 months, and 83.92 at 18 months post-TBI. Structural equation modeling supports PTSD as driving pain. PTSD predicted pain, but only in the short term. |
1 |
| McLeod, Bay and Snyder, 2010, USA | Retrospective case-control |
140/126 Football athletes with concussion matched to athletes without concussion |
Mild TBI | 5 local high schools in Arizona | 5 local high schools in Arizona | Concussion group: M = 15.0; SD = ±1.3 Control group: M = 14.6; SD = ±1.2 |
266 M | 66.1%> 1 year, 19.1% within 1 year, 7% within the past 6 months, 6.1% within the past 3 months, and 1.4% within 1 month | Medical Outcomes Short Form-36 (child report): | The concussion group reported significantly lower mean scores/higher pain (p < 0.001) on the bodily pain (52.86 ± 6.9) compared to the control group (55.56 ± 7.3), with an effect size of 0.35. | 3 |
| Symptom questionnaire (n = 3) | |||||||||||
| Haran and colleagues, 2016, Australia | Prospective cohort | 93/NA | Mild TBI | Emergency department at The Royal Children's Hospital Melbourne | NA | M = 12.7; SD = ±0.27 |
77/16 | Not specified but average follow-up at 32 (± 5.2) days after initial ED visit | Follow-up phone call assessing post-concussive symptoms. The exact questionnaire for assessing “Concussive signs and symptoms” is not specified, but is said to be adapted from validated measures. Not specified whether child or parent report | 85 children completed follow-up; 65 children experienced post-concussive symptoms; fatigue (57.6%), headache (56.5%), and neck pain (25.9%) were the top three main symptoms. | 2 |
| Heyer and colleagues, 2016, USA | Retrospective cohort | 1953/NA | Mild TBI | Pediatric Sports Medicine Clinic | NA | M = 14.1; SD = ±2.09 |
1229/724 | Median 9 days, range 1–30 days |
Symptom questionnaire (includes headache and neck pain) adapted from other sources Rate severity on a scale from 0 (not present) to 6 (severe) Child report |
Neck pain reported by 37.1% of the sample, with a mean score of 0.86, and a related headache probability of 0.89. Principal component analysis found strong correlations for a “cephalic” component consisting of neck pain, headache, nausea, photophobia, phonophobia, and dizziness. |
2 |
| Necajauskaite and colleagues, 2005, Europe | Retrospective case- control | 102/102 Matched to other mild body injury |
Mild TBI | Kaunas University of Medicine Hospital and Kaunas Red Cross Hospital | Kaunas University of Medicine Hospital and Kaunas Red Cross Hospital | M = 11; SD = ±3.1 |
74/28 | Median = 27 months and not shorter than 1 year | Standardized questionnaire of previous (year before inquiry) and present (last month before inquiry) health status and symptoms concomitant to headaches and dizziness Parent report |
6 children with mild TBI (9.4%) reported pain felt with headache in other body sites. This was higher than the control group of children with other mild body injury without head trauma (n = 4, 8%), although not significant (p > 0.05). | 3 |
| Unspecified clinical assessment (e.g., based on patient report or physical exam; n = 7) | |||||||||||
| Ellis and McDonald, 2015, Canada | Case study | 1/NA | Not specified | Pan Am Clinic/Concussion Program | NA | 13 years old | 1 M | 2 days, 2 weeks, 6 weeks, 2 months |
Unclear. Patient endorsement |
3 days post-injury, patient endorsed headache and neck pain. Full and painless range of motion achieved. Cleared for return to sports at 2 months. Diagnosis was coexistent sports-related concussion and cervical spinal cord injury without radiographic abnormality. | 4 |
| Kwon and Jang, 2014, Korea | Case study | 1/NA | Mild TBI | Yeungnam University | NA | 14 years old | 1 F | Initial, 29 days, 2 months, 10 weeks | Not specified, patient report | Patient involved in car accident, began to suffer posterior neck pain and back pain radiating to the right leg. Neurological examination at 10 weeks post-injury revealed quadriparesis and memory impairment. Diffusion tensor tractography showed discontinuation of both corticoreticular pathways at the midbrain level. | 4 |
| Lim and colleagues, 2007, Ukraine | Case study | 1/NA | Severe TBI | Department of Pediatric Neurosurgery, Republic Crimea Children Clinical Hospital | NA | 5 years old | 1 M | 2 weeks | Patient history examination; clinical assessment | Patient had mandibular fractures and underwent Maxillo-mandibular surgery with a Kirschner's knitting needle. After the surgery, the patient reported throbbing generalized headache, abrupt pain on his face and jaw, which was described as a burning and stabbing sensation. Radiologic films showed that Kirschner's knitting needle mobilized from the extracranial cavity into the middle cranial cavity, resulting in iatrogenic TBI. | 4 |
| Litt, 1995, USA | Case study | 1/NA | Not specified. GCS score of 5 at 17 days post-collision | Columbus State Community College |
NA | 16 years old | 1 M | Findings approx. 17 days after the first TBI and on the same day as the second TBI | Physical examination | The patient complained of left elbow pain, dizziness, and headache with vomiting, pallor, and unresponsiveness shortly after. CT scan showed an acute right-sided subdural hematoma with a midline shift. | 4 |
| Logan and colleagues, 2001, USA | Case study | 1/NA | Not specified. GCS score of 15 upon arrival at ED | University of Illinois; Carle Sports Medicine | NA | 18 years old | 1 M | Initial assessment, 24 h, 1 week, 12 days, 1 month post-injury | Clinical exam; patient report | 12 days post-TBI, patient complained of nocturnal neck pain. Clinical exam revealed an area of tenderness beneath the left occipital protuberance. Pain increased when patient turned his head to the right. One month later, the nocturnal neck pain from the cervical ligament sprain had ceased. | 4 |
| Meoded and colleagues, 2011, USA | Retrospective cohort (n = 5) |
10/NA | Mild to severe TBI | Johns Hopkins Hospital, Baltimore, Maryland | NA | M = 7.18; SD = ±4.37 |
4/6 | Not specified | Clinical assessment on admission | A 6-year-old female with GCS = 15 presented with cervical pain. MRI showed retroclival epidural hematoma, and associated tectorial membrane disruption, and minor compression of the brainstem; 5 other patients in the cohort had “minor pain.” | 4 |
| Zaremski and colleagues, 2015, USA | Case study | 1 | “Concussion” | Sports Medicine Clinic | NA | 17 years old | 0/1 | Continuous follow-up | Physical examination and patient report | Patient developed neck pain 5 weeks after concussion and showed a positive Tinel sign at the left craniocervical junction. Interventional pain management (e.g., greater occipital nerve [ON], lesser ON, and third ON block) relieved the pain, but the procedures needed to be repeated and managed long term. | 4 |
Level of evidence was assessed using “Levels of Evidence for Primary Research Question (prognostic studies)” by the Center for Evidence-Based Medicine (Oxford, UK). The scores range from 1 to 5, where a higher score indicates a weaker/lower level of evidence. Level 1 corresponds to high-quality prospective studies with sufficiently high follow-up rate (≥70%); level 2 for retrospective studies of lower quality or lesser-quality prospective studies; level 3 for case-control studies; and level 4 for case studies (Howick and colleagues, 2011). Level 5 is expert opinion, which was not encompassed by the reviewed studies.
NA, not applicable; TBI, traumatic brain injury; M-AIS, Maximum Abbreviated Injury Scale; GCS, Glasgow Coma Scale; ED, emergency department; M, mean; SD, standard deviation; M/F, male/female; approx., approximately; PTSD, post-traumatic stress disorder; HRQOL, health-related quality of life; CHQ, Child Health Questionnaire; CT, computed tomography; MRI, magnetic resonance imaging.
One study assessed pain intensity by body region (level 1), three studies measured pain using an overall score spanning the entire body (two level 1 and one level 3), three studies used a symptom questionnaire or checklist to assess specific types of pain such as neck pain (two level 2 and one level 3), and seven case studies relied on unspecified clinical assessment (seven level 4 evidence).
Pain rating by body region (n = 1)
A cohort study of 144 adolescents between the ages of 0 and 17 years with TBI of all severities provided pain ratings using the Numerical Rating Scale-11 (anchors, 0 = no pain; 10 = worst pain imaginable).1 Moderate pain was defined as a rating of 3 or higher out of 10. This definition was chosen because it is comparable to pain experienced by children immediately after major surgery.15 Results from this study showed that pain ratings in adolescents with mild TBI remained stable from 3 (55% reported significant pain) to 36 months (57.1% reported significant pain). Adolescents with moderate or severe TBI showed a significant increase in pain prevalence from 3 (36% reported significant pain) to 36 months (68% reported significant pain).
Persistent pain was defined as pain rated as ≥3 of 10 at all assessment time points (i.e., at enrollment and 3, 12, 24, and 36 months post-TBI). Among the participants, 24% (n = 35) met the criteria for persistent pain. In this subgroup, sites of pain most commonly reported were the head (85.7%), back (42.9%), lower limb (11.4%), neck (8.6%), upper limb (5.7%), and stomach (2.9%). Among the persistent pain group (n = 20), 57.1% of youth reported pain involving more than one anatomic region. To the best of our knowledge, this is the only study examining pain by location continuously over the long-term (e.g., >3 months) in youth post-TBI.
Overall pain rating (n = 3)
Three studies examined overall pain. Two of the studies used the parent form of the Child Health Questionnaire (CHQ) to assess overall bodily pain (intensity and frequency).16,17 The CHQ assesses pain intensity (“During the past four weeks, how often has your child had bodily pain or discomfort?”) and pain frequency (“During the past four weeks, how often has your child had bodily pain or discomfort?”) on a scale from 1 to 6. Research has shown acceptable internal consistency, negligible floor and ceiling effects for the bodily pain subscale, convergent and discriminant validity, and equal item variance for the CHQ.18
In the study by Batailler and colleagues (2014), which included 127 children ages 0–16 with mild or moderate TBI, parents rated the recovery status of their child with five response options: “fully recovered,” “improved but not recovered,” “stabilized,” “deteriorated,” or “unknown.” Results showed that 77.5% of the children who had not fully recovered had bodily pain.16 Further, a significant correlation was found between the CHQ bodily pain subscale and recovery status (r = 0.482; p < 0.001) at 1 year post-injury. Recovery status correlated with the CHQ pain subscale more strongly than all other CHQ subscales (physical functioning, general health, mental health, social limitation, parental impact-time, parental impact-emotional, self-esteem, behavior, and family cohesion) but one (social-physical; r = 0.502; p < 0.001).16
In the other study using the CHQ, Brown and colleagues (2014) recoded the CHQ score into a transformed score ranging from 0 to 100, where lower scores indicate higher levels of pain (intensity and frequency) on the CHQ bodily pain or discomfort scale.17 Among 195 children ages 6–15 with mild, moderate, or severe TBI, mean ratings were 77.23 at 3 months, 80.01 at 6 months, and 83.92 at 18 months post-TBI. The researchers also analyzed the bodily pain scale score data using structural equation modeling to investigate the relationship between pain (CHQ pain subscale) and post-traumatic stress (Clinician Administered Posttraumatic Stress Disorder [PTSD] Scale for Children and Adolescents).19 The “Perpetual Avoidance Model,” where PTSD is theorized to facilitate the maintenance of pain, which, in turn, prolongs PTSD, fit the data well (root mean square error of approximation = 0.05 and x2(3) = 4.57; p = 0.206).
The other study of overall pain ratings utilized the Medical Outcomes Short Form (MOSF), which assesses pain intensity (“How much bodily pain have you had during the past 4 weeks?”) and pain-related impact (“During the past 4 weeks, how much did pain interfere with your normal work?”).20 The adolescents rated their pain intensity on a scale from 1 (“none”) to 6 (“very severe”) and the impact of pain on functioning on a scale from 1 (“not at all”) to 5 (“extremely”). A bodily pain subscale score is then computed, ranging from 0 to 100, with a lower score indicating lower health-related quality of life. The psychometric properties of the MOSF-36 have revealed strong convergent validity in the bodily pain scale with physical-health factorial tests as expected.21 McLeod and colleagues (2010) found that in 140 high school athletes (age range not reported), those with a history of concussion reported a significantly lower score (higher pain intensity and pain-related impact) than those without a history of concussion (52.86 ± 6.9 in concussion group and 55.56 ± 7.3 in control group; p < 0.001), with a medium effect size of d = 0.35.20
Symptom questionnaire (n = 3)
Three studies used nonstandardized or unreferenced symptom checklists or questionnaires to assess various symptoms of pain.22–24 The psychometric properties of these questionnaires were not reported. The questionnaires included neck pain as a possible symptom, in addition to headache, similar to other standardized concussion assessment tools (e.g., symptom checklist included in the Sport Concussion Assessment Tool).25 Most other symptom questionnaires used in TBI only assesses headache pain (e.g., Post-concussive Symptoms Scale,26 Post-Concussive Symptom Inventory,27 and Health and Behavior Inventory)28 and not pain in other anatomic regions.
Two studies reported on neck pain after mild TBI within the first month of injury. Haran and colleagues (2016) reported neck pain in 25.9% of 93 children between the ages of 5 and 18 years approximately 1 month after mild TBI (parent or child report not specified),22 and Heyer and colleagues (2016) reported child endorsement of neck pain in 37.1% of 1953 children ages 6–22 at a median of 9 days after mild TBI.23 This rate of neck pain (25.9% to 37.1%) is higher than the rate (1.8% to 8.6%) reported by Tham and colleagues (2013) at 36 months post-TBI (all injury severities)1; the difference may be attributed to the timing of assessment of pain, as well as the source of participants (prospective emergency department vs. clinic-based).
Studies using symptom questionnaires also provided information about how symptoms cluster together. Heyer and colleagues (2016) found that six symptoms (neck pain, headache, sensitivity to light, sensitivity to noise, nausea, and dizziness) loaded onto a “cephalic” component.23 When neck pain was present, the probability of headache was 0.89 (lower probability than most of the other post-concussion symptoms evaluated). When a subcohort of patients with pre-morbid headaches were analyzed, neck pain no longer loaded onto the cephalic component. Instead, neck pain clustered with a “somatic” (rather than emotional or cognitive) component along with numbness in extremities and weakness in extremities.23 Necajauskaite and colleagues (2005) also examined symptom clusters. Headache was associated with pain in other body sites in 9.4% (n = 6) of the mild TBI group and 8% (n = 4) of the matched control group (without head trauma).24
Unspecified pain assessment (n = 7)
Many of the case studies described pain (along with other clinical features) as a sign of more serious central nervous system or cranial injury. For example, a case of an individual with neck pain was posited to be related to spinal cord injury, although no radiographic abnormality was detected.29 In other cases, neck and back pain radiating to the right leg presented with impairments in the corticoreticular pathways30; jaw and face pain was related to dislocation of a neurosurgical needle leading to iatrogenic TBI,31 and nocturnal neck pain was related to cervical ligament sprain.32
Discussion
To our knowledge, this is the first scoping review on nonheadache and overall pain in children post-TBI. Of studies that examined pain post-TBI, 95 of 109 (87%) reported on headache pain, but not on any other types of pain. Among the 14 included studies that reported on nonheadache pain, half were case studies (7 of 14; 50%). The level of evidence was generally low, with few high-quality prospective studies. Overall, three main findings are supported by this review: 1) Despite emerging evidence that nonhead pain is common in children post-TBI,1 few studies (n = 7) have been published on nonheadache or overall pain; 2) the assessment of pain in the TBI literature is problematic; and 3) how pain is assessed in children post-TBI needs improvement given research showing that pain is linked to worse recovery, poorer quality of life, and can be long-lasting.1,2,16,17,20 Thus, more rigorous examination of nonheadache pain and its role in impeding recovery in children post-TBI is imperative.
The neural mechanisms for nonheadache pain in children are not clearly understood, although some insights are available from research on adult TBI. A study of 15 adult patients post-TBI with chronic pain noted interesting features. These included: pain that tended to be unilateral/nonsymmetric, delayed development of pain (average = 6.6 months), reduced threshold for sensation of thermal and tactile stimulation, allodynia (normally nonpainful stimulation leading to pain), and hyperpathia (abnormally elevated levels of pain).33 Interestingly, touch and graphesthesia remained intact, as compared to pain-free patients post-TBI and healthy controls. These findings suggest selective damage to the pain and temperature systems underlying the development of central pain in TBI patients.33 This is supported by a recent diffusion tensor tractography study of the spinothalamic tract, which found decreased fractional anisotropy, increased mean diffusivity, and a lower tract volume in a mild TBI group presenting with neuropathic pain (must have developed after the TBI) as compared to two control groups (mild TBI without pain and healthy controls).11
Concurrent orthopedic injuries likely also play a role. Heterotopic ossification (i.e., presence of bone in soft tissue where bone does not normally exist), neuromuscular spasticity, peripheral nerve injuries, and fractures have been reported in adults post-TBI. An estimated 21–50% of individuals with a TBI also have concurrent orthopedic injuries, thus pain attributed to orthopedic injuries may be present in TBI patients.34–36 Unfortunately, of the seven cohort studies, only one study by Necajauskaite and colleagues (2005) clearly differentiated isolated TBI from injuries to other parts of the body and no TBI.24 All other studies did not clearly state whether concurrent orthopedic injuries were also present in their sample.
Measurement issues
In studies investigating pain in children post-TBI, numerous measurement issues are evident. These include a lack of systematic assessment of pain in different parts of the body, reliance on parental report of pain in young children who are capable of providing self-report (i.e., after 4 years of age), and the use of symptom rating scales that have not yet been shown to be reliable or valid in youth post-TBI.
The diversity of commonly painful locations post-TBI (headache, back, lower limb, neck, upper limb, and stomach)1 are not reflected by many rating scales routinely administered in the pediatric TBI population. Most symptom rating scales assess headache pain only (e.g., Post-concussive Symptoms Scale,37 Post-Concussive Symptom Inventory,38 and the Health and Behavior Inventory).28 Although overall pain ratings are simpler, they do not allow for a fine-grained understanding of what types of pain are contributing to the overall score. This point is not trivial. Research shows that individuals with chronic generalized or widespread pain differ significantly from those with located/localized pain. Multiple pain sites imply higher pain intensity, longer pain duration, and reduced working capacity (i.e., more sick leave).39 Thus, the assessment of pain using an overall measure is problematic because it omits important information about pain localization, which is likely to be highly relevant to recovery post-TBI.
The two studies reviewed that included children <8 years of age16, 17 relied on parent ratings rather than validated child pain assessment tools (e.g., Faces Pain Scale Revised for children ≥4 years).40 Other simplified tools have been recently developed to facilitate self-report in young children.41 Previous research has found that parents can misperceive their child's pain and may overestimate or underestimate their child's pain experience.42 Thus, although little is known about pain after childhood TBI, this is especially true for young children <8 years of age.
In studies using symptom-rating scales, many of the scales used were not standardized, not referenced, and not included as supplementary material. The scales were described rather vaguely as: “Concussive signs and symptoms”; “Symptom questionnaire”; and “Standardized questionnaire of previous and present health status.” Thus, it is unclear as to what exactly is being assessed in these scales, and whether all items were reported.
Recommendations for pain assessment
We believe that thorough pain assessment should encompass: 1) pain intensity, 2) pain duration and frequency, 3) pain location, and 4) impact of pain on functioning. Prospective assessment of pain intensity is ideal (e.g., The Headache Diary and The Pain Diary)43,44 to minimize recall bias. The Pain Questionnaire is frequently used in chronic pain studies and assesses pain frequency, location, duration, intensity, emotional distress, and interference.45 Two other pain assessment questionnaires frequently used in chronic pain literature are the Abu-Saad Pediatric Pain Assessment Tool (PPAT)46 and the Varni/Thompson Pediatric Pain Questionnaire (PPQ).47 The PPAT assesses multiple aspects of pain (e.g., triggers, medication, descriptors of pain qualities, present pain, and worse pain).46 The PPQ measures pain intensity, location, and the sensory, evaluative, and affective qualities of pain.47 Both are well-established pain instruments.48 The Patient-Reported Outcomes Measurement Information System also recently developed a new Pediatric Pain Interference Scale, which assesses the impact of pain on daily activities over the past week.49
Response bias is important to consider as part of assessment and score interpretation. Young children, especially <5 years of age, tend to use the bottom and top extremes of scales rather than rate in the middle.50 Whenever possible, steps should be taken to mitigate response biases. Overall, pain assessment can be strengthened through: using multiple methods, obtaining data from multiple informants, and assessing multiple dimensions of pain (intensity, affective reaction, cognitive appraisal, sensory qualities, impairment, location, etc.). We recommend choosing age-appropriate and validated tools51 and interpreting ratings by children <5 years of age with caution (e.g., for research, results could be analyzed with and without ratings from participants <5 years of age). The anchors in any given scale may be a major influence on a child's pain rating and should be taken into consideration for score interpretation. Scales with lower anchors depicting a neutral face prompt lower pain ratings than scales with lower anchors depicting a smiling face.52 In addition, it is advisable to complement self-report ratings with observational measures (e.g., Face Legs Activity Cry and Consolability scale53) and to consider the context for pain assessment (i.e., a child's motivation and expectations—do they think a high pain rating will result in more time away from school and sports?). When a child's ability to use a pain scale is unclear, offering the child a chance to practice using the scale for painful and nonpainful hypothetical situations (e.g., opening a birthday present vs. stepping on a sharp nail) may be helpful.50,54 Obtaining scores from multiple informants (e.g., child, parent, and clinician) makes it possible to observe, discuss, and resolve any discrepancies that may arise across the scores.54, 55
Future research on pain in traumatic brain injury
This review highlights important avenues for future research on pain and TBI in the pediatric population. Future research should explore whether different aspects of recovery post-TBI differ depending on whether children experience only headache pain as compared to nonheadache pain, or pain in multiple locations. This is supported by past research that found noncephalic pain to be a risk factor for the onset of chronic and persistent migraine.2 Other research shows that recurrent complaints of pain in children were associated with chronic health problems, frequent change of residence, poor school performance, frequent television watching, and rarely playing with other children.56 In a general population of 6636 Dutch children between the ages of 0 and 18 years, multiple pains were common (endorsed by half of respondents who experienced pain) and also related to higher pain intensity.57
Longitudinal research to study changes in pain, pre- and post-injury, should be prioritized. Mounting evidence suggests that headache post-TBI often involves a continuation or exacerbation of pre-existing headache. Among children who experienced chronic headaches after mild TBI, 47% had a previous history of migraines and 82% either had a pre-existing headache disorder or a family history of migraine.58 It is unknown whether nonheadache pain, or pain in multiple locations, is also likely to have pre-existed the TBI.
Future research with orthopedic injury controls and chronic pain controls (e.g., with no TBI history) will enhance TBI studies. We do not currently know whether individuals that have the same type of pain (e.g., shoulder), but from different injury mechanisms (e.g., TBI vs. orthopedic injury), are impacted equally. Based on ongoing work by the authors, however, the impact of pain may differ in these populations. In a large prospective study of children after mild TBI or orthopedic injury recruited from the emergency department, preliminary analyses showed that higher pain ratings were more strongly related to post-concussive symptoms in children after mild TBI than in children following mild orthopedic injury.59
The study of psychosocial factors is also essential for understanding how acute pain transitions into chronic pain post-TBI. This is supported by findings in childhood TBI showing that PTSD symptoms facilitate continued pain.17 Pain catastrophizing in children and adolescents has been found to be a strong predictor of pain-related disability, over and above characteristics of pain.60 Research has also shown that maternal catastrophizing is significantly related to the child's pain intensity.61 Thus psychosocial factors, including internalizing behaviors and parent's response to their child's pain, may drive the interference and chronicity of symptoms and are therefore an important area for future research in pediatric TBI.
Limitations
Half of our included articles were case studies, which may not necessarily generalize to the wider population. Although the focus of the review is on nonheadache pain, we included studies assessing overall pain, where pain attributed to headaches can be expected to contribute to the rating.
Conclusion
Pain left untreated post-TBI may have negative and long-lasting consequences on pain sensitivity, immune functioning, neurophysiology, attitudes, and healthcare behavior. Findings of this scoping review suggest that nonheadache pain is present in youth post-TBI and is linked to poorer recovery and outcomes. Yet, we found almost no studies that assessed pain comprehensively in terms of intensity, location, frequency, and interference with the child's daily functioning post-TBI. Future research examining how different profiles of pain are related to other outcomes has the potential to improve the care and management of children with TBI.
Appendix
MEDLINE/OvidSP (1946 to present; 667 results)
1. exp brain injuries/
2. brain injur*.mp.
3. exp brain concussion/
4. concuss*.mp.
5. exp post-concussion syndrome/
6. post-concuss*.mp.
7. postconcuss*.mp.
8. exp pain/
9. pain*.mp.
10. exp Headache Disorders/ or exp Headache/ or exp Post-Traumatic Headache/
11. headache*.mp.
12. exp Migraine Disorders/
13. migraine*.mp.
14. exp child/
15. child*.mp.
16. exp adolescent/
17. adolescen*.mp.
18. exp infant/
19. infant*.mp.
20. exp pediatrics/
21. pediatric*.mp.
22. paediatric*.mp.
23. exp child, preschool/
24. preschool*.mp.
25. toddler*.mp.
26. youth*.mp.
27. teen*.mp.
28. TBI*.mp.
29. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 28
30. 8 or 9 or 10 or 11 or 12 or 13
31. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
32. 29 and 30 and 31
33. remove duplicates from 32
34. limit 33 to english language
EMBASE/OvidSP (1974 to June 2016; 1611 vs 8403 prior limit)
1. exp brain injuries/
2. brain injur*.mp.
3. exp brain concussion/
4. concuss*.mp.
5. exp post-concussion syndrome/
6. post-concuss*.mp.
7. postconcuss*.mp.
8. exp pain/
9. pain*.mp.
10. exp Headache Disorders/ or exp Headache/ or exp Post-Traumatic Headache/
11. headache*.mp.
12. exp Migraine Disorders/
13. migraine*.mp.
14. exp child/
15. child*.mp.
16. exp adolescent/
17. adolescen*.mp.
18. exp infant/
19. infant*.mp.
20. exp pediatrics/
21. pediatric*.mp.
22. paediatric*.mp.
23. exp child, preschool/
24. preschool*.mp.
25. toddler*.mp.
26. youth*.mp.
27. teen*.mp.
28. TBI*.mp.
29. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 28
30. 8 or 9 or 10 or 11 or 12 or 13
31. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
32. 29 and 30 and 31
33. remove duplicates from 32
34. limit 33 to english language
PsycINFO/OvidSP (1806 to June 2016; 171 vs 1299)
1. exp traumatic brain injury/
2. traumatic brain injur*.mp.
3. exp brain concussion/
4. concuss*.mp.
5. post-concuss*.mp.
6. postconcuss*.mp.
7. TBI*.mp.
8. exp pain/
9. pain*.mp.
10. exp MIGRAINE HEADACHE/ or exp HEADACHE/
11. headache*.mp.
12. migraine*.mp.
13. child*.mp.
14. adolescen*.mp.
15. infant*.mp.
16. exp pediatrics/
17. pediatric*.mp.
18. paediatric*.mp.
19. exp preschool students/
20. preschool*.mp.
21. toddler*.mp.
22. youth*.mp.
23. teen*.mp.
24. 1 or 2 or 3 or 4 or 5 or 6 or 7
25. 8 or 9 or 10 or 11 or 12
26. 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
27. 24 and 25 and 26
28. remove duplicates from 27
29. limit 28 to english language
CINAHL/EBSCO (1937 to June 2016; 282 vs 1010)
S32 S29 AND S30 AND S31
S31 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27
S30 S8 OR S9 OR S10 OR S11 OR S12 OR S13
S29 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S28
S28 TBI*
S27 teen*
S26 youth*
S25 toddler*
S24 preschool*
S23 (MH “Child, Preschool”)
S22 paediatric*
S21 pediatric*
S20 (MH “Pediatrics+”)
S19 infant*
S18 (MH “Infant+”)
S17 adolescen*
S16 (MH “Adolescence+”)
S15 child*
S14 (MH “Child+”)
S13 migraine*
S12 (MH “Migraine”)
S11 headache*
S10 (MH “Headache+”)
S9 pain*
S8 (MH “Pain+”)
S7 postconcuss*
S6 post-concuss*
S5 (MH “Postconcussion Syndrome”)
S4 concuss*
S3 (MH “Brain Concussion+”)
S2 “brain injur*”
S1 (MH “Brain Injuries+”)
SportDiscus/EBSCO (1985 to June 2016; 89 vs 614)
S39 AND S40 AND S41 Search modes - Find all my search terms
S41 S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37
S40 S26 OR S27 OR S28
S39 S22 OR S23 OR S24 OR S25 OR S38
S38 TBI*
S37 teen*
S36 youth
S35 toddler*
S34 preschool*
S33 paediatric*
S32 pediatric*
S31 infant*
S30 adolescen*
S29 child*
S28 migraine*
S27 headache*
S26 pain*
S25 post-concuss*
S24 postconcuss*
S23 concuss*
S22 “traumatic brain injur*”
S21 S18 AND S19 AND S20
S20 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16
S19 S5 OR S6 OR S7
S18 S1 OR S2 OR S3 OR S4 OR S17
S17 TBI*
S16 teen*
S15 youth
S14 toddler*
S13 preschool*
S12 paediatric*
S11 pediatric*
S10 infant*
S9 adolescen*
S8 child*
S7 migraine*
S6 headache*
S5 pain*
S4 post-concuss*
S3 postconcuss*
S2 concuss*
S1 “traumatic brain injur*”
Cochrane Central Register of Controlled Trials/OvidSP (Dates? 47 vs 121)
1. exp Brain Injuries/
2. exp Brain Concussion/
3. exp Post-Concussion Syndrome/
4. brain injur*.mp.
5. concuss*.mp.
6. postconcuss*.mp.
7. post-concuss*.mp.
8. exp Pain/
9. exp Post-Traumatic Headache/ or exp Headache/ or exp Tension-Type Headache/
10. exp Migraine Disorders/
11. pain*.mp.
12. headache*.mp.
13. migraine*.mp.
14. exp Child/
15. exp Adolescent/
16. exp Infant/
17. exp Pediatrics/
18. exp Child, Preschool/
19. child*.mp.
20. adolescen*.mp.
21. infant*.mp.
22. pediatric*.mp.
23. paediatric*.mp.
24. preschool*.mp.
25. youth.mp.
26. teen*.mp.
27. toddler*.mp.
28. TBI*.mp.
29. 28 or 1 or 2 or 3 or 4 or 5 or 6 or 7
30. 8 or 9 or 10 or 11 or 12 or 13
31. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
32. 29 and 30 and 31
33. remove duplicates from 32
34. limit 33 to english language
Cochrane Database of Systematic Reviews/OvidSP (Dates? 67 vs 118)
1. concuss*.mp.
2. postconcuss*.mp.
3. post-concuss*.mp.
4. pain*.mp.
5. headache*.mp.
6. migraine*.mp.
7. child*.mp.
8. adolescen*.mp.
9. infant*.mp.
10. pediatric*.mp.
11. paediatric*.mp.
12. preschool*.mp.
13. youth.mp.
14. teen*.mp.
15. toddler*.mp.
16. traumatic brain injur*.mp.
17. TBI*.mp.
18. 1 or 2 or 3 or 16 or 17
19. 4 or 5 or 6
20. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
21. 18 and 19 and 20
22. remove duplicates from 21
Acknowledgments
The authors thank Dr. Alix Hayden for her help with developing and adapting the search strategy. V.K. is supported by funding from the University of Calgary Integrated Concussion Research Program and the Alberta Children's Hospital Research Institute. K.O.Y. is supported by the Robert and Irene Ward Chair in Pediatric Brain Injury, funded by the Alberta Children's Hospital Foundation, as well as by a Foundation Grant from the Canadian Institutes of Health Research. M.N. is supported by the Vi Riddell Pediatric Pain Initiative of the Alberta Children's Hospital Research Institute.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Tham S.W., Palermo T.M., Wang J., Jaffe K.M., Temkin N., Durbin D., and Rivara F.P. (2013). Persistent pain in adolescents following traumatic brain injury. J. Pain 14, 1242–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scher A.I., Buse D.C., Fanning K.M., Kelly A.M., Franznick D.A., Adams A.M., and Lipton R.B. (2017). Comorbid pain and migraine chronicity: The Chronic Migraine Epidemiology and Outcomes Study. Neurology 89, 461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nampiaparampil D. (2011). Chronic headache after pediatric brain injury: a systematic review. J. Behav. Brain Sci. 1, 81–86 [Google Scholar]
- 4.Walker L.S., Sherman A.L., Bruehl S., Garber J., and Smith C.A. (2012). Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain 153, 1798–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shelby G.D., Shirkey K.C., Sherman A.L., Beck J.E., Haman K., Shears A.R., Horst S.N., Smith C.A., Garber J., and Walker L.S. (2013). Functional abdominal pain in childhood and long-term vulnerability to anxiety disorders. Pediatrics 132, 475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groenewald C.B., Essner B.S., Wright D., Fesinmeyer M.D., and Palermo T.M. (2014). The economic costs of chronic pain among a cohort of treatment-seeking adolescents in the United States. J. Pain 15, 925–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palermo T.M. (2000). Impact of recurrent and chronic pain on child and family daily functioning: a critical review of the literature. J. Dev. Behav. Pediatr. 21, 58–69 [DOI] [PubMed] [Google Scholar]
- 8.Forgeron P.A., Evans J., McGrath P.J., Stevens B., and Finley G.A. (2013). Living with difference: exploring the social self of adolescents with chronic pain. Pain Res. Manag. 18, e115–e123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker W.C. (2004). Pain pathoetiology after TBI: neural and nonneural mechanisms. J. Head Trauma Rehabil. 19, 72–81 [DOI] [PubMed] [Google Scholar]
- 10.Nampiaparampil D.E. (2008). Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA 300, 711–719 [DOI] [PubMed] [Google Scholar]
- 11.Kim J.H., Ahn S.H., Cho Y.W., Kim S.H., and Jang S.H. (2015). The relation between injury of the spinothalamocortical tract and central pain in chronic patients with mild traumatic brain injury. J. Head Trauma Rehabil. 30, E40–E46 [DOI] [PubMed] [Google Scholar]
- 12.Giza C.C., Mink R.B., and Madikians A. (2007). Pediatric traumatic brain injury: not just little adults. Curr. Opin. Crit. Care 13, 143–152 [DOI] [PubMed] [Google Scholar]
- 13.Carroll L.J., Cassidy J.D., Holm L., Kraus J., and Coronado V.G.; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. (2004). Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 43 Suppl., 113–125 [DOI] [PubMed] [Google Scholar]
- 14.Howick J., Chalmers I., Glasziou P., Greenhalgh T., Heneghan C., Liberati A., Moschetti I., Phillips B., and Thornton H. (2011). The 2011 Oxford CEBM Levels of Evidence (Introductory Document). Oxford Centre for Evidence-Based Medicine: Oxford, UK [Google Scholar]
- 15.Demyttenaere S., Finley G.A., Johnston C.C., and McGrath P.J. (2001). Pain treatment thresholds in children after major surgery. Clin. J. Pain 17, 173–177 [DOI] [PubMed] [Google Scholar]
- 16.Batailler P., Hours M., Maza M., Charnay P., Tardy H., Tournier C., and Javouhey E. (2014). Health status recovery at one year in children injured in a road accident: a cohort study. Accid. Anal. Prev. 71, 267–272 [DOI] [PubMed] [Google Scholar]
- 17.Brown E.A., Kenardy J.A., and Dow B.L. (2014). PTSD perpetuates pain in children with traumatic brain injury. J. Pediatr. Psychol. 39, 512–520 [DOI] [PubMed] [Google Scholar]
- 18.Landgraf J.M., Maunsell E., Speechley K.N., Bullinger M., Campbell S., Abetz L., and Ware J.E. (1998). Canadian-French, German and UK versions of the Child Health Questionnaire: methodology and preliminary item scaling results. Qual. Life Res. 7, 433–445 [DOI] [PubMed] [Google Scholar]
- 19.Nader K., Kriegler J.A., Blake D.D., Pynoos R.S., Newman E., and Weather F.W. (1996). Clinician Administered PTSD Scale, Child and Adolescent Version. National Center for PTSD: White River Junction, VT [Google Scholar]
- 20.Valovich-McLeod T.C., Bay C., and Snyder A.R. (2010). Self-reported history of concussion affects health-related quality of life in adolescent athletes. Athl. Train. Sports Health Care 2, 219–226 [Google Scholar]
- 21.McHorney C.A., Ware J.E., and Raczek A.E. (1993). The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 31, 247–263 [DOI] [PubMed] [Google Scholar]
- 22.Haran H.P., Bressan S., Oakley E., Davis G.A., Anderson V., and Babl F.E. (2016). On-field management and return-to-play in sports-related concussion in children: are children managed appropriately? J. Sci. Med. Sport 19, 194–199 [DOI] [PubMed] [Google Scholar]
- 23.Heyer G.L., Young J.A., Rose S.C., McNally K.A., and Fischer A.N. (2016). Post-traumatic headaches correlate with migraine symptoms in youth with concussion. Cephalalgia 36, 309–316 [DOI] [PubMed] [Google Scholar]
- 24.Necajauskaite O., Endziniene M., and Jurieniene K. (2005). Prevalence, clinical features and accompanying signs of post-traumatic headache in children. Medicina (Kaunas) 41, 100–108 [PubMed] [Google Scholar]
- 25.Echemendia R.J., Meeuwisse W., McCrory P., Davis G.A., Putukian M., Leddy J., Makdissi M., Sullivan S.J., Broglio S.P., Raftery M., Schneider K., Kissick J., McCrea M., Dvorak J., Sills A.K., Aubry M., Engebretsen L., Loosemore M., Fuller G., Kutcher J., Ellenbogen R., Guskiewicz K., Patricios J., and Herring S. (2017). The Sport Concussion Assessment Tool 5th Edition (SCAT5). Br. J. Sports Med. 51, 848–850 [DOI] [PubMed] [Google Scholar]
- 26.Joyce A.S., Labella C.R., Carl R.L., Lai J.S., and Zelko F.A. (2015). The Postconcussion Symptom Scale: utility of a three-factor structure. Med. Sci. Sports Exerc. 47, 1119–1123 [DOI] [PubMed] [Google Scholar]
- 27.Lawler K.A., and Terrigino C.A. (1996). Guidelines for evaluation and education of adult patients with mild traumatic brain injuries in an acute care hospital setting. J. Head Trauma Rehabil. 11, 11 [Google Scholar]
- 28.Ayr L.K., Yeates K.O., Taylor H.G., and Browne M. (2009). Dimensions of postconcussive symptoms in children with mild traumatic brain injuries. J. Int. Neuropsychol. Soc. 15, 19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis M.J., and McDonald P.J. (2015). Coexistent sports-related concussion and cervical SCIWORA in an adolescent: a case report. Curr. Sports Med. Rep. 14, 20–22 [DOI] [PubMed] [Google Scholar]
- 30.Kwon H.G., and Jang S.H. (2014). Delayed gait disturbance due to injury of the corticoreticular pathway in a patient with mild traumatic brain injury. Brain Inj. 28, 511–514 [DOI] [PubMed] [Google Scholar]
- 31.Lim L.W., Molchanov V.I., and Volkodav O.V. (2007). Iatrogenic traumatic brain injury: penetration of Kirschner's knitting needle into the middle cranial cavity. J. Craniofac. Surg. 18, 674–679 [DOI] [PubMed] [Google Scholar]
- 32.Logan S.M., Bell G.W., and Leonard J.C. (2001). Acute subdural hematoma in a high school football player after 2 unreported episodes of head trauma: a case report. J. Athl. Train. 36, 433–436 [PMC free article] [PubMed] [Google Scholar]
- 33.Ofek H., and Defrin R. (2007). The characteristics of chronic central pain after traumatic brain injury. Pain 131, 330–340 [DOI] [PubMed] [Google Scholar]
- 34.Roden-Foreman K., Solis J., Jones A., Bennett M., Roden-Foreman J.W., Rainey E.E., Foreman M.L., and Warren A.M. (2017). Prospective evaluation of posttraumatic stress disorder and depression in orthopaedic injury patients with and without concomitant traumatic brain injury. J. Orthop. Trauma 31, e275–e280 [DOI] [PubMed] [Google Scholar]
- 35.Uhl R.L., Rosenbaum A.J., Czajka C., Mulligan M., and King C. (2013). Minor traumatic brain injury: a primer for the orthopaedic surgeon. J. Am. Acad. Orthop. Surg. 21, 624–631 [DOI] [PubMed] [Google Scholar]
- 36.Read K.M., Kufera J.A., Dischinger P.C., Kerns T.J., Ho S.M., Burgess A.R., and Burch C.A. (2004). Life-altering outcomes after lower extremity injury sustained in motor vehicle crashes. J. Trauma 57, 815–823 [DOI] [PubMed] [Google Scholar]
- 37.Pardini D., Stump J., Lovell M.R., Collins M.W., and Moritz K.F. (2004). The post-concussion symptom scale (PCSS): a factor analysis. Br. J. Sports Med. 38, 1114751937 [Google Scholar]
- 38.Gioia G., Janusz J., Isquith P., and Vincent D. (2008). Psychometric properties of the parent and teacher Post-Concussion Symptom Inventory (PCSI) for children and adolescents. J. Int. Neuropsychol. Soc. 14, 118078527 [Google Scholar]
- 39.Andersson H.I., Ejlertsson G., Leden I., and Rosenberg C. (1996). Characteristics of subjects with chronic pain, in relation to local and widespread pain report. A prospective study of symptoms, clinical findings and blood tests in subgroups of a geographically defined population. Scand. J. Rheumatol. 25, 146–154 [DOI] [PubMed] [Google Scholar]
- 40.Hicks C.L., von Baeyer C.L., Spafford P.A., van Korlaar I., and Goodenough B. (2001). The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain 93, 173–183 [DOI] [PubMed] [Google Scholar]
- 41.von Baeyer C.L., Jaaniste T., Vo H.L.T., Brunsdon G., Lao H.C. and Champion G.D. (2017). Systematic review of self-report measures of pain intensity in 3- and 4-year-old children: bridging a period of rapid cognitive development. J. Pain 18, 1017–1026 [DOI] [PubMed] [Google Scholar]
- 42.Chambers C.T., Giesbrecht K., Craig K.D., Bennett S.M., and Huntsman E. (1999). A comparison of faces scales for the measurement of pediatric pain: children's and parents' ratings. Pain 83, 25–35 [DOI] [PubMed] [Google Scholar]
- 43.Hunfeld J.A., Perquin C.W., Duivenvoorden H.J., Hazebroek-Kampschreur A.A., Passchier J., van Suijlekom-Smit L.W., and van der Wouden J.C. (2001). Chronic pain and its impact on quality of life in adolescents and their families. J. Pediatr. Psychol. 26, 145–153 [DOI] [PubMed] [Google Scholar]
- 44.Richardson G.M., McGrath P.J., Cunningham S.J., and Humphreys P. (1983). Validity of the headache diary for children. Headache 23, 184–187 [DOI] [PubMed] [Google Scholar]
- 45.Palermo T.M., Valenzuela D., and Stork P.P. (2004). A randomized trial of electronic versus paper pain diaries in children: impact on compliance, accuracy, and acceptability. Pain 107, 213–219 [DOI] [PubMed] [Google Scholar]
- 46.Abu-Saad H.H., Kroonen E., and Halfens R. (1990). On the development of a multidimensional Dutch pain assessment tool for children. Pain 43, 249–256 [DOI] [PubMed] [Google Scholar]
- 47.Varni J.W., Thompson K.L., and Hanson V. (1987). The Varni/Thompson Pediatric Pain Questionnaire. I. Chronic musculoskeletal pain in juvenile rheumatoid arthritis. Pain 28, 27–38 [DOI] [PubMed] [Google Scholar]
- 48.Cohen L.L., Lemanek K., Blount R.L., Dahlquist L.M., Lim C.S., Palermo T.M., McKenna K.D., and Weiss K.E. (2008). Evidence-based assessment of pediatric pain. J. Pediatr. Psychol. 33, 939–955; discussion, 956–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varni J.W., Stucky B.D., Thissen D., Dewitt E.M., Irwin D.E., Lai J.S., Yeatts K., and Dewalt D.A. (2010). PROMIS Pediatric Pain Interference Scale: an item response theory analysis of the pediatric pain item bank. J. Pain 11, 1109–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Baeyer C.L. (2009). Children's self-report of pain intensity: what we know, where we are headed. Pain Res. Manag. 14, 39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stinson J.N., Kavanagh T., Yamada J., Gill N., and Stevens B. (2006). Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain 125, 143–157 [DOI] [PubMed] [Google Scholar]
- 52.Chambers C.T., and Craig K.D. (1998). An intrusive impact of anchors in children's faces pain scales. Pain 78, 27–37 [DOI] [PubMed] [Google Scholar]
- 53.Malviya S., Voepel-Lewis T., Burke C., Merkel S., and Tait A.R. (2006). The revised FLACC observational pain tool: improved reliability and validity for pain assessment in children with cognitive impairment. Paediatr. Anaesth. 16, 258–265 [DOI] [PubMed] [Google Scholar]
- 54.von Baeyer C.L. (2006). Children's self-reports of pain intensity: scale selection, limitations and interpretation. Pain Res. Manag. 11, 157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Baeyer C.L., and Spagrud L.J. (2007). Systematic review of observational (behavioral) measures of pain for children and adolescents aged 3 to 18 years. Pain 127, 140–150 [DOI] [PubMed] [Google Scholar]
- 56.Bakoula C., Kapi A., Veltsista A., Kavadias G., and Kolaitis G. (2006). Prevalence of recurrent complaints of pain among Greek schoolchildren and associated factors: a population-based study. Acta Paediatr. 95, 947–951 [DOI] [PubMed] [Google Scholar]
- 57.Perquin C.W., Hazebroek-Kampschreur A.A., Hunfeld J.A., Bohnen A.M., van Suijlekom-Smit L.W., Passchier J., and van der Wouden J.C. (2000). Pain in children and adolescents: a common experience. Pain 87, 51–58 [DOI] [PubMed] [Google Scholar]
- 58.Kuczynski A., Crawford S., Bodell L., Dewey D., and Barlow K.M. (2013). Characteristics of post-traumatic headaches in children following mild traumatic brain injury and their response to treatment: a prospective cohort. Dev. Med. Child Neurol. 55, 636–641 [DOI] [PubMed] [Google Scholar]
- 59.Kwan V., Kowalski K., Noel M., Taylor G., Bigler E., Cohen D., Mihalov L., Zumberge N., Bacevice A., Bangert B., and Yeates K. Pain in Children Following Mild Traumatic Brain Injury: A Longitudinal Analysis in Association with Post-Concussive Symptoms (unpublished master's thesis). The University of Calgary: Calgary, Alberta: Canada [Google Scholar]
- 60.Asmundson G.J., Noel M., Petter M., and Parkerson H.A. (2012). Pediatric fear-avoidance model of chronic pain: foundation, application and future directions. Pain Res. Manag. 17, 397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hechler T., Vervoort T., Hamann M., Tietze A.L., Vocks S., Goubert L., Hermann C., Wager J., Blankenburg M., Schroeder S., and Zernikow B. (2011). Parental catastrophizing about their child's chronic pain: are mothers and fathers different? Eur. J. Pain 15, 515..e1–e9. [DOI] [PubMed] [Google Scholar]